Abstract
Plasma leakage in severe dengue has been postulated to be associated with skewed cytokine immune responses. In this study, the association of cytokines with vascular permeability in dengue patients was investigated. Human serum samples collected from 48 persons (13 with dengue fever, 29 with dengue hemorrhagic fever, and 6 healthy) were subjected to cytokines analysis by using Luminex Multiplex Technology. Selected serum samples from patients with dengue hemorrhagic fever sera and recombinant human cytokines were then tested for roles on inducing vascular permeability by treatment of human umbilical vein endothelial cells. Confocal immunofluorescence staining indicated morphologic alteration of human umbilical vein endothelial cells treated with serum samples from patients with dengue hemorrhagic fever compared with serum samples from healthy persons. The findings suggest that cytokines produced during dengue hemorrhagic infections could induce alterations in the vascular endothelium, which may play a fundamental role in the pathophysiology of dengue.
Introduction
Dengue is the most extensive vector borne infectious disease and a major source of public health concern in tropical and sub-tropical regions of the world. The Pediatric Dengue Vaccine Initiative has estimated that globally there are some 3.61 billion persons (55% of the world's population) are at risk for dengue, and there are approximately 36 million cases of dengue fever (DF) and 2.1 million severe dengue infections every year.1 In Southeast Asia, by the late 1990s, dengue had become the most important mosquito-borne disease affecting humans after malaria, and there were approximately 40 million cases of DF and several hundred thousand cases of dengue hemorrhagic fever (DHF) each year.2,3 Because this disease affects persons of all ages, it has inevitably contributed to a substantial economic burden to society.4
Dengue virus comprises four genetically distinct serotypes: dengue virus serotype 1, 2, 3, and 4 (DENV-1–DENV-4). Infection by any one of the dengue serotypes can cause a spectrum of illnesses ranging from clinically silent infection, mild febrile illness, and DF, to life-threatening DHF (grades I and II) or dengue shock syndrome (DSS) (grades III and IV).5,6
Increased vascular permeability, which leads to plasma leakage, has been demonstrated as the fundamental feature in DHF that implies damage to the vascular endothelium and induces major clinical complications; it is the critical stage of the disease that can cause hypovolemic shock (DSS).5 Plasma leakage in DHF generally lasts no more than 48 hours and is usually follow by rapid complete recovery.7 Consistent with the clinical course, plasma leakage in DHF occurs with a relative lack of tissue inflammation, suggesting that a transient change in factors that regulate vascular permeability in the physiologic state may be the mechanism of plasma leakage in the disease.8
The pathogenesis of dengue is not fully understood, even though many studies have been conducted on its pathogenesis in the past few decades. This lack of understanding is caused mainly by the lack of an appropriate animal model that can precisely simulate dengue virus infections in humans. Recently, human umbilical vein endothelial cells (HUVEC) were identified as the central model used to understand infection and pathologic events during severe dengue.8 Although increased levels of cytokines, such as interferons (IFNs), interleukin-2 (IL-2), IL-8, tumor necrosis factor-α (TNFα), and vascular endothelial growth factor A (VEGF-A), have been reported to be associated with enhancement of vascular permeability, the relative role of these cytokines in plasma leakage is not known.9,10
In this study, we investigated the effect of cytokines in dengue patient serum by using an in vitro model with HUVEC. We also performed confocal immunofluorescence analysis on the morphologic changes of HUVEC treated with serum samples from dengue patients and compared them with those of healthy donors. Our data suggest that cytokines produced during dengue hemorrhagic infections participate in the regulation of vascular permeability.
Materials and Methods
Study participants.
Forty-two persons (13 with DF and 29 with DHF) who were admitted to University Malaya Medical Center, Kuala Lumpur, Malaysia, for acute dengue infection, were recruited in this preliminary study of cytokine profiling in dengue patients. Blood samples were collected during acute, defervescence, and convalescence phases. Laboratory tests, including a dengue polymerase chain reaction, virus isolation, hemagglutination, IgM-capture enzyme-linked immunosorbent assay, and test for non-structural protein 1, were conducted for confirmation of dengue virus infection.11 Persons were subsequently clinically diagnosed as either having DF or DHF by clinicians on the basis of World Health Organization criteria.2 Ethical clearance (Ref. no.: 321.4) for the study was approved by the Scientific and Ethical Committee of University Malaya Medical Center, and procedures were conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000.12
Detection of cytokines by bead-based enzyme-linked immunosorbent assay.
Multiple cytokines in patient serum samples were identified and quantified by using the human cytokine 27-plex panel kit (catalog no. 171-A11127; Bio-Rad, Hercules, CA) and the 2-plex panel kit (catalog no. XF0000ZG2Y) with the Bio-Plex suspension array system (Bio-Rad) in accordance to the manufacturer's protocol. Briefly, diluted serum samples (1:3 dilutions) were added into each of the 96-well filter plates containing multiplex beads. Plates were then placed on a micro-plate shaker and gently shaken in the dark for 30 minutes at room temperature. Plates were then washed three times with 100 μL of Bio-Plex wash buffer by vacuum filtration. Twenty-five microliters of antibodies to various cytokines was added and the plates were incubated in the dark for 30 minutes with gently shaking. After three washes, 50 μL of phycoerythrin-conjugated streptavidin was added and the plates were incubated for 10 minutes. Fluorescent signals were read by using a Luminex Machine (Bio-Rad). The analyte concentration was calculated by using software provided by the manufacturer.
Raw data was initially measured as the relative fluorescence intensity and then converted to cytokine concentration on the basis of a standard curve generated from reference concentrations supplied in the kit. The following cytokines were measured: IL-1β, IL-1 receptor antagonist (IL-1Ra), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, eotaxin, basic fibroblast growth factor (FGF-basic), granulocyte colony-stimulating factor (G-CSF), granulocyte–macrophage colony-stimulating factor (GM-CSF), IFN-γ, IFN-γ–induced protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), MIP-1β, platelet-derived growth factor-ββ (PDGF-ββ), regulated-on-activation normal T-cell expressed and secreted (RANTES), TNF-α, and VEGF in the 27-plex assay; and IL-18 and intercellular adhesion molecule 1 (ICAM-1) in the 2-plex assay.
Cell culture.
Human umbilical vein endothelial cells (American Type Culture Collection, Manassas, VA) were maintained in endothelial cell–based medium 2 (Lonza, Basel, Switzerland) supplemented with 10% fetal calf serum, and cultured at 37°C in an atmosphere of 5% CO2. For sub-culturing or experiments, cells were seeded into culture flasks or chamber slides that were pre-coated with 50 μg/mL of fibronectin (Roche, Mannheim, Germany). Pre-coating was performed by incubating flasks with 100 μL/cm2 of 50 μg/mL of fibronectin and incubated at room temperature for 45 minutes. The solution was then removed and flasks were used immediately for cell culture.
Recombinant human cytokines.
Recombinant human (rh) cytokines (rhIL-1ra, rhIL-9, rhMCP-1, rhRANTES, rhEotaxin, and rhIP-10) (R&D Systems, Minneapolis, MN) were used to test their effects on inducing endothelial cells permeability changes. All of these cytokines except rhIL-9 were selected on the basis of our findings that their levels were significantly increased (P < 0.05) in DHF patients compared with healthy controls, and on their availability in the laboratory when the assay was performed. Although the level of IL-9 was increased in a DHF patient, there was no significant association (Appanna R and others, unpublished data).
Assay for endothelial cell permeability changes.
The assay was performed to determine alterations in the distribution of two junctional complexes: adherens junction protein vascular endothelial cadherin (VE-cadherin) and endothelial tight junction protein zonula occludens-1 (ZO-1) after treatment of HUVEC with dengue patient serum samples and recombinant human cytokines. Briefly, endothelial cells were seeded onto eight-well chamber slides coated with fibronectin. The next day, serum samples from dengue patients (diluted in endothelial cell–based medium 2 at a ratio of 1:3) and rh cytokines (rhIL-1ra, rhIL-9, rhMCP-1, rhRANTES, rhEotaxin, and rhIP-10) at appropriate working concentrations were added and incubated for 3 hours at 37°C in an atmosphere of 5% CO2.
Cells were then subjected to immunofluorescence staining of VE-cadherin and ZO-1. For testing the effects of cytokine-neutralizing antibodies on VE-cadherin and ZO-1, anti-human cytokine monoclonal antibodies were pre-incubated with culture medium in the chamber for 3 hours at 37°C before addition of rh cytokines. Working concentrations of rh cytokines and their respective cytokine neutralizing monoclonal antibodies (αh) (R&D Systems) were rhIP-10 (200,000 pg/mL), rhRANTES (800,000 pg/mL), rhMCP-1 (3,000 pg/mL), rhIL-1ra (20,000 pg/mL), rhEotaxin (1,500 pg/mL), rhIL-9 (6,000 pg/mL), αhIP-10 (20 μg/mL), αhRANTES (32 μg/mL), αhMCP-1 (0.9 μg/mL), αhIL-1ra (12 μg/mL), αhEotaxin (0.75 μg/mL), and αhIL-9 (12 μg/mL).
Immunofluorescence staining of VE-cadherin and ZO-1.
The HUVEC were placed in cell chambers, washed three times with phosphate-buffered saline (PBS), and fixed with 2% paraformaldehyde (Sigma, St. Louis, MO) for 10 minutes. After three washes with PBS, cells were permeabilized with 0.1% Triton X-100 (BDH Laboratory Supplies, London, United Kingdom) for five minutes at room temperature. The cells were then blocked with 2% BSA for 30 minutes at room temperature before rinsing with PBS. Cells were then immunolabeled with purified mouse monoclonal anti-human ZO-1 antibody (BD Biosciences, San Jose, CA) (1:200 dilution of a 250 μg/mL stock) and rabbit monoclonal anti-human VE-cadherin antibody (Sigma) (1:100 dilution of a 1.0 mg/mL stock) and incubated for one hour at room temperature. After three washes with PBS, Alexa Fluor® 488 goat anti-mouse IgG (heavy and light chains) (Invitrogen, Carlsbad, CA) and Alexa Fluorα® 568 goat anti-rabbit IgG (heavy and light chains) with working dilution of 5 μg/mL were added to the cells and incubated for one hour. 4′,6-diamidino-2-phenylindole (0.05 μg/mL) (Sigma) was added to the chambers for 5 minutes to stain nuclei, and the chambers were washed three times with PBS. Cells were then mounted with fluorescent mounting medium (Dako, Carpinteria, CA), and viewed under a laser confocal microscope (TCS SP5; Leica, Wetzlar, Germany).
Results
Forty-two adults with confirmed dengue (13 with DF and 29 with DHF) and 6 healthy controls were selected for preliminary study of their cytokine profiles at different days of fever onset. All patients were given a clinical diagnosis of DF or DHF by a clinician on the basis of World Health Organization criteria.2 The mean age of the patients was 29.3 years (range = 14–67 years), and there were 22 males and 20 females. Detailed clinical and laboratory data of all dengue patients were summarized as supplementary data. Blood collection was performed at 2–14 days of illness (mean duration of illness = 6 days). The mean of the defervescence phase was day 5 of illness (range = 3–7 days). The highest (70.45%) number of patients was in defervescence on days 4–6 of illness. IgM was detected in 39 patients. Dengue virus was detected in 32 patients (13 with DENV-1, 4 with DENV-2, 7 with DENV-3, and 8 with DENV-4), and dengue virus non-structural protein 1 was detected in 27 patients.
The profile of 29 different type of cytokine levels was determined during the acute (2¬–3 days of illness), defervescence (4 – 6 days of illness) or convalescent (> 7 days of illness) phases in DF and DHF patients, as shown in Table 1. Pro-inflammatory cytokines IL-18, anti-inflammatory cytokines IL-1ra, adhesion molecule ICAM-1, and chemokines Eotaxin, IP-10, MCP-1, MIP-1β, and RANTES were significantly increased in serum samples from DHF patients (P < 0.05). However, only IP-10, MCP-1, and MIP-1β were significantly increased in serum samples from DF patients. Cytokines that were significantly reduced in serum samples from DHF patients were pro-inflammatory cytokines IFN-γ, IL-5, IL-12, anti-inflammatory cytokines IL-4, and growth factors FGF-basic, G-CSF, PDGF, and VEGF. Pro-inflammatory cytokine IL-2, anti-inflammatory cytokine IL-13, and growth factor G-CSF were significantly decreased in DF patients.
Table 1.
Cytokine levels in serum samples of dengue patients compared with those of healthy donors*
| Activity | Cytokine | Cytokine level, mean (pg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Healthy control | DF (day of illness) | DHF (day of illness) | ||||||
| 2–3 | 4–6 | > 7 | 2–3 | 4–6 | > 7 | |||
| Pro-inflammatory | IFN-γ | 157.2 | 71 | 89.8 | 87.1 | 192 | 187.9† | 184.8 |
| IL-5 | 2.6 | 1.2 | 1.3 | 1.4 | 1.8 | 1.3† | 1.8 | |
| IL-12 | 54.2 | 20.8 | 28.1 | 25.8† | 27.2 | 18.0† | 28.5† | |
| IL-18 | 102.3 | 213 | 316.3 | 170.1 | 228.2 | 392.7† | 270.2† | |
| Anti-inflammatory | IL-1ra | 504.8 | 194.6 | 591.8 | 283.4 | 3,010 | 2,294.7† | 879.6 |
| IL-4 | 26.4 | 2.3 | 3.9 | 3.3 | 7.7 | 9.4† | 9.7† | |
| IL-13 | 8.4 | 3.6 | 4.3† | 5.1 | 16.9 | 6.1 | 7.1 | |
| Adhesion molecule | ICAM-1 | 79,313 | 94,769 | 93,873 | 73,801.9 | 113,644 | 143,398† | 126,522† |
| Growth factor | FGF-basic | 114.0 | 37.3 | 61.0 | 42.9 | 51.8 | 43.1† | 98.9 |
| G-CSF | 65.3 | 33.8 | 26.5† | 24.9† | 46.7 | 30.4† | 37.6† | |
| PDGF | 8,810.6 | 7,640 | 8018 | 13,173 | 8,303.8 | 5,332.6† | 11,090 | |
| VEGF | 175.5 | 223 | 219.3 | 343.3 | 202 | 115.8† | 235.4 | |
| Chemokine | Eotaxin | 184.4 | 175.2 | 148.8 | 192.0 | 325.8† | 207.1 | 210.6 |
| IP-10 | 1,114.7 | 46,967† | 35,875† | 20,505† | 71,569† | 40,478.9† | 24,852† | |
| MCP-1 | 84.1 | 397.6† | 232.7 | 117.1 | 400.4† | 291.3† | 152.3 | |
| MIP-1β | 102.5 | 334.2† | 655.7† | 200.0 | 315.9† | 447.6† | 245.5† | |
| RANTES | 19,808 | 159,.891 | 70,887 | 71,586 | 51,325 | 5 × 106† | 59,314 | |
DF = dengue fever; DHF = dengue hemorrhagic fever; IFN-γ = interferon-γ; IL = interleukin; ICAM-1 = intercellular adhesion molecule 1; FGF-basic; = basic fibroblast growth factor; G-CSF = granulocyte colony-stimulating factor; PDGF = platelet-derived growth factor; VEGF = vascular endothelial growth factor; IP-10 = interferon-γ–induced protein 10; MCP-1 = monocyte chemoattractant protein 1; MIP-1β = macrophage inflammatory protein-β; RANTES = regulated-on-activation normal T-cell expressed and secreted.
P < 0.05 (cytokines that showed significant difference between healthy persons and dengue patients).
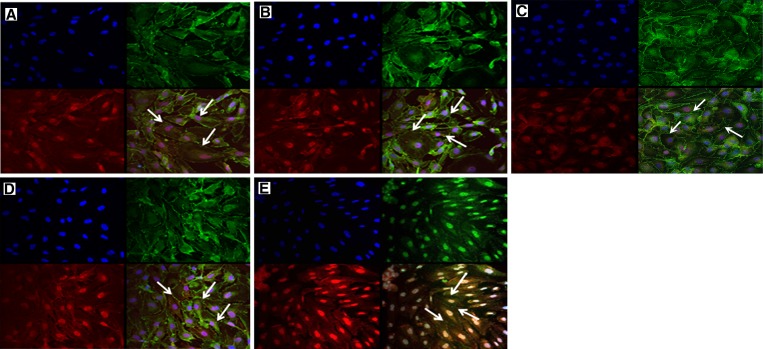
Effects of cytokines on permeability of monolayers of HUVEC were accessed by treatment of HUVEC directly with DHF patient sera. The reassembly of endothelial adherens junction proteins (VE-cadherin) and tight junction proteins (ZO-1) were observed by using confocal immunofluorescence (IF) techniques. Immunofluorescence staining showed that there were morphologic alterations of cultured monolayers of HUVEC treated with serum samples from DHF patients obtained at different phases of illness compared with serum samples from DF patients and healthy controls. (Figure 1). Monolayers of HUVEC were disrupted and had highly irregular shaped cells (Figure 1A and B) compared with normal morphology, which shows a cobblestone-like shape (Figure 1D and E). Large gaps between the cells were observed, which indicated perturbations of ZO-1 and VE-cadherin proteins. Furthermore, this perturbation was more apparent in HUVEC treated with serum from DHF patients than with HUVEC treated with pooled recombinant human cytokines (Figure 2 ). Monolayers of HUVEC treated with serum samples from DF patients had similar morphology as cells treated with serum samples from healthy controls (Figure 1C).
Figure 1.
Immunofluorescence analysis of perturbation of tight junction protein ZO-1 (Alexa 488; Invitrogen, Carlsbad, CA) and adherens junction protein VE-cadherin (Alexa 568) on the outer membrane of human umbilical vein endothelial cells. Cells were treated with A, acute-phase serum sample from patient A with dengue hemorrhagic fever (DHF); B, defervescence serum sample from DHF patient A; C, acute-phase serum from patient B with dengue fever; D, healthy donor serum; and E, culture medium only. Arrows indicate ZO-1 and VE-cadherin distributions. Assay was done in duplicate and repeated twice.
Figure 2.
Immunofluorescence analysis of cell morphology and perturbation of tight junction protein ZO-1 (Alexa 488; Invitrogen, Carlsbad, CA) and adherens junction protein VE-cadherin (Alexa 568) on the outer membrane of human umbilical vein endothelial cells treated serum samples from a patient with dengue hemorrhagic fever (DHF) and pooled recombinant human (rh) cytokines. Cells were treated with A, acute-phase serum sample from patient C with DHF; B, defervescence serum sample from DHF patient D; and C, pooled recombinant human cytokines. Arrows indicate ZO-1 and VE-cadherin distributions.
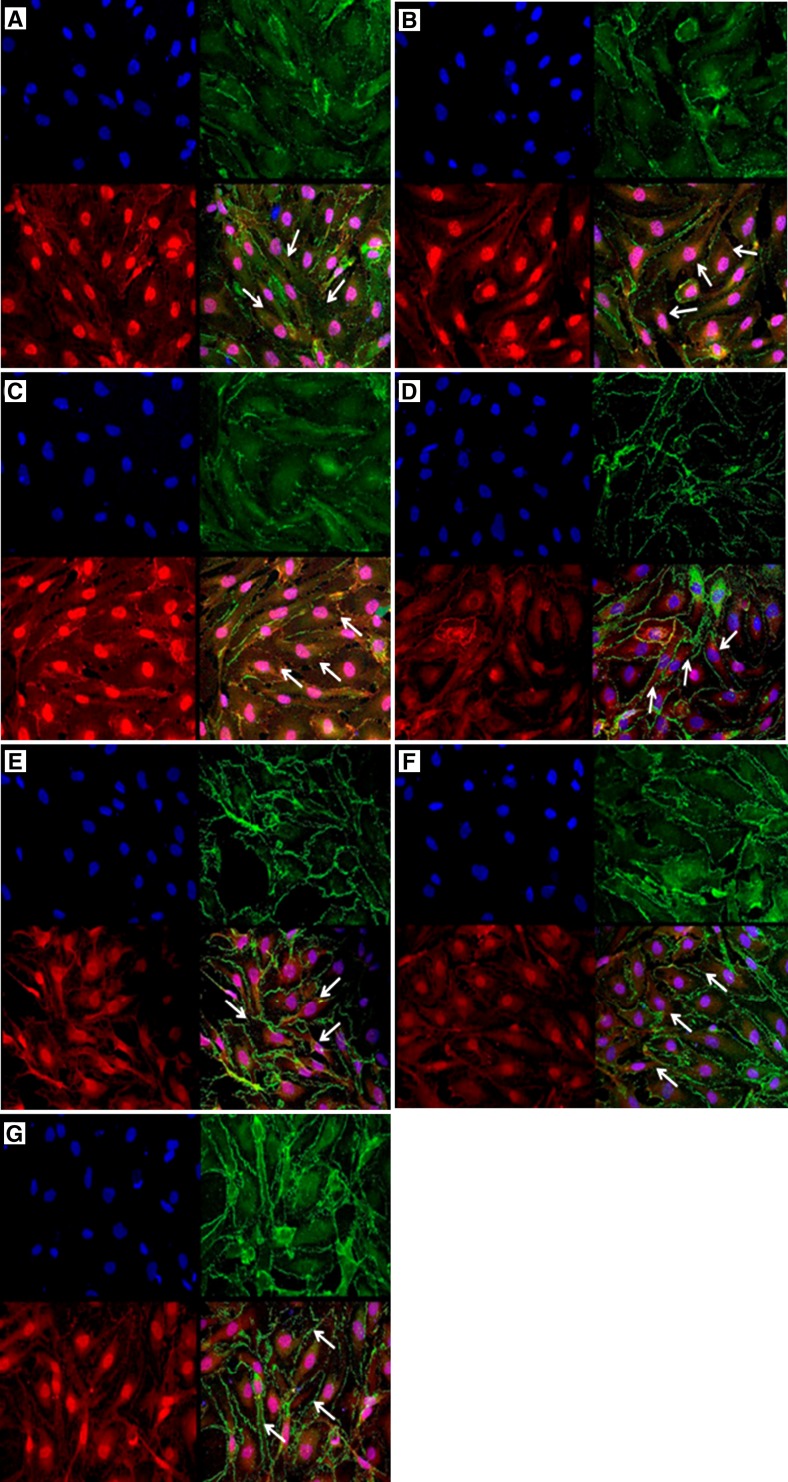
Disarray of ZO-1 and VE-cadherin proteins was also observed in HUVEC treated with individual rh cytokines on the basis of the highest in vivo concentration of these cytokines detected in DHF patients. The degree of tight junction alterations was higher in cells treated with rhIL-1ra, followed by rhIP-10, rhEotaxin, rhRANTES, rhMCP-1, and rh-IL-9 (Figure 3). However, perturbation was less apparent compared with that in HUVEC treated with serum samples from DHF patients. This finding suggests that other cytokine factors in DHF patient serum contributed to full leakage of HUVEC.
Figure 3.
Immunofluorescent analysis of perturbation of the tight junction protein ZO-1 (Alexa 488; Invitrogen, Carlsbad, CA) and adherens junction protein VE-cadherin (Alexa 568) on the outer membrane of human umbilical vein endothelial cells treated with individual recombinant human (rh) cytokines. A, rhIL-1ra; B, rhIP-10; C, rhEotaxin; D, rhRANTES; E, rhMCP-1; F, rhIL-9; and G, control treated with culture media. IL = interleukin; IP-10 = interferon-γ–induced protein 10; RANTES = regulated-on-activation normal T-cell expressed and secreted; MCP-1 = monocyte chemoattractant protein 1. Arrows indicate ZO-1 and VE-cadherin distributions.
Reassembly of endothelial junctional proteins was investigated by determining whether morphologic changes caused by cytokine factors were reversible. This was performed by conditioning HUVEC with respective cytokine-neutralizing antibodies, followed by treatment with recombinant human cytokines. Results showed that the initial distortion observed in HUVEC endothelial junctional proteins was reversible (Figure 4). This finding indicates that increased vascular permeability does not cause morphologic damage to the vascular endothelium and that leakage is transient.
Figure 4.
Immunofluorescent analysis of perturbation of tight junction protein ZO-1 (Alexa 488; Invitrogen, Carlsbad, CA) and adherens junction protein VE-cadherin (Alexa 568) on the outer membrane of human umbilical vein endothelial cells conditioned medium containing recombinant human (rh) cytokine treated with respective neutralizing antibody (ab). A, abIL-1ra; B, abIP-10, C, abEotaxin; D, abRANTES; E, abMCP-1; and F, abIL-9. IL = interleukin; IP-10 = interferon-γ–induced protein 10; RANTES = regulated-on-activation normal T-cell expressed and secreted; MCP-1 = monocyte chemoattractant protein 1. Arrows indicate ZO-1 and VE-cadherin distributions.
Discussion
Vascular leakage is a major feature in patients who have DHF. Many factors have been reported to cause plasma leakage in severe dengue; among these are the cross-reactive memory T-cells activations, which induce proliferation and production of pro-inflammatory cytokines such as IFN-γ. These cytokines interact directly with vascular endothelium, resulting in plasma leakage.13,14 Although many studies have reported that the inflammatory response, cytokine storm, and activation of the complement system play a key role in the progression of severe clinical manifestation, identification of appropriate cytokine profiles, especially during the critical period of illness (defervescence stage) and its association with the progression of severe dengue, is not well established.8,15–18
In this study, we attempted to demonstrate the effects of cytokines in serum of DHF patients by using an in vitro model of HUVEC. Although extensive studies have been conducted during the past several decades, the molecular mechanisms underlying vascular leakage in severe dengue remain unclear. The results could be extrapolated to explain the possible mechanism involved in alterations of vascular endothelium during dengue infection.
Plasma leakage often occurs in serosal tissues, which indicates that endothelial cells from the microvasculature are preferentially affected during the disease.19 Rapid recovery from plasma leakage by patients with dengue suggests that endothelial cell alterations could be induced by serum inflammatory mediators. Cytokine storm, a result of severe dengue, has been proposed to play a central role in creating an endothelial sieve, which leads to plasma leakage. Crone reported that endothelial cells can dramatically alter their permeability in response to immune responses, particularly to cytokines such as TNFα, IFNγ, IL-6, and IL-8, and other pro-inflammatory cytokines.20 Another study has also reported increased levels of VEGF-A as a potent permeability-enhancing cytokine in DHF.21 Other cytokines such as IL-2 and RANTES have been reported in patients with DHF to regulate cell permeability, although the relative role of these cytokines in plasma leakage is not known.22,23
In this study, analysis of multiple cytokines in serum obtained from DHF patients showed an association between cytokine profiles and disease severity, and the major cytokines implicated are the inflammatory group and chemokines. Our investigations show a probable role of pro-inflammatory cytokines (IL-18, IFN-γ, IL-5, and IL-12), anti-inflammatory cytokines (IL-1ra and IL-4), adhesion molecule (ICAM-1), chemokines (Eotaxin, IP-10, MCP-1, MIP-1β, and RANTES), and growth factors (FGF-basic, G-CSF, PDGF, and VEGF) in DHF patients. The effect of serum from DHF patients on monolayers of HUVEC has further suggested that these cytokines might play an important role in the pathophysiology of the vascular disorder. Using selected individual human recombinant cytokines, we showed that markedly increase levels of IL-1ra, IL-8, MCP-1, IP-10, and RANTES would have perturbed organized cells in HUVEC and cause morphologic alterations in endothelial cells.
One limitation of this study was that only six rh cytokines were analyzed. Although the degree of permeability changes in monolayers of HUVEC and reversion of permeability were not quantified, preliminary findings in this study have demonstrated the effect of cytokines in increasing vascular permeability. Observations of highly irregular-shaped cells and large gaps between cells in monolayers of HUVEC treated with serum from DHF patients indicated perturbations of ZO-1 and VE-cadherin. Endothelial integrity is maintained mainly by adherens junction protein, in which the adhesive protein cadherin form predominates, whereas the connector protein ZO-1 is a marker of a tight junction and serves as a linker between the adherens junction and the actin cytoskeleton.19 Observations of altered cells after treatment with serum from DHF serum treated HUVEC cells comparing to the untreated cells suggest that remodeling of the actin cytoskeleton, cadherin, and ZO-1 may be the molecular basis that underlies increased vascular permeability during DHF/DSS. In addition, our results also showed that the effect on vascular alteration was transient and reversible. These findings have further supported the characteristic feature of DHF, whereby capillary permeability changes occur without morphologic damage to capillary endothelium.
In summary, we showed that serum samples from DHF dengue patients induce alterations in the vascular endothelium, which may play a fundamental role in the pathophysiology of the disease. Our results also indicated that alterations were only transient. These findings suggest that production of cytokines during dengue significantly influence vascular permeability. We postulate that analysis of a broad range of cytokines and their correlation with endothelial damage would help in understanding dengue immunopathogenesis.
ACKNOWLEDGMENTS
We thank the clinicians and the nurses at the University Malaya Medical Centre, Kuala Lumpur, Malaysia, for assistance in collecting samples, and the patients for participating in the study.
Footnotes
Financial support: This study was supported by University of Malaya research grants UMRG RG081-09HTM and HIRG J-00000-73560.
Disclosure: None of the authors have any conflicts of interest.
Authors' addresses: Ramapraba Appanna and Shamala Devi Sekaran, Department of Medical Microbiology, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia, E-mails: ag_praba@yahoo.com and shamalaya@yahoo.com. Seok Mui Wang, Institute of Medical Molecular Biotechnology, Faculty of Medicine, Universiti Teknologi MARA, Selangor, Malaysia, E-mail: seokmuiwang@yahoo.com. Sasheela A. Ponnampalavanar, Department of Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia, E-mail: sasheela@um.edu.my. Lucy Chai See Lum, Department of Pediatrics, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia, E-mail: lumcs@um.edu.my.
Reprint requests: Shamala Devi Sekaran, Department of Medical Microbiology, Faculty of Medicine, University of Malaya, Kuala Lumpur 50603, Malaysia, E-mail: shamalaya@yahoo.com.
References
- 1.International Vaccine Institute . The Pediatric Dengue Vaccine Initiative. Vaccines, Children and a Better World Annual Report. Seoul: International Vaccine Institute; 2008. pp. 28–35. [Google Scholar]
- 2.World Health Organization . Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization; 2009. pp. 3–54. [PubMed] [Google Scholar]
- 3.Nielsen DG. The relationship of interacting immunological components in dengue pathogenesis. J Virol. 2009;6:211. doi: 10.1186/1743-422X-6-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singhasivanon P, Jacobson J. Dengue is a major global health problem. J Clin Virol. 2009;46:S1–S2. doi: 10.1016/S1386-6532(09)70285-9. [DOI] [PubMed] [Google Scholar]
- 5.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 6.Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22:564–581. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srikiatkhachorn A, Ajariyakhajorn C, Endy TP, Kalayanarooj S, Libraty DH, Green S, Ennis FA, Rothman AL. Virus-induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic fever. J Virol. 2007;1:1592–1600. doi: 10.1128/JVI.01642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basu A, Chaturvedi UC. Vascular endothelium: the battlefield of dengue viruses. FEMS Immunol Med Microbiol. 2008;53:287–299. doi: 10.1111/j.1574-695X.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srikiatkhachorn A, Ajariyakhajorn C, Endy TP, Kalayanarooj S, Libraty DH, Green S, Ennis FA, Rothman AL. Virus-induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic fever. J Virol. 2007;1:1592–1600. doi: 10.1128/JVI.01642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaturvedi UC, Agarwal R, Elbishbishi EA, Mustafa AS. Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol Med Microbiol. 2000;28:183–188. doi: 10.1111/j.1574-695X.2000.tb01474.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang SM, Sekaran SD. Early diagnosis of dengue infection using a commercial dengue duo rapid test kit for the detection of NS1, IgM, IgG. Am J Trop Med Hyg. 2010;83:690–695. doi: 10.4269/ajtmh.2010.10-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Medical Association Declaration of Helsinki Ethical principles for medical research involving human subjects. JAMA. 2000;24:3043–3045. [PubMed] [Google Scholar]
- 13.Rothman AL, Ennis FA. Immunopathogenesis of dengue hemorrhagic fever. Virology. 1999;257:1–6. doi: 10.1006/viro.1999.9656. [DOI] [PubMed] [Google Scholar]
- 14.Mongkolsapaya J, Duangchinda T, Dejnirattisai W, Vasanawathana S, Avirutnan P, Jairungsri A, Khemnu N, Tangthawornchaikul N, Chotiyarnwong P, Sae-Jang K, Koch M, Jones Y, McMichael A, Xu X, Malasit P, Screaton G. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J Immunol. 2006;176:3821–3829. doi: 10.4049/jimmunol.176.6.3821. [DOI] [PubMed] [Google Scholar]
- 15.Chaturvedi UC, Agarwal R, Elbishbishi EA, Mustafa AS. Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol Med Microbiol. 2000;28:183–188. doi: 10.1111/j.1574-695X.2000.tb01474.x. [DOI] [PubMed] [Google Scholar]
- 16.Mustafa AS, Elbishbishi EA, Agarwal R, Chaturvedi UC. Elevated levels of interleukin-13 and IL-18 in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol. 2001;30:229–233. doi: 10.1111/j.1574-695X.2001.tb01575.x. [DOI] [PubMed] [Google Scholar]
- 17.Priyadarshini D, Gadia RR, Tripathy A, Gurukumar KR, Bhagat A, Patwardhan S, Mokashi N, Vaidva D, Shah PS, Cecelia D. Clinical findings and pro-inflammatory cytokines in dengue patients in western India: a facility-based study. PLoS ONE. 2010;5:e8709. doi: 10.1371/journal.pone.0008709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shresta S. Role of complement in dengue virus infection: protection or pathogenesis. mBio. 2012;3:e00003–12. doi: 10.1128/mBio.00003-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanlaya R, Pattanakitsakul S, Sinchaikul S, Chen ST, Thongboonkerd V. Alterations in actin cytoskeletal assembly and junctional protein complexes in human endothelial cells induced by dengue virus infection and mimicry of leukocyte transendothelial migration. J Proteome Res. 2009;8:2551–2562. doi: 10.1021/pr900060g. [DOI] [PubMed] [Google Scholar]
- 20.Crone C. Modulation of solute permeability in microvascular endothelium. Fed Proc. 1986;45:77–83. [PubMed] [Google Scholar]
- 21.Tseng CS, Lo HW, Teng HC, Lo WC, Ker CG. Elevated levels of plasma VEGF in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol. 2005;43:99–102. doi: 10.1016/j.femsim.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Huang YH, Lei HY, Liu HS, Lin YS, Liu CC, Yeh TM. Dengue virus infects human endothelial cells and induces IL-6 and IL-8 production. Am J Trop Med Hyg. 2000;63:71–75. doi: 10.4269/ajtmh.2000.63.71. [DOI] [PubMed] [Google Scholar]
- 23.Avirutnan P, Malasit P, Seliger B, Bhakdi S, Husmann M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J Immunol. 1998;161:6338–6346. [PubMed] [Google Scholar]