Abstract
Twenty cases of Dyphillobothrium pacificum (fish tapeworm) infections were prospectively studied to determine whether this tapeworm is associated with megaloblastic anemia, as commonly reported for D. latum infections. The most frequent symptoms were fatigue and mild abdominal pain, which were identified in approximately 66.6% of the 18 patients interviewed. Fourteen patients received treatment with niclosamide and all were cured. The other six patients spontaneously eliminated the tapeworms. One patient, who also had chronic diabetes and gastric atrophy, had low vitamin B12 levels and megaloblastic anemia. In all other patients, including three other patients with anemia, baseline vitamin B12 levels were in the reference range and did not significantly change when re-assessed three months later. Unlike D. latum, infection with D. pacificum is seldom associated with megaloblastic anemia or vitamin B12 deficit.
Introduction
Human diphyllobothriasis (fish tapeworm infection) is mainly caused by two species, Dyphillobothrium latum and D. pacificum, and rarely by D. nihonkaiense, D. cordatum, D. ursi, D. dendriticum, D. lanceolatum, D. dalliae, or D. yonagoensis.1–3 Humans are an accidental definitive host of these parasites, which usually infect sea lions or fur seals,1 and usually harbor a single tapeworm, which in the case of D. latum, may reach 12 meters in length.1,4
Diphyllobothrium eggs are ovoid, operculated, and non-embryonated, and have a maximal diameter of 50–60 μm. A ciliated embryo is released in an aquatic environment and infects microcrustaceans, which serve as intermediate invertebrate hosts for the parasite. Inside microcrustaceans, the parasite develops into an infective procercoid larva. Fish become infected by ingesting microcrustaceans carrying the procercoid larva. Inside the fish, the larva will migrate to the muscles and develop into a plerocercoid larva that is infective to humans and other animals, which can serve as definitive hosts. Infection in humans is usually caused after ingestion of undercook fish infected with the plerocercoid larva.2
Diagnosis of diphyllobothriasis can be achieved by microscopic identification of operculated eggs in fecal samples.2,5 Spontaneous elimination of proglottids in feces provides histologic differentiation and occasionally might enable species confirmation when it is possible to identify specific morphologic features of the genital pore. However, molecular techniques have been extensively used to differentiate Diphyllobothrium spp.6,7 Symptoms of infection are mild and include abdominal pain, bloating, diarrhea, anorexia, headaches, constipation, and vomiting.1,2 Treatment with niclosamide or praziquantel is highly effective.8,9 Vitamin B12 deficiency resulting in megaloblastic anemia and rarely mechanic intestinal obstruction has been reported in association with D. latum.10–13
Megaloblastic anemia results from altered DNA synthesis of all blood cells, usually resulting from vitamin B and or folic acid deficiencies.14 Diphyllobothrium latum competes with the host for vitamin B12.1,2,10–13 In Nordic countries, megaloblastic anemia secondary to dyphillobothriasis has been reported.13 In these cases, oral or parenteral vitamin B12 administration after parasite expulsion brings levels back to reference range.13
Diphyllobothrium pacificum infection (Nybellin 1931, Margolis 1956)15 is endemic to the Pacific coasts of South America and southern Asia,1,6 and is the only type of diphyllobothriasis described in Peru to date. It was present before the European colonization, as demonstrated by the finding of characteristic eggs in pre-Hispanic mummies.15–17 Its prevalence may reach 2% in some coastal communities,15 and it can also be found in high mountain towns because of the widespread distribution and commercialization of marine fish for ceviche, which consists of raw fish marinated in lemon juice and other ingredients.15
Before molecular biology techniques were available, species were identified by detecting transversal sulci between the anterior border of the proglottid and the common genital pore.14,18 Unlike the Nordic species D. latum, which is a parasite of freshwater fish, D. pacificum is a parasite of saltwater fish, and it is much smaller, usually < 1 meter in length.2 Megaloblastic anemia has not been systematically assessed in persons infected with D. pacificum. We evaluated a consecutive series of patients infected with D. pacificum to assess whether this parasite was associated with vitamin B12 deficiency or megaloblastic anemia.
Materials And Methods
Study population.
The study was performed during March 2009–January 2011 and included patients with a confirmed diagnosis of diphyllobothriasis by demonstration of parasite eggs or parasite material in feces. Most patients were identified during a region-wide cysticercosis control program in northern coastal Peru. Patients were asked to provide additional fecal samples and baseline and day 90 blood samples for hematologic analysis and determination of vitamin B12 levels. Exclusion criteria included pregnancy or unwillingness to comply with the day 90 follow-up visit. Patients given a diagnosis > 15 days before initial contact with the study team were requested to provide new baseline fecal samples and were only included if current infection was proven. The study and informed consent forms were reviewed and approved by the institutional review board of the Universidad Peruana Cayetano Heredia in Lima, Peru.
Patients with Diphyllobothirum eggs in feces received anti-parasitic treatment with niclosamide, as per standard of care.2 Post-treatment fecal samples were collected for 48 hours for detection of parasite material. Cure was confirmed by microscopic analysis of feces at day 30. Patients had additional fecal examinations performed at days 60 and 90 post-treatment to rule out treatment failure or early re-infection. Fecal samples were analyzed macroscopically to detect proglottids, then preserved in phosphate-buffered saline containing 5% formaldehyde at a final dilution of 1:5. Twenty milliliters of sample was concentrated by using the spontaneous tube sedimentation technique5 and read by microscopy at 100×. Fecal examinations were performed by a microscopist in a local laboratory, and results were confirmed by a second microscopist in a reference laboratory.
After a case report questionnaire that included demographic and epidemiologic information was completed, 5-mL blood samples were collected by venipuncture at baseline (not more than 15 days after treatment or spontaneous parasite expulsion) and 90 days later for determination of hematocrit and vitamin B12 levels. Hematocrits were determined by using heparinized capillary tubes after centrifugation of blood at 10,000 rpm for 5 minutes. The reference values used for this study were 40–54% in males and 38–48% in females.19 Patients with anemia were given their hematocrit and vitamin B12 results and referred to the local health center for treatment. Serum was separated and frozen at –20°C until transport to a private reference laboratory where vitamin B12 levels were measured by using an electrochemoluminescence immunoassay in a E170 Cobas apparatus (Roche, Mannheim, Germany). Reference values for this assay ranged from 243 to 894 pg/mL for persons from the United States and from 197 to 866 pg/mL for persons from Europe.20 Because no specific reference values for the population in Peru were available, we conservatively used the reference range for persons from the United States.
Results
The study included 20 patients, mostly from the area of the cysticercosis elimination study (northern Coastal Peru, Tumbes [n = 17] and Piura [n = 2]), plus one patient who lived in Lima. There were 13 women and seven men in the study (mean age = 25 years, age range = 3–66 years). Patients had either received niclosamide treatment as part of a mass chemotherapy campaign aimed to control Taenia solium cysticercosis in their region (n = 4) or treatment at their local health centers. Dyphyllobothrium pacificum specimens or proglottid material were recovered from 15 patients either by spontaneous elimination or after anti-parasitic treatment. Three specimens were confirmed by polymerase chain reaction as D. pacificum, as part of another study that genotyped isolates obtained from parasite-positive persons.6 Recovered worms were of similar sizes, approximately 70 cm in length (Figure 1).
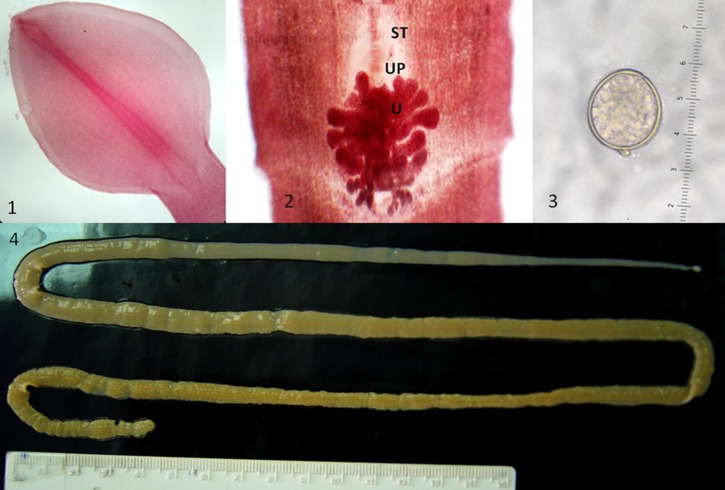
Figure 1.
Diphyllobothrium pacificum specimens. 1, Top left: heart-shaped scolex. 2, Top center: proglottid showing the uterus (U), transversal sulci (ST), and uterine pore (UP). 3, Top right: egg (operculum = 60 μm). 4, Bottom: strobila of D. pacificum (approximate length = 70 cm).
At baseline, 18 of the 20 participants answered the study survey (two refused to provide a blood sample) and two provided a baseline blood sample but did not answer the survey. Of the 18 respondents to the questionnaire, all reported frequent consumption of fish, mostly fried (94%, 17 of 18), stewed (83%, 15 of 18), or as ceviche (72%, 13 of 18). Most frequently consumed fish species were Trachurus symmetricus murphyi (jurel) by 67% of the persons, Sarda chiliensis (bonito) by 17%, Coryphaena hippurus (perico) by 17%, Mugil cephalus (lisa) by 17%, or Paralonchurus polyclemus peruanus (coco) by 11%. The most frequently reported symptoms were fatigue and mild abdominal pain (12 of 18, 66.6%), followed by constipation and diarrhea in 44% (8 of 18), weight loss (3 of 18, 16%), and loss of appetite (2 of 18, 11%).
Baseline hematologic and vitamin B12 levels.
Of 18 baseline blood samples, 16 had a hematocrit determined. The mean (SD) hematocrit value was 38.94 (4.12). Five patients had anemia, which was defined as a hematocrit < 40% for men and < 38% for women19 (Table 1).
Table 1.
Vitamin B12 levels and hematocrits in patients in Peru infected with Diphyllobothrium pacificum*
| Patient | Sex | Age, years | Serum vitamin B12 level, pg/mL | Hematocrit | Diagnostic finding | Symptoms† | Dietary habits‡ | ||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Day 90 | Baseline | Day 90 | ||||||
| 1 | M | 14 | 377 | – | 38 | – | Eggs | 1 | a, b, c |
| 2 | F | 41 | 283.1 | 480,1 | 37 | – | Eggs | 1, 2, 3 | a, b, c |
| 3 | F | 10 | 647.9 | 626,6 | 40 | 40 | Eggs | 1, 2, 3 | a, b, c |
| 4 | F | 48 | 387.1 | 352,4 | 44 | 42 | Eggs | 1 | a, b, c |
| 5 | M | 3 | 585.6 | 670,7 | 38 | 39 | Eggs | 1, 2, 4, 5 | a, b, c |
| 6 | F | 66 | 198.7 | 143,3 | 33 | 36 | Eggs | 1, 2, 3 | a, b, c |
| 7 | M | 21 | 317.1 | 348,6 | 37 | 45 | Proglottids | 1, 5 | a, b, c |
| 8 | M | 41 | 486.8 | – | 41 | – | Proglottids | 2, 4 | a, b, c |
| 9 | M | 28 | 293.8 | – | 48 | – | Proglottids | 2, 5 | a, b, c |
| 10 | M | 23 | 377.2 | 360,3 | 45 | 41 | Proglottids | 1, 2, 4, 6 | a, b, c |
| 11 | F | 8 | 1,120 | – | 35 | – | Proglottids | 6 | a, c |
| 12 | F | 17 | 399.6 | – | – | – | Proglottids | None | None |
| 13 | F | 24 | 740.7 | – | 40 | – | Proglottids | 2, 3 | a, b |
| 14 | F | 56 | 517 | 649,5 | 33 | 39 | Proglottids | 2, 4 | a, b, c |
| 15 | F | 50 | 532.2 | 620,5 | 39 | 39 | Proglottids | 1, 2, 3, 4 | a, b, c |
| 16 | F | 23 | 308.7 | – | – | – | Eggs | None | None |
| 17 | F | 14 | 929 | 1120 | 39 | 39 | Proglottids | 3, 4 | None |
| 18 | F | 33 | – | – | – | – | Proglottids | 1, 2, 3 | a, b, c |
| 19 | F | 27 | – | – | 36 | – | Proglottids | 1, 2, 4 | a, b, c |
| 20 | M | 26 | – | – | – | – | Eggs | 1, 3, 4 | a |
Patients with anemia are shown in bold.
1 = abdominal pain; 2 = fatigue; 3 = constipation; 4 = diarrhea; 5 = weight loss; 6 = loss of appetite.
Reported consumption of fish: a = fried; b = stewed; c = ceviche.
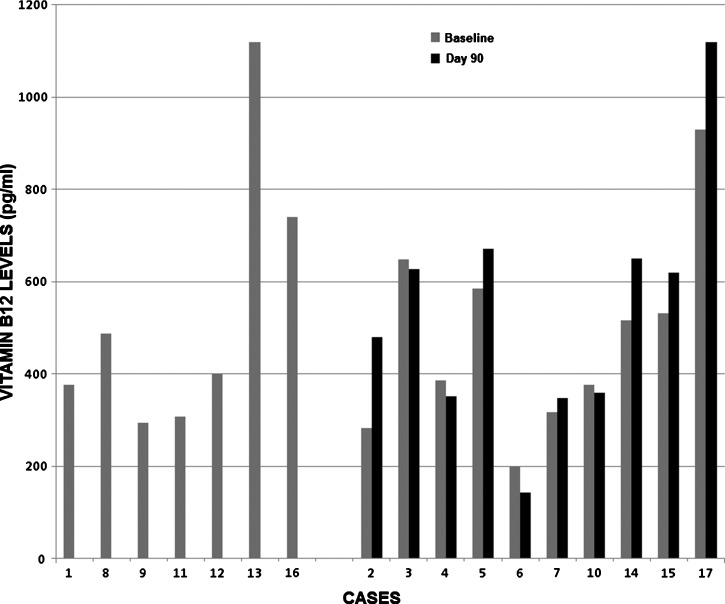
Seventeen persons had baseline vitamin B12 levels determined. All vitamin B12 levels were in reference range except for the patient above described, whose baseline level was 199 pg/mL (reference range for her age was 243–894 pg/mL). The median vitamin B12 level was 437 pg/mL and the interquartile range was 332–572.5 pg/mL (Figure 2).
Figure 2.
Serum vitamin B12 levels at baseline and 90 days after treatment in 17 patients in Peru infected with Diphyllobothrium pacificum.
The patient with a low hematocrit (33%, reference range = 36–46) and a low vitamin B12 level (198.7 pg/mL, reference range = 243–894 pg/mL) was a 66-year-old women with a 10-year history of diabetes mellitus, which caused a neurogenic bladder, repeated Escherichia coli urinary tract infections in the past five years, and endoscopy-diagnosed gastric atrophy.
Examination of a peripheral blood smear from the anemic patient showed 2–3% macroovalocytes without neutrophil hypersegmentation. A diagnosis of megaloblastic anemia was made and the patient received vitamin B12 for seven days, plus oral hypoglycemic drugs (glibenclamide) for treatment of diabetes.
Day 90 hematologic and vitamin B12 levels.
Ten participants complied with the 90-day follow-up and provided a follow-up blood sample. The hematocrit was the reference value for all persons, including the patient with anemia at baseline, who now had a borderline value of 36%. All vitamin B12 levels were in the reference range (median = 550.5 pg/mL, interquartile range = 351–638 pg/mL) except for the patient who had low vitamin B12 levels at baseline and now had a level of 143 pg/mL 90 days after successful treatment for dyphillobothriasis. A follow-up smear at this time showed erythrocytes with normal size and slight hypochromia.
Paired comparison between baseline and 90-day vitamin B12 levels in these 10 patients demonstrated an increase of borderline statistical significance (477.49 pg/mL versus 537.20 pg/mL: P = 0.075, by paired Student's t-test).
Discussion
Diphyllobothriasis is endemic to different regions in Europe, Asia, and South America. This parasitosis is caused by consumption of poorly cooked fish. The common causative agent (D. latum) is a large tapeworm that can reach a length of 20 meters. One of its more conspicuous clinical manifestations in humans is megaloblastic anemia, which is caused by the parasite intake of vitamin B12 or folic acid within the host.1–4 Clinically evident megaloblastic anemia is found in 2–3% of persons harboring a D. latum tapeworm, and > 50% of D. latum carriers have low levels of serum vitamin B12 levels (< 100 pg/mL compared with 350–450 pg/mL in non-infected persons).13 In Pacific coast regions of South America, diphyllobothriasis is usually caused by D. pacificum, which is a shorter tapeworm (approximately 1 meter in length), but can have a length of ≤ 4 meters on rare occasions.
We evaluated a series of consecutive patients with D. pacificum infections to assess whether they had vitamin B12 deficiency or megaloblastic anemia. Our study demonstrated that vitamin B12 deficiency and megalobastic anemia are not common in persons infected with D. pacificum. However, we identified a patient with a moderately low hematocrit (33%) associated with a low vitamin B12 level (199 pg/mL [reference value = 243 pg/mL], which decreased to 143 pg/mL at day 90 of follow-up). All other infected persons had levels > 290 pg/mL (median = 437 pg/mL). In the patient with anemia and low vitamin B12 levels, erythrocytes showed changes suggestive of megaloblastosis. At 90 days, despite an increase in hematocrit, disappearance of erythrocytic megaloblastic changes, and appearance of signs of iron deficiency, vitamin B12 levels for this patient decreased from 199 pg/mL to 143 pg/mL. This patient likely had combined vitamin B12 and iron deficiency, and the presence of D. pacificum was unrelated to her macrocytic anemia. The provision of exogenous B12 may have driven her into an iron deficiency state, resulting in hypochromasia.
Only a few case series of manifestations of D. pacificum infection have been reported. In 21 children infected with D. pacificum, only two cases of anemia were associated with chronic renal insufficiency and iron deficiency anemia. Unfortunately, there were no data were reported on vitamin B12 levels in these patients.21
Reduced absorption of vitamin B12 is the major pathophysiologic mechanism in megaloblastic anemia and may be caused by several mechanisms. Under usual circumstances, a protein known as intrinsic factor is secreted by the stomach. This factor is essential for absorption of vitamin B12 and combines with it to form a stable complex. Vitamin B12 deficiency anemia (classically known as pernicious anemia) usually refers to decreased vitamin B12 levels secondary to a loss of intrinsic factor secretion. Vitamin B12 deficiency anemia, once known as tapeworm anemia or bothriocephalus anemia because it is exacerbated by the worm uptake of vitamin B12, was common in Finland22 but is now rarely seen because of improved diet, prenatal care, and treatment.
As an alternative mechanism, competition for available vitamin B12 and intrinsic factor cleavage may occur in the blind loop syndrome (in which bacterial overgrowth consumes existing vitamin B12) or during tapeworm infestations, particularly diphyllobothriasis. In the case of tapeworm infections, most carriers have normal secretion of intrinsic factor. Worm extracts can degrade vitamin B12–intrinsic factor complexes.23 Other factors contributing to the development of megaloblastic anemia may include genetic components of the host, poor nutrition, gastrointestinal disorders (achlorhydria, gastritis), and biological aspects of the parasite.24 Attachment of Diphyllobothrium spp. to the intestinal wall usually occurs in the ileum, less commonly in the jejunum or other regions. It has been suggested that worm activity in competing for vitamin B12 would be more efficient when attached at the jejunal level or in large tapeworms.24
We saw no reason to apply molecular biology techniques to identify the worms to the species level in more than three patients because worms detected had the typical morphologic characteristics of D. pacificum, e.g., transversal sulci and heart-shaped scolex. More importantly, D. latum has not been reported in Peru. In two published studies using polymerase chain reaction, all specimens (n = 8) collected in Peru were D. pacificum.6,7
Spontaneous elimination of Diphyllobothrium tapeworms occurred in seven (35%) of our patients, which is consistent with the rate of 25% reported in the literature. In all patients, treatment or spontaneous worm expulsion were followed by negative fecal examination results at 30 days. Diphyllobothrium pacificum is phylogenetically distant from other species in the Diphyllobothrium genus,6 and may have first evolved as a parasite of large sea mammals before than human populations colonized the coasts of the southern Pacific region.
We found that 1 of 20 patients infected with D. pacificum had mild megaloblastic anemia, which demonstrates that it can occur, albeit at a low frequency. Thus, infection with D. pacificum infection is a benign entity, resolves by spontaneous expulsion of worms, and is not commonly associated with vitamin B12 deficiency or megaloblastic anemia. Conversely, D. latum is frequently associated with low vitamin B12 levels.13 The difference in worm size (and thus increased competition with the host for nutrients, including vitamin B2 and folic acid) and other factors such as dietary profile might contribute to the absence of megaloblastic anemia in our study.
Footnotes
Financial support: This study was supported by the John C. Fogarty Foundation–National Institutes of Health training grant TW001140. Hector H. Garcia is supported by a Wellcome Trust International Senior Research Fellowship in Public Health and Tropical Medicine.
Authors' addresses: Juan A. Jimenez and Ricardo Gamboa, Microbiology and Center for Global Health–Tumbes, Universidad Peruana Cayetano Heredia, Lima, Peru, E-mails: jajch@hotmail.com and rgamboa@peruresearch.org. Silvia Rodriguez and Hector H. Garcia, Microbiology and Center for Global Health–Tumbes, Universidad Peruana Cayetano Heredia, Lima, Peru, and Cestodes Research Department, Instituto Peruano de Parasitología Clínica y Experimental, Lima, Peru, E-mails: silvia@peruresearch.com and hgarcia@jhsph.edu. Lourdes Rodriguez, Infectious Disease, Hospital Nacional Guillermo Almenara Irigoyen, ESSALUD, Lima, Peru, E-mail: lourdesrod@gmail.com.
References
- 1.Scholz T, Garcia HH, Kuchta R, Wicht B. Update on the human broad tapeworm (genus Diphyllobothrium), including clinical relevance. Clin Microbiol Rev. 2009;22:146–160. doi: 10.1128/CMR.00033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia HH, Jimenez JA, Escalante H. Cestodes. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. Manual of Clinical Microbiology. 10th edition. Washington, DC: American Society for Microbiology Press; pp. 2222–2229. [Google Scholar]
- 3.Dupouy-Camet J, Peduzzi R. Current situation of human Diphyllobothrium in Europe. EuroSurveilli. 2004;9:31–35. [PubMed] [Google Scholar]
- 4.Sagua H, Neira I, Araya J, Gonzalez J. New cases of Diphyllobothrium pacificum (Nybelin, 1931) Margolis, 1956 human infection in northern Chile, probably related with the El Niño phenomenon, 1975–2000 [in Spanish] Bol Chil Parasitol. 2001;56:22–25. [PubMed] [Google Scholar]
- 5.Terashima A, Marcos L, Maco V, Canales M, Samalvides F, Tello R. Spontaneous sedimentation in tube technique (SSTT) for diagnosis of intestinal parasites [in Spanish] Rev Gastroenterol Peru. 2009;29:305–310. [PubMed] [Google Scholar]
- 6.Skeríková A, Brabec J, Kuchta R, Jimenez JA, Garcia HH, Scholz T. Is the human-infecting Diphyllobothrium pacificum a valid species or just a South American population of the holarctic fish broad tapeworm, D. latum? Am J Trop Med Hyg. 2006;75:307–310. [PubMed] [Google Scholar]
- 7.Wicht B, Yanagida T, Scholz T, Ito A, Jimenez JA, Brabec J. Multiplex PCR for differential identification of broad tapeworms (Cestoda: Diphyllobothrium) infecting humans. J Clin Microbiol. 2010;48:3111–3116. doi: 10.1128/JCM.00445-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearson RD, Hewlett EL. Niclosamide therapy for tapeworm infections. Ann Intern Med. 1985;102:550–551. doi: 10.7326/0003-4819-102-4-550. [DOI] [PubMed] [Google Scholar]
- 9.Lumbreras H, Terashima A, Alvarez H, Tello R, Guerra H. Single dose treatment with praziquantel (Cesol R, EmBay 8440) of human cestodiasis caused by Diphyllobothrium pacificum. Tropenmed Parasitol. 1982;33:5–7. [PubMed] [Google Scholar]
- 10.Anonymous Pathogenesis of the tapeworm anaemia. BMJ. 1976;2:1028. [PMC free article] [PubMed] [Google Scholar]
- 11.Vuylsteke P, Bertrand C, Verhoef GE, Vandenberghe P. Case of megaloblastic anemia caused by intestinal taeniasis. Ann Hematol. 2004;83:487–488. doi: 10.1007/s00277-003-0839-2. [DOI] [PubMed] [Google Scholar]
- 12.Osorio G, Daiber A, Donckaster R, Ubilla M, Con I, Anguita T, Pinto R. Severe megaloblastic anemia due to Diphyllobotrium latum. First case identified in Chile [in Spanish] Rev Med Chil. 1974;102:700–703. [PubMed] [Google Scholar]
- 13.Nyberg W, Grasbeck R, Saarni M, Von Bonsdorff B. Serum vitamin B12 levels and incidence of tapeworm anemia in a population heavily infected with Diphyllobothrium latum. Am J Clin Nutr. 1961;9:606–612. doi: 10.1093/ajcn/9.5.606. [DOI] [PubMed] [Google Scholar]
- 14.Aslinia F, Mazza JJ, Yale SH. Megaloblastic anemia and other causes of macrocytosis. Clin Med Res. 2006;4:236–241. doi: 10.3121/cmr.4.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cabrera R, Tantalean M, Rojas R. Diphyllobothrium pacificum (Nybelin, 1931) Margolis, 1956 en Canis familiaris de la ciudad de Chincha, Perú. Bol Chil Parasitol. 2001;56:26–28. [PubMed] [Google Scholar]
- 16.Araujo A, Ferreira LF, Confalonieri UE, Nunez L, Cruz FO. Eggs of Diphyllobothrium pacificum in precolumbian human coprolites. Paleopathology Newsletter. 1983;41:11–13. [PubMed] [Google Scholar]
- 17.Reinhard K, Urban O. Diagnosing ancient diphyllobothriasis from Chinchorro mummies. Mem Inst Oswaldo Cruz. 2003;98((Suppl 1)):191–193. doi: 10.1590/s0074-02762003000900028. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira LF, de Araújo AJ, Confalonieri UE, Nuñez L. The finding of eggs of Diphyllobothrium in human coprolites (4,100–1,950 bc) from northern Chile. Mem Inst Oswaldo Cruz. 1984;79:175–180. doi: 10.1590/s0074-02761984000200004. [DOI] [PubMed] [Google Scholar]
- 19.Muñoz ME, Moron CG. Manual de Procedimientos de Laboratorio en Tecnicas Basicas de Hematologia. Lima: Ministerio de Salud del Peru; 2005. pp. 40–88. Instituto Nacional de Salud, Serie de Normas Técnicas. [Google Scholar]
- 20.Anonymous . Mannheim, Germany: Roche Diagnostics; 2001. (Vitamina B12. Elecsys® Sistemas 2010/Modular Analytics E170). Code 1.820.753. [Google Scholar]
- 21.Medina JP, Tantalean M, Cano M. Diphyllobothrium pacificum en niños del Peru. Diagnostico (Peru) 2002;41:161–164. [Google Scholar]
- 22.Heyneman D. Chapter 89. Cestodes. In: Baron S, editor. Medical Microbiology. Fourth edition. Galveston, TX: University of Texas Medical Branch at Galveston; 1996. [PubMed] [Google Scholar]
- 23.Nyberg W. Diphyllobothrium latum and human nutrition, with particular reference to vitamin B12 deficiency. Proc Nutr Soc. 1963;22:8–14. doi: 10.1079/pns19630004. [DOI] [PubMed] [Google Scholar]
- 24.Donoso-Scroppo M, Raposo L, Reyes H, Godorecci S, Castillo G. Megaloblastic anemia induced by Diphyllobothrium latum infection. Rev Med Chil. 1986;114:1171–1174. [PubMed] [Google Scholar]