Abstract
Gnathostomiasis is now recognized as a zoonosis with a worldwide distribution. In the Americas, it is caused by the third-stage larvae of Gnathostoma binucleatum and in Asia mainly by G. spinigerum. The availability and preparation of specific antigens are among the main obstacles for developing reliable immunodiagnostic tests. In this study, six immunodominant peptides were identified and characterized from G. binucleatum, somatic antigens (AgS: 24, 32, and 40 kDa) and excretory-secretory antigens (AgES: 42, 44, and 56 kDa) by two-dimensional immunoblot analysis. Among those immunodominant peptides, two AgS spots were characterized by mass spectrometric analysis (32 kDa; pI 6.3 and 6.5) and identified as type 1 galectins. In accordance with this finding, a fraction of AgS exhibited affinity to lactose and displayed a 100% specificity and sensitivity for the diagnosis of human gnathostomiasis.
Introduction
Human gnathostomiasis is one of the emerging parasitic zoonoses caused by several species of the genus Gnathostoma.1–5 In Mexico, Gnathostoma binucleatum is the parasite responsible for at least 9,000 cases of the disease documented mainly in laboratories and research centers.5 The disease is acquired by ingestion of raw or undercooked freshwater fish parasitized with the advanced third stage larvae (L3A). Clinical manifestations are varied and include cutaneous, ocular, visceral, and neurologic symptoms.5–7
To date, definitive diagnosis of the disease depends on recovery and identification of L3A. However, recovery of larvae from infected humans is often difficult because of the migratory nature of the parasite. Immunologic diagnostic methods are considered useful, but these methods can show cross-reactivity with other parasites such as Strongyloides stercoralis and Angiostrongylus cantonensis.3,5,8 Other diagnostic methods such as double immunodiffusion, indirect hemagglutination, immunofluorescence, or Western blotting have similar limitations.2,4,5,9
False-positive results often obtained with these methods are believed to be caused by similarity of antigens that can be shared by closely related phylogenetic species.10 Conversely, false-negative results are probably related to modulation of the host immune response when stimulated by parasite antigens.8 For this reason, identification and use of specific immunodominant antigens is considered to be a better approach for the diagnosis of human gnathostomiasis.3,5,11–14 The first immunodominant antigen described was a 24-kDa peptide from G. spinigerum, a species endemic to Asia, mainly Thailand.14 Other antigens, such as metalloproteases (24 kDa), serine proteases (approximately 35 kDa), and cathepsins (24 kDa), have also been identified in this species.12,15
A number of proteins have been identified in G. binucleatum (40, 50, 80, 115, 120, and 208 kDa) that were recognized by serum samples from 9 patients with human gnathostomiasis and 1 patient with confirmed disease. Two of the peptides were metalloproteases (80 and 208 kDa).16,17
The purpose of this study was to identify and characterize G. binucleatum immunodominant antigens derived from somatic antigens (AgS) and excretory-secretory antigens (AgES) that could be useful for immunodiagnosis of human gnathostomiasis cases.
Materials And Methods
Gnathostoma binucleatum L3A.
Larvae were collected in Ojo de Agua (22°45′28″N, 105°40′25″W), Tecualilla, Sinaloa, México, from the muscle masses of the fish species Dormitator latifrons and Eleotris picta, which are natural hosts of these parasites. Specimens were recovered and identified by the method described by Diaz-Camacho and others,18 and were processed to obtain AgS and AgES.
Antigen preparation.
Somatic antigens.
Somatic antigens were obtained from 350 L3A frozen in liquid nitrogen. Larval tissues were homogenized in 40 mM Tris-HCl, and the mixture was sonicated (20 60-Hz pulses/5 minutes, with 1-minute intervals between each pulse) by using a ultrasonic homogenizer (no. 4710; Cole Parmer Instruments Co., Vernon Hills, IL), centrifuged at 21,910 × g for 25 minutes at 18°C, and the supernatant was stored at –80°C until used.
Excretory-secretory antigens.
Excretory-secretory antigens were prepared from larvae incubated at 37°C in RPMI 1640 medium containing 0.1% NaHCO3, 30 μg/mL of chloramphenicol, 50 μg/mL of gentamicin, and 2.5 μg/mL of amphotericin B. Culture media were collected every 48 hours, concentrated, and stored at –80°C. Protein content was analyzed by using the method of Bradford.19
Electrophoresis.
Unidimensional electrophoresis.
Somatic antigens (5 μg/lane) and AgES (0.33 μg/lane) were separated on 12% acrylamide gels (100 V, 30 mA, and 7 W for 2.5 hours) by using the method of Laemmli and Favre.20 Gels were stained with a 0.1% Coomassie brilliant blue R-250 solution (Bio-Rad, Hercules, CA), and gel images were documented by using the XRS system (Bio-Rad).
Two-dimensional electrophoresis.
Two-dimensional electrophoresis was performed by using the method of O'Farrell.21 For the first dimension, AgS (300 μg) or AgES (90 μg) were mixed in 300 μL of rehydration buffer (8 M urea, 2% CHAPS buffer, 50 mM dithiothreitol, 0.4% ampholytes, 0.001% bromophenol blue) and subjected to electrophoresis on immobilized pH gradient gels (no. 1632011, 17 cm, pH 5–8; Bio-Rad) in the Protean isoelectric focusing system (Bio-Rad) at 250 V for 20 min, 10,000 V for 2.5 hr, and 40,000 V for 1 hour. The immobilized pH gradient gels were then incubated for 15 minutes in a solution containing 6 M urea, 0.375 M Tris-HCl, pH 8.8, 2% sodium dodecyl sulfate (SDS), 20% glycerol, 2% dithiothreitol, followed by a 15-minute incubation in 6 M urea, 0.375 M Tris-HCl, pH 8.8, 2% SDS, 20% glycerol, 2% iodoacetamide.
The second-dimension electrophoresis was carried out by using 12% gels and sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (100 V, 30 mA, and 7 W). Gels were stained with 0.1% Coomassie brilliant blue R-250 and documented by using the XRS system.
Ethics.
The Ethics Committee of the Autonomous University of Sinaloa reviewed and approved this study (Identification of Immunodominant Peptides from Gnathostoma binucleatum; July 2009). Informed consent was obtained from all study participants.
Serum samples.
Serum samples were collected from persons who were examined at the Unidad de Investigaciones en Salud Pública Louis Pasteur, Facultad de Ciencias Químico Biológicas de la Universidad Autónoma de Sinaloa. Serum samples were obtained from 16 patients with gnathostomiasis diagnosed either by recovering the larva or by a positive enzyme-linked immunosorbent assay (ELISA) result. Thirty-five serum samples were also obtained from persons with other parasite infections diagnosed by stool examination (3 with S. stercoralis, 1 with Ascaris lumbricoides, 1 with Trichiuris trichiura, 10 with Giardia lamblia, 10 with Hymenolepis nana, and 10 with Endolimax nana), and 15 serum samples were obtained from healthy persons. The number of serum samples used depending on their availability.
ELISA standardization for gnathostomiasis.
Serum samples used in this study were tested by an ELISA, which was standardized by using 63 serum samples from of healthy persons (10 children 5–7 years of age and 53 adults 19–62 years of age) and 30 serum samples from patients with gnathostomiasis, who were given this diagnosis on the basis of their clinical and epidemiologic histories.
The ELISA was performed with AgS of L3A larvae of G. binucleatum, and the conditions used were established in previous analyses according to the methods of Diaz-Camacho et al.7 In brief, larvae were obtained from seven freshwater fish species, which were identified as intermediate hosts of G. binucleatum in Sinaloa. Flat-well microplates were used and AgS (200 ng/well) was adsorbed for 24 hours at 4°C. Wells were blocked by incubation with phosphate buffered saline (PBS), 10% bovine serum albumin, and 0.5% Tween 20 for 30 minutes at room temperature. Serum samples were diluted 1:200 in PBS. Two hundred microliters was used per well, microplates were incubated for 90 minutes at 37°C, and then incubated with goat anti-human IgG-peroxidase diluted 1:1,000 in PBS, 10% bovine serum albumin, and 0.5% Tween 20. Wells were rinsed three times for five minutes after each incubation in PBS-Tween, and incubated with 2,2′-azino-bis-(3-ethylbenzothiazolin-6-sulfonic acid) substrate for 30 minutes at 37°C. The reaction was stopped by addition of 1% SDS.
Samples were read at 405 nm in a Titerteck Multiscan MCC (Titerteck Instruments, Inc., Huntsville, AL). Mean values followed a normal pattern, and the cutoff value for the assay was 0.5 (mean plus 3 SD). Sensitivity and specificity were calculated to be 90.9% and 95.4%, respectively. An ELISA to validate the immunodominance of lactose-binding antigen was performed by using the protein fraction with lactose affinity (approximately 0.001 ng/50 μL/well). A lower cutoff value (0.23) was obtained by using the lower protein concentration.
Immunoelectrotransfer of antigens.
Antigens (AgS and AgES) were transferred to nitrocellulose membranes by using a humid transfer module (Mini Trans-Blot Electrophoretic Transfer Cell; Bio-Rad). Membranes were immersed for 10 minutes in 40% methanol (v/v) and separated at 100 V and 500 mA for 5 hours according to the method of Towbin and others.22 After blotting the antigens, the membranes were incubated for 1.5 hours at room temperature with serum samples diluted 1:1,000 in PBS, 0.1% Tween 20, and 5% fat-free milk. Membranes were then incubated with goat anti-human IgG-peroxidase (AbD Serotec, Raleigh, NC) diluted 1:2,000. After each incubation, membranes were rinsed three times for five minutes in PBS and 0.1% Tween 20. Membranes were further incubated for 15 minutes in a solution of 25 mg diaminobenzidine and 5 μg of hydrogen peroxide in PBS.
Mass spectrometric analysis of immunodominant antigens.
Protein spots were excised from the Coomassie-stained SDS gel, destained, reduced, carbamidomethylated, and digested with modified porcine trypsin (Promega, Madison, WI). Peptide mass spectrometric analysis was conducted by using a 3200 Q TRAP hybrid tandem mass spectrometer (Applied Biosystems/MDS Sciex, Concord, Ontario, Canada) equipped with a nanoelectrospray ion source (NanoSpray II) and a MicroIonSpray II head as described by González-Zamorano and others.23 The instrument was coupled on line to a nanoAcquity Ultra Performance LC system (Waters Corporations, Milford, MA). In brief, spectra were acquired in automated mode using information dependent acquisition. Precursor ions were selected in Q1 using the enhanced MS mode as survey scan. The enhanced MS mode was followed by an enhanced resolution scan of the three most intense ions at the low speed of 250 amu/second to determine the ion charge states and then by an enhanced product ion scan. The precursor ions were fragmented by collision-induced dissociation in the Q2 collision cell. The fragment ions generated were captured and mass analyzed in the Q3 linear ion trap.
Database searching and protein identification were performed with the MS/MS spectra data sets using the MASCOT search algorithm (version 1.6b9; Matrix Science, London, United Kingdom; www.matrixscience.com). Mass tolerances of 0.5 Da for the precursor and 0.3 Da for the fragment ion masses were used. Carbamidomethyl–cysteine was the fixed modification and one missed cleavage for trypsin was allowed. Searches were conducted by using the metazoa subset of the NCBInr database (www.ncbi.nih.gov). Peptide sequences obtained by mass spectrometry were compared with sequences from the Blast database and a multiple alignment was conducted by using the MultiAlin program.24
Lactose affinity purification of AgS.
Somatic antigens from approximately 300 L3A were applied to an α-lactose agarose affinity column (5 mL; Sigma, St. Louis, MO) equilibrated with 40 mM Tris-HCl buffer, pH 9.4, at 24°C. A sample of AgS (1 mL, approximately 3.6 μg/mL of protein) was deposited in the column and incubated for 1 hour at room temperature. Unbound proteins were eluted with 40 mL of buffer, collected as 1-mL fractions, and those bound to lactose were eluted with 30 mL of buffer containing 0.2 M lactose. The presence of AgS proteins was verified by SDS-PAGE and staining with 0.1% (w/v) Coomassie R-250 and silver stain (PROT-SIL 1; Sigma) and by immunoelectrotransfer (IET) with serum from a patient with a confirmed diagnosis of gnathostomiasis.
Validation of immunodominance of lactose-binding antigen.
Specificity and sensitivity was estimated by testing serum samples from 11 patients with a confirmed diagnosis of gnathostomiasis by ELISA and IET, 8 serum samples from patients infected with other parasites (S. stercoralis, G. lamblia, H. nana, Entamoeba coli, Enterobius vermicularis) and 1 serum sample from a healthy person.
Results
Protein profiles of G. binucleatum L3A antigens.
Somatic antigens.
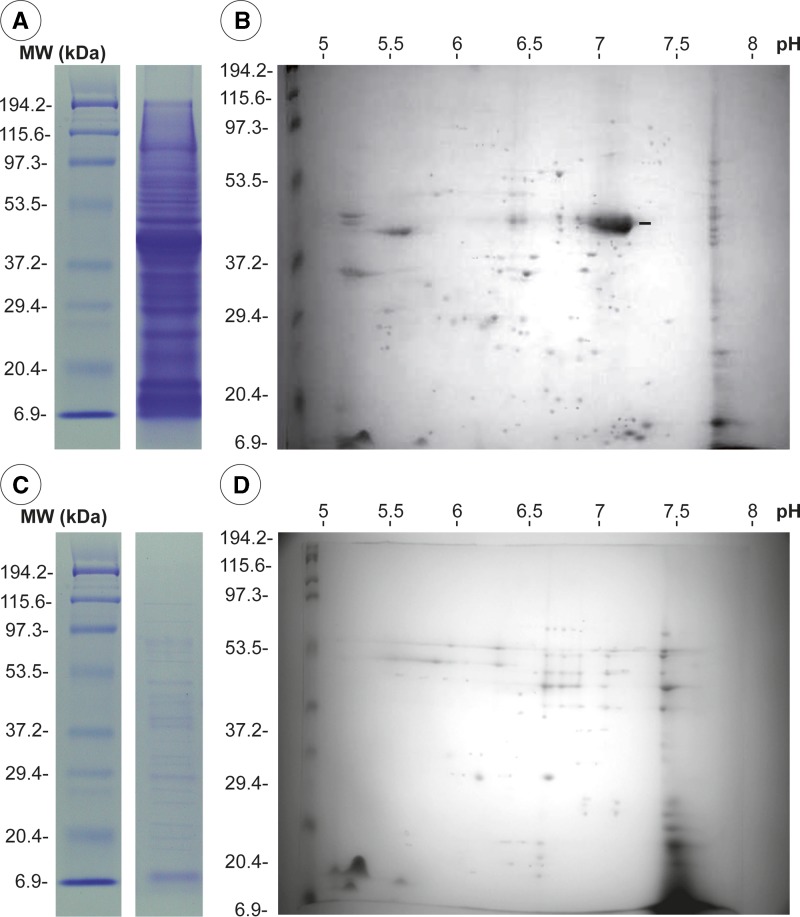
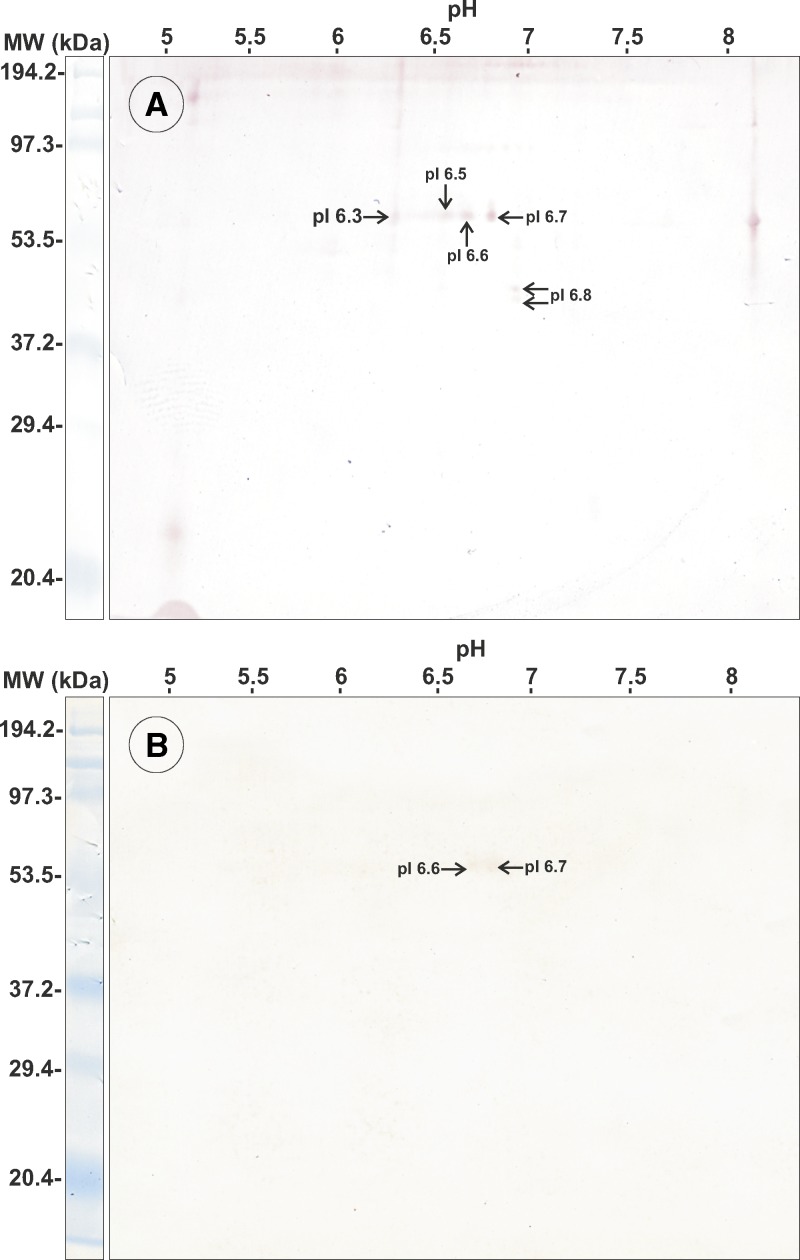
Somatic antigens showed 36 bands between 6 and 194.4 kDa by SDS-PAGE (Figure 1A). Two-dimensional immunoblot analysis showed 119 protein spots (7–110 kDa, pI 5–8); 68.1% had a pI in the range of 6–7 and most (79%) had an average of three proteins with the same molecular weight. One protein of 45 kDa, pI 6.8, had a higher concentration (Figure 1B).
Figure 1.
Electrophoretic patterns of proteins of third-stage larvae of Gnathostoma binucleatum. Somatic antigens: A, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; B, two-dimensional immunoblot analysis. Excretory-secretory antigens: C, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; D, two-dimensional immunoblot analysis. MW = molecular weight; kDa = kilodaltons.
Excretory-secretory antigens.
Excretory-secretory antigens showed 22 bands by SDS-PAGE with the molecular ranges between 6 and 190 kDa (Figure 1C) and 59 spots by two-dimensional immunoblot analysis; 69.59% of the spots had molecular weights in the range of 9–75 kDa and most had at least three proteins with similar molecular weights (Figure 1D).
Identification of immunodominant antigens by SDS-PAGE.
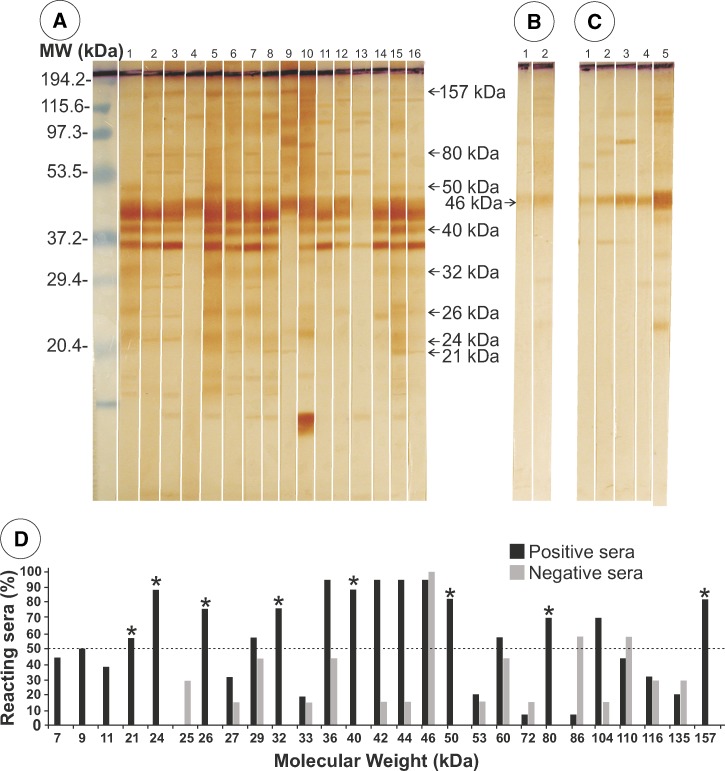
Immunoelectrotransfer of AgS separated by SDS-PAGE had 36 peptides (Figure 2A), of which a 46-kDa peptide cross-reacted with two serum samples (100%) from patients infected with S. stercoralis (Figure 2B), and with five serum samples from healthy persons (Figure 2C). Immunoreactivity of each band to patient serum samples was analyzed. Fifteen proteins were recognized by serum samples from more than 50% of patients with gnathostomiasis, and eight of these bands (21, 24, 26, 32, 40, 50, 80, and 157 kDa) did not cross-react with control serum samples (Figure 2D). Specificity of these bands ranged between 56% and 88%.
Figure 2.
Immunoelectrotransfer of somatic antigens separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis against serum samples from A, 16 patients with gnathostomiasis (arrows indicate specific protein bands); B, 2 patients infected with Strongyloides stercoralis; and C, 5 healthy persons (arrow indicates the protein with 100% cross-reactivity). D, Positive and negative results according to molecular weight (MW). Asterisks indicate specific peptides and the dotted line indicates the minimum value for considering a result specific for gnathostomiasis. kDa = kilodaltons.
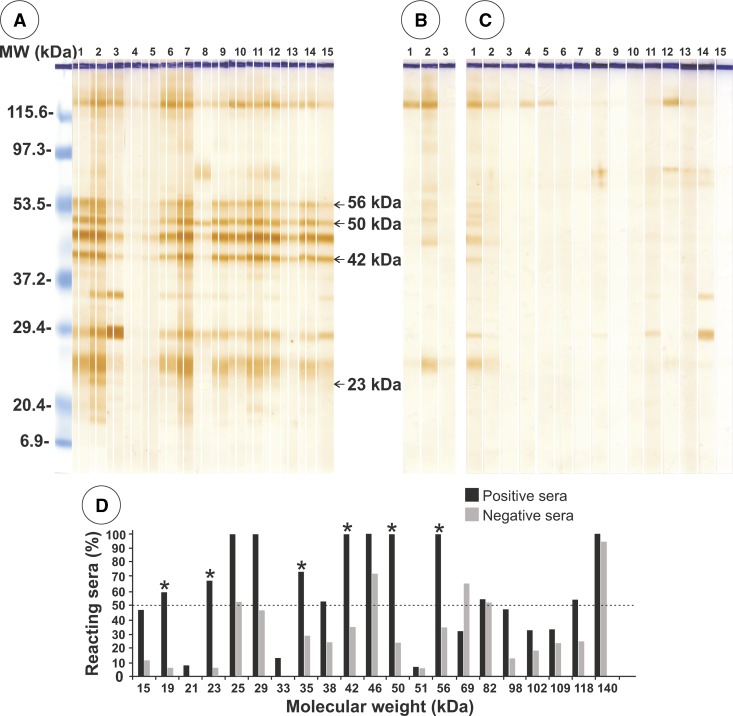
Immunoelectrotransfer of AgES after SDS-PAGE identified 21 antigenic bands, 13 of which reacted with serum samples from patients with gnathostomiasis (reaction range = 50–100%) (Figure 3A). All peptides showed cross-reactivity with negative control serum samples. Three of these peptides (42, 50, and 56 kDa) showed 100% reactivity with the patient serum samples but also had cross-reactivity values with control serum samples of 35.3%, 23.5%, and 35.3%, respectively (Figure 3B–3D).
Figure 3.
Immunoelectrotransfer of excretory-secretory antigens separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis against serum samples from A, 5 patients positive by enzyme-linked immunosorbent assay for gnathostomiasis; B, 3 patients infected with Strongyloides stercoralis; and C, 15 serum samples from healthy persons. D, Positive and negative results according to molecular weight (MW). Asterisks indicate peptides with the highest specificity and the dotted line indicates the minimum value for considering a result specific for gnathostomiasis. kDa = kilodaltons.
Identification of immunodominant antigens by two-dimensional immunoblot analysis.
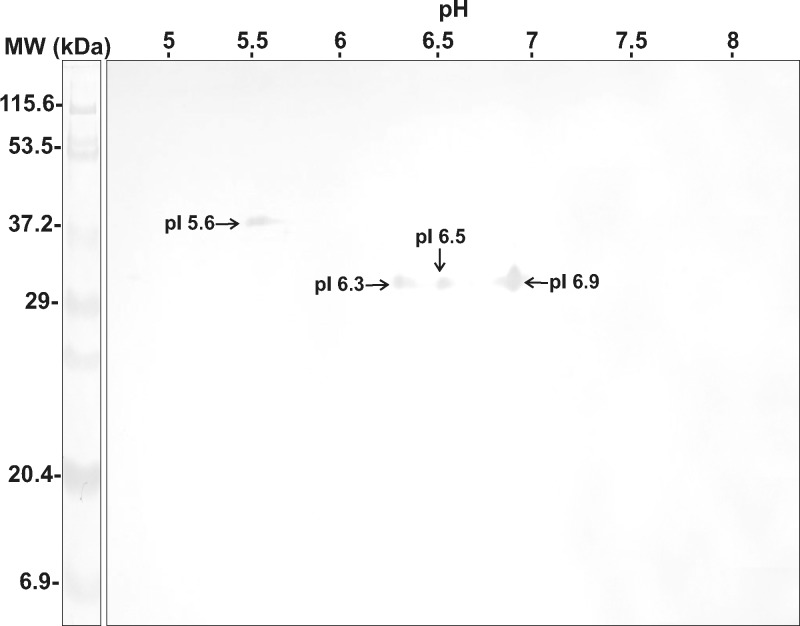
Two-dimensional immunoblot analysis of AgS identified four protein spots that reacted with a mixture of 10 serum samples from patients with gnathostomiasis. Three antigens had molecular weights of 32 kDa (pI 6.3, 6.5, 6.9) and one antigen had a molecular weight of 40 kDa (pI 5.6) (Figure 4). None of these peptides cross-reacted with control serum samples from healthy persons.
Figure 4.
Immunoelectrotransfer of excretory-secretory antigens separated by two-dimensional immunoblot analysis against mixed serum samples from 10 patients positive by enzyme-linked immunosorbent assay for gnathostomiasis. MW = molecular weight; kDa = kilodaltons.
Similarly for AgES, two-dimensional immunoblot analysis identified six spots (42 kDa, pI 6.8; 44 kDa, pI 6.8; and 4 of 56 kDa, pI 6.3, 6.5, 6.6 and 6.7) that were recognized by pooled serum samples from the patients (Figure 5A). However, 2 of the 56 kDa spots (pI 6.6 and 6.7) also reacted with a mixture of three serum samples from patients infected with S. stercoralis (Figure 5B).
Figure 5.
Immunoelectrotransfer of excretory-secretory antigens separated by two-dimensional immunoblot analysis against mixed serum samples from A, 10 patients with gnathostomiasis and B, 3 patients infected with Strongyloides stercoralis. MW = molecular weight; kDa = kilodaltons.
Mass spectrometric analysis.
Amino acid sequences were obtained from two-dimensional immunoblot analysis spots of two proteins/peptides (AgSGb1, 32 kDa, pI 6.3; AgSGb2, 32 kDa, pI 6.5). For AgSGb1, three peptides were obtained (FTINLHSK, IEPGQTLIVK and NGDIALHFNPR). For AgSGb2, eight peptides were obtained (SYPIPYR, KGTPFDIR, NGDIALHFNPR, NALQAGNWGNEER, YYPVPYESGISQGLSVGR, MPFEK, IEPGTLIVK and RNGDIALHFNPR).
Sequence analysis showed that AgSGb1 and AgSGb2 belong to the galectin family of peptides. By aligning the peptides with known sequences from nematodes (Figure 6), we found similarities of those two peptides with galectin-1 from Haemonchus contortus, Trichostrongylus colubriformis, Ostertagia circumcincta, Brugia malayi, Onchocerca volvulus, Caenorhabditis briggsae, and Dirofilaria immitis (for AgSGb1, score = 48.4–54.8%, 55% identity, 79% homology; for AgSGb2, score = 25.6–31.1%, 45% identity, 66% homology).
Figure 6.
Sequence alignment for somatic antigens Gb1 and Gb2 of Gnathostoma binucleatum. Homologous sequences from galectin-1 of other nematodes are enclosed in boxes: Haemonchus contortus (AAD11972.1), Trichostrongylus colubriformis (AAD11971.1), Ostertagia circumcincta (AAC47547.1), Brugia malayi (AAF37721.1), Dirofilaria immitis (AAF37720.1), Onchocerca volvulus (AAA20541.1), and Caenorhabditis briggsae (XP 001679448.1). Asterisks show identical residues in the compared sequences.
Specificity and immunoreactivity of the lactose-binding fraction in AgS.
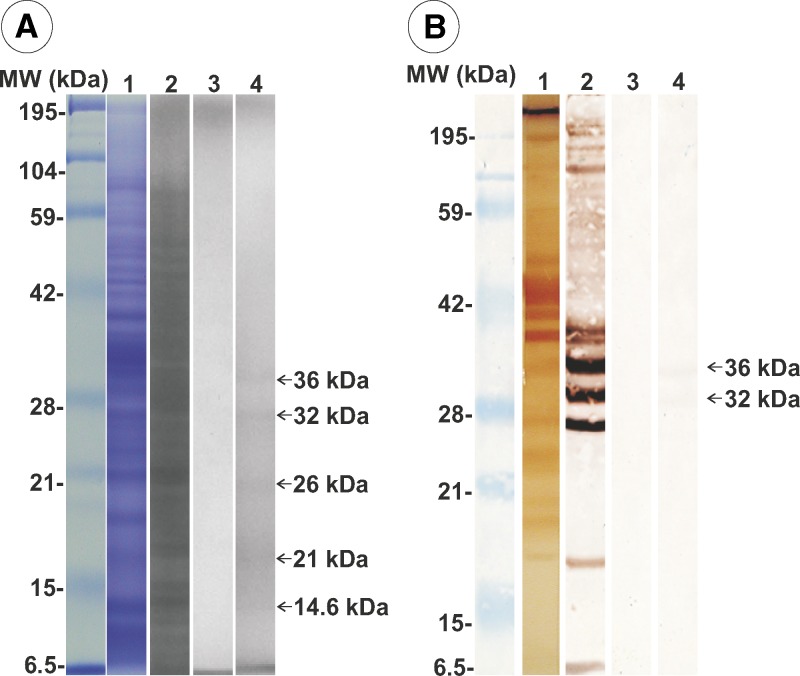
Most AgS proteins were eluted in the unbound fraction 1 from lactose affinity chromatography (Figure 7A, lane 2), and fraction 2 did not show any protein (Figure 7A, lane 3). Conversely, fraction 3 of the lactose bound proteins showed only five peptides (14.6, 21, 26, 32, and 36 kDa) (Figure 7A, lane 4). The IET of total AgS showed a large number of antigenic peptides (Figure 7B, lane 1) and in unbound fraction 1 (Figure 7B, lane 2). Fraction 2 did not show any antigenic peptide bands (Figure 7B, lane 3). In fraction 3, which was composed of bound column proteins eluted with lactose buffer, 32-kDa and 36-kDa bands reacted with antibodies in serum from a patient with confirmed gnathostomiasis (by ELISA and recovery of larva from a biopsy) (Figure 7B, lane 4).
Figure 7.
Analysis of fractions from lactose affinity chromatography of somatic antigens of Gnathostoma binucleatum. A, Lane 1, sodium dodecyl sulfate–polyacrylamide gel electrophoresis of total somatic antigens; lane 2, unbound proteins (fraction 1); lane 3, proteins not detected in fraction 2; lane 4, fraction with lactose affinity. B. Lane 1, Immunoelectrotransfer of total somatic antigens; lane 2, fraction 1; lane 3, fraction 2; lane 4, two proteins with lactose affinity (fraction 3). MW = molecular weight; kDa = kilodaltons.
In the evaluation of the galectin-like fraction as an immunodominant antigen for gnathostomiasis, the ELISA showed that AgS was recognized by serum samples from all patients (11 of 11) with gnathostomiasis. However, seven of eight serum samples from patients infected with other parasites also showed positive results. The fraction with lactose affinity showed 100% specificity and sensitivity.
Discussion
One of the main problems with immunodiagnosis has been the limitation of obtaining enough antigen, particularly in areas to which diseases are not endemic.5 Antigens are mostly obtained from the larval stages in the natural intermediate hosts found in infected fresh water fish in disease-endemic zones.18
This study further characterized protein antigens of a larval parasite stage. The SDS-PAGE profile of AgS was complex (molecular weight range = 6–194.4 kDa) (Figure 1A). These results are consistent with reported SDS-PAGE patterns of antigens of G. binucleatum larvae and adults in which the protein molecular weight range was < 20–208 kDa.17 We did not find bands with high molecular weights. One possible reason for such differences could be the source of the larvae. In the present study, we used larvae from intermediate host fishes, but larvae used by Caballero-Garcia and others17 were from experimentally infected mice. Continuous growth of G. spinigerum L3A in mice has been reported.10,25
When the SDS-PAGE pattern of G. binucleatum AgS in our study was compared with those of other Gnathostoma species (G. spinigerum, G. hispidum, and G. doloresi), they were also complex, but similar with bands with a molecular weight range of 15 kDa to 106 kDa.3 In addition, G. binucleatum had bands with higher molecular weights (Figure 1A).
Electrophoretic patterns of AgS by two-dimensional immunoblot analysis were even more complex. We found 119 spots with a molecular weight range of 7–110 kDa, pI 5-8 (Figure 1B). This number of spots was larger than those reported for larvae and male and female adults of G. spinigerum, which showed 75, 44, and 53 prominent spots, respectively.26 Wongkam and others26 reported that G. spinigerum larvae have more proteins spots in the range of 30–43 kDa and pI 4.6–5.7 than adult specimens, and adult female antigen has protein spots in the range of 20.1–30 kDa and pI 6–7, which were not present in adult males.26 Similar to our results, most proteins of G. spinigerum were neutral or acidic.26 The SDS-PAGE patterns for AgES, with 22 bands 6–190 kDa (Figure 1C), were more complex and different from those in L3A recovered from experimentally infected mice (28–208 kDa).16 Two-dimensional immunoblot analysis patterns from this study (Figure 1D) were even more complex, showing 59 spots (9–75 kDa, pI 6–7.5).
The results of IET of AgS showed a large number of antigenic proteins, most of which were cross-reactive and only eight (21, 24, 26, 32, 40, 50, 80, and 157 kDa) were specifically recognized by serum samples from patients infected with G. binucleatum (Figure 2). Previous IET analysis using a total G. binucleatum extract17 showed only four antigenic proteins (40, 60, 80, and 115 kDa), and a total adult worm extract showed that only the 80-kDa and the 115-kDa two proteins were recognized, but the 115 kDa protein was cross-reactive with A. lumbricoides, Toxocara canis, and Ancylostoma caninum. In the same study, it was also reported that the 40-kDa protein did not cross-react with serum samples of patients with other parasitic diseases.
Using G. spinigerum L3 antigen and the serum of infected patients, Tapchaisri and others14 identified more than 20 antigenic bands (13–150 kDa), 16 of which were prominent (21, 22, 24, 32, 33, 34, 35, 38, 42, 43, 49, 94, 120, 135, and 150 kDa), that were recognized commonly by serum samples from most patients with gnathostomiasis. Proteins with molecular weights > 30 kDa cross-reacted with serum samples from patients with other parasitic diseases, and some high molecular weight proteins reacted nonspecifically with serum samples from healthy persons. In this study, the authors proposed that the 24-kDa protein was a specific diagnostic tool for gnathostomiasis.14 The same antigen was also found in the somatic extract of G. spinigerum L3 collected from eels27 and was confirmed by using a monoclonal antibody against the protein.28 Larvae from G. spinigerum (recovered from infected mice) also contained the 24-kDa antigen, which was localized mainly in the esophagus and intestine of L3 larva of G. spinigerum.29 In patients with neurognathostomiasis caused by G. spinigerum, 13 SDS-PAGE bands were suggested to be diagnostic (21, 24, 29, 31, 33, 44.5, 49, 52, 60, 85, 88, 92, and 110 kDa), among which the 21-kDa and 24-kDa proteins were the most sensitive and 100% specific for diagnosis.11 This antigen was not found in L3A from G. binucleatum obtained from experimentally infected mice.17 However, in the present study, using larvae recovered from infected fish, we detected an antigenic band of 24 kDa (Figure 2).
In the present study, using L3A from G. binucleatum, we identified a specific protein of 40 kDa (Figure 2). This band was identified in Gnathostoma (spinigerum, hispidum, and doloresi) adults and was immunogenic by IET with serum samples from patients infected with one of these three species.3 In a previous report,17 the 40-kDa protein was not found in the adult stage of G. binucleatum.
In this study, AgES showed three antigenic proteins (42, 50, and 56 kDa) by IET with serum samples from patients with gnathostomiasis. However, the 50-kDa and 56 kDa proteins cross-reacted with serum samples from patients infected with S. stercoralis. In addition, the 42-kDa protein also reacted nonspecifically with serum samples of healthy persons (Figure 3). Our results contrasted with those reported for AgES of L3 obtained from infected mice in that three bands (80, 120, and 208 kDa) were found by using serum samples from infected mice, and one reactive band (120 kDa) was detected with a human serum sample from an infected person.16
We report IET patterns and two-dimensional immunoblot analysis of AgS (Figure 4) and AgES (Figure 5) obtained from L3A of G. binucleatum. Four peptides in AgS, three with molecular weights of 32 kDa (pI 6.3, 6.5 and 6.9) and one with a molecular weight of 40 kDa (pI 5.6), were identified as specific antigens (Figure 4) that showed no cross-reactivity with serum samples from healthy persons. In the two-dimensional immunoblot analysis results with L3 of G. spinigerum, AgS showed 30–70 antigen spots (14.4–94 kDa, pI 4.65–9.6), spots with molecular weights > 30 kDa cross-reacted with serum samples from patients with angiostrongyliasis, cysticercosis, and healthy persons. Spots with lower molecular weights (23–25 kDa, pI 8.3–8.5) were more specific for the diagnosis of gnathostomiasis (80–83%).26 Immunoelectrotransfer showed that AgES had six antigen spots (42 kDa, pI 6.8; 44 kDa, pI 6,8; 56 kDa, pI 6.3, 6.5, 6.6, and 6.7) (Figure 5A): four were specific for gnathostomiasis and two cross-reacted with serum samples from patients infected with S. stercoralis (56 kDa, pI 6.6 and 6.7) (Figure 5B).
Sequence analysis performed on two of the four proteins from AgS separated by two-dimensional immunoblot analysis (Figure 4) showed that the 32-kDa protein (pI 6.3 and 6.5) aligned with galectin-1 (Figure 6). This molecule has been identified in a free-living nematode (Caenorhabditis elegans).30 Moreover, this molecule was also found in an array of parasitic nematodes such as B. malayi,31 D. immitis, A. caninum, T. colubriformis, A. cantonensis, and Teladorsagia circumcincta.8,32–34 Galectin proteins show β-galactoside affinity, and they are structurally characterized by a carbohydrate recognition domain, which codes information for cell-to-cell and cell-to-extracellular matrix interactions;35 they also mediate the invasion into host, and participate in maintaining infection and modulating the host immune response.36 Although galectins of the parasitic helminthes have been poorly studied, two recombinant galectins of Haemonchus contortus (rHco-gal-m and rHco-gal-f) were used for vaccination of 9–10-month-old goats against infection by this parasite; the application of 100 μg of protein reduced fecal egg output and worm burdens by 37.25% and 41.1%, respectively.37
Because galectins were identified as the immunodominant antigens in AgS G. binucleatum L3, AgS was fractionated by lactose affinity chromatography and two proteins were obtained (32-kDa and 36 kDa), which bound with serum antibody from a patient with confirmed gnathostomiasis (Figure 7, lane 4), and it is assumed that the 32-kDa protein might correspond to one of the sequences of AgSGb1 or AgSGb2 (Figure 6).
In the present study, total AgS exhibited 100% sensitivity, but reduced specificity because of cross-reactivity with serum samples from patients with other parasitic diseases and also because of non-specific reactions with serum samples from healthy persons. This finding suggests that the main problem in using crude AgS from G. binucleatum larvae is cross-reactivity or nonspecific reactions, which was not seen when lactose affinity–purified protein antigens were used for IET. In conclusion, the present results demonstrate that a fraction with lactose affinity obtained from L3A larvae of G. binucleatum has a 100% sensitivity and specificity for the immunodiagnosis of human gnathostomiasis.
ACKNOWLEDGMENTS
We thank María del Carmen de la Cruz Otero and Dr. Jose Angel López Valenzuela (Facultad de Ciencias Químico Biológicas de la Universidad Autónoma de Sinaloa) for providing technical support, and Dr. Yukifumi Nawa (Department of Parasitology, Faculty of Medicine of the Khon Kaen University, Khon Kaen Thailand) for critically reviewing the manuscript.
Footnotes
Financial support: This study was supported by the Consejo Nacional de Ciencia y Tecnología (CO1-48161) and the Programa de Fomento y Apoyo a la Investigación de la Universidad Autonoma de Sinaloa (PROFAPI-UAS, 2007/171).
Authors' addresses: Samuel Campista-León, Francisco Delgado-Vargas, Héctor Samuel López-Moreno, Julian Ríos-Sicairos, Ángel Noel Bojórquez-Contreras, and Sylvia Páz Díaz-Camacho, Facultad de Ciencias Químico Biológicas, Universidad Autónoma de Sinaloa, Culiacán, Sinaloa, México, E-mails: calsa68@hotmail.com, fdelgado@uas.edu.mx, hsamlo@uas.edu.mx, julianriossicairos@gmail.com, angelnbc@uas.edu.mx, and spdiazc@uas.edu.mx. Abraham Landa, Kaethe Willms, and Guillermo Mendoza-Hernández, Facultad de Medicina, Universidad Nacional Autónoma de México, México City D.F., México, E-mails: landap@servidor.unam.mx, kaethe@servidor.unam.mx, and menher@servidor.unam.mx.
References
- 1.Almeyda-Artigas RJ, Bargues MD, Mas-Coma S. ITS-2 rDNA sequencing of Gnathostoma species (Nematoda) and elucidation of the species causing human gnathostomiasis in the Americas. J Parasitol. 2000;86:537–544. doi: 10.1645/0022-3395(2000)086[0537:IRSOGS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Díaz-Camacho SP, De la Cruz-Otero MC, Willms K. Gnathostomosis. Revista de la Facultad de Medicina de la UNAM. 2000;43:192–201. [Google Scholar]
- 3.Ishiwata K, Diaz-Camacho SP, Ogata K, Nakamura-Uchiyama F, Hiromatsu K, Nawa Y. Evaluation of the antigenic similarities of adult-worm extracts from three Gnathostoma species, using sera from Mexican and Japanese patients with Gnathostoma infections. Ann Trop Med Parasitol. 2003;97:629–637. doi: 10.1179/000349803225001490. [DOI] [PubMed] [Google Scholar]
- 4.Tada I, Araki T, Matsuda H, Araki K, Akahane H, Mimori T. A study on immunodiagnosis of gnathostomiasis by ELISA and double diffusion with special reference to the antigenicity of Gnathostoma doloresi. Southeast Asian J Trop Med Public Health. 1987;18:444–448. [PubMed] [Google Scholar]
- 5.Waikagul J, Diaz-Camacho SP. Gnathostomiasis. In: Murrell KW, Fried B, editors. World Class Parasites: Food-Borne Parasitic Zoonoses. Fish and Plant Borne Parasites. New York: Springer-Verlag; 2007. pp. 235–261. [Google Scholar]
- 6.Diaz-Camacho SP, Willms K, de la Cruz-Otero M del C, Zazueta-Ramos ML, Baylis-Gaxiola S, Castro-Velazquez R, Osuna-Ramirez I, Bojorquez-Contreras A, Torres-Montoya EH, Sanchez-Gonzales S. Acute outbreak of gnathostomiasis in a fishing community in Sinaloa, Mexico. Parasitol Int. 2003;52:133–140. doi: 10.1016/s1383-5769(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 7.Diaz-Camacho SP, Zazueta-Ramos M, Ponce-Torrecillas E, Osuna-Ramirez I, Castro-Velazquez R, Flores-Gaxiola A, Baquera-Heredia J, Willms K, Akahane H, Ogata K, Nawa Y. Clinical manifestations and immunodiagnosis of gnathostomiasis in Culiacan, Mexico. Am J Trop Med Hyg. 1998;59:908–915. doi: 10.4269/ajtmh.1998.59.908. [DOI] [PubMed] [Google Scholar]
- 8.Mulvenna J, Hamilton B, Nagaraj SH, Smyth D, Loukas A, Gorman JJ. Proteomics analysis of the excretory/secretory component of the blood-feeding stage of the hookworm Ancylostoma caninum. Mol Cell Proteomics. 2009;8:109–121. doi: 10.1074/mcp.M800206-MCP200. [DOI] [PubMed] [Google Scholar]
- 9.Morakote N, Nateewatana N, Maleewong W, Uthong T. Anatomical localization of Gnathostoma spinigerum larval antigens by an indirect fluorescent antibody test. Southeast Asian J Trop Med Public Health. 1989;20:291–295. [PubMed] [Google Scholar]
- 10.Rojekittikhun W, Pubampen S. Morphological variation and abnormality of cephalic hooklets of Gnathostoma spinigerum hepatic stage larvae from laboratory infected mice. Southeast Asian J Trop Med Public Health. 1998;29:118–122. [PubMed] [Google Scholar]
- 11.Intapan PM, Khotsri P, Kanpittaya J, Chotmongkol V, Sawanyawisuth K, Maleewong W. Short report: immunoblot diagnostic test for neurognathostomiasis. Am J Trop Med Hyg. 2010;83:927–929. doi: 10.4269/ajtmh.2010.10-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kongkerd N, Uparanukraw P, Morakote N, Sajid M, McKerrow JH. Identification and characterization of a cathepsin L-like cysteine protease from Gnathostoma spinigerum. Mol Biochem Parasitol. 2008;160:129–137. doi: 10.1016/j.molbiopara.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Laummaunwai P, Intapan PM, Wongkham C, Lulitanond V, Tayapiwatana C, Maleewong W. Gnathostoma spinigerum: molecular cloning, expression and characterization of the cyclophilin protein. Exp Parasitol. 2010;126:611–616. doi: 10.1016/j.exppara.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Tapchaisri P, Nopparatana C, Chaicumpa W, Setasuban P. Specific antigen of Gnathostoma spinigerum for immunodiagnosis of human gnathostomosis. Int J Parasitol. 1991;21:315–319. doi: 10.1016/0020-7519(91)90033-4. [DOI] [PubMed] [Google Scholar]
- 15.Uparanukraw P, Morakote N, Harnnoi T, Dantrakool A. Molecular cloning of a gene encoding matrix metalloproteinase-like protein from Gnathostoma spinigerum. Parasitol Res. 2001;87:751–757. doi: 10.1007/s004360100440. [DOI] [PubMed] [Google Scholar]
- 16.Caballero-Garcia ML, Almeyda-Artigas RJ, Mosqueda-Cabrera MA, Jimenez-Cardoso E. Gnathostoma binucleatum: excretion-secretion antigen analysis obtained from advanced third-stage larvae in in vitro culture. Exp Parasitol. 2005;110:140–145. doi: 10.1016/j.exppara.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 17.Caballero-Garcia ML, Almeyda-Artigas RJ, Mosqueda-Cabrera MA, Jimenez-Cardoso E. Protein profile analysis from advanced third-stage larvae (AdvL3) and adult worms of Gnathostoma binucleatum (Nematoda: Spirurida) Parasitol Res. 2007;100:555–560. doi: 10.1007/s00436-006-0354-1. [DOI] [PubMed] [Google Scholar]
- 18.Díaz-Camacho SP, de la Cruz Otero MC, Zazueta Ramos ML, Bojórquez-Contreras Á, Sicairos-Félix J, Campista-León S, Guzmán-Loreto R, Delgado-Vargas F, León-Règagnon V, Nawa Y. Identification of estuarine fish Dormitator latifrons as an intermediate host and Eleotris picta as a paratenic host for Gnathostoma binucleatum. Parasitol Res. 2008;106:1421–1425. doi: 10.1007/s00436-008-1151-9. [DOI] [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli UK, Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973;80:575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- 21.O'Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 22.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Zamorano M, Mendoza-Hernandez G, Xolalpa W, Parada C, Vallecillo AJ, Bigi F, Espitia C. Mycobacterium tuberculosis glycoproteomics based on ConA-lectin affinity capture of mannosylated proteins. J Proteome Res. 2009;8:721–733. doi: 10.1021/pr800756a. [DOI] [PubMed] [Google Scholar]
- 24.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anantaphruti M, Waikagul J, Nithi-uthai S, Pubampen S, Rojekittikhun W. Detection of humoral immune response to Gnathostoma spinigerum in mice. Southeast Asian J Trop Med Public Health. 1986;17:172–176. [PubMed] [Google Scholar]
- 26.Wongkham C, Maleewong W, Leamviteevanich K, Intapan PM, Morakote N. Antigenic components of Gnathostoma spinigerum recognized by infected human sera by two-dimensional polyacrylamide gel electrophoresis and immunoblotting. Asian Pac J Allergy Immunol. 2000;18:47–52. [PubMed] [Google Scholar]
- 27.Nopparatana C, Setasuban P, Chaicumpa W, Tapchaisri P. Purification of Gnathostoma spinigerum specific antigen and immunodiagnosis of human gnathostomiasis. Int J Parasitol. 1991;21:677–687. doi: 10.1016/0020-7519(91)90079-m. [DOI] [PubMed] [Google Scholar]
- 28.Chaicumpa W, Ruangkunaporn Y, Nopparatana C, Chongsa-Nguan M, Tapchaisri P, Setasuban P. Monoclonal antibody to a diagnostic Mr 24,000 antigen of Gnathostoma spinigerum. Int J Parasitol. 1991;21:735–738. doi: 10.1016/0020-7519(91)90089-p. [DOI] [PubMed] [Google Scholar]
- 29.Nopparatana C, Chaicumpa W, Tapchaisri P, Setasuban P, Ruangkunaporn Y. Towards a suitable antigen for diagnosis of Gnathostoma spinigerum infection. Int J Parasitol. 1992;22:1151–1156. doi: 10.1016/0020-7519(92)90034-i. [DOI] [PubMed] [Google Scholar]
- 30.Itagaki T, Nishizaki S, Sekihashi K, Kobayashi H, Kidokoro S, Kezuka Y, Arata Y, Hirabayashi J, Kasai K, Nonaka T. Crystallization and preliminary x-ray crystallographic analysis of galectin LEC-1 from Caenorhabditis elegans. Protein Pept Lett. 2008;15:419–422. doi: 10.2174/092986608784246407. [DOI] [PubMed] [Google Scholar]
- 31.Hewitson JP, Harcus YM, Curwen RS, Dowle AA, Atmadja AK, Ashton PD, Wilson A, Maizels RM. The secretome of the filarial parasite Brugia malayi: proteomic profile of adult excretory-secretory products. Mol Biochem Parasitol. 2008;160:8–21. doi: 10.1016/j.molbiopara.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Athanasiadou S, Pemberton A, Jackson F, Inglis N, Miller HR, Thevenod F, Mackellar A, Huntley JF. Proteomic approach to identify candidate effector molecules during the in vitro immune exclusion of infective Teladorsagia circumcincta in the abomasum of sheep. Vet Res. 2008;39:58. doi: 10.1051/vetres:2008035. [DOI] [PubMed] [Google Scholar]
- 33.Kiel M, Josh P, Jones A, Windon R, Hunt P, Kongsuwan K. Identification of immuno-reactive proteins from a sheep gastrointestinal nematode, Trichostrongylus colubriformis, using two-dimensional electrophoresis and mass spectrometry. Int J Parasitol. 2007;37:1419–1429. doi: 10.1016/j.ijpara.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Pou-Barreto C, Quispe-Ricalde MA, Morchon R, Vazquez C, Genchi M, Postigo I, Valladares B, Simon F. Galectin and aldolase-like molecules are responsible for the specific IgE response in humans exposed to Dirofilaria immitis. Parasite Immunol. 2008;30:596–602. doi: 10.1111/j.1365-3024.2008.01060.x. [DOI] [PubMed] [Google Scholar]
- 35.Hirabayashi J, Kasai K-i. The family of metazoan metal-independent b-galactoside-binding lectins: structure, function and molecular evolution. Glycobiology. 1993;3:297–304. doi: 10.1093/glycob/3.4.297. [DOI] [PubMed] [Google Scholar]
- 36.Álvarez-Fernández G, Bustos-Jaimes I, Castañeda-Patlán C, Guevara-Fonseca J, Romero-Álvarez I, Vázquez-Meza H. Aspectos bioquimicos, estructurales y funcionales de las galectinas en la inmunidad. Mensaje Bioquímico, UNAM XXXIV: 2010:17–30. [Google Scholar]
- 37.Yanming S, Ruofeng Y, Muleke CI, Guangwei Z, Lixin X, Xiangrui L. Vaccination of goats with recombinant galectin antigen induces partial protection against Haemonchus contortus infection. Parasite Immunol. 2007;29:319–326. doi: 10.1111/j.1365-3024.2007.00949.x. [DOI] [PubMed] [Google Scholar]