Abstract
Vibriocidal antibody is a marker of recent exposure to Vibrio cholerae O1 infection. We examined vibriocidal titers for 1 year after an episode of severe cholera in patients in Dhaka, Bangladesh; 16 of 53 (30%) patients had a fourfold or greater increase in vibriocidal titer between 6 and 12 months after an episode of severe cholera, suggesting reexposure to the organism. Among patients with rises in titers during follow-up, the patients initially infected with serotype Ogawa had earlier rises in titer than the patients initially infected with serotype Inaba. These data and others suggest that an episode of severe cholera protects against symptomatic disease for several years, but reexposure to the organism occurs frequently in an endemic area, with immunological boosts beginning as early as 6 months after severe disease. Repeated exposures to V. cholerae in endemic areas may be a necessary component for long-lasting protection against severe disease.
Introduction
Cholera is an acute, dehydrating diarrheal illness that affects millions of people each year.1 The O1 serogroup of Vibrio cholerae is the predominant cause of human disease worldwide, and it occurs in two biotypes, El Tor and classical. The antigenic determinants of the lipopolysaccharide (LPS) O antigen allow for additional classification of these biotypes into serotypes Ogawa and Inaba. Natural infection with V. cholerae confers a substantial period of protection from recurrent symptomatic disease. On rechallenge with classical V. cholerae O1, North American volunteers showed 100% protection from symptoms for at least 3 years.2 Epidemiologic studies in cholera-endemic areas suggest that protection from symptomatic disease after an episode of cholera may last even longer than 3 years.2–4 In a recent study with age-matched controls in a cholera-endemic area, an episode of El Tor cholera conferred a 65% lower risk of a subsequent episode of symptomatic El Tor cholera over 3 years.5 After V. cholerae O1 serotype Ogawa infection, protection from reinfection is longer lasting with serotype Ogawa V. cholerae compared with serotype Inaba, but after serotype Inaba infection, patients are equally protected from both serotype Ogawa and Inaba subsequent infections.3,5
The vibriocidal antibody is the best-studied marker of protection from cholera, and it is frequently used as a measure of immunity. The majority of vibriocidal antibodies, which are complement-fixing bacteriocidal antibodies, can be absorbed with V. cholerae LPS.6 Susceptibility to V. cholerae infection is greater in persons with lower baseline vibriocidal titers. However, there is no threshold level of vibriocidal titer that confers complete protection from infection or symptoms, and the vibriocidal antibody is thought to be a surrogate marker of a protective mucosal immune response.7–10 In areas where cholera is endemic, most residents have detectable vibriocidal antibodies by the teenage years, and titers increase with age.10,11 Because of the background rate of vibriocidal antibodies in these populations, there is no threshold cutoff diagnostic of infection in an endemic area. Rather, a fourfold or greater increase between paired acute and convalescent measurements of the serogroup-specific vibriocidal titer is preferred for documentation of recent V. cholerae exposure in endemic areas.7,12
In the high-risk cholera settings of Dhaka, Bangladesh, exposure to V. cholerae is common. In this prospective study, we followed a cohort of patients after an episode of symptomatic cholera to characterize the frequency of reexposure to the organism over a 1-year period using a fourfold or greater rise in vibriocidal titer during follow-up to identify exposure sufficient to generate an immune response.
Materials and Methods
This study was conducted at the International Center for Diarrhoeal Disease Research, Bangladesh (icddr, b) Dhaka Hospital, which cares for more than 120,000 patients per year, including approximately 20,000 with cholera. Most of the patients live in the urban high-risk cholera areas of Dhaka. Patients presenting to the hospital between 2006 and 2010 with acute watery diarrhea were eligible for inclusion in this study if stool cultures were subsequently positive for V. cholerae as the sole pathogen, they were between the ages of 2 and 60 years, they resided in or around Dhaka city, they were without significant comorbid conditions, and they consented for a study with a 1-year follow-up period and periodic blood draws. The patients enrolled represent a convenience sample of those patients meeting the inclusion criteria. At the time of enrollment, suspected V. cholerae colonies were serologically confirmed by slide agglutination, with specific monoclonal antibody for Ogawa or Inaba serotypes.13 After obtaining informed, written consent from patients, venous blood draws were performed on the second day of hospitalization and days 7, 30, 90, 180, 270, and 360 after the onset of illness. At each time point, serum was assayed for the vibriocidal and cholera antigen-specific antibodies described below. At each study visit, the level of dehydration was assessed according to the World Health Organization (WHO) dehydration scale. For 1 year after discharge, participants were questioned monthly about diarrhea symptoms between study visits, whether care was sought for diarrhea during the previous month, need for intravenous rehydration solutions that would connote moderate or severe dehydration related to diarrhea, and use of antibiotics.14 Per study protocol, repeat stool cultures were obtained only in patients with moderate or severe dehydrating diarrhea; none of the patients followed in this study had moderate or severe dehydrating diarrhea during the 1 year follow-up period. The Research and Ethical Review Committees of the icddr, b and the Institutional Review Board of the Massachusetts General Hospital approved this study.
Vibriocidal antibody responses of both serotypes were measured in serum samples at each time point of follow-up as previously described using guinea pig complement and V. cholerae O1 Ogawa (X-25049) or Inaba (T-19479) as the target organism.15 Heat-inactivated serum was diluted fivefold, and serial twofold dilutions were examined. We defined the vibriocidal titer as the reciprocal of the highest dilution resulting in > 50% reduction of the optical density compared with the control wells without serum. Positive and negative controls with samples from infected and uninfected individuals were used to provide consistency across plates. We classified individuals with a fourfold or greater rise in serotype-specific vibriocidal antibody during follow-up as reexposed to V. cholerae O1. Immunoglobulin G (IgG), IgA, and IgM antibodies to the B subunit of cholera toxin (CtxB) and V. cholerae O1 LPS were also measured at each time point by kinetic enzyme-linked immunosorbent assay (ELISA) as previously described, and the results were expressed in ELISA units against a positive standard on each plate.15,16
The term initial infection is used in this study to refer to the first confirmed episode of V. cholerae infection for which the patient presented to the icddr, b between the years 2006 and 2010. Participants in this study have likely had previous infections before enrollment in this study. Baseline characteristics of different groups were analyzed using the Fisher's exact test for categorical variables and the Mann–Whitney U test for continuous variables, including age in years. Among patients with a vibriocidal rise during follow-up, the time to a fourfold or greater rise in vibriocidal titer in those patients with initial Ogawa infections was compared with the time in those patients with initial Inaba infections using the Mann–Whitney U test. For this comparison, only those patients with a fourfold or greater rise in titer were included. All reported P values are two-tailed, and the predetermined threshold for statistical significance was P < 0.05. Statistical analyses were performed using Stata version 9.0 (Stata Corporation, Inc., College Station, TX) and GraphPad Prism 4 (GraphPad Software, Inc., La Jolla, CA).
Results
Fifty-four patients with serogroup O1 El Tor V. cholerae infection hospitalized at the icddr, b between 2006 and 2010 were prospectively entered into the study and followed for 1 year. One patient did not have an Ogawa serology vibriocidal titer on day 90, and this patient was excluded from the analysis. For the remaining 53 participants, all blood samples at all time points were collected. The number of patients enrolled each year was 2 patients in 2006, 26 patients in 2007, 15 patients in 2008, 9 patients in 2009, and 1 patient in 2010. Twenty patients (20/53; 38%) were female, and the median age was 27 years (range = 4–59), which is shown in Table 1. Among the 53 patients, 37 (70%) patients were initially infected with V. cholerae serotype Ogawa, and 16 (30%) patients were initially infected with serotype Inaba. All patients had a fourfold or greater increase in the vibriocidal antibody titer between the measurements on days 2 and 7 or day 30 after infection for the same serotype as the admission stool culture. On presentation, all patients were treated with antibiotics active against V. cholerae.
Table 1.
Demographic, clinical, and immunologic characteristics of study participants
| Patients without a fourfold or more increase in vibriocidal antibody during days 90 to 360 (N = 37) | Patients with a fourfold or more increase in vibriocidal antibody during days 90 to 360 (N = 16) | |
|---|---|---|
| Age in years (GM, range) | 27 (8–59) | 20 (5–45) |
| Sex (female) | 13/37 (35%) | 7/16 (44%) |
| Number requiring IV fluids at admission | 11/37 (30%) | 5/16 (31%) |
| Reported diarrhea during days 90 to 360 of follow-up | 21/37 (57%) | 12/16 (75%) |
| Reported ORS use during days 90 to 360 of follow-up | 33/37 (90%) | 14/16 (88%) |
| Serotype of initial infection | Ogawa 26/37 (70%) | Ogawa 11/16 (69%) |
| Vibriocidal titer baseline* at day 2 (GM, 95% CI) | 58 (33–103) | 49 (26–93) |
| Vibriocidal titer at day 7 | 3,900 (2,400–6,500) | 5,700 (3,700–9,000) |
| Vibriocidal titer at day 30 | 1,700 (1,200–2,200) | 2,250 (1,200–3,700) |
| Vibriocidal titer at day 90 | 310 (210–460) | 470 (220–1,000) |
CI = confidence interval; IV = intravenous; GM = geometric mean.
All vibriocidal titer results are matched to serotype of initial infection.
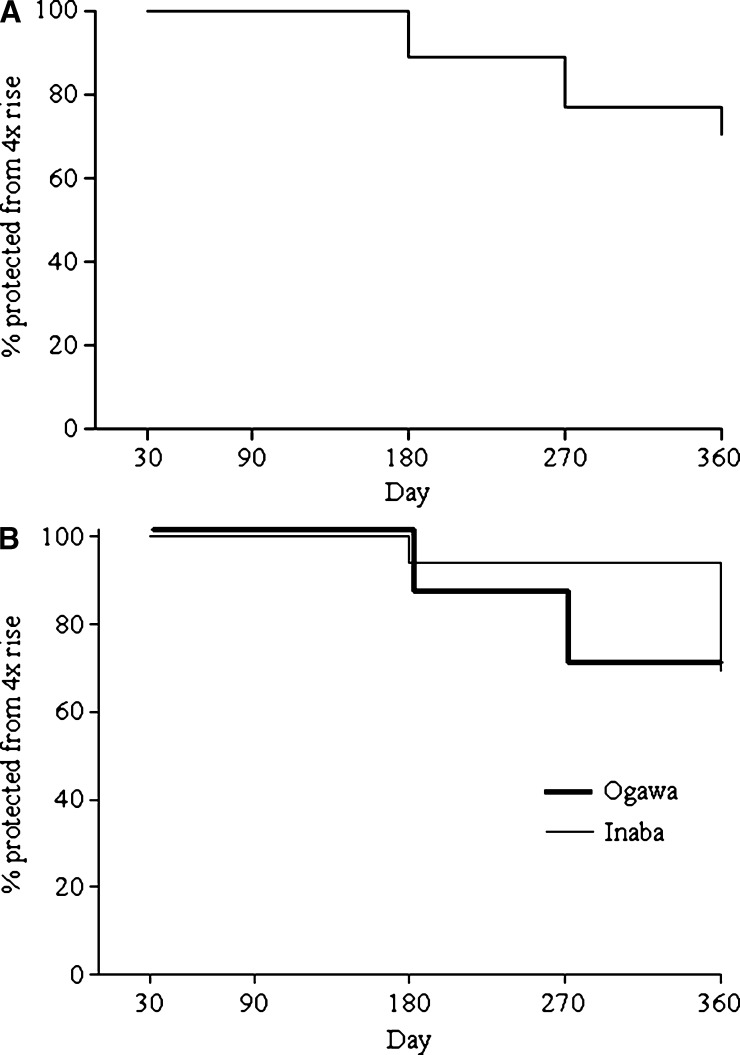
Between days 90 and 360 of follow-up, 16 of 53 (30%) patients had a fourfold or greater increase in vibriocidal titer, which represented a deviation from the otherwise downward slope of titers after the first infection (shown in the time to event curve in Figure 1A). None of the patients had a fourfold or greater increase between days 30 and 90 of follow-up. Between days 90 and 360 of follow-up, diarrhea, oral rehydration solution (ORS) use, and antibiotic use were reported by most patients, but there were no statistically significant differences between those patients with and without fourfold vibriocidal titer increases. Between days 90 and 360 of follow-up, there were no episodes of dehydrating diarrhea detected at follow-up visits. Between follow-up visits, none of the patients enrolled in the study reported seeking medical care or required intravenous rehydration solution for dehydrating diarrhea. Of the 16 patients with vibriocidal rises during follow-up, 2 patients had a fourfold rise in titer during follow-up on more than one occasion; the time to first titer rise is used in Figure 1A.
Figure 1.
(A) Time to event curve of the proportion of patients who have not yet had a subsequent fourfold or greater increase in vibriocidal titer during follow-up after severe cholera. (B) Time to event curve of the proportion of patients who have not yet had a subsequent fourfold or greater increase in vibriocidal titer during follow-up after severe cholera stratified by serotype of initial infecting strain. The Mann–Whitney U test was used for comparison of time in days to fourfold or greater rise between the two groups (P = 0.01).
There was a statistically significant inverse association between age in years and the likelihood of a fourfold or greater increase in vibriocidal titer during the days 90 to 360 follow-up period (P = 0.04). Among the 16 patients with fourfold or greater increases, 3 patients were children < 10 years of age; among the 37 patients who did not have a fourfold increase, only 1 patient was < 10 years of age. Among patients with a vibriocidal rise during the follow-up period, patients initially infected with V. cholerae serotype Ogawa were more likely to have a fourfold or greater increase earlier in follow-up than patients initially infected with an Inaba strain (P = 0.01); the time to vibriocidal rise for the two groups is shown in Figure 1B. We found no differences in vibriocidal peak titers, nadirs, timing of peaks, or rate of vibriocidal titer decline after initial infection between patients who had a subsequent fourfold or greater increase in vibriocidal titer during follow-up and patients who did not. We also did not find significant increases in serum IgG or IgA antibodies to CtxB or LPS or serum IgM antibodies to LPS at the same time point as the vibriocidal titer increases in patients with vibriocidal increases compared with those patients without increases at that time point.
For the 16 patients with a fourfold vibriocidal titer rise during follow-up, the serotype-specific vibriocidal titer on day 7 after acute infection was between 8- and 1,200-fold greater than the day 2 titer, with a mean 264-fold increase. The magnitude of vibriocidal titer rises during the follow-up period, when that occurred, was lower, ranging from 4- to 64-fold increases, with a mean 11-fold increase. During the day 90 to 360 period, 75% (12/16) of patients with vibriocidal titer rises had fourfold or greater increases in only one serotype-specific vibriocidal titer (as opposed to both serotypes).
Among the 11 patients with initial serotype Ogawa infection and a subsequent rise in vibriocidal titer, 9 patients had a fourfold or greater increase in serotype Inaba titers during follow-up, shown schematically in Figure 2. Of these nine patients, one patient also had a fourfold rise in Ogawa titer at the same time point as the Inaba rise, and two patients had a less than fourfold increase in Ogawa vibriocidal titers at the time point of the Inaba rise. Six of nine patients had an unchanged or decreasing Ogawa titer at the time of the Inaba rise. Two patients with initial Ogawa infection had a fourfold or greater rise in Ogawa-specific vibriocidal titers during follow-up; at the same time point, one patient had an Inaba titer decrease, and one patient had a less than fourfold increase in Inaba titer.
Figure 2.
Schematic of the fourfold or greater increases in serotype-specific vibriocidal titer for 53 patients after severe cholera, including measurements of heterologous serotype titer at the same time point. Patients with multiple rises in vibriocidal titer during follow-up are classified by the serotype of first rise.
Among the five patients with initial Inaba infection, three patients had fourfold or greater rises in Ogawa titers between days 90 and 360. Among these three patients, one patient had an equal increase in Inaba titer at the same time point as the Ogawa titer rise, and two patients had decreases in Inaba titers at the time of the Ogawa titer rise. Two patients with initial Inaba infection had fourfold or greater increases in Inaba titers during follow-up; at that same time point, one patient had no change in the Ogawa titer; and one patient had a less than fourfold increase in Ogawa titer. Vibriocidal titer rises during follow-up were more common for the serotype discordant to the initial infection, with a trend toward significance (P = 0.12).
Two patients experienced more than one fourfold or greater rise in vibriocidal titer during 1 year of follow-up. The first patient was a man 21 years of age who was initially infected with Inaba serotype. He had a 64-fold rise in serotype Ogawa vibriocidal antibody on day 90 and an 8-fold rise in serotype Inaba titer at day 180. The second patient, a 5-year-old girl initially infected with Ogawa serotype, had a 32-fold rise in Inaba vibriocidal titer on day 90, and a second 8-fold rise in serotype Inaba titer on day 360 accompanied by a 4-fold rise in Ogawa vibriocidal titer on the same day.
Discussion
In cholera-endemic areas, individuals develop vibriocidal antibody responses early in life, and titers increase with age.10,17,18 Presumably, this increase over a lifetime is caused by frequent exposure to V. cholerae. The present study suggests that, in a highly cholera-endemic area, patients have exposures to V. cholerae sufficient to generate a vibriocidal response that begins as early as 6 months after an episode of severe cholera. If routine stool cultures had been done as part of our protocol, it is possible that even more patients would have shown transient colonization with V. cholerae insufficient to generate a vibriocidal antibody response. Protection from a fourfold increase in vibriocidal titer is of shorter duration in younger patients and patients initially infected with serotype Ogawa V. cholerae. Although the higher risk associated with younger age may be partially explained by lower average vibriocidal titers at younger ages, our group has shown previously that younger age is a risk factor for V. cholerae infection independent of vibriocidal titer,7 suggesting that immune-mediated mechanisms independent of vibriocidal titer may accumulate over time. We are not certain why there were not antibody responses to LPS at the same time points as the vibriocidal increases, but it is possible that the serum vibriocidal response is more sensitive than other serum antibody responses to this organism. Episodes of diarrhea in this population were extremely common and likely caused by a range of pathogens; it was not possible to correlate symptoms with the times of vibriocidal titer increases given that patients were seen only monthly during follow-up. Therefore, we cannot determine if the fourfold rises in titer represent asymptomatic colonization or infection with mild symptoms. None of the patients developed moderate to severe symptoms during the follow-up period.
Patients with serotype O1 V. cholerae infection as well as brucellosis or Citrobacter spp. infections19 may show fourfold or greater increases in vibriocidal titers, but vibriocidal titers do not increase significantly after other infections, including other Vibrio spp. and non-O1 V. cholerae.20–23 The increases in vibriocidal titers described here, therefore, are most likely caused by reexposure to V. cholerae O1. As described previously in household contacts of cholera patients, a range of possible outcomes can occur after exposure to V. cholerae, including colonization (either transient or longer lasting), a fourfold or greater rise in vibriocidal titer, increases in other immune responses, or symptomatic diarrhea with no, mild, moderate, or severe dehydration.7,24 Many patients in this study had increases in vibriocidal titer, but they were fully protected from symptomatic disease requiring intravenous rehydration. After cholera, healthy volunteer studies have shown that, on rechallenge with V. cholerae, vibriocidal titer rises are of a lower magnitude compared with those rises observed after initial infection.2,12 The diminished vibriocidal response with repeat exposure seen in this study may be caused by a low inoculum exposure or may be secondary to partial immunity after the recent episode of severe cholera, with neutralization of the inoculum by pre-existing mucosal antibodies in the gut.
In endemic areas, the predominance of El Tor serotype Ogawa versus Inaba fluctuates, and both are present during most cholera epidemics.25–27 Studies of recurrent symptomatic cholera in endemic areas and other data suggest that infection with serotype Inaba confers more complete and longer lasting protection than infection with serotype Ogawa.5,26 We have previously shown that patients infected with serotype Ogawa O1 V. cholerae are younger, have more severe disease, and have higher vibriocidal titer elevations in acute infection than patients with serotype Inaba.28,29 The Ogawa serotype has antigenic determinants A and B on the O side chain of the LPS, and the Inaba serotype has determinants A and C.30 The serotype Ogawa antigenic determinant B contains a 2-O-methyl group in the non-reducing terminal sugar of the O-specific polysaccharide (O-SP) of the LPS. How this structural distinction translates to different levels of protection is unknown. Our results support the finding that, among patients with evidence of reexposure to V. cholerae soon after severe cholera, serotype Inaba infection may confer a longer duration of protection than Ogawa infection. It is also possible that the likelihood of reinfection at a specific time point may be influenced by local serotype predominance at that time, which varies from year to year. In the year after one episode of severe cholera, several patients initially infected with serotype Inaba had subsequent fourfold or greater rises in vibriocidal titers, showing that any protection conferred by serotype Inaba V. cholerae O1, at least to asymptomatic reinfection, is incomplete.
Recognized asymptomatic or subclinical colonization after reexposure to V. cholerae7,24 as well as the present study suggest a model in which an episode of severe cholera in an endemic area may confer protection against moderate to severe disease for an extended period, but reexposure to V. cholerae accompanied by rises in vibriocidal antibodies may be more common than previously recognized and occur early after severe disease. This frequent reexposure may be a necessary component of acquiring longer lasting protection against recurrent dehydrating disease.
ACKNOWLEDGMENTS
The authors thank the patients for their participation in this study. We also appreciate the dedication of the field and laboratory workers of the Protective Immunity to Cholera Study at the International Centre for Diarrhoeal Disease Research, Bangladesh.
Footnotes
Financial support: This work was supported by a Partners Healthcare Center of Expertise in Global and Humanitarian Health Travel Grant (to A.A.W.); Fogarty International Clinical Research Scholars Award R24 TW007988 (to F.C. and A.I.K.) from the Fogarty International Center; American Recovery and Reinvestment Act (ARRA) Post-Doctoral Fellowship in Global Infectious Diseases TW05572 (to D.T.L.); a Postdoctoral Fellowship in Tropical Infectious Diseases from the American Society for Tropical Medicine and Hygiene—Burroughs Wellcome Fund (to D.T.L.); the Harvard Initiative for Global Health Post-Doctoral Fellowship in Global Infectious Diseases (to D.T.L); a Training Grant in Vaccine Development and Public Health TW005572 (to T.U., E.T.R., and F.Q.); Career Development Awards K08 AI089721 (to R.C.C.), K01 TW07144 (to R.C.L.), and K01 TW07409 (to J.B.H.); a Physician Scientist Early Career Award from the Howard Hughes Medical Institute (to R.C.L.); National Institutes of Health Grants U01 AI077883 (to E.T.R.), AI058935 (to E.T.R. and S.B.C.), and R03 AI063079 (to F.Q.); and the Swedish Agency for International Development and Cooperation (F.Q.).
Authors' addresses: Ana A. Weil, Department of Medicine, Massachusetts General Hospital, Boston, MA, E-mail: aweil@partners.org. Fahima Chowdhury, Ashraful I. Khan, Daniel T. Leung, Taher Uddin, Yasmin Ara Begum, Nirod Chandra Saha, and Firdausi Qadri, Centre for Vaccine Sciences, International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh, E-mails: fchowdhury@icddrb.org, ashrafk@icddrb.org, dleung@partners.org, taher_imm@icddrb.org, yasmin@icddrb.org, nirod@icddrb.org, and fqadri@icddrb.org. Richelle C. Charles, Regina C. LaRocque, Jason B. Harris, Edward T. Ryan, and Stephen B. Calderwood, Division of Infectious Diseases, Massachusetts General Hospital, Boston, MA, E-mails: rccharles@partners.org, rclarocque@partners.org, jbharris@partners.org, etryan@partners.org, and scalderwood@partners.org.
References
- 1.Zuckerman JN, Rombo L, Fisch A. The true burden and risk of cholera: implications for prevention and control. Lancet Infect Dis. 2007;7:521–530. doi: 10.1016/S1473-3099(07)70138-X. [DOI] [PubMed] [Google Scholar]
- 2.Levine MM, Black RE, Clements ML, Cisneros L, Nalin DR, Young CR. Duration of infection-derived immunity to cholera. J Infect Dis. 1981;143:818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- 3.Woodward WE. Cholera reinfection in man. J Infect Dis. 1971;123:61–66. doi: 10.1093/infdis/123.1.61. [DOI] [PubMed] [Google Scholar]
- 4.Glass RI, Becker S, Huq MI, Stoll BJ, Khan MU, Merson MH, Lee JV, Black RE. Endemic cholera in rural Bangladesh, 1966–1980. Am J Epidemiol. 1982;116:959–970. doi: 10.1093/oxfordjournals.aje.a113498. [DOI] [PubMed] [Google Scholar]
- 5.Ali M, Emch M, Park JK, Yunus M, Clemens J. Natural cholera infection-derived immunity in an endemic setting. J Infect Dis. 2011;204:912–918. doi: 10.1093/infdis/jir416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmgren J, Svennerholm AM. Mechanisms of disease and immunity in cholera: a review. J Infect Dis. 1977;136((Suppl)):S105–S112. doi: 10.1093/infdis/136.supplement.s105. [DOI] [PubMed] [Google Scholar]
- 7.Harris JB, Larocque RC, Chowdhury F, Khan AI, Logvinenko T, Faruque AS, Ryan ET, Qadri F, Calderwood SB. Susceptibility to Vibrio cholerae infection in a cohort of household contacts of patients with cholera in Bangladesh. PLoS Negl Trop Dis. 2008;2:e221. doi: 10.1371/journal.pntd.0000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tacket CO, Cohen MB, Wasserman SS, Losonsky G, Livio S, Kotloff K, Edelman R, Kaper JB, Cryz SJ, Giannella RA, Schiff G, Levine MM. Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El tor inaba three months after vaccination. Infect Immun. 1999;67:6341–6345. doi: 10.1128/iai.67.12.6341-6345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saha D, LaRocque RC, Khan AI, Harris JB, Begum YA, Akramuzzaman SM, Faruque AS, Ryan ET, Qadri F, Calderwood SB. Incomplete correlation of serum vibriocidal antibody titer with protection from Vibrio cholerae infection in urban Bangladesh. J Infect Dis. 2004;189:2318–2322. doi: 10.1086/421275. [DOI] [PubMed] [Google Scholar]
- 10.Glass RI, Svennerholm AM, Khan MR, Huda S, Huq MI, Holmgren J. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J Infect Dis. 1985;151:236–242. doi: 10.1093/infdis/151.2.236. [DOI] [PubMed] [Google Scholar]
- 11.Mosley WH, Ahmad S, Benenson AS, Ahmed A. The relationship of vibriocidal antibody titre to susceptibility to cholera in family contacts of cholera patients. Bull World Health Organ. 1968;38:777–785. [PMC free article] [PubMed] [Google Scholar]
- 12.Clements ML, Levine MM, Young CR, Black RE, Lim YL, Robins-Browne RM, Craig JP. Magnitude, kinetics, and duration of vibriocidal antibody responses in North Americans after ingestion of Vibrio cholerae. J Infect Dis. 1982;145:465–473. doi: 10.1093/infdis/145.4.465. [DOI] [PubMed] [Google Scholar]
- 13.Qadri F, Azim T, Chowdhury A, Hossain J, Sack RB, Albert MJ. Production, characterization, and application of monoclonal antibodies to Vibrio cholerae O139 synonym Bengal. Clin Diagn Lab Immunol. 1994;1:51–54. doi: 10.1128/cdli.1.1.51-54.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization . The Treatment of Diarrhea: A Manual for Physicians and Other Senior Health Workers. 4th Ed. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- 15.Qadri F, Wenneras C, Albert MJ, Hossain J, Mannoor K, Begum YA, Mohi G, Salam MA, Sack RB, Svennerholm AM. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect Immun. 1997;65:3571–3576. doi: 10.1128/iai.65.9.3571-3576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kendall EA, Tarique AA, Hossain A, Alam MM, Arifuzzaman M, Akhtar N, Chowdhury F, Khan AI, Larocque RC, Harris JB, Ryan ET, Qadri F, Calderwood SB. Development of immunoglobulin M memory to both a T-cell-independent and a T-cell-dependent antigen following infection with Vibrio cholerae O1 in Bangladesh. Infect Immun. 2010;78:253–259. doi: 10.1128/IAI.00868-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosley WH, Benenson AS, Barui R. A serological survey for cholera antibodies in rural east Pakistan. 1. The distribution of antibody in the control population of a cholera-vaccine field-trial area and the relation of antibody titre to the pattern of endemic cholera. Bull World Health Organ. 1968;38:327–334. [PMC free article] [PubMed] [Google Scholar]
- 18.Mosley WH, McCormack WM, Ahmed A, Chowdhury AK, Barui RK. Report of the 1966–67 cholera vaccine field trial in rural East Pakistan. 2. Results of the serological surveys in the study population—the relationship of case rate to antibody titre and an estimate of the inapparent infection rate with Vibrio cholerae. Bull World Health Organ. 1969;40:187–197. [PMC free article] [PubMed] [Google Scholar]
- 19.Gangarosa EJ, DeWitt WE, Feeley JC, Adams MR. Significance of vibriocidal antibodies with regard to immunity to cholera. J Infect Dis. 1970;121((Suppl 121)):36. doi: 10.1093/infdis/121.supplement.s36. [DOI] [PubMed] [Google Scholar]
- 20.Morris JG, Jr, Takeda T, Tall BD, Losonsky GA, Bhattacharya SK, Forrest BD, Kay BA, Nishibuchi M. Experimental non-O group 1 Vibrio cholerae gastroenteritis in humans. J Clin Invest. 1990;85:697–705. doi: 10.1172/JCI114494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefkowitz A, Fout GS, Losonsky G, Wasserman SS, Israel E, Morris JG., Jr A serosurvey of pathogens associated with shellfish: prevalence of antibodies to Vibrio species and Norwalk virus in the Chesapeake Bay region. Am J Epidemiol. 1992;135:369–380. doi: 10.1093/oxfordjournals.aje.a116298. [DOI] [PubMed] [Google Scholar]
- 22.Chowdhury F, Begum YA, Alam MM, Khan AI, Ahmed T, Bhuiyan MS, Harris JB, LaRocque RC, Faruque AS, Endtz H, Ryan ET, Cravioto A, Svennerholm AM, Calderwood SB, Qadri F. Concomitant enterotoxigenic Escherichia coli infection induces increased immune responses to Vibrio cholerae O1 antigens in patients with cholera in Bangladesh. Infect Immun. 2008;78:2117–2124. doi: 10.1128/IAI.01426-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qadri F, Mohi G, Hossain J, Azim T, Khan AM, Salam MA, Sack RB, Albert MJ, Svennerholm AM. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin Diagn Lab Immunol. 1995;2:685–688. doi: 10.1128/cdli.2.6.685-688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weil AA, Khan AI, Chowdhury F, Larocque RC, Faruque AS, Ryan ET, Calderwood SB, Qadri F, Harris JB. Clinical outcomes in household contacts of patients with cholera in Bangladesh. Clin Infect Dis. 2009;49:1473–1479. doi: 10.1086/644779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalsgaard A, Skov MN, Serichantalergs O, Echeverria P, Meza R, Taylor DN. Molecular evolution of Vibrio cholerae O1 strains isolated in Lima, Peru, from 1991 to 1995. J Clin Microbiol. 1997;35:1151–1156. doi: 10.1128/jcm.35.5.1151-1156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longini IM, Jr, Yunus M, Zaman K, Siddique AK, Sack RB, Nizam A. Epidemic and endemic cholera trends over a 33-year period in Bangladesh. J Infect Dis. 2002;186:246–251. doi: 10.1086/341206. [DOI] [PubMed] [Google Scholar]
- 27.Harris AM, Chowdhury F, Begum YA, Khan AI, Faruque AS, Svennerholm AM, Harris JB, Ryan ET, Cravioto A, Calderwood SB, Qadri F. Shifting prevalence of major diarrheal pathogens in patients seeking hospital care during floods in 1998, 2004, and 2007 in Dhaka, Bangladesh. Am J Trop Med Hyg. 2008;79:708–714. [PMC free article] [PubMed] [Google Scholar]
- 28.Khan AI, Chowdhury F, Harris JB, Larocque RC, Faruque AS, Ryan ET, Calderwood SB, Qadri F. Comparison of clinical features and immunological parameters of patients with dehydrating diarrhoea infected with Inaba or Ogawa serotypes of Vibrio cholerae O1. Scand J Infect Dis. 2010;42:48–56. doi: 10.3109/00365540903289688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung DT, Rahman MA, Mohasin M, Riyadh MA, Patel SM, Alam MM, Chowdhury F, Khan AI, Kalivoda EJ, Aktar A, Bhuiyan MS, LaRocque RC, Harris JB, Calderwood SB, Qadri F, Ryan ET. Comparison of memory B cell, antibody-secreting cell, and plasma antibody responses in young children, older children, and adults with infection caused by Vibrio cholerae O1 El Tor Ogawa in Bangladesh. Clin Vaccine Immunol. 2011;18:1317–1325. doi: 10.1128/CVI.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stroeher UH, Karageorgos LE, Morona R, Manning PA. Serotype conversion in Vibrio cholerae O1. Proc Natl Acad Sci USA. 1992;89:2566–2570. doi: 10.1073/pnas.89.7.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]