Abstract
The pathogenesis of plasma leakage during dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) is largely unknown. Angiopoietins are key regulators of vascular integrity: Angiopoietin-1 is stored in platelets and maintains vascular integrity, and endothelium-derived angiopoietin-2 promotes vascular leakage. We determined angiopoietin-1 and angiopoietin-2 levels in a cohort of children in Indonesia with DHF/DSS and related them to plasma leakage markers. Patients with DHF/DSS had reduced angiopoietin-1 and increased angiopoietin-2 plasma levels on the day of admission when compared with levels at discharge and in healthy controls. There was an inverse correlation between angiopoietin-1 and markers of plasma leakage and a positive correlation between angiopoietin-2 and markers of plasma leakage. Angiopoietin-1 levels followed the same trend as the soluble platelet activation marker P-selectin and correlated with platelet counts. Dengue-associated thrombocytopenia and endothelial activation are associated with an imbalance in angiopoietin-2: angiopoietin-1 plasma levels. This imbalance may contribute to the transient plasma leakage in DHF/DSS.
Introduction
The clinical course of dengue virus infection ranges from asymptomatic infection to severe disease, known as dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS). The latter is characterized by a transient endothelial hyperpermeability, of which the pathogenesis is still incompletely understood.1,2
Inflammatory cytokines and angiogenic proteins are important mediators of vascular integrity.3 The angiogenic protein vascular endothelial growth factor (VEGF) is a strong inducer of vascular permeability and several studies have reported circulating VEGF levels in dengue patients with contradicting results.4–8 However, the role of another class of angiogenic proteins, known as angiopoietins, is unknown in dengue.
Angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2) and their endothelial tyrosine kinase receptor Tie-2 form a central signaling system in endothelial permeability.9 Angiopoietin-1–mediated Tie2 activation maintains the quiescent state of the endothelium by stabilizing endothelial cell-cell junctions and by countering the permeabilizing effects of VEGF.10 Angiopoietin-2 antagonizes the effects of Ang-1; it destabilizes the endothelium by disrupting cell-cell adhesion and primes the endothelial cells to the effects of pro-inflammatory cytokines and VEGF.11 Angiopoietin-2 is almost exclusively produced in endothelial cells and stored in Weibel-Palade bodies (WPBs), from which it can be rapidly released upon activation of the endothelium.12 Angiopoietin-1 is produced in pericytes and smooth muscle cells, but platelets also contain high quantities of Ang-1.13 Thus, the number and activation status of circulating platelets may influence plasma Ang-1 levels. Evidence is increasing that platelets are important cells for maintaining vascular stability and platelet-derived Ang-1 may be one of the factors involved.14
Dengue hemorrhagic fever/dengue shock syndrome is associated with thrombocytopenia, inflammation, and endothelial cell activation, and these processes might lead to significant alterations in the balance between Ang-1 and Ang-2, favoring plasma leakage and hemorrhage.15 We therefore studied Ang-1 and Ang-2 levels children with DHF/DSS and related these levels to markers of plasma leakage.
Methods
This study was part of a larger cohort study that investigated pathophysiologic mechanisms of severe dengue. Consecutive children with DHF/DSS ≤ 15 years of age who were admitted to the pediatric ward or intensive care unit of the Dr. Kariadi University Hospital in Semarang, Indonesia, during 2005–2006 were enrolled in the study. Study details and clinical characteristics have been reported.16 All patients had positive results for dengue-specific IgM, as determined by enzyme-linked immunosorbent assay (Focus Technologies, Chanhassen, MN). The Ethics Committee of Diponegoro University in Indonesia approved the study. Written informed consent was obtained from the parents or legal guardians of the children.
Forty-nine children, for whom sufficient blood sample volume was available, were randomly selected from this larger cohort and classified as DHF or DSS according to World Health Organization criteria.17 Twenty-five healthy children from Indonesia served as controls. Demographic and clinical characteristics of study participants are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the patients studied*
| Characteristic | DHF (DHF I and II), n = 26 (53%) | DSS (DHF III and IV), n = 23 (47%) |
|---|---|---|
| Male sex | 12 (46) | 7 (30) |
| Age, years | 8 (6–10) | 8 (6–10) |
| Body weight, kg | 20 (17–28) | 24 (18–30) |
| Body height, cm | 125 (111–135) | 120 (113–140) |
| Body temperature,°C | 37.6 (37.0–38.4) | 38.0 (37.0–38.5) |
| Duration of fever until admission, days | 4.0 (3.0–5.0) | 4.0 (4.0–5.0) |
| Tourniquet test positive | 17/23 (74) | 10/16 (63) |
| Petechiae | 1/17 (6) | 1/15 (7) |
| Epistaxis | 3 (12) | 0 (0) |
| Gum bleeding | 0 (0) | 0 (0) |
| Hematemesis | 2 (8) | 0 (0) |
| Melena | 0 (0) | 1 (4) |
| Hemoglobin, g/dL | 13.1 (12.7–14.0) | 13.5 (12.3–14.2) |
| Hematocrit, % | 38.0 (37.9–41.3) | 40.0 (36.0–43.5) |
| Platelet count × 109/L | 62 (39–88) | 39 (26–68) |
| Leukocyte count, × 103 cells/mL | 5.0 (3.1–7.8) | 5.0 (3.0–9.5) |
| Albumin serum, g/dL, | 3.3 (2.9–3.8) | 2.8 (2.5–3.4)† |
| Alanine aminotransferase, U/dL | 48 (31–82) | 46 (32–71) |
| Pleural effusion index | 8 (0–22) | 18 (11–28)† |
Values are medians (interquartile ranges) or absolute no. (%). All data are data at enrollment, except for bleeding manifestations, which are presented for the whole duration of admission. DHF = dengue hemorrhagic fever; DSS = dengue shock syndrome.
P < 0.05, by Mann-Whitney U test.
Fluid resuscitation was performed in all patients according to World Health Organization–based protocols. Blood was drawn the days of enrollment and of discharge by using a syringe and directly transferred to vacutainers containing sodium citrate as an anticoagulant. Sodium citrate was used because other anticoagulants or serum result in platelet activation and release of platelet-derived molecules such as Ang-1. Short application of a tourniquet for blood drawing was often inevitable. Blood samples were processed as soon as possible and handled with care to avoid in vitro platelet activation. Blood was centrifuged for 20 minutes at 1,600 × g, and plasma was stored at −80°C. Plasma levels of Ang-1, Ang-2, and P-selectin were measured by using commercial enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN).
Results
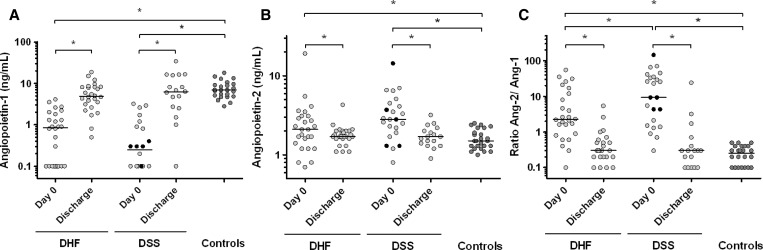
Patients with DHF/DSS had lower Ang-1 levels at enrollment compared with corresponding discharge levels and levels in controls, and had the lowest median level in children with DSS (Figure 1A). Nineteen patients had an Ang-1 level below the detection limit of 0.1 ng/mL at enrollment. In contrast, children with DHF/DSS had higher Ang-2 levels at enrollment than at discharge and compared with healthy controls (Figure 1B). Taken together, DHF/DSS was associated with a clear increase in Ang-2/Ang-1 ratios, and those with DSS had the highest ratios (Figure 1C). Five children died and although their Ang-1 levels were in the lowest range, their corresponding Ang-2 levels were more evenly distributed (Figure 1). There was no difference in Ang-1 and Ang-2 levels between children with and without bleeding.
Figure 1.
Plasma levels of (A) angiopoietin-1 and (B) angiopoietin-2 and corresponding angiopoietin-2:angiopoietin-1 ratios (C) in children in Indonesia with dengue hemorrhagic fever (DHF) (n = 26) and dengue shock syndrome (DSS) (n = 23) on day 0 (day of admission), day of discharge (n = 44) and in a control group of healthy children from Indonesia (n = 25). The minimum detection limit of angiopoietin-1 was 0.1 ng/mL. Black points indicate non-survivors. Horizontal lines represent median values. P values were determined by Wilcoxon signed-rank test for data in time and Mann-Whitney U test for comparison with the control group. *P < 0.05.
The association of Ang-1 and Ang-2 levels with plasma leakage was determined by using serum albumin level and the pleural effusion index (PEI). This index was defined as 100 times the maximum width of the (right or left) pleural effusion on a chest radiograph (right lateral decubitus position) divided by the maximum width of the ipsilateral hemi-thorax. The Ang-1 levels at enrollment correlated positively with albumin levels (Spearman R [Rs] = 0.55, P < 0.0001) and negatively with the PEI (Rs = −0.39, P = 0.005). The Ang-2 levels correlated inversely with serum albumin (Rs = −0.38; P = 0.009) but not with the PEI. The Ang-2:Ang-1 ratio was related with the PEI (Rs = 0.43, P = 0.002) and albumin levels (Rs = −0.60, P < 0.0001). The Ang-1 or Ang-2 levels did not correlate significantly with hematocrit values. Although platelet numbers correlated with serum albumin levels (Rs = 0.44, P < 0.05), there was no significant correlation with the PEI (Rs = −0.26, P = 0.07).
As mentioned, platelets contain high quantities of Ang-1 and release Ang-1 during platelet activation.13 The Ang-1 levels correlated with the platelet count (Rs = 0.44, P < 0.002). Plasma levels of platelet activation marker P-selectin followed the trend of Ang-1 levels and showed a low median level at admission (45.3 ng/mL; interquartile range = 29.4–66.3 ng/mL) that had increased by the day of discharge (120.7 ng/mL; interquartile range = 91.6–187.5 ng/mL). Nonetheless, Ang-1 levels only weakly correlated with P-selectin levels (Rs = 0.30, P < 0.04) at enrollment, but when the 19 samples with Ang-1 levels below the detection limit were not analyzed, this correlation improved considerably (Rs = 0.69, P < 0.0001).
Discussion
This study shows that DHF/DSS is associated with reduced Ang-1 plasma levels and increased Ang-2 levels. These proteins and their endothelial receptor are important mediators of vascular integrity, and we speculate that this imbalance in the Ang/Tie system contributes to the transient increase in vascular permeability. Platelets contain Ang-1, and we hypothesize that dengue-associated thrombocytopenia explains the low Ang-1 levels. Increasing evidence suggests a role for platelets in regulation of vascular integrity with Ang-1 and other platelet-derived angiogenic proteins as central mediators.14 As suggested by others, platelet count and activation status should be taken into account when plasma levels of platelet-derived proteins are measured.18,19
Angiopoietin-2 is produced in endothelial cells and stored in WPBs.12 The finding of high levels of von Willebrand factor, the most abundant WPB constituent, in dengue patients, supports the notion that endothelial cell activation with WPB exocytosis is an important feature of dengue infection.15,20 Although the exact mechanisms of increased WPB exocytosis with subsequent Ang-2 release in dengue are unknown, we hypothesize that endothelial activation caused by increased pro-inflammatory cytokines is centrally involved.21 In addition to the effect of inflammatory cytokines, other mechanisms may also be involved, including direct interaction of dengue virus with endothelial cells, release of mast cell products, and pro-coagulant factors such as thrombin.21–25
No definite conclusion on a causal relationship of Ang-1 and Ang-2 levels and plasma leakage could be made in this observational study. Similar trends in Ang-1 and Ang-2 levels have been found in severe malaria and sepsis,26,27 but in contrast to DHF/DSS and sepsis, plasma leakage is not a prominent feature of malaria. Differences in other mediators of vascular permeability, such as pro-inflammatory cytokines and angiogenic proteins and their soluble receptors, might account for these differences in vascular permeability.
Our study had some limitations. First, no values for the platelet count at discharge and in the control group were available. Second, despite our efforts to limit ex vivo platelet activation, some degree of activation is often inevitable and this should be taken into account when interpreting plasma Ang-1 and P-selectin levels. Angiopoietin-1 levels across different studies seem to vary more than Ang-2 levels in controls.16,28–31 Angiopoietin-1 levels in the controls of our study were comparable with those of other studies, which also found relatively higher Ang-1 levels.29,30 Therefore, we suggest that relative changes in Ang-1 levels within one study may be more informative than comparing absolute Ang-1 levels between studies. Third, the dengue diagnosis was based on the presence of dengue-specific IgM in a single sample. False-positive IgM results caused by cross-reactivity may occur in patients with infectious diseases other than dengue, although the fact that all children in our study population had proven plasma leakage (pleural effusion on chest radiograph) around defervescence, a finding specific for dengue, makes alternative diagnoses unlikely.
In conclusion, DHF/DSS is associated with an imbalance in the Ang/Tie system favoring plasma leakage. Dengue-associated thrombocytopenia may play a so far overlooked role in the transient plasma leakage seen in DHF/DSS. No specific treatment for dengue is available. Interventions aimed at the prevention of thrombocytopenia or the correction of the imbalance in the Ang/Tie system might offer a valuable adjunctive treatment of this devastating disease.
ACKNOWLEDGMENTS
We thank all children and their parents/caretakers for participating in this study. This study was presented in part during the Fourth International Meeting on Angiogenesis, Amsterdam, March 2–4, 2011.
Disclaimer: The sponsor of this study is a public or nonprofit organization that supports science in general. It had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Financial support: M. Michels is supported by a junior researcher grant from the Radboud University Nijmegen Medical Centre.
Disclosure: None of the authors has any commercial or other associations that might pose a conflict of interest.
Authors' addresses: Meta Michels, André J. A. M. van der Ven, and Quirijn de Mast, Department of Internal Medicine, Radboud University Medical Centre, Nijmegen, The Netherlands, E-mails: m.michels@aig.umcn.nl, a.vanderven@aig.umcn.nl, and q.demast@aig.umcn.nl. Kis Djamiatun, Department of Parasitology, Faculty of Medicine, Diponegoro University, Semarang, Indonesia, E-mail: ramus64@yahoo.com. Rob Fijnheer, Philip G. de Groot, and Silvie Sebastian, Department of Clinical Chemistry and Haematology, University Medical Center Utrecht, Utrecht, The Netherlands, E-mails: r.fijnheer@umcutrecht.nl, ph.g.degroot@umcutrecht.nl, and s.a.e.sebastian@umcutrecht.nl. Arjan W. Griffioen, Angiogenesis Laboratory, Department of Medical Oncology, VUMC-Cancer Centre, VU University Medical Center, Amsterdam, The Netherlands, E-mail: aw.griffioen@vumc.nl. Sultana M. H. Faradz, Division of Human Genetics, Center for Biomedical Research, Kariadi Hospital and Faculty of Medicine, Diponegoro University, Semarang, Indonesia, E-mail: smhfaradz@yahoo.com.
References
- 1.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 2.Noisakran S, Chokephaibulkit K, Songprakhon P, Onlamoon N, Hsiao HM, Villinger F, Ansari A, Perng GC. A re-evaluation of the mechanisms leading to dengue hemorrhagic fever. Ann N Y Acad Sci. 2009;1171((Suppl 1)):E24–E35. doi: 10.1111/j.1749-6632.2009.05050.x. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi UC, Agarwal R, Elbishbishi EA, Mustafa AS. Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol Med Microbiol. 2000;28:183–188. doi: 10.1111/j.1574-695X.2000.tb01474.x. [DOI] [PubMed] [Google Scholar]
- 4.Becquart P, Wauquier N, Nkoghe D, Ndjoyi-Mbiguino A, Padilla C, Souris M, Leroy EM. Acute dengue virus 2 infection in Gabonese patients is associated with an early innate immune response, including strong interferon alpha production. BMC Infect Dis. 2010;10:356. doi: 10.1186/1471-2334-10-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sathupan P, Khongphattanayothin A, Srisai J, Srikaew K, Poovorawan Y. The role of vascular endothelial growth factor leading to vascular leakage in children with dengue virus infection. Ann Trop Paediatr. 2007;27:179–184. doi: 10.1179/146532807X220280. [DOI] [PubMed] [Google Scholar]
- 6.Seet RC, Chow AW, Quek AM, Chan YH, Lim EC. Relationship between circulating vascular endothelial growth factor and its soluble receptors in adults with dengue virus infection: a case-control study. Int J Infect Dis. 2009;13:e248–e253. doi: 10.1016/j.ijid.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Srikiatkhachorn A, Ajariyakhajorn C, Endy TP, Kalayanarooj S, Libraty DH, Green S, Ennis FA, Rothman AL. Virus-induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic fever. J Virol. 2007;81:1592–1600. doi: 10.1128/JVI.01642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng CS, Lo HW, Teng HC, Lo WC, Ker CG. Elevated levels of plasma VEGF in patients with dengue hemorrhagic fever. FEMS Immunol Med Microbiol. 2005;43:99–102. doi: 10.1016/j.femsim.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 9.van Meurs M, Kumpers P, Ligtenberg JJ, Meertens JH, Molema G, Zijlstra JG. Bench-to-bedside review: angiopoietin signalling in critical illness - a future target? Crit Care. 2009;13:207. doi: 10.1186/cc7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 11.Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 12.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 13.Li JJ, Huang YQ, Basch R, Karpatkin S. Thrombin induces the release of angiopoietin-1 from platelets. Thromb Haemost. 2001;85:204–206. [PubMed] [Google Scholar]
- 14.Nachman RL, Rafii S. Platelets, petechiae, and preservation of the vascular wall. N Engl J Med. 2008;359:1261–1270. doi: 10.1056/NEJMra0800887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sosothikul D, Seksarn P, Pongsewalak S, Thisyakorn U, Lusher J. Activation of endothelial cells, coagulation and fibrinolysis in children with dengue virus infection. Thromb Haemost. 2007;97:627–634. [PubMed] [Google Scholar]
- 16.Michels M, Djamiatun K, Faradz SM, Koenders MM, de Mast Q, van der Ven AJ. High plasma mid-regional pro-adrenomedullin levels in children with severe dengue virus infections. J Clin Virol. 2011;50:8–12. doi: 10.1016/j.jcv.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Anonymous . Dengue Haemorrhagic Fever: Diagnosis, Treatment, Prevention and Control. Second edition. Geneva: World Health Organization; 1997. http://www.who.int/csr/resources/publications/dengue/itoviii.pdf Available at. [Google Scholar]
- 18.Panzer S, Rosales S, Gisslinger H, Jungbauer L, Kaider A, Knöbl P, Sillaber C, Pabinger I. Plasma levels of P-selectin are determined by platelet turn-over and the P-selectin Thr715Pro polymorphism. Thromb Res. 2008;121:573–579. doi: 10.1016/j.thromres.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 19.Hormbrey E, Gillespie P, Turner K, Han C, Roberts A, McGrouther D, Harris AL. A critical review of vascular endothelial growth factor (VEGF) analysis in peripheral blood: is the current literature meaningful? Clin Exp Metastasis. 2002;19:651–663. doi: 10.1023/a:1021379811308. [DOI] [PubMed] [Google Scholar]
- 20.Djamiatun K, van der Ven AJ, de Groot PG, Faradz SM, Hapsari D, Dolmans WM, Sebastian S, Fijnheer R, de Mast Q. Severe dengue is aasociated with consumption of von Willebrand factor and its cleaving enzyme ADAMTS-13. PLoS Negl Trop Dis. 2012;6:e1628. doi: 10.1371/journal.pntd.0001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rondaij MG, Bierings R, Kragt A, van Mourik JA, Voorberg J. Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1002–1007. doi: 10.1161/01.ATV.0000209501.56852.6c. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg BE, Goldenberg NM, Lee WL. Do viral infections mimic bacterial sepsis? The role of microvascular permeability: a review of mechanisms and methods. Antiviral Res. 2012;93:2–15. doi: 10.1016/j.antiviral.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Dalrymple N, Mackow ER. Productive dengue virus infection of human endothelial cells is directed by heparan sulfate-containing proteoglycan receptors. J Virol. 2011;85:9478–9485. doi: 10.1128/JVI.05008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown MG, Hermann LL, Issekutz AC, Marshall JS, Rowter D, Al-Afif A, Anderson R. Dengue virus infection of mast cells triggers endothelial cell activation. J Virol. 2011;85:1145–1150. doi: 10.1128/JVI.01630-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuta T, Murao LA, Lan NT, Huy NT, Huong VT, Thuy TT, Tham VD, Nga CT, Ha TT, Ohmoto Y, Kikuchi M, Morita K, Yasunami M, Hirayama K, Watanabe N. Association of mast cell-derived VEGF and proteases in dengue shock syndrome. PLoS Negl Trop Dis. 2012;6:e1505. doi: 10.1371/journal.pntd.0001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conroy AL, Lafferty EI, Lovegrove FE, Krudsood S, Tangpukdee N, Liles WC, Kain KC. Whole blood angiopoietin-1 and -2 levels discriminate cerebral and severe (non-cerebral) malaria from uncomplicated malaria. Malar J. 2009;8:295. doi: 10.1186/1475-2875-8-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giuliano JS, Jr, Lahni PM, Harmon K, Wong HR, Doughty LA, Carcillo JA, Zingarelli B, Sukhatme VP, Parikh SM, Wheeler DS. Admission angiopoietin levels in children with septic shock. Shock. 2007;28:650–654. [PMC free article] [PubMed] [Google Scholar]
- 28.Giuliano JS, Jr, Lahni PM, Bigham MT, Manning PB, Nelson DP, Wong HR, Wheeler DS. Plasma angiopoietin-2 levels increase in children following cardiopulmonary bypass. Intensive Care Med. 2008;34:1851–1857. doi: 10.1007/s00134-008-1174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KW, Lip GY, Blann AD. Plasma angiopoietin-1, angiopoietin-2, angiopoietin receptor tie-2, and vascular endothelial growth factor levels in acute coronary syndromes. Circulation. 2004;110:2355–2360. doi: 10.1161/01.CIR.0000138112.90641.7F. [DOI] [PubMed] [Google Scholar]
- 30.Lim HS, Blann AD, Chong AY, Freestone B, Lip GY. Plasma vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in diabetes: implications for cardiovascular risk and effects of multifactorial intervention. Diabetes Care. 2004;27:2918–2924. doi: 10.2337/diacare.27.12.2918. [DOI] [PubMed] [Google Scholar]
- 31.Van der Heijden M, Pickkers P, van Nieuw Amerongen GP, van Hinsbergh VW, Bouw MP, van der Hoeven JG, Groeneveld AB. Circulating angiopoietin-2 levels in the course of septic shock: relation with fluid balance, pulmonary dysfunction and mortality. Intensive Care Med. 2009;35:1567–1574. doi: 10.1007/s00134-009-1560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]