Abstract
Schistosomiasis control programs aim to reduce morbidity but are evaluated by infection prevalence and intensity reduction. We present baseline cross-sectional data from a nested cohort study comparing indicators of morbidity for measuring program impact. Eight hundred twenty-two schoolchildren 7–8 years of age from Nyanza Province, Kenya, contributed stool for diagnosis of Schistosoma mansoni and soil-transmitted helminths (STH) and blood smears for malaria, and were evaluated for anemia, quality of life, exercise tolerance, anthropometry, and ultrasound abnormalities. Schistosoma mansoni, STH, and malaria infection prevalence were 69%, 25%, and 8%, respectively. Only anemia and S. mansoni infection (adjusted odds ratio [aOR] = 1.70; confidence interval [CI] = 1.03–2.80), and hepatomegaly and heavy S. mansoni infection (aOR = 2.21; CI = 1.19–4.11) were associated. Though anemia and hepatomegaly appeared most useful at baseline, additional morbidity indicators may be sensitive longitudinal measures to evaluate schistosomiasis program health impact.
Introduction
Human schistosomiasis is a disease caused by any of five species of trematode flatworms, or blood flukes, in the genus Schistosoma. The World Health Organization (WHO) estimates that over 237 million persons required treatment of schistosomiasis in 20101 with estimates of up to an additional 779 million at-risk globally.2 Other authors estimate that the burden of schistosomiasis resulting from either current or former infections affects upward of 587 million persons.3 Unlike diseases such as human immunodeficiency virus (HIV) and malaria where mortality is high, schistosomiasis burden is described primarily in terms of morbidity and historically has been underreported. Recently, King and others3 attempted to present a more realistic estimate of the burden by recalculating the prevalence, time-preference discounting, and weight of disability. They estimated that schistosome infections accounted for 24–56 million disability-adjusted life years (DALYs) lost in 2010. For perspective, in the most recent WHO/World Bank Global Burden of Disease (GBD) program data from 2004 it was estimated that 34 million DALYs were lost to malaria and 58.5 million to HIV/acquired immunodeficiency syndrome (AIDS).4
In endemic areas, initial infection is acquired at a young age. Verani and others5 found that 14% of 1-year-old children along the Kenyan shores of Lake Victoria were Schistosoma mansoni positive, whereas Odogwu and others6 found a prevalence of 47.4% in children < 3 years of age around Lake Victoria in Uganda. The prevalence of infection in school-aged children in these areas can be as high as 86–90%7; in the absence of treatment, infection can persist for years. Even for those who do receive treatment, reinfection is likely.8
In children, schistosomiasis typically presents with generalized, non-specific signs and symptoms, making it difficult to identify disease-specific morbidity indicators and challenging to develop tools for assessing those indicators. Over time, morbidity may progress from subtle manifestations such as anemia, to more severe, debilitating, and irreversible conditions such as growth stunting, impaired cognitive development, increased susceptibility to co-infection, decreased quality of life, exercise intolerance, infertility, portal hypertension, and liver failure.9–15 The key determinants of morbidity progression are repeated infection, intensity of infection, and duration of infection.16 Currently, the WHO strategy for schistosomiasis control relies on mass-drug administration (MDA) of praziquantel,17 which is effective in reducing disease-associated morbidity.18–22 The impact of MDA is measured by a change in infection prevalence and intensity. Guidelines exist for schistosomiasis MDA thresholds, frequency, and target populations (school-based versus community-wide), but there are few objective data supporting them. As the number of operating control programs and people being treated continue to increase, the need for evidence-based guidelines is becoming more important.1
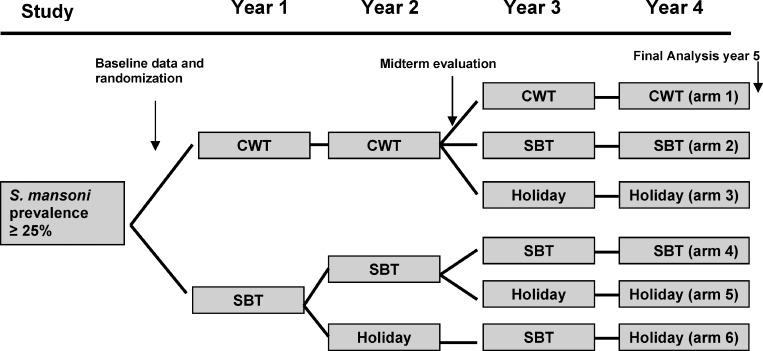
The purpose of the Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) is to provide answers to key questions about the control and prevention of schistosomiasis. One of the SCORE projects, “Comparison of School and Community-based Mass Drug Administration Delivery Strategies for Control of S. mansoni Infections in Western Kenyan in Areas with ≥ 25% Prevalence,” is a 5-year longitudinal study designed to evaluate different combinations and frequencies of school-based (SBT) and community-wide (CWT) MDA treatment strategies (Figure 1). The primary objective of this study is to determine the impact of these strategies on infection prevalence and intensity to inform programmatic decision making. However, the impact on health is also important when evaluating programs. We are conducting a 5-year nested cohort study within the parent SCORE study to evaluate the impact of treatment on morbidity reduction. To date, we have collected the baseline cross-sectional data and performed an initial evaluation of the morbidity indicators and tools.
Figure 1.
Parent Schistosomiasis Consortium for Operational Research and Evaluation (SCORE) study design. Communities were randomized into six intervention arms. CWT = community-wide treatment; SBT = school-based treatment. Arms are sequentially numbered on the right side of the figure with Arm 1 being the top arm, and Arm 6 being the bottom arm.
Methods
Ethics statement and eligibility criteria.
Ethical clearance was obtained from the Departmental and Institutional Scientific Steering Committees of KEMRI followed by the National/KEMRI Ethical Clearance Committee. The Institutional Review Boards of both the Centers for Disease Control and Prevention (CDC) and the University of Georgia also reviewed the study protocol and deferred to the KEMRI Ethical Clearance Committee. Children who were 7–8 years of age, assented to participate, and had parental or guardian consent were eligible for inclusion. Children with obvious physical disabilities that could affect their participation in some aspect of the study (e.g., the shuttle run) were excluded.
Study area and population.
This cross-sectional study was conducted in schools within 5 km of Lake Victoria in Nyanza Province of Kenya where prior studies had identified a high prevalence of schistosomiasis and STH infections.5,23 Though MDAs for STHs have been carried out in the area, no prior MDAs for schistosomiasis had been performed. As part of the larger SCORE project, 300 schools were evaluated for eligibility. A single stool was examined (two slides per stool) by the Kato-Katz fecal thick smear technique in 50 children 13–14 years of age in each school. If at least 13 children in the school were positive for S. mansoni eggs, a prevalence of > 25%, the community was eligible for entrance into the parent study. One hundred fifty communities were eligible and randomly selected into one of six different treatment arms (Figure 1) of the parent SCORE project.
From the parent SCORE study, we selected 10 communities from the study arm with the highest drug pressure, 4 years of community-wide treatment (Figure 1, Arm 1), and 10 communities from the study arm with the lowest drug pressure, two years of school-based treatment interrupted by holiday (Figure 1, Arm 6), based on proximity to our laboratory and community size. From these 20 communities, we intended to randomly select four schools from each arm and enroll 100 children from each community school for a total of 800 children.
Stool examination.
Children were given stool containers and asked to provide fresh stool samples on three consecutive days. Samples were transported to the CDC/KEMRI laboratory where they were processed and examined by the Kato-Katz technique for detection of parasite eggs, two slides per stool. The presence of S. mansoni, Ascaris lumbricoides, Trichuris trichiura, and hookworm eggs was recorded. Egg counts were quantified for S. mansoni only. The arithmetic mean of egg counts was calculated from the total slides per child, and expressed as eggs per gram (epg). Infections were categorized by intensity according to the WHO guidelines as light (1–99 epg), moderate (100–399 epg), or heavy (≥ 400 epg).24 Each stool evaluation was performed blinded to prior stool results, and all morbidity testing was performed with the operator blinded to participant infection status.
Blood collection and processing.
A 5 mL venous blood sample was collected from each enrolled individual. Hemoglobin concentration was calculated with HemoCue (Ängelholm, Sweden) in g/dL, and anemia status was categorized according to established country-specific cutoffs adjusted by age as normal (≥ 11.2), mild (8.2 to < 11.2), moderate (5.2 to < 8.2), and severe (< 5.2).25 Category cutoffs were adjusted for altitude. Plasmodium falciparum parasitemia was determined by examination of blood smears by experienced microscopists.
Anthropometric measurements.
Height was measured with a locally made stadiometer. Children were asked to remove shoes and dressing on their heads. They were then asked to stand on the base of the stadiometer with both feet together, and their head in contact with the vertical board. After proper positioning, the child was asked to stand as tall as possible and take a deep breath. A ruler was then place on their head, and precise height was measured in centimeters. Weight was measured with a scale tared each morning or at every school visited, whichever was more frequent. Children were asked to remove shoes and any excess clothing and stand on the center of the scale. Examiners recorded the results in kilograms with one decimal place. Two readings for height and weight were taken for each child, and the mean of these results was calculated. Data were entered into the WHO Anthro (version 3.2.2, January 2011) software, and Z-scores were calculated.26 Wasting was defined as a BMI-for-age Z-score of < −2, and stunting was defined as a height-for-age Z-score of < −2.
Exercise testing.
Exercise tolerance was tested using the multistage 20 m shuttle run, originally described and validated in a cohort of Canadian schoolchildren27; it is used as a measure of maximal aerobic capacity by correlating the level achieved on running to a maximal oxygen-uptake, or VO2 max. Children were organized into groups of five and asked to repeatedly run 20 m at increasing speeds designated by an audio recording. When they were no longer able to continue at the rate set by the recording, they were asked to stop. This final level achieved was recorded and correlated to a VO2 max.
Ultrasonographic evaluations.
We used an SSD-500 portable ultrasound machine (Aloka, Tokyo, Japan) with a 3.5 MHz convex probe to evaluate study participants for hepatosplenic and portosystemic morbidity according to the Niamey protocol.28 Examinations were performed by a senior radiographer (E.I.) with extensive experience in field ultrasonography of persons with schistosomiasis. Liver texture patterns graded B–F were considered abnormal. Hepatomegaly (HM), splenomegaly (SM), portal branch thickening (PBT), and increased portal vein diameter (PVD) were defined as values 2 SDs above a reference Senegalese population, adjusted for age and height.
Quality of life evaluations.
Quality of life evaluations were performed using the validated Pediatric Quality of Life Inventory PedsQL instrument for children, version 5 (MAPI Research Trust, Lyon, France). Participants were individually asked questions in four dimensions of functioning: physical, emotional, social, and school. The answers were scored and then linearly transformed to a 0–100 continuous scale. Mean scores with 95% confidence intervals (CIs) were calculated for the study sample, and compared between children with and without S. mansoni infection. Predictor estimates were calculated for multivariable analyses.
Data handling and analysis.
Demographic data were collected on smartphones and uploaded to a dedicated database maintained on a central server (EpiCollect). Laboratory and morbidity data were collected on paper forms and entered by a secure web-based portal into the same database. Data were analyzed using SAS version 9.3 (SAS Institute Inc., Cary, NC). All tests and confidence intervals used the 5% level of significance. Univariable and multivariable models were created with S. mansoni as the exposure of interest. Logistic and linear regression was performed for dichotomous and continuous outcomes, respectively, using statistical methods that accounted for the sampling strategy. Standard errors were adjusted for stratified cluster sampling in uni- and multivariable models using Taylor series approximations.29 Tests for interactions were performed. Univariable results are expressed as proportions or means with associated P values or confidence limits, and multivariable results as adjusted odds ratios (aOR) or estimates with 95% CIs. Two multivariable models were created for each of the morbidity outcomes (anemia, stunting, wasting, shuttle run score, PedsQL, and ultrasound results [liver texture pattern, HM, SM, PBT, and PVD]), one with S. mansoni infection as a categorical value with A. lumbricoides, T. trichiura, and hookworm evaluated individually and the other with S. mansoni and STHs collapsed into binary variables. Predictors for each multivariable model were chosen based on expert opinion and model fit statistics.30 Sensitivity analyses were performed to determine if bias was introduced when excluding participants with any missing data. Multiple imputation, a recommended approach for sensitivity analysis,31 was used to check for changes in statistical inference when all participants with four or more S. mansoni slides were included.
Results
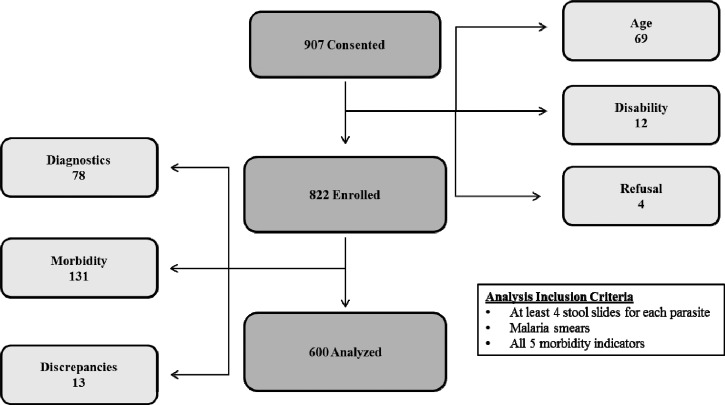
Data were collected in the dry season between February and April of 2011. We intended to enroll 800 children from a total of eight schools, but because of a lower enrollment than expected, we randomly selected two additional schools per study arm. A total of 907 children from 12 communities were consented. Of these, 69 children were excluded for age ineligibility, 12 were excluded for having a physical disability unrelated to schistosomiasis, and 4 withdrew after initially assenting (Figure 2). The remaining 822 children were enrolled in the study. For the purpose of our analyses, we only included children who had at least four stool results from a total of six possible slides per parasite (S. mansoni and the STHs), a thick and thin blood smear for P. falciparum infection, and results from each of the five morbidity tests. Of the 822 children, a total of 222 were excluded because of missing data or data entry discrepancies that we could not resolve (Figure 2). Our final analysis included 600 children.
Figure 2.
Numbers of children consented, enrolled, and included in the analysis. Light gray boxes to the right and left of the central pathway represent children excluded from the project and the reason for exclusion. The box in the bottom right of the figure indicates the inclusion criteria for the analysis.
Study sample characteristics.
Fifty-one percent of the population was female, and 47% were 7 years of age. Prevalence of S. mansoni was 69% with 33%, 23%, and 12% of the population having light, moderate, and heavy infections, respectively. Twenty-five percent of the population had at least one STH with 15%, 10%, and 5% having Trichuris, Ascaris, and hookworm infections, respectively; 8% of the participants were infected with P. falciparum (Table 1). Thirty-six percent of the children were anemic with 26% mild, 9% moderate, and 1% severe anemia. Though only 10% of the children were stunted, 26% were wasted. On ultrasound, 67% of the children had HM, 57% had SM, 34% had PBT, 14% had an increased PVD, 25% had liver pattern B, and < 1% (three children) had liver texture pattern C; no child had a liver texture pattern more advanced than C (Table 1).
Table 1.
Study sample characteristics
| N = 600 | ||
|---|---|---|
| Characteristics | n | % |
| S. mansoni | 411 | 69% |
| Infection intensity* | ||
| Light | 199 | 33% |
| Moderate | 138 | 23% |
| Heavy | 74 | 12% |
| Any STH | 151 | 25% |
| T. trichiura | 89 | 15% |
| A. lumbricoides | 60 | 10% |
| Hookworm | 32 | 5% |
| Malaria | 46 | 8% |
| Anemia | 216 | 36% |
| Degree of anemia† | ||
| Mild | 155 | 26% |
| Moderate | 54 | 9% |
| Severe | 6 | 1% |
| Anthropometry | ||
| Stunted | 59 | 10% |
| Wasted | 157 | 26% |
| Ultrasound findings | ||
| Pattern A | 450 | 75% |
| Pattern B | 147 | 25% |
| Pattern ≥ C | 3 | < 1% |
| HM | 400 | 67% |
| SM | 342 | 57% |
| PBT | 204 | 34% |
| PVD | 82 | 14% |
| Mean | ||
| Shuttle run‡ | 47.5 | |
| PedsQL‡ | 85.7 | |
Light = 1–99 eggs per gram (epg); moderate = 100–399 epg; Heavy = ≥ 400 epg.
Mild = 8.2 to < 11.2 g/dL; moderate = 5.2 to < 8.2 g/dL; severe = < 5.2 g/dL.
Shuttle run and PedsQL scores are continuous values expressed as means.
STH = soil-transmitted helminths; hepatomegaly; SM = splenomegaly; PBT = portal branch thickening; PVD = portal vein diameter.
Univariable relationships with S. mansoni infection.
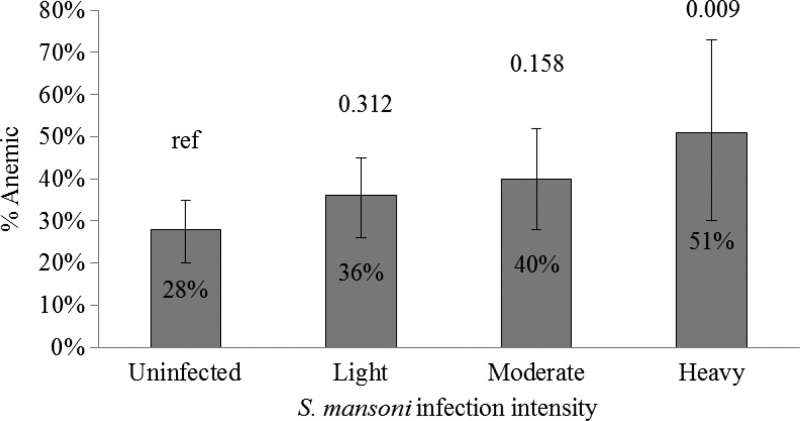
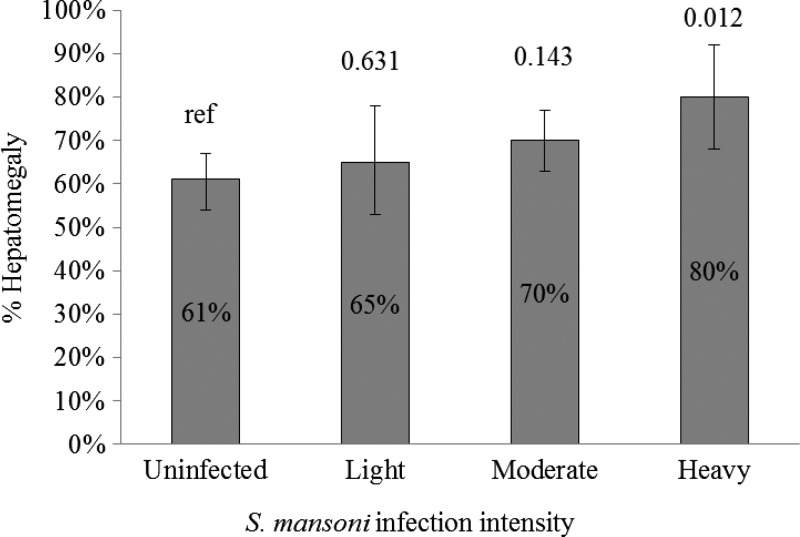
Children infected with S. mansoni were more likely to be infected with P. falciparum (9%) than those not infected with S. mansoni (5%, P = 0.003). As a group, infection with any STH (28% versus 20%, P = 0.16) was not associated with being positive for schistosomiasis. Ascaris (12% versus 6%, P = 0.006) and Trichuris (18% versus 9%, P = 0.03) infections were more common, whereas hookworm (4% versus 9%, P = 0.01) infections were less common in children with schistosomiasis. Anemia was more common in children with S. mansoni infection (P = 0.02), including moderate and severe anemia, and the percent anemic increased with intensity of S. mansoni infection stratum, becoming significant in the heavy intensity stratum (P = 0.009) (Table 2, Figure 3 ). Children with liver texture pattern B were less likely to be infected with S. mansoni (22% versus 30%), though this did not reach statistical significance (P = 0.08). Because of the small number of children with liver texture pattern C, we collapsed the outcomes of liver texture patterns B and C in our models. Worth noting is that all three of the children with liver texture pattern C were infected with S. mansoni. A large percentage of children with schistosomiasis had hepatomegaly (69%), but the underlying prevalence of hepatomegaly in children without schistosomiasis was almost as high (61%). This association was not statistically significant (P = 0.06). When stratified by infection intensity, the prevalence of hepatomegaly increased in children with increasing intensity of S. mansoni infection, becoming statistically significant in heavily infected children (P = 0.01) (Figure 4 ).
Table 2.
Univariable associations with S. mansoni infection
| S. mansoni (+) | S. mansoni (−) | P value | |||
|---|---|---|---|---|---|
| N = 411 | N = 189 | ||||
| n | % | n | % | ||
| Malaria | 36 | 9% | 10 | 5% | 0.003 |
| Any STH | 114 | 28% | 37 | 20% | 0.160 |
| A. lumbricoides | 48 | 12% | 12 | 6% | 0.006 |
| T. trichiura | 72 | 18% | 17 | 9% | 0.030 |
| Hookworm | 15 | 4% | 17 | 9% | 0.010 |
| Anemia* | 164 | 40% | 52 | 28% | 0.020 |
| Normal Hb | 247 | 60% | 137 | 72% | Ref |
| Mild | 114 | 28% | 41 | 22% | 0.140 |
| Moderate | 44 | 11% | 11 | 6% | 0.000 |
| Severe | 6 | 1% | 0 | 0% | N/A |
| Antropometry | |||||
| Wasting | 111 | 27% | 46 | 24% | 0.668 |
| Stunting | 40 | 10% | 19 | 10% | 0.869 |
| Ultrasound findings† | |||||
| Pattern A | 317 | 77% | 133 | 70% | Ref |
| Patter B | 91 | 22% | 56 | 30% | 0.080 |
| Pattern ≥ C | 3 | 1% | 0 | 0% | N/A |
| HM | 284 | 69% | 116 | 61% | 0.060 |
| SM | 236 | 57% | 106 | 56% | 0.875 |
| PBT | 141 | 34% | 63 | 33% | 0.800 |
| Increased PVD | 57 | 14% | 26 | 14% | 0.908 |
| Mean | 95% CI | Mean | 95% CI | ||
| Shuttle run | 47.5 | (47.3–47.7) | 47.5 | (47.2–47.8) | 0.851 |
| PedsQL | 85.7 | (85.3–86.1) | 85.8 | (85.1–86.5) | 0.924 |
Normal ≥ 11.2 g/dL; Mild = 8.2 to < 11.2 g/dL; moderate = 5.2 to < 8.2 g/dL; severe = < 5.2 g/dL.
Pattern A, Pattern B, and Pattern C refers to liver texture pattern.
STH = soil-transmitted helminths; HM = hepatomegaly; SM = splenomegaly; PBT = portal branch thickening PVD = portal vein diameter. All were defined as values > 2 standard deviations above a reference Senegalese population, adjusted for heights and age.
Shuttle run and PedsQL scores are continuous values expressed as means.
Figure 3.
Proportion of children with anemia by intensity of Schistosoma mansoni infection. Gray bars represent the percent of children with anemia stratified by infection intensity. Whiskers above the bars represent 95% confidence intervals around the percent anemic. Numbers above the whiskers represent P values comparing the stratum value to the uninfected population.
Figure 4.
Proportion of children with hepatomegaly by intensity of Schistosoma mansoni infection. Gray bars represent the percent of children with hepatomegaly stratified by infection intensity. Whiskers above the bars represent 95% confidence intervals around the percent with hepatomegaly. Numbers above the whiskers represent P values comparing the stratum value to the uninfected population.
We did not find statistically significant associations between schistosomiasis and age, gender, stunting, wasting, shuttle run outcome, PedsQL score, SM, PBT, PVD, hookworm, or Trichuris infection (Table 3).
Table 3.
Predictors of anemia from multivariable-adjusted logistic model accounting for clustering
| Predictors | aOR | 95% CI |
|---|---|---|
| S. mansoni uninfected* | Ref | Ref |
| Light | 1.31 | (0.77–2.23) |
| Moderate | 1.59 | (0.84–3.01) |
| Heavy | 2.47 | (1.25–4.88) |
| Malaria | 1.81 | (1.26–2.60) |
| A. lumbricoides | 1.52 | (0.99–2.35) |
| T. trichiura | 1.44 | (0.97–2.12) |
| Hookworm | 0.57 | (0.29–1.11) |
| Age | 1.32 | (0.997–1.74) |
| Female | 0.84 | (0.70–1.02) |
| S. mansoni infection | 1.7 | (1.03–2.80) |
| Any STH | 1.07 | (0.74–1.54) |
Light = 1–99 eggs per gram (epg); moderate = 100–399 epg; Heavy = ≥ 400 epg.
Schistosoma mansoni infection and any soil-transmitted helminths (STH) infection are results from a model with these variables collapsed into binary outcomes.
Multivariable models.
When controlling for other predictors, anemia was associated with P. falciparum infection (adjusted odds ratio [aOR] = 1.81; 95% CI = 1.26–2.60). We found a dose-response relationship between anemia and increasing intensities of S. mansoni infection that, like in the univariable model, became statistically significant in heavy intensity infections (aOR = 2.47; 95% CI = 1.25–4.88). As a binary predictor, the relationship between anemia and S. mansoni infection (aOR = 1.70; 95% CI = 1.03–2.80) remained statistically significant when controlling for P. falciparum infection (Table 3).
When controlling for other variables on abdominal ultrasonography, the only outcomes associated with schistosomiasis were hepatomegaly and liver texture pattern B. We found a trend between hepatomegaly and increasing intensities of S. mansoni infection that became statistically significant in high intensity infections (aOR = 2.21; 95% CI = 1.19–4.11). When collapsed into a binary predictor, the relationship between hepatomegaly and S. mansoni infection was no longer statistically significant (aOR = 1.34; 95% CI = 0.90–2.00). Hepatomegaly was also associated with wasting (aOR = 0.66; 95% CI; 0.47–0.92) and stunting (aOR = 2.10; 95% CI = 1.30–3.40) (Table 4). Likewise, there appeared to be a dose-response effect such that children with increasing intensities of S. mansoni infection were less likely to have a liver texture pattern B, reaching significance in heavy intensity infections (aOR 0.59; 95% CI = 0.29–0.999). Stunting was negatively associated (aOR = 0.56; 95% CI = 0.33–0.96) while female gender (aOR = 2.02; 95% CI = 1.44–2.83) was positively associated with liver texture pattern B. In the multivariable model with S. mansoni and STH infections collapsed into binary outcomes, S. mansoni (aOR = 0.75; 95% CI = 0.57–0.997) and any STH (aOR = 0.55; 95% CI = 0.36–0.84) infection were negatively associated with liver texture pattern B (Table 5). We did not find statistically significant associations between PBT, PVD, or SM and S. mansoni infection. Furthermore, we did not find statistically significant associations between our anthropometric measurements (stunting and wasting), shuttle run or PedsQL outcomes and S. mansoni infection in the multivariable model.
Table 4.
Predictors of hepatomegaly from multivariable-adjusted logistic model accounting for clustering
| Predictors | aOR | 95% CI |
|---|---|---|
| S. mansoni uninfected* | Ref | Ref |
| Light | 1.14 | (0.67–1.95) |
| Moderate | 1.36 | (0.90–2.04) |
| Heavy | 2.21 | (1.19–4.11) |
| Malaria | 1.23 | (0.71–2.11) |
| A. lumbricoides | 0.81 | (0.47–1.39) |
| T. trichiura | 1.11 | (0.56–2.20) |
| Hookworm | 0.68 | (0.38–1.22) |
| Age | 1.2 | (0.93–1.53) |
| Normal Hb† | Ref | Ref |
| Mild | 1.34 | (0.87–2.07) |
| Moderate/Severe | 1.16 | (0.58–2.31) |
| Wasting | 0.66 | (0.47–0.92) |
| Stunting | 2.1 | (1.30–3.40) |
| Female | 0.73 | (0.52–1.03) |
| S. mansoni infection | 1.38 | (0.95–1.99) |
| Any STH | 0.92 | (0.58–1.47) |
Light = 1–99 eggs per gram (epg); moderate = 100–399 epg; Heavy = ≥ 400 epg.
Normal ≥ 11.2 g/dL; Mild = 8.2 to < 11.2 g/dL; moderate = 5.2 to < 8.2 g/dL; severe = < 5.2 g/dL.
Schistosoma mansoni infection and any soil-transmitted helminths (STH) infection are results from a model with these variables collapsed into binary outcomes.
Table 5.
Predictors of liver texture pattern > A from multivariable-adjusted logistic model accounting for clustering
| Predictors | aOR | 95% CI |
|---|---|---|
| S. mansoni uninfected* | Ref | Ref |
| Light | 0.77 | (0.51–1.17) |
| Moderate | 0.72 | (0.37–1.39) |
| Heavy | 0.59 | (0.29–0.999) |
| Malaria | 1.31 | (0.62–2.77) |
| A. lumbricoides | 0.49 | (0.21–1.13) |
| T. trichiura | 0.97 | (0.54–1.74) |
| Hookworm | 0.57 | (0.23–1.45) |
| Age | 1.02 | (0.66–1.58) |
| Normal Hb† | ||
| Mild | 1.05 | (0.78–1.41) |
| Moderate/Severe | 1.04 | (0.57–1.90) |
| Wasting | 1.03 | (0.63–1.70) |
| Stunting | 0.56 | (0.33–0.96) |
| Female | 2.02 | (1.44–2.83) |
| S. mansoni infection | 0.75 | (0.57–0.997) |
| Any STH | 0.55 | (0.36–0.84) |
Light = 1–99 eggs per gram (epg); moderate = 100–399 epg; Heavy = ≥ 400 epg.
Normal ≥ 11.2 g/dL; Mild = 8.2 to < 11.2 g/dL; moderate = 5.2 to < 8.2 g/dL; severe = < 5.2 g/dL.
Schistosoma mansoni infection and any soil-transmitted helminths (STH) infection are results from a model with these variables collapsed into binary outcomes.
Sensitivity analyses and multiple imputation.
We compared the data from the population of 600 children that we included in our analyses to the total population of 720 children who provided at least four slides for S. mansoni but were missing at least one morbidity measure to assess for introduced bias by analytic exclusion. Statistically significant differences between the populations of 600 children and 720 children were found for S. mansoni infection (68.5% versus 55.8%, P = 0.007), and the outcomes of liver pattern B (24.5% versus 14.5%, P = 0.007), and wasting (26.2% versus 41.6%, P = 0.0005). We found a change in statistical inference for the outcome of liver texture pattern B such that the associations with S. mansoni infection as a categorical or binary variable, stunting, and female gender were no longer significant. Statistical inference did not change in any other model.
Discussion
The objectives of our study were to describe the baseline morbidity in our cohort, and to evaluate morbidity indicators and tools for their use in assessing program impact. Direct measurement of schistosomiasis morbidity reduction associated with MDA implementation is challenging. School-aged children are the population targeted by control programs and treatment is to prevent or reduce morbidity, with special emphasis on averting irreversible disease consequences. Morbidity is frequently unapparent in this population, and so morbidity proxies, or indicators, are used. These indicators should be directly associated with the disease and morbidity outcome such that by treating the disease, the value of the indicator will change in a measurable way that signifies that morbidity has been decreased or prevented. Indicators should be specific to the disease such that the subsequent change in the indicator value is a function of treating the disease and not some unrelated, unmeasured factor. Furthermore, sensitive tools that are technically and logistically facile for detecting the indicators must be field applicable. The results of our study provide a baseline assessment of morbidity indicators and tools for schistosomiasis in young school children.
Parasitologic findings.
Schistosoma mansoni and STH prevalence in our study sample were high and comparable to levels found in other studies of similarly aged children in areas where praziquantel MDA has not been implemented5,6,23; although infection intensity is not a direct indicator of morbidity, its consistent association with disease consequences and easily quantified outcomes maintain its use in monitoring program impact.
Malaria prevalence was lower than other studies in this region and may partially be explained by timing of the study outside of the seasonal peaks.5,32 The positive association between S. mansoni and P. falciparum infections has been found in many studies5,33,34 and may be driven by immunologic factors.35–37
Anemia.
The high prevalence of anemia we observed was expected in a region endemic for schistosomiasis, intestinal parasitosis, and malaria. Neither Trichuris nor hookworm infections were independently associated with anemia, however S. mansoni infection was associated with anemia, consistent with other studies both before and after controlling for other predictors.11,19,38–40
Though it may lack specificity in relation to other conditions common in developing countries, our data support the role of anemia as a good morbidity indicator for schistosomiasis, given that it can be easily measured and there was a large absolute difference in prevalence of anemia in children infected and uninfected with S. mansoni.
Ultrasound findings.
Because malaria is holoendemic in Nyanza Province, it is possible that the surprisingly high prevalence of hepatomegaly (61%) and splenomegaly (56%) in children who were not infected with S. mansoni could be partly explained by resolved malaria infection. However, 50% of malaria-associated hepatomegaly typically resolves within 2 weeks of parasite clearance.41,42 We did not test for viral hepatitis infections or vaccination status nor were we able to find data on the prevalence of viral hepatitis or hepatitis B vaccine coverage rates in the region. Though we did not test for HIV, a prevalence of 3.6% was found in children 1.5–2 years of age in a recent study near our site.43 Because there are presumably few risk factors for HIV between the ages of 2 and 8 years, we would expect the HIV prevalence in our age group to be similar. This suggests that even if HIV was the cause, it would only explain identified liver findings in a small fraction of our population. We did find a high prevalence of stunting and wasting, suggesting that this population may be at risk for protein wasting, but we did not evaluate children for this. More recently, authors have found an association between diet and hepatomegaly, which may contribute to this background finding.44 Further investigation into the significance of these and other potential etiologies of hepatomegaly and splenomegaly in this region is warranted.
Our initial models suggested a statistically significant negative association between schistosomiasis and liver texture pattern B. However, after performing sensitivity analyses with multiple imputation, the initial negative association was no longer significant. This result is more intuitively logical and consistent with prior studies, as the etiology and association with schistosomiasis of liver texture pattern B remains unclear. For this reason, most studies of liver pathology consider only patterns of C and higher as schistosomiasis-associated morbidity.18,45–47 Nevertheless the high prevalence of liver texture pattern B found in our study sample warrants further investigation into its etiology and clinical significance.
Liver texture patterns, PBT, and PVD do not fit our criteria as good indicators of morbidity in children because they were not associated with schistosomiasis in this age group. This does not appear to be a flaw in the tool (ultrasound examination), but rather reflects the point that these morbidity indicators are typically associated with S. mansoni infection in adults who have more chronic infections47,48 and are rare in school-aged children.18,45,49 This suggests that the Niamey protocol, which was designed for assessing morbidity in adult populations, is not adequate for assessing morbidity in children and that development of more age-appropriate tools should be considered.
However, hepatomegaly fit our criteria as a good indicator. High prevalence of hepatomegaly in S. mansoni-infected children is linked to morbidity and effective treatment reduces hepatomegaly.18,34,45 Furthermore, the earlier in life that these treatments occur, as disease is acquired at a young age, the greater the extent of reversibility as chronic infection is interrupted.22 The specificity and attributable contribution of schistosomiasis to hepatomegaly is not clear given the high prevalence of hepatomegaly in our uninfected population. This suggests that hepatomegaly as an assessment tool will perform better in certain settings than others. Access to portable and relatively inexpensive ultrasound machines, and the relative ease of measuring hepatomegaly, makes this an attractive indicator for use in young children.
Anthropometry.
Though both stunting and wasting were prevalent in our study, we did not find the associations with schistosomiasis that other investigators have shown.50–52 Our inability to reproduce the association is likely caused by residual confounding from unmeasured variables. The overwhelming support from other studies argues that anthropometry should remain in the toolkit for morbidity assessments.
Shuttle run.
Like two recently published studies evaluating the 20-m shuttle run as a tool for measuring exercise tolerance in polyparasitized children and other studies that tested running performance in children,53–55 we did not find an association with S. mansoni infection. The 20-m shuttle run was originally developed and validated in children from developed countries as a tool to measure maximal exercise capacity by correlation to VO2 max.27 Like most ultrasound investigations, studies that showed decreased physical fitness in association with schistosomiasis focused on adults, specifically their working capacity.13–15,56 In these studies, productivity was measured over the course of a day. We did not find an association with schistosomiasis and the 20-m shuttle run, suggesting that the impact of schistosome infections associated with exercise tolerance may be from lack of stamina rather than an effect on shorter duration intense exertion. Alternatively, it could be that the duration of infection necessary to develop schistosomiasis-associated exercise intolerance is longer than the exposure these children have experienced. This hypothesis could be tested by evaluating work and shuttle run performances in infected and uninfected adults. Both of these concerns suggest that the specific morbidity indicator for exercise intolerance associated with schistosomiasis may need to be further defined, as well as the tool to assess that indicator.
Quality of life.
We did not find associations between quality of life and schistosomiasis in our study population. Like the shuttle run, the PedsQL tool was developed and validated in developed countries to measure morbidity in four dimensions as noted in the methods section. The PedsQL tool may not capture the specific experience associated with schistosomiasis; there may be cultural or language barriers that limit the assessment of infection impact and chronic manifestations of schistosomiasis may be accepted as normal health status.57–60 Therefore, a person might not perceive themselves to have a decreased quality of life on questioning or language may interfere with the interpretation of the impact. These concerns suggest that qualitative studies aimed at determining if and in what capacity quality of life in children is affected by schistosomiasis are warranted. At that point we can focus on developing the appropriate tool and quantifying morbidity.
Limitations.
Many of our results may have been influenced by misclassification bias. Infection status was based on stool examination, which is known to have limited sensitivity, and S. mansoni infections may have been missed as a result. Additionally, we included children who only contributed two stools as opposed to three, the gold standard for coproparasitologic evaluation. As a cross-sectional study, we were only able to test for current infection status, and therefore could not temporally associate disease and subsequent morbidity, nor could we assess the impact of duration of infection or repeated infections on morbidity. These limitations may have biased our results toward the null hypothesis. Furthermore, because we were only able to choose a total of 12 clusters our power to detect differences may have been reduced.
Conclusions.
Though infection prevalence in our population was comparable to levels in older age groups where morbidity has been documented, the paucity of statistically significant associations between schistosomiasis and many of the classic morbidity indicators for schistosomiasis suggests that other factors such as duration and intensity of infection are critical for morbidity development. Our data highlight the importance of evaluating schistosomiasis-associated morbidity in schoolchildren and the need to identify the appropriate morbidity indicators and specific tools for measuring those indicators. Like other authors, our data suggest that anemia and hepatomegaly may be useful components of a morbidity toolkit. Additional markers of morbidity may become more apparent when these baseline data are coupled with the longitudinal measurements that are scheduled at years 3 and 5 of the overall study. Using morbidity impact as a measure of program evaluation would make an important contribution toward the design of effective MDA interventions.
ACKNOWLEDGMENTS
We thank the principals and teachers of the schools where we worked and all of the children who participated and their parents. We extend a special thanks to all of the hard working people at KEMRI in the laboratories and the field for their assistance, and to the SCORE Secretariat for making this research possible.
Footnotes
Financial support: This study received financial support from the University of Georgia Research Foundation, Inc., which is funded by the Bill and Melinda Gates Foundation for this SCORE project.
Disclosure: This work is published with the permission of the Director, Kenya Medical Research Institute. The findings and conclusions of this work are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Authors' addresses: Aaron M. Samuels, Ryan E. Wiegand, Molly Hyde, Susan P. Montgomery, and W. Evan Secor, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: IYP2@cdc.gov, FWK2@cdc.gov, Molly1013@hotmail.com, ZQU6@cdc.gov, and WAS4@cdc.gov. Elizabeth Matey, Pauline N. M. Mwinzi, Geoffrey Muchiri, Edmund Ireri, and Diana M. S. Karanja, Kenya Medical Research Institute, Kisumu, Kenya, E-mails: Ematey@kemricdc.org, PMwinzi@kemricdc.org, Geoffmosh@yahoo.com, Eikareko@hotmail.com, and DKaranja@kemricdc.org.
References
- 1.WHO Schistosomiasis: population requiring preventive chemotherapy and number of people treated in 2010. Wkly Epidemiol Rec. 2012;87:37–44. [PubMed] [Google Scholar]
- 2.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 3.King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO . The Global Burden of Disease: 2004 Update. Geneva: World Health Organization; 2008. [Google Scholar]
- 5.Verani JR, Abudho B, Montgomery SP, Mwinzi PN, Shane HL, Butler SE, Karanja DM, Secor WE. Schistosomiasis among young children in Usoma, Kenya. Am J Trop Med Hyg. 2011;84:787–791. doi: 10.4269/ajtmh.2011.10-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odogwu SE, Ramamurthy NK, Kabatereine NB, Kazibwe F, Tukahebwa E, Webster JP, Fenwick A, Stothard JR. Schistosoma mansoni in infants (aged < 3 years) along the Ugandan shoreline of Lake Victoria. Ann Trop Med Parasitol. 2006;100:315–326. doi: 10.1179/136485906X105552. [DOI] [PubMed] [Google Scholar]
- 7.Stothard JR, Kabatereine NB, Tukahebwa EM, Kazibwe F, Mathieson W, Webster JP, Fenwick A. Field evaluation of the Meade Readiview handheld microscope for diagnosis of intestinal schistosomiasis in Ugandan school children. Am J Trop Med Hyg. 2005;73:949–955. [PubMed] [Google Scholar]
- 8.Satayathum SA, Muchiri EM, Ouma JH, Whalen CC, King CH. Factors affecting infection or reinfection with Schistosoma haematobium in coastal Kenya: survival analysis during a nine-year, school-based treatment program. Am J Trop Med Hyg. 2006;75:83–92. [PMC free article] [PubMed] [Google Scholar]
- 9.Smith JH, Christie JD. The pathobiology of Schistosoma haematobium infection in humans. Hum Pathol. 1986;17:333–345. doi: 10.1016/s0046-8177(86)80456-7. [DOI] [PubMed] [Google Scholar]
- 10.Warren KS. The pathology, pathobiology and pathogenesis of schistosomiasis. Nature. 1978;273:609–612. doi: 10.1038/273609a0. [DOI] [PubMed] [Google Scholar]
- 11.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 12.Jukes MC, Nokes CA, Alcock KJ, Lambo JK, Kihamia C, Ngorosho N, Mbise A, Lorri W, Yona E, Mwanri L, Baddeley AD, Hall A, Bundy DA. Partnership for Child Development Heavy schistosomiasis associated with poor short-term memory and slower reaction times in Tanzanian schoolchildren. Trop Med Int Health. 2002;7:104–117. doi: 10.1046/j.1365-3156.2002.00843.x. [DOI] [PubMed] [Google Scholar]
- 13.Ndamba J, Makaza N, Munjoma M, Gomo E, Kaondera KC. The physical fitness and work performance of agricultural workers infected with Schistosoma mansoni in Zimbabwe. Ann Trop Med Parasitol. 1993;87:553–561. doi: 10.1080/00034983.1993.11812810. [DOI] [PubMed] [Google Scholar]
- 14.Collins KJ, Brotherhood RJ, Davies CT, Dore C, Hackett AJ, Imms FJ, Musgrove J, Weiner JS, Amin MA, El Karim M, Ismail HM, Omer AH, Sukkar MY. Physiological performance and work capacity of Sudanese cane cutters with Schistosoma mansoni infection. Am J Trop Med Hyg. 1976;25:410–421. doi: 10.4269/ajtmh.1976.25.410. [DOI] [PubMed] [Google Scholar]
- 15.Fenwick A, Figenschou BH. The effect of Schistosoma mansoni infection of the productivity of cane cutters on a sugar estate in Tanzania. Bull World Health Organ. 1972;47:567–572. [PMC free article] [PubMed] [Google Scholar]
- 16.King CH, Sturrock RF, Kariuki HC, Hamburger J. Transmission control for schistosomiasis - why it matters now. Trends Parasitol. 2006;22:575–582. doi: 10.1016/j.pt.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 17.WHO . Preventive Chemotherapy in Human Helminthiasis. Geneva: World Health Organization; 2006. Coordinated use of anthelmintic drugs in control interventions: guidelines for health professionals and programme managers. [Google Scholar]
- 18.Vennervald BJ, Booth M, Butterworth AE, Kariuki HC, Kadzo H, Ireri E, Amaganga C, Kimani G, Kenty L, Mwatha J, Ouma JH, Dunne DW. Regression of hepatosplenomegaly in Kenyan school-aged children after praziquantel treatment and three years of greatly reduced exposure to Schistosoma mansoni. Trans R Soc Trop Med Hyg. 2005;99:150–160. doi: 10.1016/j.trstmh.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Kabatereine NB, Brooker S, Koukounari A, Kazibwe F, Tukahebwa EM, Fleming FM, Zhang Y, Webster JP, Stothard JR, Fenwick A. Impact of a national helminth control programme on infection and morbidity in Ugandan schoolchildren. Bull World Health Organ. 2007;85:91–99. doi: 10.2471/BLT.06.030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koukounari A, Gabrielli AF, Toure S, Bosque-Oliva E, Zhang Y, Sellin B, Donnelly CA, Fenwick A, Webster JP. Schistosoma haematobium infection and morbidity before and after large-scale administration of praziquantel in Burkina Faso. J Infect Dis. 2007;196:659–669. doi: 10.1086/520515. [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Rahman TA, Collins KJ, Dore C. Oxylog studies of energy expenditure and schistosomiasis in the Sudan. J Trop Med Hyg. 1990;93:365–371. [PubMed] [Google Scholar]
- 22.Richter J. The impact of chemotherapy on morbidity due to schistosomiasis. Acta Trop. 2003;86:161–183. doi: 10.1016/s0001-706x(03)00032-9. [DOI] [PubMed] [Google Scholar]
- 23.Handzel T, Karanja DM, Addiss DG, Hightower AW, Rosen DH, Colley DG, Andove J, Slutsker L, Secor WE. Geographic distribution of schistosomiasis and soil-transmitted helminths in western Kenya: implications for anthelmintic mass treatment. Am J Trop Med Hyg. 2003;69:318–323. [PubMed] [Google Scholar]
- 24.Committee WHOE Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rep Ser. 2002;912:i–vi. 1–57 back cover. [PubMed] [Google Scholar]
- 25.Kimathi NAMJ. In: Clinical Guidelines for Diagnosis and Treatment of Common Conditions in Kenya. GoK MoH., editor. Nairobi: The Regal Press Kenya Lett; 2002. pp. 190–202. [Google Scholar]
- 26.WHO Child Growth Standards. 2012. http://www.who.int/childgrowth/software/en/ Available at. Accessed September 12, 2011.
- 27.Leger LA, Mercier D, Gadoury C, Lambert J. The multistage 20 metre shuttle run test for aerobic fitness. J Sports Sci. 1988;6:93–101. doi: 10.1080/02640418808729800. [DOI] [PubMed] [Google Scholar]
- 28.Richter J, Domingues AL, Barata CH, Prata AR, Lambertucci JR. Report of the second satellite symposium on ultrasound in schistosomiasis. Mem Inst Oswaldo Cruz. 2001;96((Suppl)):151–156. doi: 10.1590/s0074-02762001000900023. [DOI] [PubMed] [Google Scholar]
- 29.Binder DA. On the variances of asymptotically normal estimators from complex surveys. Int Stat Rev. 1983;51:279–292. [Google Scholar]
- 30.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer; 2001. [Google Scholar]
- 31.Groenwold RH, Donders AR, Roes KC, Harrell FE, Jr, Moons KG. Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol. 2012;175:210–217. doi: 10.1093/aje/kwr302. [DOI] [PubMed] [Google Scholar]
- 32.Hamel MJ, Adazu K, Obor D, Sewe M, Vulule J, Williamson JM, Slutsker L, Feikin DR, Laserson KF. A reversal in reductions of child mortality in western Kenya, 2003–2009. Am J Trop Med Hyg. 2011;85:597–605. doi: 10.4269/ajtmh.2011.10-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sokhna C, Le Hesran JY, Mbaye PA, Akiana J, Camara P, Diop M, Ly A, Druilhe P. Increase of malaria attacks among children presenting concomitant infection by Schistosoma mansoni in Senegal. Malar J. 2004;3:43. doi: 10.1186/1475-2875-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson S, Vennervald BJ, Dunne DW. Chronic hepatosplenomegaly in African school children: a common but neglected morbidity associated with schistosomiasis and malaria. PLoS Negl Trop Dis. 2011;5:e1149. doi: 10.1371/journal.pntd.0001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muok EM, Mwinzi PN, Black CL, Carter JM, Ng'ang'a ZW, Gicheru MM, Secor WE, Karanja DM, Colley DG. Short report: Childhood coinfections with Plasmodium falciparum and Schistosoma mansoni result in lower percentages of activated T cells and T regulatory memory cells than schistosomiasis only. Am J Trop Med Hyg. 2009;80:475–478. [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson S, Jones FM, Mwatha JK, Kimani G, Booth M, Kariuki HC, Vennervald BJ, Ouma JH, Muchiri E, Dunne DW. Hepatosplenomegaly associated with chronic malaria exposure: evidence for a pro-inflammatory mechanism exacerbated by schistosomiasis. Parasite Immunol. 2009;31:64–71. doi: 10.1111/j.1365-3024.2008.01078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson S, Dunne DW. Advances in our understanding of the epidemiology of Plasmodium and schistosome infection: informing coinfection studies. Curr Opin HIV AIDS. 2012;7:225–230. doi: 10.1097/COH.0b013e328351b9fb. [DOI] [PubMed] [Google Scholar]
- 38.Ajanga A, Lwambo NJ, Blair L, Nyandindi U, Fenwick A, Brooker S. Schistosoma mansoni in pregnancy and associations with anaemia in northwest Tanzania. Trans R Soc Trop Med Hyg. 2006;100:59–63. doi: 10.1016/j.trstmh.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 39.Brito LL, Barreto ML, Silva Rde C, Assis AM, Reis MG, Parraga IM, Blanton RE. Moderate- and low-intensity co-infections by intestinal helminths and Schistosoma mansoni, dietary iron intake, and anemia in Brazilian children. Am J Trop Med Hyg. 2006;75:939–944. [PubMed] [Google Scholar]
- 40.Koukounari A, Fenwick A, Whawell S, Kabatereine NB, Kazibwe F, Tukahebwa EM, Stothard JR, Donnelly CA, Webster JP. Morbidity indicators of Schistosoma mansoni: relationship between infection and anemia in Ugandan schoolchildren before and after praziquantel and albendazole chemotherapy. Am J Trop Med Hyg. 2006;75:278–286. [PubMed] [Google Scholar]
- 41.Sowunmi A. Hepatomegaly in acute falciparum malaria in children. Trans R Soc Trop Med Hyg. 1996;90:540–542. doi: 10.1016/s0035-9203(96)90313-2. [DOI] [PubMed] [Google Scholar]
- 42.Sowunmi A, Adedeji AA, Sowunmi CO, Falade CO, Falade AG, Ohaeri B, Happi TC, Oduola AM. Clinical characteristics and disposition kinetics of the hepatomegaly associated with acute, uncomplicated, Plasmodium falciparum malaria in children. Ann Trop Med Parasitol. 2001;95:7–18. doi: 10.1080/00034980020030939. [DOI] [PubMed] [Google Scholar]
- 43.Feikin DR, Laserson KF, Ojwando J, Nyambane G, Ssempijja V, Audi A, Nyakundi D, Oyieko J, Dallas MJ, Ciarlet M, Neuzil KM, Breiman RF. Efficacy of pentavalent rotavirus vaccine in a high HIV prevalence population in Kenya. Vaccine. 2012;30((Suppl 1)):A52–A60. doi: 10.1016/j.vaccine.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 44.Gong YY, Wilson S, Mwatha JK, Routledge MN, Castelino JM, Zhao B, Kimani G, Kariuki HC, Vennervald BJ, Dunne DW, Wild CP. Aflatoxin exposure may contribute to chronic hepatomegaly in Kenyan school children. Environ Health Perspect. 2012;120:893–896. doi: 10.1289/ehp.1104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vennervald BJ, Kenty L, Butterworth AE, Kariuki CH, Kadzo H, Ireri E, Amaganga C, Kimani G, Mwatha J, Otedo A, Booth M, Ouma JH, Dunne DW. Detailed clinical and ultrasound examination of children and adolescents in a Schistosoma mansoni endemic area in Kenya: hepatosplenic disease in the absence of portal fibrosis. Trop Med Int Health. 2004;9:461–470. doi: 10.1111/j.1365-3156.2004.01215.x. [DOI] [PubMed] [Google Scholar]
- 46.Kariuki HC, Mbugua G, Magak P, Bailey JA, Muchiri EM, Thiongo FW, King CH, Butterworth AE, Ouma JH, Blanton RE. Prevalence and familial aggregation of schistosomal liver morbidity in Kenya: evaluation by new ultrasound criteria. J Infect Dis. 2001;183:960–966. doi: 10.1086/319247. [DOI] [PubMed] [Google Scholar]
- 47.Malenganisho WL, Magnussen P, Friis H, Siza J, Kaatano G, Temu M, Vennervald BJ. Schistosoma mansoni morbidity among adults in two villages along Lake Victoria shores in Mwanza District, Tanzania. Trans R Soc Trop Med Hyg. 2008;102:532–541. doi: 10.1016/j.trstmh.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Silva CC, Domingues AL, Lopes EP, Morais CN, Santos RB, Luna CF, Nader HB, Martins JR. Schistosomiasis mansoni: ultrasound-evaluated hepatic fibrosis and serum concentrations of hyaluronic acid. Ann Trop Med Parasitol. 2011;105:233–239. doi: 10.1179/136485911X12987676649629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Scheich T, Hofer L, Kaatano G, Foya J, Odhiambo D, Igogote J, Lwambo N, Ekamp H, Karst K, Haussinger D, Richter J. Hepatosplenic morbidity due to Schistosoma mansoni in schoolchildren on Ukerewe Island, Tanzania. Parasitol Res. 2012;110:2515–2520. doi: 10.1007/s00436-011-2793-6. [DOI] [PubMed] [Google Scholar]
- 50.Zhou H, Watanabe C, Ohtsuka R. Impacts of dietary intake and helminth infection on diversity in growth among schoolchildren in rural south China: a four-year longitudinal study. Am J Hum Biol. 2007;19:96–106. doi: 10.1002/ajhb.20588. [DOI] [PubMed] [Google Scholar]
- 51.Assis AM, Prado MS, Barreto ML, Reis MG, Conceicao Pinheiro SM, Parraga IM, Blanton RE. Childhood stunting in northeast Brazil: the role of Schistosoma mansoni infection and inadequate dietary intake. Eur J Clin Nutr. 2004;58:1022–1029. doi: 10.1038/sj.ejcn.1601926. [DOI] [PubMed] [Google Scholar]
- 52.Olds GR, King C, Hewlett J, Olveda R, Wu G, Ouma J, Peters P, McGarvey S, Odhiambo O, Koech D, Liu CY, Aligui G, Gachihi G, Kombe Y, Parraga I, Ramirez B, Whalen C, Horton RJ, Reeve P. Double-blind placebo-controlled study of concurrent administration of albendazole and praziquantel in schoolchildren with schistosomiasis and geohelminths. J Infect Dis. 1999;179:996–1003. doi: 10.1086/314686. [DOI] [PubMed] [Google Scholar]
- 53.Walker AR, Walker BF, Richardson BD, Smit PJ. Running performance in South African Bantu children with schistosomiasis. Trop Geogr Med. 1972;24:347–352. [PubMed] [Google Scholar]
- 54.Bustinduy AL, Thomas CL, Fiutem JJ, Parraga IM, Mungai PL, Muchiri EM, Mutuku F, Kitron U, King CH. Measuring fitness of Kenyan children with polyparasitic infections using the 20-meter shuttle run test as a morbidity metric. PLoS Negl Trop Dis. 2011;5:e1213. doi: 10.1371/journal.pntd.0001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muller I, Coulibaly JT, Furst T, Knopp S, Hattendorf J, Krauth SJ, Stete K, Righetti AA, Glinz D, Yao AK, Puhse U, N'Goran EK, Utzinger J. Effect of schistosomiasis and soil-transmitted helminth infections on physical fitness of school children in Cote d'Ivoire. PLoS Negl Trop Dis. 2011;5:e1239. doi: 10.1371/journal.pntd.0001239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parker M. Re-assessing disability: the impact of schistosomal infection on daily activities among women in Gezira Province, Sudan. Soc Sci Med. 1992;35:877–890. doi: 10.1016/0277-9536(92)90102-v. [DOI] [PubMed] [Google Scholar]
- 57.Bowden A, Fox-Rushby JA. A systematic and critical review of the process of translation and adaptation of generic health-related quality of life measures in Africa, Asia, Eastern Europe, the Middle East, South America. Soc Sci Med. 2003;57:1289–1306. doi: 10.1016/s0277-9536(02)00503-8. [DOI] [PubMed] [Google Scholar]
- 58.Ukwandu NC, Nmorsi OP. The perception, beliefs and practices toward genitourinary schistosomiasis by inhabitants of selected endemic areas (Edo/Delta States) in south-eastern Nigeria. Rev Inst Med Trop Sao Paulo. 2004;46:209–216. doi: 10.1590/s0036-46652004000400007. [DOI] [PubMed] [Google Scholar]
- 59.Wagatsuma Y, Aryeetey ME, Nkrumah FK, Sack DA, Kojima S. Highly symptom-aware children were heavily infected with urinary schistosomiasis in southern Ghana. Cent Afr J Med. 2003;49:16–19. [PubMed] [Google Scholar]
- 60.Amazigo UO, Anago-Amanze CI, Okeibunor JC. Urinary schistosomiasis among school children in Nigeria: consequences of indigenous beliefs and water contact activities. J Biosoc Sci. 1997;29:9–18. doi: 10.1017/s0021932097000096. [DOI] [PubMed] [Google Scholar]