Abstract
México has cities (e.g., México City and Puebla City) located at elevations > 2,000 m and above the elevation ceiling below which local climates allow the dengue virus mosquito vector Aedes aegypti to proliferate. Climate warming could raise this ceiling and place high-elevation cities at risk for dengue virus transmission. To assess the elevation ceiling for Ae. aegypti and determine the potential for using weather/climate parameters to predict mosquito abundance, we surveyed 12 communities along an elevation/climate gradient from Veracruz City (sea level) to Puebla City (∼2,100 m). Ae. aegypti was commonly encountered up to 1,700 m and present but rare from 1,700 to 2,130 m. This finding extends the known elevation range in México by > 300 m. Mosquito abundance was correlated with weather parameters, including temperature indices. Potential larval development sites were abundant in Puebla City and other high-elevation communities, suggesting that Ae. aegypti could proliferate should the climate become warmer.
Introduction
The mosquito Aedes aegypti, a primary vector of dengue, yellow fever, and chikungunya viruses, is widely distributed in the subtropics and tropics.1,2 Latin America, including México, has experienced increasing dengue case numbers in recent decades, and dengue is now hyperendemic in many areas, with cocirculation of multiple and sometimes all four dengue virus serotypes.3 Moreover, chikungunya presents a major threat to Latin America should the causative virus emerge there.4 Some settings in Latin America present an intriguing situation, where Ae. aegypti mosquitoes are abundant and endemic dengue virus transmission occurs in low-elevation areas but where a large proportion of the human population lives in high-elevation cities located above the elevation ceiling below which local climates allow for proliferation of the mosquito vector and endemic dengue virus transmission. This includes, for example, México City and Puebla City in México and Quito in Ecuador. An important question is whether ongoing climate warming potentially could lead to the elevation ceiling for Ae. aegypti moving up to an extent where currently unaffected high-elevation cities are threatened by mosquito vector proliferation and establishment of local dengue virus transmission cycles. Although the mosquito has been studied intensely in dengue-endemic areas at the core of its geographic range, virtually nothing is known of its natural history at the cool margins of its range.
Ae. aegypti is closely associated with humans and human habitation. The female is primarily an indoor day-biter that feeds almost exclusively on humans and exploits artificial containers as sites to deposit her eggs.5–7 The geographic distribution of Ae. aegypti is considered to, in part, be limited by cold temperatures: the low-latitude areas equatorward of the average 10°C winter isotherms in the northern and southern hemispheres approximate the climatic boundary for establishment of the mosquito.8–13 Eggs of Ae. aegypti can be transported over long distances in artificial containers through human activities, including to areas outside the established range, but the innate climate tolerance precludes establishment in colder areas at middle and high latitudes and at high elevations at lower latitudes. The highest previously published elevation records for Ae. aegypti in the Americas are 1,630 m for México14 and 2,200 m for Colombia.15 Moreover, the work by Herrera-Basto and others16 reported a dengue outbreak in the Méxican city of Taxco, located at 1,700 m, but the collected mosquitoes were only reported as Aedes species. Although these mosquitoes likely included Ae. aegypti, they also may have included specimens of another container-inhabiting mosquito Ae. epactius, which occurs at high elevation in México.17,18
There is a dearth of field studies aiming to determine the specific climate conditions under which Ae. aegypti is capable of establishment at the cool margins of its range. This dearth has (1) prevented the development of robust climate-based models for the distribution and abundance of the mosquito at the cool margins of its range and (2) limited our ability to assess the potential for climate warming to lead to changes in the geographic distribution of the mosquito, which could place additional human populations at risk for exposure to this important arbovirus vector in the Americas. The latter includes not only high-elevation urban areas in México and other parts of Latin America but potentially, also high-latitude urban centers in the United States.
Studies of associations between climate parameters and Ae. aegypti are complicated by the dependence of the mosquito on humans, especially its preference for human blood and its use of artificial containers as larval development sites.5,19 Socioeconomic conditions and human behavior (for example, water storage practices or use of air conditioning or mosquito screening to prevent intrusion of mosquitoes into homes) can confound basic associations between climate parameters and mosquito abundance.20–25 It is, therefore, especially challenging to study associations between climate and Ae. aegypti along transects that include high variability in socioeconomic conditions, such as transects extending south to north from México to the United States. To minimize the potential confounding effect of socioeconomic conditions, we focused on an elevation and climate gradient within central México, where the targeted communities included neighborhoods of comparable socioeconomic status. These communities extended from Veracruz City on México's eastern seaboard to high-elevation communities (> 2,000 m) in the central highlands of México, including Puebla City. We report the collection of Ae. aegypti from several communities located above 1,600 m (1,690–2,130 m), extending the known elevation range of this important mosquito arbovirus vector in México by several hundred meters.
Materials And Methods
Study environment.
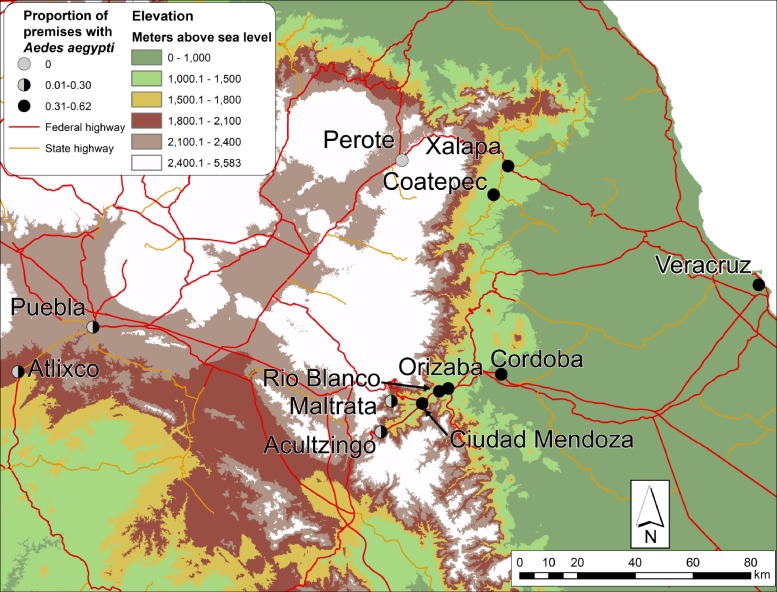
The study included 12 communities located along an elevation and climate gradient ranging from sea level in Veracruz State to high elevation (> 2,000 m) in Veracruz and Puebla States (Figure 1 and Table 1). To facilitate comparison among communities, we focused on low- to middle-income homes with small- to medium-sized yards. The following premises types were excluded from the study: high-income premises and low-income fraccionamiento-style premises, which typically are small homes clustered closely together with very small yards.
Figure 1.
Locations of study communities in Veracruz State and Puebla State, México, in relation to elevation and the proportion of examined premises with Ae. aegypti present.
Table 1.
General characteristics of study communities along an elevation and climate gradient in Veracruz and Puebla States, México
| State and community | Population estimate* | No. of examined clusters/individual premises | Mean elevation of premises (m) | Maximum/minimum temperature (°C)† | Mean annual rainfall (mm)† | Time period for surveys of mosquito immatures in 2011 | |
|---|---|---|---|---|---|---|---|
| July | January | ||||||
| Veracruz State | |||||||
| Veracruz City | 428,000 | 3/54 | 11 | 31.3/23.5 | 25.2/18.0 | 1,274 | July 11–13 |
| Córdoba | 141,000 | 3/51 | 853 | 29.2/18.5 | 24.6/13.3 | 2,082 | July 18 and 19 |
| Coatepec | 53,000 | 3/48 | 1,198 | 27.8/14.3 | 21.7/8.9 | 894 | August 31 and September 1 |
| Orizaba | 121,000 | 4/51 | 1,227 | 25.9/14.2 | 21.5/9.5 | 923 | July 25–27 |
| Rio Blanco | 40,000 | 3/54 | 1,251 | ND‡ | ND‡ | ND‡ | August 2 and 3 |
| Ciudad Mendoza | 35,000 | 4/48 | 1,334 | ND‡ | ND‡ | ND‡ | August 3–8 |
| Xalapa | 425,000 | 4/51 | 1,416 | 25.5/15.2 | 20.8/10.3 | 731 | August 23 and 24 |
| Acultzingo | 7,040 | 4/50 | 1,693 | 24.8/12.4 | 20.6/7.1 | 581 | August 11–16 |
| Maltrata | 11,840 | 3/51 | 1,713 | 22.5/11.1 | 21.5/6.6 | 606 | August 8 and 9 |
| Perote | 38,000 | 4/51 | 2,417 | 21.6/5.3 | 19.2/0.4 | 476 | August 29 and 30 |
| Puebla State | |||||||
| Puebla City | 1,434,000 | 4/48 | 2,133 | 23.0/8.5 | 19.7/1.7 | 860 | August 17 and 18 |
| Atlixco | 87,000 | 4/50 | 1,825 | 25.9/12.4 | 24.2/7.7 | 530 | August 19 and 20 |
Based on data for 2010 obtained from México's Instituto Nacional de Estadística y Geografía.
Based on data obtained from México's Servicio Meteorológico Nacional for 1975–2005 (except 1957–1987 for Córdoba, 1961–1979 for Acultzingo, and 1970–2000 for Maltrata).
No long-term data available.
Selection of study premises and temporal sampling scheme.
Google Earth (Google, Mountain View, CA) imagery, typically 1–3 years old, was used to select four clusters within each community to target for surveys of mosquito immatures in artificial containers. A cluster was defined as an area of approximately 1 km2 including blocks (groups of houses surrounded by streets or roads) considered suitable for inclusion in the study. In most communities, with the exception of the small towns of Acultzingo and Maltrata, clusters were separated by a distance of at least 1 km, which exceeds the typical Ae. aegypti flight range (< 100 m).26 The target number of premises to examine per community was 50; the actual number fell within three to four different clusters per community (Table 1). No more than five premises were examined within a single block. The locations of the examined premises were recorded with a Global Positioning System receiver (Garmin eTrex Vista H; Garmin, Olathe, KS).
Because of the intensive sampling effort and the large geographic area covered, we were only able to examine the study premises on a single occasion within the perceived July to September peak period for abundance of mosquito immatures in the study area (July to September is the warmest part of the rainy season). To minimize the effect of seasonal changes in mosquito abundance occurring over the July 11 to September 1, 2011 sampling period, we started the sampling in the community with the lowest elevation (Veracruz City) and worked up in elevation along the core of the transect, which also included Córdoba, Orizaba, Rio Blanco, Ciudad Mendoza, Acultzingo, Maltrata, Puebla City, and Atlixco (Figure 1 and Table 1). The sampling in these core communities started on July 11 and concluded by August 20. Three additional communities along another elevation gradient farther north in Veracruz State (Coatepec, Xalapa, and Perote) (Figure 1 and Table 1) were sampled from August 23 to September 1.
Temperature is an important driver for population growth of Ae. aegypti, particularly during rainy parts of the year (primarily June to October in our study area) when water-filled containers are most abundant.9–12,27,28 Our temporal sampling scheme within the rainy season, with lower-elevation communities sampled before higher-elevation communities, was designed to minimize the potential confounding effect of increasing mosquito numbers over time when comparing presence and abundance of Ae. aegypti among communities. We used the cumulative number of growing degree days (GDDs) (10°C base) from June 1 to the specific mosquito survey dates in a given community to assess whether there were major differences in accumulated temperature among communities at the time when the mosquito surveys were conducted. The number of days during which GDDs were accumulated before the mosquito surveys ranged from 42 in Veracruz City and 48.5 in Córdoba at the lowest elevations (GDDs = 715 and 670, respectively) to 78.5–80.5 in Puebla City and Atlixco at high elevations (GDDs = 640 and 779, respectively). By the time that mosquito surveys were conducted in a given community, the cumulative number of GDDs was reasonably uniform among the study communities located along the core of the transect (range = 634–779). Moreover, the observed variability among communities was not associated with their elevation (analysis of variance [ANOVA], P = 0.99), which indicates that data from the mosquito surveys were not skewed for communities located at lower versus higher elevations along the transect. We, therefore, conclude that the core communities were sampled at points in time, within the 2011 rainy season, that were reasonably comparable with regards to cumulative degree days. The additional communities to the north (Coatepec, Perote, and Xalapa) exhibited more variation in cumulative GDDs because of the later sampling.
Premises characteristics.
We recorded basic characteristics of the study premises: (1) elevation, (2) number of people that slept in the home the previous night, (3) approximate yard size (m2), (4) vegetation present in the yard (percentage grass cover and number of shrubs and trees), (5) roof type (metal, plastic, concrete, clay tile, asbestos, or other/mixed), (6) presence of rain gutters, (7) presence of open spaces between the top of the wall and the roof, (8) wall type (concrete, wood, brick, cinder block, or other/mixed), (9) floor type (concrete, brick, tile, soil, or other/mixed), (10) number of rooms, (11) total numbers of windows and doors and numbers of windows and doors with intact screens, (12) presence and use of air conditioning, (13) availability and regularity of piped water, (14) frequency of trash collection and whether tires are removed as part of the trash collection, (15) numbers and status with regards to water fill level and use of lids of different types of large water-holding structures located on the roof (plastic roof tanks), outdoors or indoors at ground level (swimming pools, concrete tanks, or barrels/drums made of metal or plastic), or below ground level (wells, septic tanks, or cisterns), and (16) total numbers of smaller containers and numbers of these containers with water in them.
Mosquito collection.
Surveys for immatures were conducted in all study communities. Water-holding containers located indoors or outdoors on the study premises were examined for presence of mosquito larvae and pupae. The following container types were excluded from the examination based on safety concerns or difficulty of access: plastic roof water tanks, rain gutters, and septic tanks. All mosquito immatures were collected from small- to medium-sized containers. Using the methodology described in the work by Romero-Vivas and others,29 a sweep net mounted on a pole was used to sample large containers, including barrels/drums, cement tanks, cement cisterns, and wells, in which it is difficult to see the immatures without emptying the containers fully. This methodology estimates the total number of pupae in a large container based on the immature collected with a single sweep of the net and a multiplication factor determined by the container water capacity (less or more than 1,000 L) and the water fill level (one-third full, two-thirds full, or full).29
Collected immatures were separated by premises of collection and life stage (larvae or pupae). They were transported to the laboratory and reared to adults. Adults were identified, using the key in the work by Darsie and Ward,30 as males or females belonging to the following taxonomic entities: (1) Ae. aegypti, (2) Ae. epactius, or (3) a grouping consisting of any other mosquito species (referred to as other mosquito species). No special efforts were made to identify immatures, because only fourth-instar larvae are consistently distinguishable as Ae. aegypti versus Ae. epactius.
Estimation of percentage of premises with Ae. aegypti present or abundance of Ae. aegypti pupae per premises.
Data from the surveys for immatures were used to estimate the percentage of premises with Ae. aegypti present and the abundance of Ae. aegypti pupae per premises. Abundance was estimated only for pupae, because a greater proportion of collected pupae (73%) was reared to adults and identified compared with collected larvae (16%).
Percentage of premises with Ae. aegypti present.
Of 607 examined premises, Ae. aegypti was present on 160 (i.e., identified as adults resulting from immatures collected from these premises) and absent from 349 (i.e., either no immatures were observed or all specimens belonged to Ae. epactius or other mosquito species). The remaining 98 premises (16% of total premises) produced field observations of immatures that were not identified to species as adults and therefore, potentially could include Ae. aegypti. These premises were proportionally allocated to the presence versus absence categories for Ae. aegypti by cluster or community based on data for premises with definitive presence versus absence within the same cluster or community. For instance, if a community had 10 unassigned premises, 20 premises with Ae. aegypti present, and 30 premises with Ae. aegypti absent, the proportion of the 10 unassigned premises for that community classified as likely having Ae. aegypti present would be 10 × (20/(20 + 30)) = 4.
Abundance of Ae. aegypti pupae per premises.
Field-observed pupae that were not subsequently identified as adults were assigned to Ae. aegypti, Ae. epactius, or other mosquito species in accordance with the data for pupae that could be assigned to these taxonomic classifications. This assignment was based on data from (1) the same container type on the same premises (if such data were available), (2) other container types on the same premises, (3) the cluster in which the premises was located, or (4) the community in which the premises was located. Using scenario 2 as an example, if a single taxonomic entity was identified from a given premises, then all unassigned pupae from that specific premises were assumed to belong to the same taxonomic entity. If multiple taxonomic entities were identified, then the unassigned pupae were proportionately allocated among them. The final step in estimating the abundance of pupae for a given premises and taxonomic entity involved applying a multiplication factor by the container type that the pupae were observed in to account for complete sampling of small- and medium-sized containers versus partial sampling of very large containers. Container types with complete sampling were uniformly assigned a neutral multiplication factor of one. Multiplication factors ranging from 1.9 to 3.5 were used, using the information in the work by Romero-Vivas and others,29 for very large container types, with partial sampling based on their water volume and water fill level.
Weather data.
Determination of correlations between presence or abundance of Ae. aegypti and the local climate focused primarily on weather data for the 30-day period preceding the survey for immatures in a given community in 2011. Using shorter (7 or 15 days) or longer (60 days) time periods produced similar results (data not shown). Weather parameters under consideration, with relevance for the biology of Ae. aegypti and perceived a priori importance along the targeted sampling transect, which extends into cool and dry areas at high elevation, included (1) average minimum daily temperature, (2) daily temperature range, (3) cumulative GDDs (10°C base), (4) average minimum daily relative humidity (RH), and (5) total rainfall. This study was complemented by examining correlations between presence or abundance of Ae. aegypti and average minimum daily winter temperature during the previous winter (December of 2010 to February of 2011).
Temperature and RH data for the 30-day period preceding the survey for immatures in a given community were obtained from HOBO (Onset Computer Corporation, Bourne, MA) data loggers set up in each community along the transect. Temperature and RH observations from the closest HOBO site were adjusted for elevation to each residence location. Rainfall data were obtained from the 0.07°-gridded Climate Prediction Center Morphing Technique (CMORPH) dataset,31 which uses precipitation estimates derived exclusively from low orbiter satellite microwave observations and features transported by spatial propagation information obtained from geostationary satellite infrared (IR) data. CMORPH provides some of the most reliable estimates for tropical summer rainfall compared with other satellite- and model-based rainfall products.32 Average minimum daily winter temperature from December of 2010 to February of 2011 was based on output from the North American Regional Reanalysis (NARR) dataset provided by the National Centers for Environmental Prediction (Silver Spring, MD). CMORPH and NARR data were bilinearly interpolated from surrounding grid points to each residence location, and NARR temperature values were adjusted to 1 arc-second Advanced Spaceborne Thermal Emission and Reflection Radiometer (ASTER) terrain height data representative of each residence location. The household-level data were then averaged across clusters and communities.
Statistical analyses.
Statistical analyses were carried out using the JMP statistical package,33 and results were considered significant when P < 0.05. Statistical tests used are noted in the text. Correlations between environmental factors (elevation or weather parameters) and the estimated proportion of homes with Ae. aegypti present or the abundance of Ae. aegypti pupae per premises were examined at the cluster level and included only clusters with ≥ 15 premises examined. Bonferroni correction was used to account for multiple comparisons.
Results
A total of 43,921 immatures was observed in containers on the study premises. This total included 40,722 larvae and 3,199 pupae. Approximately 20% (8,833/43,921) of the field-observed immatures were successfully reared to adults and identified, including 15.9% of 40,722 larvae and 73.4% of 3,199 pupae. Identification of adult specimens produced 5,758 Ae. aegypti (2,770 females, 2,957 males, and 31 adults not identifiable to sex), 2,703 Ae. epactius, and 372 mosquitoes of other species.
Ae. aegypti mosquitoes were collected from 11 of 12 study communities (Figure 1 and Table 2); the lone exception was the community at the highest elevation (Perote; > 2,400 m). Ae. aegypti was, thus, collected at elevations ranging from near sea level in Veracruz City on the Gulf of México to > 2,100 m in Puebla City in the central highlands of México. The estimates for percentage of premises in the study communities with Ae. aegypti present and abundance of Ae. aegypti pupae on the study premises (Table 2) indicate that, along our elevation and climate gradient in central México, the mosquito is abundant at elevations up to 1,300 m, moderately abundant from 1,300 to 1,700 m, and still present but rare from 1,700 to 2,150 m.
Table 2.
Collections of Ae. aegypti from communities in Veracruz and Puebla States, México, during surveys for immatures in artificial containers from July to September of 2011
| Community (mean elevation of premises; m) | No. of Ae. aegypti identified to species* | Estimated proportion of premises with Ae. aegypti† | Estimated no. Ae. aegypti pupae per premise† | Selected weather data for the 30-day period before the survey for immatures‡ | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Average daily temperature (°C) | Average daily RH (%) | Total rainfall (mm) | |||
| Veracruz City (11) | 792 | 0.52 | 4.91 (11.11) | 0 | 28.9 | 79.3 | 146 |
| Córdoba (853) | 570 | 0.36 | 6.04 (30.26) | 0 | 23.5 | 83.9 | 321 |
| Coatepec (1,198) | 602 | 0.46 | 3.26 (9.82) | 0 | 21.6 | 82.9 | 96 |
| Orizaba (1,227) | 1,369 | 0.39 | 14.29 (38.24) | 0 | 20.5 | 87.2 | 292 |
| Rio Blanco (1,251) | 1,540 | 0.62 | 10.43 (26.35) | 0 | 20.3 | 86.0 | 279 |
| Ciudad Mendoza (1,334) | 350 | 0.43 | 2.63 (8.04) | 0 | 19.8 | 86.0 | 236 |
| Xalapa (1,416) | 149 | 0.36 | 0.98 (2.46) | 0 | 20.6 | 81.4 | 73 |
| Acultzingo (1,693) | 212 | 0.26 | 1.68 (6.24) | 0 | 18.5 | 84.7 | 164 |
| Maltrata (1,713) | 7 | 0.07 | 0.06 (0.40) | 0 | 19.4 | 81.0 | 190 |
| Atlixco (1,825) | 164 | 0.17 | 0.28 (1.19) | 0 | 19.5 | 72.1 | 43 |
| Puebla City (2,133) | 3 | 0.05 | 0.04 (0.27) | 0 | 17.8 | 71.6 | 94 |
| Perote (2,417) | 0 | 0.00 | 0.00 (0) | 0 | 13.6 | 85.6 | 53 |
Collected as larvae or pupae from artificial containers and reared to adults before species identification. Not all observed immatures (which also included Ae. epactius and other mosquito species) were reared successfully to adults.
The process for estimating these numbers is explained in Materials and Methods.
Calculated based on the specific sampling dates for each community shown in Table 1.
Elevation was negatively correlated with the estimated proportion of homes with Ae. aegypti present or the abundance of Ae. aegypti pupae per premises along the sampling transect (Table 3). Positive correlations with the estimated proportion of homes with Ae. aegypti present or the abundance of Ae. aegypti pupae per premises were recorded for the average minimum daily temperature during the preceding winter period (December to February) and the average minimum daily temperature, cumulative GDDs, average daily minimum RH, and total rainfall during the 30-d period preceding the mosquito survey in a given community (Table 3). For the average daily temperature range, there was a strong but non-significant trend (P = 0.06) to a negative correlation with the abundance of Ae. aegypti pupae per premises (Table 3).
Table 3.
Correlations between elevation or selected weather parameters and the estimates for percentage of premises with Ae. aegypti present or abundance of Ae. aegypti pupae per premises at the cluster level
| Dependent and independent parameters | Spearman's rank correlation* | |
|---|---|---|
| ρ | P | |
| Percentage of premises with Ae. aegypti present | ||
| Elevation | –0.736 | 0.002† |
| Winter average minimum daily temperature‡ | 0.785 | 0.001† |
| 30-day average minimum daily temperature§ | 0.682 | 0.005† |
| 30-day average daily temperature range§ | −0.349 | 0.202 |
| 30-day cumulative GDDs§ | 0.721 | 0.002† |
| 30-day average minimum daily RH§ | 0.680 | 0.005† |
| 30-day total rainfall§ | 0.607 | 0.016 |
| Abundance of Ae. aegypti pupae per premises | ||
| Elevation | −0.713 | 0.003† |
| Winter average minimum daily temperature‡ | 0.769 | 0.001† |
| 30-day average minimum daily temperature§ | 0.722 | 0.002† |
| 30-day average daily temperature range§ | −0.495 | 0.061 |
| 30-day cumulative GDDs§ | 0.760 | 0.001† |
| 30-day average minimum daily RH§ | 0.795 | < 0.001† |
| 30-day total rainfall§ | 0.617 | 0.014 |
Based on data from eight communities (Córdoba, Orizaba, Rio Blanco, Ciudad Mendoza, Acultzingo, Maltrata, Atlixco, and Puebla City) located along the core transect at elevations from 850 to 2,130 m and including only clusters (N = 15) from these communities with ≥ 15 homes examined.
Statistically significant (P < 0.05) with Bonferroni correction for multiple comparisons.
Based on data for December of 2010 to February of 2011.
Based on data for the 30-day period preceding the mosquito survey in a given community in 2011.
We also compared selected characteristics of the study premises in nine communities with robust Ae. aegypti populations (Veracruz City, Córdoba, Coatepec, Orizaba, Rio Blanco, Ciudad Mendoza, Xalapa, Acultzingo, and Atlixco; ≥ 149 Ae. aegypti identified to species per community, estimated proportions of premises with Ae. aegypti present ≥ 0.17 per community, and estimated mean numbers of Ae. aegypti pupae per premises ≥ 0.28 per community) with those characteristics for premises in three high-elevation communities with no or minimal numbers of Ae. aegypti collected (Puebla City, Maltrata, and Perote; less than or equal to seven Ae. aegypti identified to species per community, estimated proportions of premises with Ae. aegypti present ≤ 0.07 per community, and estimated mean numbers of Ae. aegypti pupae per premises ≤ 0.06 per community) (Table 4). For the comparison between Puebla City and the nine communities with robust Ae. aegypti populations, the only significant differences were greater numbers of large containers at ground level and lower use of air conditioning in Puebla City (Table 4). These differences should promote rather than prevent the establishment of Ae. aegypti populations in Puebla City. The comparison between the small community of Maltrata and the nine communities with robust Ae. aegypti populations produced several significant differences: (1) premises in Maltrata were of larger size, (2) houses in Maltrata were less likely to have intact window/door screens, regular access to piped water, and trash removal services at least weekly, and (3) premises in Maltrata harbored greater numbers of large containers and total water-filled containers at ground level (Table 4). These differences should promote rather than prevent the establishment of Ae. aegypti populations in Maltrata. The outcome of the comparison between the small city of Perote and the nine communities with robust Ae. aegypti populations was different: houses in Perote were less likely to have intact window/door screens or use air conditioning, but the premises harbored lower numbers of total containers and total water-filled containers at ground level (Table 4). The permissiveness of the houses in Perote for entry of mosquitoes should promote the establishment of Ae. aegypti populations, whereas the lower numbers of containers present to serve as larval development sites may negatively impact population buildup. However, although the average numbers of water-filled containers at ground level per premises were lower in Perote (5.4) compared with Puebla City (6.0), Maltrata (8.0), or the nine communities with robust Ae. aegypti populations (6.6), such containers were still present in numbers that are likely to support Ae. aegypti.
Table 4.
Comparison of selected characteristics of study premises for nine communities with robust Ae. aegypti populations versus individual high-elevation communities with no or minimal numbers of Ae. aegypti collected
| Premises characteristics | Communities with robust Ae. aegypti populations (N = 457 premises)* | High-elevation communities with no or minimal numbers of Ae. aegypti collected† | ||
|---|---|---|---|---|
| Puebla City‡ (N= 48 premises) | Maltrata‡ (N = 51 premises) | Perote‡ (N = 51 premises) | ||
| Mean size (m2) of the lot (SD) | 352 (436) | 305 (228)§ | 392 (366)¶ | 222 (125)§ |
| Mean no. shrubs or trees on the lot (SD) | 9.0 (26.8) | 8.2 (10.6)§ | 6.2 (9.9)§ | 10.6 (15.9)§ |
| Mean no. rooms per home (SD) | 4.6 (2.5) | 4.9 (3.1)§ | 4.5 (2.0)§ | 4.2 (2.2)§ |
| Mean no. sleepers per home (SD) | 5.1 (2.9) | 5.2 (3.0)§ | 5.3 (2.1)§ | 6.0 (2.9)¶ |
| Mean proportion per home of windows or doors with intact screens (SD) | 0.15 (0.34) | 0.10 (0.25)§ | 0.03 (0.12)¶ | 0.03 (0.09)∥ |
| Proportion of homes where air conditioning is present and used | 0.05 | 0.00¶ | 0.02§ | 0.00¶ |
| Proportion of homes with piped water always available | 0.68 | 0.66§ | 0.37** | 0.63§ |
| Proportion of homes with trash removal at least weekly | 0.98 | 1.00§ | 0.88∥ | 1.00§ |
| Mean no. large containers at ground level per premises (SD) | 2.5 (1.4) | 3.6 (2.4)** | 3.3 (1.5)** | 2.0 (1.0)§ |
| Mean no. total containers at ground level per premises (SD) | 65 (83) | 48 (56)§ | 47 (40)§ | 39 (56)** |
| Mean no. total water-filled containers at ground level per premises (SD) | 6.6 (10.5) | 6.0 (8.5)§ | 8.0 (4.7)** | 5.4 (9.9)¶ |
Including nine communities (Veracruz City, Córdoba, Coatepec, Orizaba, Rio Blanco, Ciudad Mendoza, Xalapa, Acultzingo, and Atlixco) with ≥ 149 Ae. aegypti identified to species per community, estimated proportions of premises with Ae. aegypti present ≥ 0.17, and estimated mean numbers of Ae. aegypti pupae per premises ≥ 0.28.
Including three communities (Puebla City, Maltrata, and Perote) with less than or equal to seven Ae. aegypti identified to species per community, estimated proportions of premises with Ae. aegypti present ≤ 0.07, and estimated mean numbers of Ae. aegypti pupae per premises ≤ 0.06.
Statistical comparison between Puebla City, Maltrata, or Perote and the nine communities with robust Ae. aegypti populations based on Wilcoxon ranked sums test or contingency table analysis likelihood ratio as appropriate.
Non-significant (P > 0.05).
P < 0.05.
P < 0.01.
P < 0.001.
Discussion
The potential effects of climate and environmental change on Ae. aegypti and dengue virus transmission have generated much debate.20,34–39 Part of this controversy relates to modeling future climate-driven change for the vector or disease without accounting for human-related factors, which also impact the vector (e.g., availability of water-filled artificial containers as larval development sites) or dengue virus transmission dynamics (e.g., serotype-specific susceptibility of the human population). These confounding factors can, thus, modulate the effects of climate change. We also recognize that the effects of climate and environmental change are location-specific and likely to impact Ae. aegypti and potentially, also dengue virus transmission to a greater extent in some geographic areas than others. For example, recent studies suggest that future changes in the range of Ae. aegypti in Australia may not be directly caused by climate change but rather, human response to changing rainfall patterns by increased or decreased use of water storage containers.25,34 Our study focuses on the potential for climate change, especially increasing temperatures, to result in increased risk for future human exposure to Ae. aegypti in high-elevation Latin American cities with current temperature conditions below the mosquito's innate thresholds for survival and proliferation. Annual and seasonal temperature trends along our transect are on the order of a +0.15–0.20°C increase per decade since 1950,40 and they are projected to continue rising at a similar or greater rate throughout this century.41 A warming rate of 0.2°C per decade corresponds to a given isotherm moving up in elevation by ∼30 m per decade; stated differently, at the current rate of warming, Ae. aegypti may be able to survive at elevations nearly 300 m higher in 2100 compared with today, assuming that no other factors prevent establishment at those higher elevations.
We determined presence and abundance of Ae. aegypti along an elevation and climate gradient in central México. This gradient ranged from Veracruz City at sea level, with highly favorable climate conditions for the mosquito, through mid-range elevations (1,600–1,700 m), which previous data indicated as the upper elevation margin for Ae. aegypti mosquitoes in México,14 into high elevations (> 2,000 m) in the central highlands. The mosquito was not previously reported > 1,700 m in México, where high-elevation cities, such as México City and Puebla City, potentially are threatened if the regional climate continues to warm as projected. Our most important findings were that (1) Ae. aegypti mosquitoes were moderately abundant up to 1,700 m and still present but rare from 1,700 to 2,130 m, (2) the abundance of Ae. aegypti along the elevation and climate gradient was correlated with weather parameters, and (3) there was no evidence of characteristics of the human environment in Puebla City or other high-elevation communities, where our collections yielded no or very few Ae. aegypti, that likely would prevent the proliferation of Ae. aegypti should the local climate become warmer. Our results are in accordance with previous studies from México, which reported positive associations or correlations between minimum temperature or rainfall and monthly or annual dengue incidence42–44 or between median temperature during the rainy season and dengue virus exposure across communities.45 Furthermore, one recent study concluded that, within México, climatic parameters are more important determinants of dengue incidence than socioeconomic ones.44
Large metropolitan areas located in the comparatively cooler and drier central highlands of México, such as México City and Puebla City, have local climates that are currently poorly suited for establishment and proliferation of Ae. aegypti. However, these cities are linked through transportation routes to lower-elevation communities, where warmer and wetter local climates are suitable for the mosquito to establish and thrive. For example, we found very few specimens of Ae. aegypti in Puebla City (> 2,100 m), but the mosquito was moderately abundant in adjacent lower-elevation communities on major roads leading to Puebla City from the west (Atlixco; 1,820 m) and east (Ciudad Mendoza; 1,330 m). It is very likely that cities at high elevation in México experience repeated introductions of Ae. aegypti eggs or immatures through human activities but that the mosquito is prevented from proliferating by environmental conditions that reduce survival of the eggs or the active life stages. This outcome could be related to lack of larval development sites, or as supported by our results, it could be because of cold or dry conditions limiting population growth during the mosquito season and poor survivorship of overwintering eggs. One possible scenario for Puebla City is that survivorship of overwintering Ae. aegypti eggs is minimal and that presence of the mosquito in the summer results largely from annual introductions of eggs or immatures through human transport of infested containers. We found no evidence of larval development sites (i.e., artificial containers with water present) being less common in Puebla City than study communities at lower elevations where Ae. aegypti mosquitoes are abundant. Indeed, the study premises in Puebla City harbored an average of 48 containers and 6.0 water-filled ones, which should provide ample opportunities for mosquito females to locate suitable oviposition sites. However, our data suggest that climate parameters related to temperature, humidity, and rainfall (cold and dry conditions) during the part of the year when mosquitoes are active may play key roles in preventing buildup of populations of Ae. aegypti mosquitoes in Puebla City. Moreover, winter temperatures in Puebla City are sufficiently low (Table 1) to negatively impact the survivorship of overwintering eggs.8,46 Notably, there was a negative correlation between daily temperature range, which increased with elevation along our transect, and the abundance of Ae. aegypti pupae per premises. A large daily temperature range was previously documented to have negative effects on the survivorship of Ae. aegypti females as well as influence the efficiency with which they transmit dengue virus.47
The main weaknesses of the study were that we were not able to sample the study premises on more than one occasion within the rainy season and that we were unable to identify all observed immatures, which included surprisingly large numbers of the container-inhabiting Ae. epactius. The first issue was counteracted by our temporal sampling scheme, which led to mosquito surveys being conducted at similar accumulated temperatures within the 2011 rainy season for the core communities examined. The second issue was addressed by using a conservative process to estimate abundance of Ae. aegypti based on the specimens that were definitively identified to species in the adult stage. Moreover, the overall results of the study, with gradually declining abundance of Ae. aegypti to very low numbers at high elevations, where the local climate presumably is too cool and dry for this species to proliferate, are reasonable. The only potentially controversial result was the collection of Ae. aegypti at elevations above the previously known highest elevation for collection of this mosquito in México. There is no question about the correctness of this finding, but we caution that it should be interpreted with great care; we do not yet know whether mosquito populations in high-elevation communities can reach levels that support dengue virus transmission, even among a susceptible human population.
Another potential study confounder is that we were not able to comprehensively sample all potential larval development sites that were observed on the study premises. These sites included roof water storage tanks, which were excluded because of safety concerns, and subterranean water-holding structures, including septic tanks, which require the use of emergence traps for adults to assess their productivity for Ae. aegypti. It also should be noted that our sampling focused on immatures, and it remains to be determined how frequently these immatures emerge successfully as adults in high-elevation settings compared with lower-elevation, warmer areas. Ongoing follow-up studies in selected high-elevation communities include trapping of Ae. aegypti adults.
Our results pose the question of whether continued warming and altered rainfall patterns may eventually allow for establishment and proliferation of Ae. aegypti in México's high-elevation cities. We are addressing this question in ongoing studies by (1) developing models to explain the presence and abundance of Ae. aegypti along the sampling transect based on associations with climatic parameters, characteristics of the human domestic environment, and human behavior and (2) using these models to assess the potential for future climate and environmental change to impact the spatial patterns of Ae. aegypti in high-elevation areas in México.
ACKNOWLEDGMENTS
The authors thank Eric Hubron, Elena Rustrian, Selene Tejeda, Marco Aurelio Morales, Yair Zamora, students from Universidad Veracruzana's Geography Program, and students and personnel from Bachilleres Veracruz, Ilustre Instituto Veracruzano, Bachilleres Río Blanco, and Bachilleres Ricardo Flores Magón (Oficial B) for field and laboratory assistance. We also thank the Ministries of Public Health, Education and Civil Protection of Veracruz Government for their support. Finally, we are grateful to the involved home owners for granting us access to collect mosquitoes from their properties.
Footnotes
Financial support: This study was funded by National Science Foundation Grant GEO-1010204 to the University Corporation for Atmospheric Research.
Authors' addresses: Saul Lozano-Fuentes, Kevin C. Kobylinski, and Lars Eisen, Department of Microbiology, Immunology and Pathology, Colorado State University, Fort Collins, CO, E-mails: slozano@colostate.edu, kobylinskikevin@yahoo.com, and lars.eisen@colostate.edu. Mary H. Hayden, Luca Delle Monache, Andrew J. Monaghan, and Daniel F. Steinhoff, National Center for Atmospheric Research, Boulder, CO, E-mails: mhayden@ucar.edu, lucadm@ucar.edu, monaghan@ucar.edu, and steinhof@ucar.edu. Carlos Welsh-Rodriguez, Carolina Ochoa-Martinez, and Berenice Tapia-Santos, Centro de Ciencias de la Tierra, Universidad Veracruzana, Calle Francisco J. Moreno 207, Colonia Emiliano Zapata, Xalapa, Veracruz, Mexico, E-mails: cwelsh@uv.mx, orac8a@gmail.com, and beretap@gmail.com. Christopher K. Uejio, Department of Geography, Florida State University, Tallahassee, FL, E-mail: cuejio@admin.fsu.edu. Emily Zielinski-Gutierrez, Division of Vector-Borne Diseases, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Fort Collins, CO, E-mail: ebz0@CDC.GOV.
References
- 1.Gratz NG. Emerging and resurging vector-borne diseases. Annu Rev Entomol. 1999;44:51–75. doi: 10.1146/annurev.ento.44.1.51. [DOI] [PubMed] [Google Scholar]
- 2.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 3.San Martin JL, Brathwaite O, Zambrano B, Solorzano JO, Bouckenooghe A, Dayan GH, Guzman MG. The epidemiology of dengue in the Americas over the last three decades: a worrisome reality. Am J Trop Med Hyg. 2010;82:128–135. doi: 10.4269/ajtmh.2010.09-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Focks DA, Alexander N. A Multi-Country Study on the Methodology for Surveys of Aedes Aegypti Pupal Productivity: Findings and Recommendations. Geneva: World Health Organization; 2006. [Google Scholar]
- 6.Halstead SB. Dengue virus–mosquito interactions. Annu Rev Entomol. 2008;53:273–291. doi: 10.1146/annurev.ento.53.103106.093326. [DOI] [PubMed] [Google Scholar]
- 7.Scott TW, Chow E, Strickman D, Kittayapong P, Wirtz RA, Lorenz LH, Edman JD. Blood-feeding patterns of Aedes aegypti (Diptera: Culicidae) collected in a rural Thai village. J Med Entomol. 1993;30:922–927. doi: 10.1093/jmedent/30.5.922. [DOI] [PubMed] [Google Scholar]
- 8.Christophers SR. Aedes Aegypti (L.). The Yellow Fever Mosquito. Its Life History, Bionomics and Structure. Cambridge, UK: Cambridge University Press; 1960. [Google Scholar]
- 9.Farnesi LC, Martins AJ, Valle D, Rezende GL. Embryonic development of Aedes aegypti (Diptera: Culicidae): Influence of different constant temperatures. Mem Inst Oswaldo Cruz. 2009;104:124–126. doi: 10.1590/s0074-02762009000100020. [DOI] [PubMed] [Google Scholar]
- 10.Focks DA, Haile DG, Daniels E, Mount GA. Dynamic life table model for Aedes aegypti (Diptera: Culicidae): analysis of the literature and model development. J Med Entomol. 1993;30:1003–1017. doi: 10.1093/jmedent/30.6.1003. [DOI] [PubMed] [Google Scholar]
- 11.Richardson K, Hoffmann AA, Johnson P, Ritchie S, Kearney MR. Thermal sensitivity of Aedes aegypti from Australia: empirical data and prediction of effects on distribution. J Med Entomol. 2011;48:914–923. doi: 10.1603/me10204. [DOI] [PubMed] [Google Scholar]
- 12.Yang HM, Macoris MLG, Galvani KC, Andrighetti MTM, Wanderley DMV. Assessing the effects of temperature on the population of Aedes aegypti, the vector of dengue. Epidemiol Infect. 2009;137:1188–1202. doi: 10.1017/S0950268809002040. [DOI] [PubMed] [Google Scholar]
- 13.Hopp MJ, Foley JA. Global-scale relationships between climate and the dengue fever vector, Aedes aegypti. Clim Change. 2001;48:441–463. [Google Scholar]
- 14.Ibanez-Bernal S. Nuevo registro altitudinal de Aedes (Stegomyia) aegypti (Linnaeus, 1762) (Diptera: Culicidae) en Mexico. Folia Entomol Mex. 1987;72:163–164. [Google Scholar]
- 15.Suarez MF, Nelson MJ. Registro de altitud del Aedes aegypti en Colombia. Biomedica. 1981;1:225. [Google Scholar]
- 16.Herrera-Basto E, Prevots DR, Zarate ML, Silva JL, Sepulveda-Amor J. First reported outbreak of classical dengue fever at 1,700 meters above sea level in Guerrero State, Mexico, June 1988. Am J Trop Med Hyg. 1992;46:649–653. doi: 10.4269/ajtmh.1992.46.649. [DOI] [PubMed] [Google Scholar]
- 17.Diaz Najera A, Vargas L. Mosquitos mexicanos. Distribución geográfica actualizada. Rev Inst Salud Publ. 1973;33:111–125. [PubMed] [Google Scholar]
- 18.Heinemann SJ, Belkin JN. Collection records of the project “Mosquitoes of Middle America”. 9. Mexico (MEX, MF, MT, MX) Mosq Syst. 1977;9:483–535. [Google Scholar]
- 19.Tun-Lin W, Lenhart A, Nam VS, Rebollar-Tellez E, Morrison AC, Barbazan P, Cote M, Midega J, Sanchez F, Manrique-Saide P, Kroeger A, Nathan MB, Meheus F, Petzold M. Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: a multi-country non-inferiority cluster randomized trial. Trop Med Int Health. 2009;14:1143–1153. doi: 10.1111/j.1365-3156.2009.02341.x. [DOI] [PubMed] [Google Scholar]
- 20.Banu S, Hu WB, Hurst C, Tong SL. Dengue transmission in the Asia-Pacific region: impact of climate change and socio-environmental factors. Trop Med Int Health. 2011;16:598–607. doi: 10.1111/j.1365-3156.2011.02734.x. [DOI] [PubMed] [Google Scholar]
- 21.Hayden MH, Uejio CK, Walker K, Ramberg F, Moreno R, Rosales C, Gameros M, Mearns LO, Zielinski-Gutierrez E, Janes CR. Microclimate and human factors in the divergent ecology of Aedes aegypti along the Arizona, US/Sonora, MX border. EcoHealth. 2010;7:64–77. doi: 10.1007/s10393-010-0288-z. [DOI] [PubMed] [Google Scholar]
- 22.Padmanabha H, Soto E, Mosquera M, Lord CC, Lounibos LP. Ecological links between water storage behaviors and Aedes aegypti production: implications for dengue vector control in variable climates. EcoHealth. 2010;7:78–90. doi: 10.1007/s10393-010-0301-6. [DOI] [PubMed] [Google Scholar]
- 23.Ramos MM, Mohammed H, Zielinski-Gutierrez E, Hayden MH, Lopez JLR, Fournier M, Trujillo AR, Burton R, Brunkard JM, Anaya-Lopez L, Banicki AA, Morales PK, Smith B, Munoz JL, Waterman SH. Epidemic dengue and dengue hemorrhagic fever at the Texas-Mexico border: results of a household-based seroepidemiologic survey, December 2005. Am J Trop Med Hyg. 2008;78:364–369. [PubMed] [Google Scholar]
- 24.Reiter P, Lathrop S, Bunning M, Biggerstaff B, Singer D, Tiwari T, Baber L, Amador M, Thirion J, Hayes J, Seca C, Mendez J, Ramirez B, Robinson J, Rawlings J, Vorndam V, Waterman S, Gubler D, Clark G, Hayes E. Texas lifestyle limits transmission of dengue virus. Emerg Infect Dis. 2003;9:86–89. doi: 10.3201/eid0901.020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams CR, Bader CA, Kearney MR, Ritchie SA, Russell RC. The extinction of dengue through natural vulnerability of its vectors. PLoS Negl Trop Dis. 2010;4:e922. doi: 10.1371/journal.pntd.0000922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrington L, Scott T, Lerdthusnee K, Coleman R, Costero A, Clark G, Jones J, Kitthawee S, Kittayapong P, Sithiprasasna R. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg. 2005;72:209–220. [PubMed] [Google Scholar]
- 27.Azil AH, Long SA, Ritchie SA, Williams CR. The development of predictive tools for pre-emptive dengue vector control: a study of Aedes aegypti abundance and meteorological variables in North Queensland, Australia. Trop Med Int Health. 2010;15:1190–1197. doi: 10.1111/j.1365-3156.2010.02592.x. [DOI] [PubMed] [Google Scholar]
- 28.de Almeida Costa EAP, de Mendonca Santos EM, Correia JC, de Albuquerque CMR. Impact of small variations in temperature and humidity on the reproductive activity and survival of Aedes aegypti (Diptera, Culicidae) Rev Bras Entomol. 2010;54:488–493. [Google Scholar]
- 29.Romero-Vivas CME, Llinas H, Falconar AKI. Three calibration factors, applied to a rapid sweeping method, can accurately estimate Aedes aegypti (Diptera: Culicidae) pupal numbers in large water-storage containers at all temperatures at which dengue virus transmission occurs. J Med Entomol. 2007;44:930–937. doi: 10.1603/0022-2585(2007)44[930:tcfata]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Darsie RF, Jr, Ward RA. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. Gainesville, FL: University Press of Florida; 2005. [Google Scholar]
- 31.Joyce RJ, Janowiak JE, Arkin PA, Xie P. CMORPH: a method that produces global precipitation estimates from passive microwave and infrared data at high spatial and temporal resolution. J Hydromet. 2004;5:487–503. [Google Scholar]
- 32.Ebert EE, Janowiak JE, Kidd C. Comparison of near-real-time precipitation estimates from satellite observations and numerical models. Bull Am Meteorol Soc. 2007;88:47–64. [Google Scholar]
- 33.Sall J, Creighton L, Lehman A. JMP Start Statistics. 3rd Ed. Belmont, CA: Brooks/Cole; 2005. [Google Scholar]
- 34.Beebe NW, Cooper RD, Mottram P, Sweeney AW. Australia's dengue risk driven by human adaptation to climate change. PLoS Negl Trop Dis. 2009;3:e429. doi: 10.1371/journal.pntd.0000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hales S, de Wet N, Maindonald J, Woodward A. Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet. 2002;360:830–834. doi: 10.1016/S0140-6736(02)09964-6. [DOI] [PubMed] [Google Scholar]
- 36.Jetten TH, Focks DA. Potential changes in the distribution of dengue transmission under climate warming. Am J Trop Med Hyg. 1997;57:285–297. doi: 10.4269/ajtmh.1997.57.285. [DOI] [PubMed] [Google Scholar]
- 37.Ooi EE, Gubler DJ. Global spread of epidemic dengue: the influence of environmental change. Future Virol. 2009;4:571–580. [Google Scholar]
- 38.Patz JA, Martens WJM, Focks DA, Jetten TH. Dengue fever epidemic potential as projected by general circulation models of global climate change. Environ Health Perspect. 1998;106:147–153. doi: 10.1289/ehp.98106147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barclay E. Is climate change affecting dengue in the Americas? Lancet. 2008;371:973–974. doi: 10.1016/s0140-6736(08)60435-3. [DOI] [PubMed] [Google Scholar]
- 40.Hansen J, Ruedy R, Sato M, Lo K. Global surface temperature change. Rev Geophys. 2010;48:RG4004. [Google Scholar]
- 41.Christensen JH, Hewitson B, Busuioc A, Chen A, Gao X, Held I, Jones R, Kolli RK, Kwon W-T, Laprise R, Magaña Rueda V, Mearns L, Menéndez CG, Räisänen J, Rinke A, Sarr A, Whetton P. Regional climate projections. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, editors. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. New York, NY: Cambridge University Press; 2007. [Google Scholar]
- 42.Chowell G, Sanchez F. Climate-based descriptive models of dengue fever: the 2002 epidemic in Colima, Mexico. J Environ Health. 2006;68:40–44. [PubMed] [Google Scholar]
- 43.Colon-Gonzalez FJ, Lake IR, Bentham G. Climate variability and dengue fever in warm and humid Mexico. Am J Trop Med Hyg. 2011;84:757–763. doi: 10.4269/ajtmh.2011.10-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machado-Machado EA. Empirical mapping of suitability to dengue fever in Mexico using species distribution modeling. Appl Geogr. 2012;33:82–93. [Google Scholar]
- 45.Koopman JS, Prevots DR, Vaca Marin MA, Gomez Dantes H, Zarate Aquino ML, Longini IM, Jr, Sepulveda Amor J. Determinants and predictors of dengue infection in Mexico. Am J Epidemiol. 1991;133:1168–1178. doi: 10.1093/oxfordjournals.aje.a115829. [DOI] [PubMed] [Google Scholar]
- 46.Davis NC. The effects of heat and cold upon Aedes (Stegomyia) aegypti. Part I. The survival of Stegomyia eggs under abnormal temperature conditions. Am J Hyg. 1932;16:177–191. [Google Scholar]
- 47.Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, Scott TW. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc Natl Acad Sci USA. 2011;108:7460–7465. doi: 10.1073/pnas.1101377108. [DOI] [PMC free article] [PubMed] [Google Scholar]