Abstract
We retrospectively reviewed a 10-year experience of administration of cotrimoxazole alone in 31 patients compared with 109 patients who received conventional eradication therapy (cotrimoxazole plus doxycycline). The baseline characteristics, the clinical manifestations, the initial intravenous antibiotic treatments, and the mean duration of eradication therapy between the two groups were similar. The culture-confirmed recurrences among the patients who received cotrimoxazole alone and those who received the conventional regimen were not significantly different (1/31 [3.2%] versus 5/109 [4.5% odds ratio = 0.69 [95% confidence interval [CI] = 0.08–6.17]). Gastrointestinal side effects were more common among the conventional regimen group (28/109 [25.7%] versus 2/31 [6.5%], P = 0.02) and the proportion of patients who could complete at least 20 weeks of therapy without having switched to the other regimen was significantly lower (91/109 [83.5%] versus 31/31 [100.0%] P = 0.01). Cotrimoxazole alone is as effective as and better tolerated than cotrimoxazole plus doxycycline for the eradication treatment of melioidosis.
Introduction
Melioidosis is a Burkholderia pseudomallei bacterial infection endemic to northern Australia and Southeast Asia. A mortality rate as high as 43% was reported from northeast Thailand where it constitutes the third most common cause of death from infectious diseases, after human immunodeficiency virus (HIV) and tuberculosis.1,2 Of those patients who survived, 13–23% had disease recurrences with a further 15–20% fatality.3–5 The ability of B. pseudomallei to survive in phagocytes and to produce glycocalyx are thought to be the major reasons for the recurrence.6,7 Prolonged oral therapy is required to control the infection and has been identified as the major determinant factor associated with reduction of this risk.3 The current recommendation for eradication treatment of melioidosis is a three-drug regimen that includes oral cotrimoxazole (trimethoprim/sulfamethoxazole, TMP/SMX) and doxycycline for at least 12–20 weeks.3,8 Despite this treatment, culture-confirmed recurrences still occurred in ∼6% of patients at 1-year follow-up.8 The efficacy of cotrimoxazole plus doxycycline was questioned after an in vitro study indicated that doxycycline antagonized the antibacterial activity of trimethoprim and sulfamethoxazole against B. pseudomallei.9 Furthermore, 19% of the patients had to switch treatment because of adverse drug events from the three drugs.8 Because poor adherence to treatment are associated with recurrence of the disease,5 other less toxic, alternative eradication regimens including amoxicillin-clavulanate with supplemental amoxicillin, quinolone monotherapy, and a combination of azithromycin and ciprofloxacin, have been tried.10–12 However, all these regimens were associated with recurrence rates exceeding 15% and another simple but more effective regimen is needed.10–12 Cotrimoxazole alone had been successfully used to treat an acute form of melioidosis in the Philippines and is used for eradication therapy in Australia.5,13 However, there are no published studies that have compared the effectiveness and side-effect profile of cotrimoxazole monotherapy with that of the co-trimoxazole plus doxycycline for the eradication treatment of this condition. In this report, we present such a study.
Materials and Methods
The study was conducted at Songklanagarind Hospital, an 800-bed tertiary care university hospital in southern Thailand. All patients who had melioidosis confirmed by culture from January 2000 through December 2009 were retrospectively reviewed. At our institution, treatment usually consisted of an intensive phase (at least 10 days) of intravenous antibiotics, mostly ceftazidime or, in some critically ill patients, imipenem or meropenem. This was then followed by an eradication phase with oral conventional regimen treatment of cotrimoxazole (8 mg of trimethoprim and 40 mg of sulfamethoxazole/kg/d; usual dosage, 160/800 mg. twice daily, plus doxycycline (4 mg/kg/d; usual dosage, 100 mg twice daily,) for at least 20 weeks. Patients were prescribed with cotrimoxazole alone if they were children < 8 years of age, were pregnant or lactating, or had known allergies to tetracycline drugs. “Alternative” eradication regimens including amoxicillin-clavulanate (co-amoxiclav) monotherapy, or a combination of azithromycin and ciprofloxacin were used in cases of known cotrimoxazole drug allergy, or in cases infected with a strain of B. pseudomallei resistant to TMP-SMX by an E-test strip. Patients were followed up every 4 to 6 weeks until oral treatment were completed, and every 3–4 months thereafter for at least 1 year from the beginning of eradication therapy.
In this study, we retrospectively reviewed the treatment record of all patients with melioidosis who survived to discharge from the hospital. We compared two groups of patients: the cotrimoxazole monotherapy group, which consisted of patients who received eradication therapy with cotrimoxazole alone, and the conventional regimen group that received cotrimoxazole plus doxycycline. We specifically compared the clinical presentations and the outcomes, which include the disease recurrence and mortality, drug adverse events, and treatment intolerance requiring a change in therapy. Follow-up data to October 2011 were included in this study. Melioidosis was classified as bacteremic (a positive blood culture result), disseminated (two or more organs involvement), and localized (single focus of infection). The duration of eradication therapy of each patient was measured from the date of starting until discontinuation of oral treatment. Mortality was analyzed as attributable to melioidosis or to other causes. Disease recurrence was reported as both a microbiological-confirmed recurrence and a “clinical recurrence.” Microbiological-confirmed recurrent melioidosis was defined as the development of new symptoms and signs of infection in association with a culture positive for B. pseudomallei. Clinical recurrence was defined as having new clinical features compatible with melioidosis that was treated with antibiotics active against B. pseudomallei but was culture negative. Time to recurrence and/or death was measured from the start of oral eradication therapy.
Statistical analysis was performed with Stata 12 (College Station, TX). All analyses were done on an intention-to-treat basis and then repeated “per protocol.” In the “per-protocol” analyses, patients who switched treatment to the other study group were reallocated according to the final treatment they received. For comparisons of proportions, Fisher's exact test was used. Continuous data not conforming to a normal distribution were compared by means of the Mann Whitney U test. Time to recurrence and/or death were compared using the log-rank test and were depicted graphically using a Kaplan-Meier graph. Statistical differences were considered significant at the 0.05 level.
This study was approved by the Research Ethics Committee of the Faculty of Medicine of Prince of Songkla University
Results
Patients and treatment.
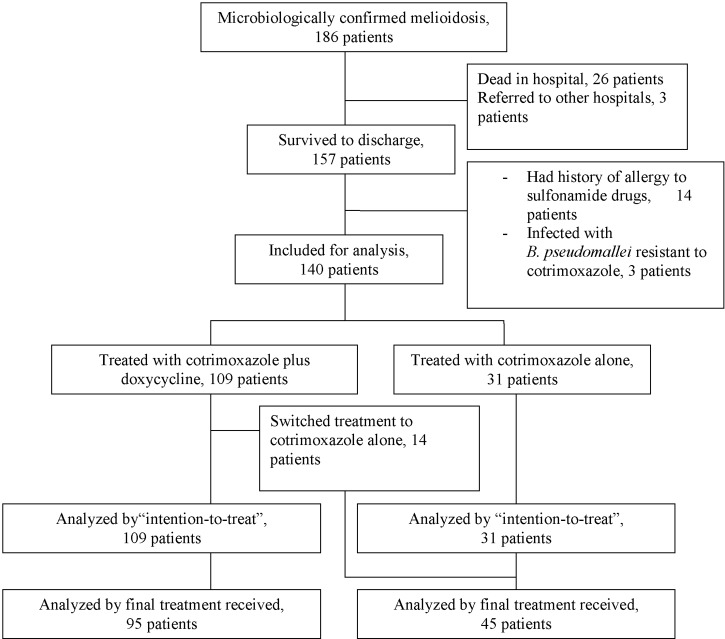
Between January 2000 and December 2009, a total of 186 patients presented to Songklanagarind Hospital with a first episode of culture-confirmed melioidosis. Twenty-six (13.9%) died during hospitalization. Of those who survived to discharge, three were referred for follow-up in other hospitals. These cases and those who died in the hospital were excluded from further study leaving 157 patients, who attended the follow up clinic at least once, eligible for analysis (Figure 1). Fourteen patients had history of allergy to sulfonamide drugs and three were infected with a strain of B. pseudomallei resistant to TMP-SMX. These seventeen patients received an “alternative” eradication treatment regimen, including ciprofloxacin plus azithromycin (N = 9), or amoxicillin/clavulanic acid with additional amoxicillin (N = 8). Of the remaining 140 patients, 109 (69.4%) received oral eradication treatment with cotrimoxazole plus doxycycline (conventional regimen group), whereas cotrimoxazole alone was prescribed in 31 patients who had a history of doxycycline or tetracycline allergy (cotrimoxazole monotherapy group).
Figure 1.
Flow chart illustrating patients included at each stage of the study.
Baseline characteristics.
Patients in the two groups were similar in terms of age, gender, and underlying disease (Table 1). Their age ranged from 6 months to 89 years of age (mean, 45 years; median, 45.4 years). Seven (5%) patients were children < 15 years of age who were similarly distributed in the two groups (5/109 versus 2/31 P = 0.30). The severity of melioidosis in terms of proportion of bacteremia and disseminated organ involvement were also comparable (31/109 versus.10/31 P = 0.159 and 41/109 versus.13/31 P = 0.149, respectively) (Table 1). Most (86.4%) of the patients had been similarly treated with intravenous ceftazidime during the initial intensive phase before beginning the oral eradication drugs.
Table 1.
Characteristic of patients
| Characteristics | Conventional regimen group | Cotrimoxazole group | P |
|---|---|---|---|
| No. of patients | 109 | 31 | – |
| No. of males | 88 (80.7%) | 21 (67.7%) | 0.06 |
| Median age, yrs (range) | 45.1 (5–89 yr) | 45.4 (6 mo–89 yr) | 0.95 |
| Underlying disease | |||
| None | 30 (27.5%) | 8 (25.8%) | 0.85 |
| Diabetes mellitus | 49 (45.0%) | 13 (41.9%) | 0.77 |
| Chronic renal failure | 24 (22.0%) | 9 (29.0%) | 0.42 |
| Hemoglobinopathy | 10 (9.2%) | 6 (19.4%) | 0.12 |
| No. with high-risk occupations for melioidosis | 38 (34.9%) | 12 (38.7%) | 0.69 |
| Initial manifestations | |||
| Disseminated | 31 (28.4%) | 10 (32.3%) | 0.16 |
| Bacteremic | 41 (37.6%) | 13 (41.9%) | 0.15 |
| Localized | 38 (34.9%) | 13 (41.9%) | 0.47 |
| Pneumonia | 19 (17.4%) | 8 (25.8%) | 0.30 |
| Liver/splenic abscess | 21 (19.3%) | 8 (25.8%) | 0.43 |
| Genitourinary infection | 12 (11.0%) | 4 (12.9%) | 0.76 |
| Septic arthritis | 19 (17.4%) | 6 (19.4%) | 0.81 |
| Intensive treatment | |||
| No. received ceftazidime intensive treatment (%) | 94 (86.2%) | 27 (87.1%) | 1.00 |
| Median duration (days) of intensive therapy (range) | 14 (9–30) | 14 (10–28) | 0.22 |
Follow-up.
The median duration of follow-up of patients without recurrence was 70 weeks (range, 53–309 weeks) in the oral conventional regimen group compared with 73 weeks (range, 53–300 weeks) in the cotrimoxazole monotherapy group (P = 0.68) (Table 2).
Table 2.
Eradication therapy, adverse drug reactions, and outcomes of patients
| Treatment/outcomes | Conventional regimen group (109) | Cotrimoxazole group (31) | Odds ratio (95% CI) | P |
|---|---|---|---|---|
| Median duration (weeks) of follow-up of patients without recurrences (range) | 70 (53–309) | 73 (53–300) | – | 0.68 |
| Eradication therapy | ||||
| Median duration (weeks) (range) | 29 (8–52) | 32 (12–52) | – | 0.92 |
| Proportion of patients receiving treatment≥ 20 weeks without treatment switching. | 91(83.4%) | 31 (100%) | – | 0.01 |
| Adverse drug reactions | ||||
| No. developed gastrointestinal side effects (%) | 28 (25.7%) | 2 (6.5%) | 0.20 (0.05–0.89) | 0.02 |
| Switched treatment | 14 (12.8%) | 0 | – | 0.02 |
| Discontinued maintenance therapy | 3 (2.8%) | 0 | – | 1.00 |
| Rash | 9 (8.3%) | 2 (6.5%) | 0.77 (0.16–3.75) | 1.00 |
| Outcomes | ||||
| Microbiologically confirmed recurrences | 5 (4.6%) | 1 (3.2%) | 0.69 (0.08–6.17) | 1.00 |
| Clinical recurrences | 2 (1.8%) | 1 (3.2%) | 1.78 (0.16–20.35) | 0.53 |
| Death | 1 (0.9%) | 0 (0.0%) | – | 1.00 |
Duration of treatment.
Eradication treatment durations are shown in Table 2. The proportion of patients who could complete at least 20 weeks of therapy without having switched to the other regimen was significantly lower in the conventional regimen group (91/109 [83.5%] versus 31/31 [100%] P = 0.01).The total duration of eradication treatment in the conventional regimen group, (including the duration of subsequent switched regimen) was similar to those of cotrimoxazole monotherapy group (median, 29 versus 32 weeks; P = 0.92) (Table 2).
Adverse drug reactions and switched treatment.
A higher proportion of patients reported gastrointestinal adverse drug events in the conventional regimen group (28/109 [25.7%] versus 2/31 [6%] P = 0.02), whereas allergic phenomena were similar (Table 2). Doxycycline-associated gastrointestinal intolerance led to cessation of the eradication therapy in 3 (2.8%) patients at 8, 8, and 10 weeks, respectively. In addition, 14 patients (12.8%) were switched to oral cotrimoxazole alone within the first 3 weeks because of these side effects. None of the patients in the cotrimoxazole monotherapy group changed treatment (14/109 versus 0/31 P = 0.02).
Recurrences.
Of the 140 patients studied, 6 (4.3%) had documented culture-proven recurrences of melioidosis. All the recurrent B. pseudomallei isolates were still susceptible to ceftazidime, cotrimoxazole, and tetracycline. Three (2.6%) patients had culture-negative clinical recurrences characterized by reappearances of ceftazidime-responsive hepatosplenic abscesses, at 175, 180, and 140 days after having completed a 20-week course of the conventional treatment (two patients) and cotrimoxazole monotherapy (one patient), respectively. All of them had imaging studies that are characteristic for melioidosis (target or cartwheel lesions or Swiss cheese appearance).
There was no significant difference between the two treatment groups with regard to the number of patients experiencing culture-confirmed recurrences in the “intention-to-treat” analysis (5 [4.5%] of 109 patients in the conventional regimen group versus 1 [3.2%] of 31 in the cotrimoxazole monotherapy group, odds ratio [OR] = 0.69 [95% confidence interval [CI] = 0.08–6.17]) (Table 2). None of the 14 patients in the conventional regimen group who switched treatment to cotrimoxazole monotherapy developed recurrence. A second analysis, in which these 14 patients were reallocated to the cotrimoxazole monotherapy group (“per protocol” analysis) also failed to show a significant difference in the recurrence rates (5/95 versus 1/45 OR = 0.41 [95% CI = 0.05–3.61]). The incidence of culture-negative, clinical recurrence was also similar (Table 2).
Time to recurrence.
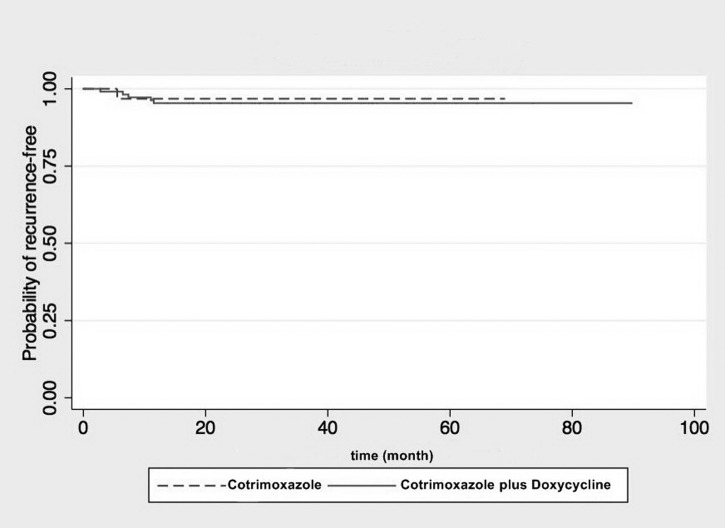
The median time to recurrence was 45 weeks (range, 28–60 weeks). There was no significant difference in time to culture-proven recurrence and/or death between the two groups in the Kaplan-Meier plot (P = 0.75 by log-rank test) (Figure 2). One-third of the recurrences occurred during the eradication phase, documented by positive B. pseudomallei cultures at 24 weeks of cotrimoxazole monotherapy, and 28 and 32 weeks of the conventional treatment, respectively. The other five patients suffered recurrences at 140, 175, 190, 196, and 280 days, after completing a 20-week course of eradication treatment, respectively. The incidence of all (culture-proven and culture-negative) melioidosis recurrence after having completed at least 20 weeks of treatment was 2 (6.4.%) of 31 in the cotrimoxazole monotherapy group and 7 (7.4%) of 95 patients in the conventional regimen group (excluding those switched to cotrimoxazole alone; OR = 0.87 [95% CI = 0.17–4.41]).
Figure 2.
Kaplan-Meier plot analysis illustrating time to recurrence after start of eradication therapy for cotrimoxazole alone versus cotrimoxazole plus doxycycline (Hazard ration 1.42; 95% confidence interval [CI] = 0.17–12.13, P = 0.750 by log rank test).
Mortality.
All of the recurrences were characterized by reappearance of fever with hepatosplenic abscesses. These were responsive to retreatment with intravenous ceftazidime in all, except one patient who recurred at 12 weeks, only 2 weeks after cessation of cotrimoxazole and doxycycline caused by side effects of the drugs. He developed pneumonia with liver and splenic abscesses and died of multiorgan failure. The blood and sputum cultures were positive for ceftazidime-sensitive B. pseudomallei.
Discussion
The regimen and duration of eradication therapy for melioidosis were the most important factors predicting recurrence.3,4 Earlier studies indicated that 13–23% of patients with melioidosis had such a recurrence and as high as one-fifth of these recurrences could be fatal.4,5 However, the choice and duration of antibiotic treatment to prevent these recurrences remains unsettled, as evidenced by several different regimen reported in the literatures during the last two decades.5,8,10–12,14 The currently recommended eradication therapy with the combination of cotrimoxazole and doxycycline, given for 12–20 weeks, has decreased the 1-year recurrence rate of culture-proven melioidosis to ∼6%.8 In our study, the 5% recurrence rates in patients who had received this combination regimen drugs is comparable to that of the original clinical trial and the 3.2% culture-proven recurrence rate in recipients of cotrimoxazole alone is quite low. Our observational study is the first comparative report to show that a treatment regimen of cotrimoxazole monotherapy is not less efficacious than the traditional combination antibiotics. For the validity of the results, we have shown that recipients of cotrimoxazole monotherapy were similar to those treated with the conventional regimen in terms of age, gender, underlying diseases, occupation, and other clinical characteristics known to be associated with recurrence risks.3,4 These include the proportion of bacteremia or multifocal distribution of the primary disease, the initial parenteral treatment with ceftazidime, and the duration of the two oral eradication regimens.3,4
Similar to the original clinical trial of the conventional regimen,8 we have found that the conventional drugs are associated with a high rate of adverse events, leading to either cessation of treatment or a change to oral cotrimoxazole monotherapy within the first 3–10 weeks. Because failure to complete at least 12 weeks of therapy is associated with a 5.7-fold increase of recurrence or death, a premature discontinuation of the eradication treatment predisposes the patient to serious consequences.5,8 The only single mortality in our study occurred in a patient who rapidly developed fatal recurrence after discontinuing the conventional drugs caused by side effects at 10 weeks of treatment. None of the other 14 patients who switched treatment to cotrimoxazole monotherapy had recurrences. A non-comparative trial from Australia reported that this monotherapy was associated with low recurrence rate < 2%.5 In addition, cotrimoxazole alone had been used in the eradication phase for four Thai children with septicemia. None had recurrence of melioidosis after 34–79 months of follow-up.15
The treatment intolerance in the recipients of cotrimoxazole plus doxycycline is most likely attributable to doxycycline as the side effects in the cotrimoxazole alone group were mild and none required a change in therapy. Furthermore, a previous clinical trial of doxycycline alone for the eradication therapy of melioidosis has documented a high rate of gastrointestinal adverse effects.14 In that study, only half of the doxycycline recipients could complete at least 12 weeks of therapy and one-third of patients required a switch of treatment.14 The recurrence rate was so high (25.6%) that the authors recommended against using doxycycline alone for a first-line regimen of oral eradication treatment of melioidosis.14 Low efficacy of doxycycline was also observed in northern Australia with mention of patients who continued to have positive cultures for B. pseudomallei for more than 3 months despite continuing eradication therapy with doxycycline.5,16 Acquired resistance to doxycycline were found in post-treatment isolates that were finally eradicated by cotrimoxazole.16 In our study, there was, however, no development of drug resistance to either cotrimoxazole or doxycycline in the recurrent isolates. Infrequent acquired resistance was also observed in northeast Thailand suggesting that development of resistance is not the main factor accounting for the majority of recurrences in Thailand.14
There are speculations that doxycycline may antagonize the antibacterial activity of cotrimoxazole against B. pseudomallei.9 However, we are unable to show the clinical significance of this in vitro interaction because the recurrence rates among our patients who had received the conventional combination drugs and those treated with cotrimoxazole alone were similar. Recurrent melioidosis is associated with the ability of B. pseudomallei to invade, survive, and proliferate for prolonged periods within the phagocytic cells.17 Untreated, B. pseudomallei can persist in the body for up to 62 years.18 Current recommendations suggest a minimum of 12–20 weeks duration of therapy but the optimal total duration remains to be determined.3,8,10 In our patients, one-third of recurrences occurred during prolonged treatment beyond the 20th week.
Recently, there are concerns that the usual adult dosing of cotrimoxazole (TMP/SMX, 160/800 mg twice daily) used in Thailand may have been suboptimal accounting for the previously high recurrence rates.5,19 However, our recurrence rate after cotrimoxazole monotherapy is comparable to that reported from Australia where twice a dosage of cotrimoxazole is used.5
There are limitations to our study. Because of the nature of a retrospective study, other potential causal factors, which may have influenced recurrence, such as the drug compliance, was difficult to analyze. The adherence to therapy was not formally assessed by pill counts or drug levels at our follow-up clinic. However, the objective endpoint of culture-confirmed recurrences in our study is quite low compared with other studies.4,8,10,12,14 In addition, compliance with follow-up was good. All of our patients had attended the follow-up clinics for at least 1 year and most of them received oral therapy for longer than 20 weeks. The total completed duration of treatment in our patients was longer than those achieved in previous studies.8,10,12,14 Because duration of standard oral treatment is inversely associated with risk of recurrence,3 these may account for the low rates of recurrence in our patients. However, a clinically meaningful difference between the recurrence rate among the patients receiving cotrimoxazole plus doxycycline and cotrimoxazole monotherapy could not be excluded because of a small number of patients in this study.
In our retrospective study, the B. pseudomallei isolates are not available for genotypic study to determine whether the recurrent strains are actually relapse or reinfected strains. However, previous studies from Thailand and Australia have demonstrated that the majority of recurrence melioidosis are caused by recrudescence of the original infecting strain (relapse).3–5,20 All of the recurrences in our patients had developed within 1 year after the primary disease. This is more compatible with relapse rather than reinfection.3
In conclusion, the addition of doxycycline to cotrimoxazole therapy does not confer an additional therapeutic benefit over cotrimoxazole monotherapy. Instead, the use of doxycycline leads to more adverse effects and may predispose for drug non-compliance, incomplete treatment, or fatal premature termination of the eradication therapy.
ACKNOWLEDGMENTS
We thank Sarayut Lucien Geater, Chaitong Churuangsuk, and Piyarat Nikomrat for assistance with data collection and statistical analysis. We also thank Alan Lucien Geater and Glenn Kern Shnigledecker.
Footnotes
Authors' addresses: Sarunyou Chusri, Songklanagarind Hospital, Hatyai, Songkhla, Songkhla, E-mail: sarunyouchusri@hotmail.com. Thanaporn Hortiwakul, Boonsri Charoenmak, and Khachornsakdi Silpapojakul, Prince of Songkla University, Internal Medicine, Hatyai, Songkhla, Thailand, E-mails: hratee@medicine.psu.ac.th, cbunsri@medicine.psu.ac.th, and skhachor@medicine.psu.ac.th.
References
- 1.Cheng AC, Currie BJ. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, Chaowagul W, Day NP, Peacock SJ. Increasing incidence of human melioidosis in northeast Thailand. Am J Trop Med Hyg. 2010;82:1113–1117. doi: 10.4269/ajtmh.2010.10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Limmathurotsakul D, Chaowagul W, Chierakul W, Stepniewska K, Maharjan B, Wuthiekanun V, White NJ, Day NP, Peacock SJ. Risk factors for recurrent melioidosis in northeast Thailand. Clin Infect Dis. 2006;43:979–986. doi: 10.1086/507632. [DOI] [PubMed] [Google Scholar]
- 4.Chaowagul W, Suputtamonkol Y, Dance DA, Rajchanuwong A, Pattaraarechachai J, White NJ. Relapse in melioidosis: incidence and risk factors. J Infect Dis. 1993;168:1181–1185. [PubMed] [Google Scholar]
- 5.Currie BJ, Fisher DA, Anstey NM, Jacups SP. Melioidosis: acute and chronic disease, relapse and re-activation. Trans R Soc Trop Med Hyg. 2000;94:301–304. doi: 10.1016/s0035-9203(00)90333-x. [DOI] [PubMed] [Google Scholar]
- 6.Pruksachartvuthi S, Aswapokee N, Thankerngpol K. Survival of Pseudomonas pseudomallei in human phagocytes. J Med Microbiol. 1990;31:109–114. doi: 10.1099/00222615-31-2-109. [DOI] [PubMed] [Google Scholar]
- 7.Vorachit M, Lam K, Jayanetra P, Costerton JW. Electronmicroscopy study of the mode of growth of Pseudomonas pseudomallei in vitro and in vivo. J Trop Med Hyg. 1995;98:379–391. [PubMed] [Google Scholar]
- 8.Chaowagul W, Chierakul W, Simpsom AJ, Short JM, Stepniewska K, Maharjan B, Rajchanuvong A, Busarawong D, Limmathurotsakul D, Cheng AC, Wuthiekanun V, Newton PN, White NJ, Day NP, Peacock SJ. Open-label randomized trial of oral trimethoprim-sulfamethoxazole, doxycycline, and chloramphenicol compared with trimethoprim-sulfamethoxazole and doxycycline for maintenance therapy of melioidosis. Antimicrob Agents Chemother. 2005;49:4020–4025. doi: 10.1128/AAC.49.10.4020-4025.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dance DA, Wuthiekanun V, Chaowagul W, White NJ. Interactions in vitro between agents used to treat melioidosis. J Antimicrob Chemother. 1989;24:311–316. doi: 10.1093/jac/24.3.311. [DOI] [PubMed] [Google Scholar]
- 10.Rajchanuvong A, Chaowagul W, Suputtamongkol Y, Smith MD, Dance DA, White NJ. A prospective comparison of co-amoxiclav and the combination of chloramphenicol, doxycycline, and co-trimoxazole for the oral maintenance treatment of melioidosis. Trans R Soc Trop Med Hyg. 1995;89:546–549. doi: 10.1016/0035-9203(95)90104-3. [DOI] [PubMed] [Google Scholar]
- 11.Chaowagul W, Suputtamongkol Y, Smith MD, White NJ. Oral fluoroquinolones for maintenance treatment of melioidosis. Trans R Soc Trop Med Hyg. 1997;91:599–601. doi: 10.1016/s0035-9203(97)90044-4. [DOI] [PubMed] [Google Scholar]
- 12.Chetchotisakd P, Chaowagul W, Mootsikapun P, Budhsarawong D, Thinkamrop B. Maintenance therapy of melioidosis with ciprofloxacin plus azithromycin compared with cotrimoxazole plus doxycycline. Am J Trop Med Hyg. 2001;64:24–27. doi: 10.4269/ajtmh.2001.64.24. [DOI] [PubMed] [Google Scholar]
- 13.John JF., Jr Trimethoprim- sulfamethoxazole therapy of pulmonary melioidosis. Am Rev Respir Dis. 1976;114:1021–1025. doi: 10.1164/arrd.1976.114.5.1021. [DOI] [PubMed] [Google Scholar]
- 14.Chaowagul W, Simpson AJ, Suputtamongkol Y, Smith MD, Angus BJ, White NJ. A comparison of chloramphenicol, trimethoprim-sulfamethoxazole, and doxycycline with doxycycline alone as maintenance therapy for melioidosis. Clin Infect Dis. 1999;29:375–380. doi: 10.1086/520218. [DOI] [PubMed] [Google Scholar]
- 15.Lumbiganon P, Chotechuangnirun N, Kosalaraksa P. Clinical experience with treatment of melioidosis in children. Pediatr Infect Dis J. 2004;23:1165–1166. [PubMed] [Google Scholar]
- 16.Jenney AW, Lum G, Fisher DA, Currie BJ. Antibiotic susceptibility of Burkholderia pseudomallei from tropical northern Australia and implications for therapy of melioidosis. Int J Antimicrob Agents. 2001;17:109–113. doi: 10.1016/s0924-8579(00)00334-4. [DOI] [PubMed] [Google Scholar]
- 17.Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol. 2006;4:272–282. doi: 10.1038/nrmicro1385. [DOI] [PubMed] [Google Scholar]
- 18.Ngauy V, Lemeshev Y, Sadkowski L, Crawford G. Cutaneous melioidosis in a man who was taken as a prisoner of war by the Japanese during World War II. J Clin Microbiol. 2005;43:970–972. doi: 10.1128/JCM.43.2.970-972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mays EE, Ricketts EA. Melioidosis: recrudescence associated with bronchogenic carcinoma twenty-six years following initial geographic exposure. Chest. 1975;68:261–263. doi: 10.1378/chest.68.2.261. [DOI] [PubMed] [Google Scholar]
- 20.Cheng AC, McBryde ES, Wuthiekanun V, Chierakul W, Amornchai P, Day NPJ, Peacock SJ. Dosing regimens of cotrimoxazole (trimethoprim-sulfamethoxazole) for melioidosis. Antimicrob Agents Chemother. 2009;53:4193–4199. doi: 10.1128/AAC.01301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]