Abstract
Objective
Amyotrophic Lateral Sclerosis (ALS) is associated with impaired executive control. The aim of the current research was to test the hypothesis that concept formation deficits associated with an extra-motor neurocognitive network involving executive and semantic resources can be found in some ALS patients.
Methods
Forty-one patients with clinically-definite ALS were assessed with Delis Kaplan Executive Function System Sorting Test (D-KEFS), a measure of concept formation requiring patients to manipulate verbal and visual semantic information and neuropsychological tests measuring naming, semantic memory, and executive control. Using D-KEFS scale scores, a k-mean cluster analysis specifying a 3-group solution was able to classify ALS patients into groups presenting with mildly impaired, average, and above average sorting test performance. High resolution T1 structural MRI was used to examine cortical thickness in a subset of 16 ALS patients.
Results
Step-wise regression analyses related free and recognition sorting test performance to measures of action naming, single word semantic knowledge, and mental search/working memory. MRI studies found widespread cortical thinning involving bilateral frontal, temporal and parietal regions. Regression analyses related recognition sorting performance to reduced MRI cortical thickness involving the left prefrontal and left parietal cortex.
Conclusions
An extra-motor cognitive network is associated with impaired concept formation in ALS.
Keywords: Amyotrophic Lateral Sclerosis (ALS), executive control, prefrontal cortex, neuropsychology, Delis-Kaplan Executive Function System (D-KEFS) Sorting Test
Introduction
Recent work suggests that transactive DNA binding protein of ~43 kDa (TDP-43) is an important histopathologic abnormality in the vast majority of sporadic Amyotrophic Lateral Sclerosis (ALS) patients placing ALS within the same neurodegenerative spectrum of disease as frontotemporal lobar degeneration (FTLD; Geser et al., 2008, 2009; Neumann et al., 2006). Although ALS has traditionally been viewed as a motor disorder with few cognitive deficits, it is now understood that neuropsychological and behavioral impairment can be present in ALS (Irwin et al., 2007; Lomen-Hoerth et al., 2003; Phukan et al., 2007; Strong et al., 1999). Moreover, recent research suggests that performance on tests assessing executive control is particularly impaired in ALS (Abrahams et al., 1996, 2004; 2005; Volpato et al., 2010). Other cognitive deficits associated with ALS include impaired performance on tests of memory and language (Briettschneider et al, 2012; Mantovan et al., 2003; Grossman et al, 2008). In this study, we examined the contribution of executive and language-related deficits to concept formation in ALS.
Concept formation or the ability to categorize objects into meaningful or novel groups enables individuals to react flexibly to demands from the environment in order to make appropriate and effective decisions about objects (Milton, Willis & Hodgson, 2009). Prior work has demonstrated the need for significant executive skills when subjects are asked to group objects. For example, recent event-related fMRI study demonstrated considerable brain activation subserving executive components during concept formation, including activation involving the ventrolateral frontal cortex (VLFC) and precuneus (Milton, & Willis, 2004).
Deficits in concept formation seen in dementia have also been associated with considerable executive resources involving patients’ ability to retrieve and/or manipulate semantically-related information (Giovannetti et al., 2002; Grossman et al., 2008). Giovannetti et al., (2002) found gross impairment in establishing mental set on concept formation tests in dementia patients and demonstrated that this was related to a dysexecutive cognitive disorder. In another group of dementia patients, errors produced on concept formation tests appeared to be related lexical retrieval and/or semantic knowledge deficits. We investigated concept formation in ALS, in part, because of previous research demonstrating impaired executive and language-related functioning in dementia patients.
Deficits in concept formation in ALS have been most often assessed with the Wisconsin Card Sort Test (Grant & Berg, 1948) where patients need to exert inhibitory control over perseveration, successfully sort cards into categories, and then shift card sorting strategy (Abe et al., 1997; Strong et al., 1999). The Delis-Kaplan Executive Function System (D-KEFS) Sorting Test (Delis, Kramer & Kaplan, 2001) is another measure of concept formation. As compared to the WCST, the D-KEFS Sorting Test contains a number of unique features. First, the D-KEFS Sorting Test was purposefully constructed to minimize the confounding role of inhibitory control during concept formation that is such a prominent characteristic of the WCST. Second, independent of the sorts generated by patients, the D-KEFS Sorting Test also assesses the basis for patients’ sorting strategy, such as focusing on word meaning or the font used to print the words. Third, the D-KEFS Sorting Test contains both free and recognition sorting test conditions, where the recognition condition permits the examiner to assess sorting strategies in a manner that is entirely independent of a motor component. Thus, the D-KEFS Sorting Test allows for an analysis of executive and material-specific knowledge components that potentially underlie sorting/concept formation deficits associated with ALS.
The current research used the D-KEFS Sorting Test to test the hypothesis that deficits in an extra-motor, neurocognitive network involving executive and semantic resources exists in some ALS patients. Three predictions were tested. First, free and recognition sorting test performance was expected to be impaired in ALS. Second, performance on neuropsychological tests related to executive and language-related skills were expected to contribute to concept formation deficits in ALS. Third, using MRI studies measuring cortical thickness, we expected concept formation deficits in ALS to be associated with cortical areas extending beyond motor regions to include prefrontal cortical regions.
Methods
Subjects
Forty-one right-handed, high school-educated, native English-speakers with clinically-definite ALS, diagnosed according to El Escorial revised criteria (Brooks, Miller, Swash, & Munsat, 2000) were studied. These patients participated in an informed consent procedure approved by the IRB of the University of Pennsylvania. In prior research (Elman et al., 2009) patients were assigned to ALS-cognitively impaired versus ALS-cognitively normal subgroups on the basis of (1) a clinical evaluation conducted by an experienced neurologist (LM, LE, MG), and (2) a cognitive screening protocol consisting of the following tests: digits backwards, ‘animal’ fluency, the oral trail-making test, Visual-Verbal Test, and the Frontal Behavioral Inventory. ALS test performance was assessed relative to 25 healthy matched controls matched for age and education. A patient was judged to be cognitively impaired if performance on 3 of the 5 tests described above where ≤1.5sd units below normative values.
This screening protocol has been validated using receiver operator curve (ROC) analyses that discriminated between ALS-cognitively impaired versus ALS- cognitively normal patients (AUC= 0.84; Elman et al., 2009). This screening protocol was also validated using MRI-VBM analyses that related reduced ‘animal’ fluency to greater left prefrontal/premotor atrophy and attenuated digits backwards to greater right prefrontal/ventral frontal atrophy in ALS-cognitively impaired patients (Elman et al., 2009). ALS-cognitively impaired patients were further diagnosed for the presence of frontotemporal lobar degeneration (FTLD) based on a modification of published criteria (McKhann et al., 2001; Neary et al., 1998).
Using these criteria Elman et al., (2009) found that 20 (48.8%) patients were judged to be without cognitive deficits (ALS-nl); 12 (29.3%) patients were judged to have mild cognitive deficits (ALS-mild), and 9 patients were diagnosed with co-occurring behavioral variant FTLD (21.9%; ALS-FTLD). Exclusion criteria included evidence of another neurologic condition (e.g. head trauma, hydrocephalus), a primary psychiatric disorder (e.g. schizophrenia, bipolar disorder, major depression) or other medical conditions. Participants were not taking sedating medications that could interfere with neuropsychological performance.
The screening protocol used to classify ALS patients is heavily reliant on executive measures. Therefore, to avoid issues of circularity and as an alternative to the procedures described above, a k-means cluster analysis (SPSS v19) specifying a 3-group solution was calculated to see if selected D-KEFS sorting measures could segregate patients into groups reflecting intact, mildly impairment, or significantly impaired test behavior. The three D-KEFS sorting variables used in the k-means cluster analysis were (1) the age-corrected scale score for total free sorts, (2) the age-corrected scale score for patient’s description of their sorts, and (3) the age-corrected scale score for patient’s recognition of sorting strategies. The mean age of our ALS patients was 58.60 (±9.73) and ranged between 37 and 77 years. As age is known to affect performance on executive tests, we used age-corrected scores for this and all subsequent analyses because of the wide age range in our cohort. This analysis resulted is a group where sorting test performance was at around the 5th percentile (borderline range; n= 9), a group where sorting test performance was at around the 50th percentile (average range; n= 20); and a group were sorting behavior was at around the 84st percentile (high average range; n= 12; Table 1). The three groups created with the k-means cluster analysis was the independent variable used in any subsequent between-group analyses.
Table 1.
Delis Kaplan Sorting Test k-Means Cluster Solution
| Group 1 (average performance; n= 20) | Group1 (impaired performance; n= 9) | Group 3 (above average performance; n =15) | |
|---|---|---|---|
| Scale Score: Combined Free Sorts 1 & 2 | 10.70 | 6.00 | 13.27 |
| Scale Score Combined Sorts 1 & Description | 9.45 | 5.22 | 13.67 |
| Scale Score: Combined Recognition Sorts 1 & 2 | 9.30 | 6.00 | 15.40 |
Notes: mean= 10; standard deviation= 3
Clinical and demographic characteristics are summarized in Table 2. The three groups did not differ with respect to age, education, disease duration, MMSE, illness severity/functional disability assessed with ALSFRS-R (Cedarbaum et al., 1999), depression (Beck Depression Inventory-II), and the extent of upper motor neuron (UMN) involvement. To assess upper motor neuron involvement we constructed a 32-point scale by quantifying the presence of UMN signs in the bulbar, cervical, and lumbosacral segments. UMN signs were defined by segment as the presence of pathological reflexes (bulbar: jaw jerk, facial reflex, palmomental sign, pseudobulbar affect; cervical: biceps, triceps, finger flexors, clonus, Hoffman sign; lumbosacral: patellar, crossed adduction, ankle clonus, Babinski response). Spasticity was assessed with the Ashworth Spasticity Scale (Ashworth, 1964). Groups did not differ on these measures (Table 2).
Table 2.
Demographic and Clinical Information (means & standard deviations)
| Group 1 (average performance; n= 20) | Group 2 (impaired performance; n= 9) | Group 3 (above average performance; n= 15) | |
|---|---|---|---|
| Age | 60.10 (12.02) | 62.22 (8.39) | 58.13 (5.43) |
| Education | 14.25 (2.86) | 14.00 (2.00) | 14.80 (2.42) |
| MMSE | 26.85 (2.90) | 25.88 (2.84) | 28.13 (1.64) |
| Illness Duration | 102.13 (113.59) | 56.50 (44.94) | 41.53 (22.82) |
| Ashworth Scale | 12.69 (8.75) | 14.00 (5.44) | 15.12 (9.80) |
| ALSFRS-R Scale | 37.00 (7.12) | 31.57 (8.75) | 33.62 (7.85) |
| Upper Motor Signs (bulbar involvement) | 11.28 (9.53) | 15.50 (7.40) | 14.25 (10.59) |
Notes: MMSE= Mimi-Mental State Examination; ALSFRS-R= The ALS Functional Rating Scale-Revised
The Delis Kaplan Executive Function System Sorting Test (D-KEFS)
All protocols were administered and scored using standardized procedures described in the Delis-Kaplan Executive Function System manual (Delis, Kramer & Kaplan, 2001). The standard D-KEFS sorting test consists of two test procedures, administered in the following order – The Free Sort Test Condition consists of standard form card set 1 and 2. Each set contained six cards. For this test condition patients were asked to sort the six cards into as many groups each containing three cards. Eight sorts are possible. Three of the eight sorts can be made on the basis of verbal/semantic information derived from the stimulus words printed on the cards; and five of the eight sorts can be made on the basis of visuoperceptual features including the color, size, and shape of the stimulus cards. No right or wrong feedback is provided.
In addition to scoring whether sorts were correctly or incorrectly made, patients were then asked to describe the sorting rule used to group the cards. The patients’ description of their free sorts was scored using a 0–2 point scale. A 2-point response is assigned when an appropriate general concept is identified (e.g., “they are vehicles”); a 1-point response is assigned when patients describe specific features of the test stimuli (e.g., “they all have legs”). Zero-point responses could include responses such as “I don’t know”, “they are happy” (say, for an animal group), or a correct descriptor for only a subgroup of the test stimuli. This 0–2 point scoring system is similar to the scoring criteria for the Similarities subtest from the Wechsler Intelligence Test corpus.
For the Recognition Sort Test Condition the same set of cards were sorted by the examiner into two groups of three cards, according to the eight target sorts. Patients were asked “to tell me how the cards are the same in each group.” Recognition sorts were scored using the same 0–2 point scoring system described above. Thus, in the current research three age-corrected scale scores (mean= 10; standard deviation= 3) were analyzed: combined free sort scale score for card sets 1 and 2, the combined description of the free sorts, and patient’s recognition of the examiner’s card sorts.
For descriptive purposes, separate 1-way analyses of variance were conducted for these three D-KEFS sorting measures. Significant between-group differences were found for all three sorting variables (free sort [F(2, 41)= 103.06, p< .001); free sort description [F(2, 41)= 103.48, p< .001); recognition ([F(2, 41)= 65.64, p< .001). As expected, the three groups differed from each other. The cluster analysis-determined low average sorting group scored lower than the average and above average sorting groups; the cluster determined-average sorting group scored lower compared to the above average sorting group (p< .001, all analyses, Bonferroni corrected post-hoc tests).
Neuropsychological Assessment
We also administered a brief neuropsychological protocol to patients to measure performance on measures of language and executive functioning. All tests were converted to standardized z-scores using the group of 25 age matched normal control described above.
) Letter fluency (Spreen & Struss, 1990): Patients were given 60s to generate words, excluding proper nouns and numbers, beginning with a specified letter (‘FAS’). The dependent variable was the number of responses summed across the three letters.
) Oral Trail-Making (Ricker & Axelrod, 1994): Patients are asked to alternate production of letters and numbers starting in ascending order (i.e., 1-A-2-B) for 60 seconds. The dependent variable is the number of correct responses.
) Object Naming (Kaplan, Goodglass & Weintraub, 1983): This was assessed with the Boston Naming Test (BNT). The dependent variable was number of correct responses.
) Action Naming (Obler & Albert, 1990): Patients were shown a picture depicting an activity (e.g., a boy running) and were asked to name the action. The dependent variable was number of correct responses.
) Test of the Reception of Grammar (Bishop, 1990): This test consists of a four-alternative, forced-choice sentence-picture matching task. A subset of items requiring agreements for gender, number, and grammatically complex phrasing were administered.
) Pyramid and Palm Tree Test (Howard & Patterson, 1992): Patients are shown a series of target line drawings and words accompanied by two choices. Patients are asked to select the choice with the greatest semantic association to the target (Table 3).
Table 3.
Neuropsychological Test Results
| Group 1 (impaired performance; n= 9) | Group 2 (average performance; n= 20) | Group 3 (above average performance; n= 15) | |
|---|---|---|---|
|
| |||
| Scale Score: Combined Free Sorts 1 & 2 | 6.00 (1.00) | 10.70 (1.08) | 13.26 (1.43) |
|
| |||
| Scale Score Combined Sorts 1 & 2 Description | 5.22 (0.97) | 9.45 (1.05) | 13.66 (1.95) |
|
| |||
| Scale Score: Combined Recognition Sorts 1 & 2 | 6.00 (2.34) | 9.30 (2.29) | 15.40 (1.54) |
|
| |||
| Letter Fluency (FAS; raw score) | 9.93 (2.88) | 10.38 (2.54) | 13.30 (2.68) |
| 1 z-score | −1.03 (0.74) | −1.02 (0.68) | −0.16 (0.75) |
|
| |||
| Oral Trails (raw score) | 49.50 (0.70) | 49.53 (3.54) | 51.00 1.34) |
| 1 z-score | −2.29 (0.87) | −1.29 (1.53) | −0.55 (1.69) |
|
| |||
| Boston Naming Test (raw score) | 44.87 (10.20) | 49.76 (9.55) | 57.59 (2.28) |
| 1 z-score | −4.10 (1.00) | −2.30 (1.63) | 0.71 (0.86) |
|
| |||
| Action Naming Test (raw score) | 46.00 (3.87) | 52.61 (3.83) | 54.75 (1.65) |
| 1 z-score | −1.42 (1.10) | 0.24 (1.00) | 0.84 (0.44) |
|
| |||
| Pyramid & Palm Tree Test (words; raw score) | 47.33 (1.80) | 48.52 (2.64) | 50.92 (1.14) |
| 1 z-score | −1.52 (−0.95) | −0.92 (1.46) | 0.49 (0.60) |
|
| |||
| Pyramid & Palm Tree Test (pictures; raw score) | 47.00 (2.64) | 50.25 (2.08) | 51.18 (0.87) |
| 1 z-score | −2.84 (2.09) | −0.36 (1.69) | 0.39 (0.69) |
age corrected z-score
Imaging methods and procedures
High-resolution structural MRI scans were available in 16 ALS patients (6 ALS-nl; 7 ALS-mild; 3 ALS-FTLD). Images were acquired by a Siemens Trio 3T MRI scanner. Each study began with a rapid sagittal T1-weighted image to determine patient position. Next, high-resolution T1-weighted 3D spoiled gradient echo images were acquired with TR= 1620 msec, TE= 3 msec, slice thickness 1.0mm, flip angle 15°, matrix= 192 × 256, and in-plane resolution 0.9 × 0.9mm. Regions of cortical atrophy relative to 28 healthy controls were identified using voxel-based cortical thickness analyses of structural MRI data. We used the openly available tools PipeDream, (https://sourceforge.net/projects/neuropipedream/) and Advanced Normalization Tools (ANTS, http://www.picsl.upenn.edu/ANTS/) to perform multivariate normalization and structure-specific processing of our data (Avants, Epstein, Grossman & Gee, 2008; Klein et al., 2009). PipeDream’s cross-sectional studies deform each individual dataset into a standard local template space as well as a canonical stereotactic coordinate system. The core processing is based on mapping T1 structural MRI to an optimal template space, which is defined as the population-specific, unbiased average shape and appearance image derived from a representative population (Avants & Gee, 2004; Kim et al., 2008). The local template consists of 25 healthy seniors and 25 FTLD patients. The coordinate deformations themselves are smooth and invertible - that is, diffeomorphic - so that neuroanatomical neighbors remain neighbors under the mapping. The algorithms are also symmetric so that they are not biased towards the reference space chosen to compute the mappings. Moreover, these topology-preserving maps capture the large deformation necessary to aggregate populations of images in a common space. Recent evaluation studies suggest that ANTS-based normalization is the most stable and reliable currently available. These algorithms allow template-based priors to guide cortical segmentation and compute cortical thickness (Das, Avants, Grossman, & Gee, 2009). Cortical thickness images were smoothed using a 2mm full-width half-maximum Gaussian kernel to minimize individual gyral variations. In SPM5, two sample t-tests contrasted cortical thickness between patients and controls. An explicit mask defined by a gray matter prior probability map in SPM5 limited the analysis to voxel-wise comparisons within gray matter. The analysis included all clusters surviving a cluster level criterion of p< .001 corrected for false discovery rate (FDR), 100 voxel extent criterion, and a height threshold of p< .001 FDR-corrected. Regression analyses attempted to related free and recognition sorting test performance using the regression module of SPM5, and we accepted clusters with a peak voxel z-score ≥3.09 (p< .001).
Results
Regression Analysis for Neuropsychological Tests
We found no relationship between sorting performance and demographic variables or motor characteristics of ALS (Table 1). The relationship between sorting performance and executive and language-related measures was assessed with a series of step-wise regression analyses. For these analyses the free and recognition D-KEFS sorting measures were the dependent variables. Separate step-wise analyses were conducted for executive and language z-scores.
For language tests, scores from the Action Naming Test, the Boston Naming Test, the TROG, and Pyramid and Palms-words, and Pyramid and Palms-pictures were independent variables. For free sorting test behavior, only performance on the Action Naming Test remained in the model (r= .663 R2= .440, p< .001; F[1, 21]= 19.62, p< .001). For recognition sorting behavior, only performance on the Pyramid and Palms -word test condition remained in the model (r= .667; R2= .445; F[1, 24]= 19.21; p< .001). For executive tests, scores from the letter fluency and oral trails tests were independent variables. For regression analyses assessing both free and recognition sorting performance, only output on the letter fluency test entered into the model (free sorting: r= .505; R2= .255; F[1, 22]= 7.51, p< .021; recognition sorting: r= .638; R2= .407; F[1, 21]= 14.43, p< .001).
MRI Cortical Thickness Analysis
The analysis of cortical thickness and free sorting test performance was not significant for any cortical region. Figure 1 displays regional cortical thickness in ALS as related to recognition sort performance. Table 4 summarizes the attributes of atrophic clusters. For recognition sorting cortical thinning involving bilateral motor, premotor and dorsolateral/prefrontal, anterior/lateral temporal, and anterior parietal cortical thinning was found. Using age-corrected recognition sorting scores regression analysis was significantly related to cortical thinning involving the left dorsolateral prefrontal (Brodmann area 46) and left parietal (Brodmann area 7) cortices
Figure 1.
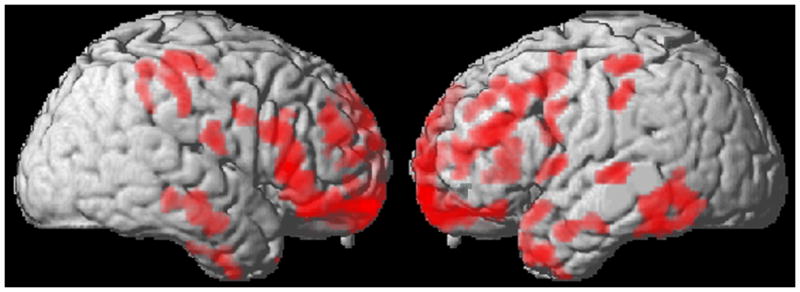
Statistically significant areas of cortical thinning (red) correlated with recognition sorting test performance (blue).
Table 4.
Peak coordinates of significantly atrophic clusters in ALS patients compared 25 healthy seniors, and peak coordinates of regression analysis relating age-corrected recognition sorting performance to cortical atrophy.
| Anatomic region (Brodmann area) | Coordinates | z-score | Cluster size (voxels) | ||
|---|---|---|---|---|---|
| X | Y | z | |||
| ATROPHY | |||||
| FRONTAL | |||||
| L dorsolateral frontal (46) | −48 | 34 | 24 | 5.08 | 1142 |
| L inferior frontal (44) | −62 | 10 | 28 | 4.57 | 123 |
| L premotor (6) | −60 | 2 | 8 | 4.47 | 108 |
| L ventral frontal (11) | −18 | 52 | −18 | 5.36 | 1709 |
| R dorsolateral frontal (10) | 40 | 50 | 22 | 5.06 | 337 |
| R anterior frontal (9) | 4 | 62 | 30 | 4.87 | 407 |
| R motor (4) | 66 | −8 | 18 | 4.51 | 104 |
| R ventral frontal (11) | 44 | 34 | −18 | 5.23 | 2079 |
| TEMPORAL | |||||
| L anterior temporal (22) | −68 | −26 | 2 | 4.84 | 141 |
| L anterior temporal (38) | −58 | 14 | −14 | 5.15 | 141 |
| L anterior temporal (21) | −64 | 2 | −22 | 5.04 | 164 |
| L anterior temporal (38) | −44 | 18 | −38 | 4.63 | 291 |
| L inferior temporal (20) | −64 | −46 | −22 | 5.12 | 641 |
| R anterior temporal (21) | 64 | −14 | −16 | 4.59 | 261 |
| PARIETAL | |||||
| L anterior parietal (40) | −52 | −32 | 56 | 4.82 | 125 |
| L anterior parietal (40) | −60 | −30 | 46 | 4.61 | 112 |
| R anterior parietal (40) | 60 | −28 | 46 | 4.71 | 477 |
| REGRESSION | |||||
| L dorsolateral prefrontal (46) | −48 | 46 | −4 | 3.65 | 11 |
| L parietal (7) | −34 | −52 | 70 | 3.32 | 12 |
Discussion
In the current research, we found that some patients with ALS produce comparatively low scores on measures of free and recognition sorting. Regression analyses found that reduced output of the letter fluency test is related lower free and recognition sorting test performance. We also found that reduced sorting performance is related to lower performance on tests that assess both action naming and word meaning. Finally, regression analysis related lower recognition sorting performance to greater cortical thinning in prefrontal and parietal cortex.
The traditional view is that ALS is a motor disorder without neuropsychological deficits. This perspective has been challenged with increasing numbers of reports demonstrating a variety of cognitive deficits and the presence of dementia in ALS (Lomen-Hoerth, 2004). Past research has reported that up to 15% of ALS patients present with FTLD; and up to 35% of patients demonstrate at least mild to moderate cognitive impairment (Ringholz et al., 2005; Strong et al., 2009). A large autopsy series showed that 12.5% of ALS patients have co-occurring FTLD (Hu et al., 2009). Executive deficits have been frequently been reported in ALS (Fein et al., 2009). Although TDP-43 histopathologic abnormalities are found throughout the brains of patients with ALS (Geser et al., 2008), direct assessments of the neuroanatomic basis for cognitive difficulty in ALS have been rare (Abrahams, Goldstein & Simmons, 2004; Briettschneider et al, 2012).
Letter fluency test performance is resource-demanding, involving both working memory and mental search skills. Prior research with letter fluency tests in dementia patients suggests that better test performance is associated with frontal lobe integrity (Libon et al., 2009). The sorting task used in the current research requires the appreciation of mutually exclusive categories and the ability to recombine cards into mutually exclusive categories. These are cognitive operations that likely require working memory and mental search abilities.
Regression analyses also related free and recognition sorting performance to language-related measures associated with semantic memory. Recent work has demonstrated language difficulties in ALS such that patients with ALS appear to be impaired in their naming and comprehension of action words (Grossman et al., 2008). The relationship between reduced free and recognition sorting difficulty and reduced performance on the Pyramid and Palms Test and the Action Naming Test suggests that semantically-mediated knowledge contributes to optimal sorting test performance and that sorting performance in ALS declines in association with either limited access to or degraded semantic memory. Findings such as these can be interpreted as reflecting the vulnerability of an action/semantic network in ALS.
Sorting is a traditional paradigm used to assess concept formation or the ability to assume what has been termed an abstract attitude (Goldstein & Scheerer, 1941). Prior research examining sorting in ALS has employed the WCST. However, differences on this test between patients with ALS and various comparison groups have been inconsistent, with some studies showed only borderline differences between ALS and controls (Abrahams, Goldstein & Kew, 1996; Talbot et al., 1995). In a study similar to the current research, ALS patients judged to be cognitively intact were compared to ALS patients with mild cognitive deficits and ALS patients with dementia. The cognitively normal subgroup performed better on the WCST than ALS patients with milder impairment and dementia. Discrepancies between these findings could be due to differences in the measures used to assess sorting and the patient groups that were studied. Despite these differences the sorting impairment we found with the recognition procedure clearly emphasizes that concept formation deficits in ALS cannot be easily attributed to the motor disorder.
Prior imaging research in ALS suggests the presence of atrophy in motor, prefrontal, and posterior cortical regions in ALS (Chang et al., 2005; Minnerop et al., 2009). Using quantitative techniques that measure cortical thickness, the current study confirmed the presence of considerable cortical thinning in ALS patients. In addition to thinning in motor cortex, we observed statistically robust cortical thinning in prefrontal, temporal and anterior regions, bilaterally. This may reflect the neuroanatomically widespread TDP-43 histopathologic abnormalities seen in autopsy-confirmed cases (Geser et al., 2008).
Neuropsychological impairment has been related directly to cortical atrophy in ALS only rarely. Abrahams et al., (2004) found limited prefrontal cortical activation in an fMRI study of lexical retrieval. Deficits in processing action words compared to object words has been previously reported in ALS and has been shown to be related to frontal cortical atrophy (Grossman et al., 2008). In the present study, we did not find a relationship between cortical thinning free sorting test behavior. This could have been due, in part, to differences between the recognition and free sorting procedures. For example, the motor component of the free sorting procedure may have confounded the sorting component of the task (e.g. motor performance is resource-demanding for ALS patients) and thus limited the ability to identify a robust relationship between behavior and cortical thinning.
Regression analysis was able to related impaired recognition sorting directly to left-sided dorsolateral prefrontal and parietal thinning in ALS. This is consistent with research associating frontal-parietal connections with complex spatial and motor activities (Anderson et al., 2012; Stepniewska et al., 2011; Yan & He, 2011). Clearly, this is an area for future research. fMRI studies of healthy adults have relate prefrontal activation to complex executive demands during sorting tasks (Badre & D’Esposito, 2007). Also, prior neuropsychological studies have associate sorting deficits with damage in this area (Fein et al., 2009). The regression analysis described above associating cortical thinning with recognition sorting deficits is consistent with a cognitive model where executive resources contribute to the retrieval and manipulation of lexical and semantic knowledge. The breakdown of this network interferes with cognitive performance in ALS.
The current research is not without limitations. While the cognitive abilities of ALS patients were carefully assessed, only a comparatively small number of patients were studied in a cross-sectional manner. Only selected executive measures were available for the current research. Different measures may have yielded different results. Also, there was not a large enough sample of ALS patients to assess regression in imaging analyses for each of three cluster-determined groups. With these caveats in mind, the data described above adds to a growing literature demonstrating that patients with ALS can present with cognitive deficits and that these deficits are governed by disease outside of motor cortex.
Acknowledgments
Supported in part by National Institutes of Health (AG32953, AG17586, AG15116, NS44266, and NS53488).
Footnotes
Discloser
Drs. Libon, Elman, McCluskey, McMillan and Avants and Ms. Boller, Morgan, Burkholder, and Chandrasekaran report no disclosures. Dr. Grossman is a consultant for Pfizer Pharmaceuticals, Forest Labs, and Allon Therapeutics. The authors report no financial or other conflicts of interest.
References
- Abe K, Fujimura H, Toyoka K, Sakoda S, Yorifuji S, Yanagihara T. Cognitive function in amyotrophic lateral sclerosis. Journal of the Neurological Sciences. 1997;148:95–100. doi: 10.1016/s0022-510x(96)05338-5. [DOI] [PubMed] [Google Scholar]
- Abrahams S, Goldstein LH, Kew JJ. Frontal lobe dysfunction in amyotrophic lateral sclerosis. Brain. 1996;119:2105–2120. doi: 10.1093/brain/119.6.2105. [DOI] [PubMed] [Google Scholar]
- Abrahams S, Goldstein LH, Simmons A. Word retrieval in amyotrophic lateral sclerosis: A functional magnetic resonance imaging study. Brain. 2004;127:1507–1517. doi: 10.1093/brain/awh170. [DOI] [PubMed] [Google Scholar]
- Abrahams S, Leigh PN, Goldstein LH. Cognitive change in ALS: A prospective study. Neurology. 2005;64:1222–1226. doi: 10.1212/01.WNL.0000156519.41681.27. [DOI] [PubMed] [Google Scholar]
- Anderson EJ, Jones DK, O’Gorman RL, Leemans A, Catani M, Husain M. Cortical network for gaze control in humans revealed using multimodal MRI. Cerebral Cortex. 2012;22:765–775. doi: 10.1093/cercor/bhr110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth B. Preliminary trial of carisoprodal in multiple sclerosis. Practitioner. 1964;192:540–542. [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Gee JC. Geodesic estimation for large deformation anatomical shape and intensity averaging. Neuroimage. 2004;23:S139–S150. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Badre D, D’Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. Journal of Cognitive Neuroscience. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Bishop R. Test for the Reception of Grammar. Thames Publishing Co; Bury St. Edmonds, UK: 1989. [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders. 2000;12:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Brettschneider J, Libon DJ, Toledo J, McCluskey L, Geser F, Lee V, Grossman M, Trojanowski JQ. Determinants of cognitive impairment and dementia in amyotrophic lateral sclerosis. Acta Neuropathologica. 2012 doi: 10.1007/s00401-011-0932-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) Journal of the Neurological Sciences. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Chang JL, Lomen-Hoerth C, Murphy J, Henry RG, Kramer JH, Miller BL, Gorno-Tempini ML. A voxel-based morphometry study of patterns of brain atrophy in ALS and ALS/FTLD. Neurology. 2005;65:75–80. doi: 10.1212/01.wnl.0000167602.38643.29. [DOI] [PubMed] [Google Scholar]
- Das SR, Avants BB, Grossman M, Gee JC. Registration based cortical thickness measurement. Neuroimage. 2009;45:867–879. doi: 10.1016/j.neuroimage.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E. The Delis-Kaplan Executive Functions Systems Test. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Elamin M, Phukan J, Bede P, Jordan N, Byrne S, Pender N, Hardiman O. Executive dysfunction is a negative prognostic indicator in patients with ALS without dementia. Neurology. 76:1263–1269. doi: 10.1212/WNL.0b013e318214359f. [DOI] [PubMed] [Google Scholar]
- Elman L, Xie S, Libon DJ, Hu W, Khan A, Morgan B, Richmond L, Goldmann Gross R, McCluskey L, Grossman M. Validating a brief screen for cognitive impairment in ALS. Abstract presented at the 61st annual meeting of the American Academy of Neurology; Seattle, WA. 2009. [Google Scholar]
- Fine EM, Delis DC, Dean D, Beckman V, Miller BL, Rosen HJ, Kramer JH. Left frontal lobe contributions to concept formation: A quantitative MRI study of performance on the Delis-Kaplan Executive Function System Sorting Test. Journal of Clinical and Experimental Neuropsychology. 2009;31:624–631. doi: 10.1080/13803390802419017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser F, Brandmeir NJ, Kwong LK, Martinez-Lage M, Elman L, McCluskey L, Xie SX, Lee VM-Y, Trojanowski JQ. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Archives of Neurology. 2008;65:636–641. doi: 10.1001/archneur.65.5.636. [DOI] [PubMed] [Google Scholar]
- Geser F, Martinez-Lage M, Robinson J, Uryu K, Neumann M, Brandmeir NJ, Xie S, Kwong LK, Elman L, McCluskey L, Clark CM, Malunda J, Miller BL, Zimmerman E, Qian J, Van Deerlin V, Grossman M, Lee VM-Y, Trojanowski JQ. The clinical and pathological continuum of multisystem TDP-43 proteinopathies. Archives of Neurology. 2009;66:180–189. doi: 10.1001/archneurol.2008.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannetti T, Lamar M, Cloud BS, Swenson R, Fein D, Kaplan E, Libon DJ. Different underlying mechanisms for deficits in concept formation in dementia. Archives of Clinical Neuropsychology. 2001;16:547–560. [PubMed] [Google Scholar]
- Grant DA, Berg EA. A behavioral analysis of degree of impairment and ease of shifting to new responses in a Weigl-type card sorting problem. Journal of Experimental Psychology. 1948;39:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- Goldstein K, Scheerer M. Abstract and concrete behavior. Psychological Monographs. 1941;53:329–401. [Google Scholar]
- Grossman M, Anderson C, Khan A, Avants B, Elman L, McCluskey L. Impaired action knowledge in amyotrophic lateral sclerosis. Neurology. 2008;71:1396–1401. doi: 10.1212/01.wnl.0000319701.50168.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D, Patterson K. Pyramid and Palm Trees. Bury St. Edmonds, UK: Thames Publishing Co; 1992. [Google Scholar]
- Hu WT, Seelaar H, Josephs KA, Knopman DS, Boeve BF, Sorenson EJ, McCluskey L, Elman L, Schelhaas HJ, Parisi JE, Kuesters B, Lee VM-Y, Trojanowski JQ, Petersen RC, van Swieten JC, Grossman M. Survival profiles of patients with frontotemporal dementia and motor neuron disease. Archives of Neurology. 2009;66:1359–1364. doi: 10.1001/archneurol.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin D, Lippa CF, Swearer JM. Cognition and Amyotrophic Lateral Sclerosis (ALS) American Journal of Alzheimer’s Disease and Other Dementias. 2007;22:300–312. doi: 10.1177/1533317507301613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Kim J, Avants B, Patel S, Whyte J, Coslett BH, Pluta J, Detre JA, Gee JC. Structural consequences of diffuse traumatic brain injury: A large deformation tensor-based morphometry study. Neuroimage. 2008;39:1014–1026. doi: 10.1016/j.neuroimage.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang M, Christensen GE, Collins L, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libon DJ, McMilan C, Powers C, Massimo L, Khan A, Morgan B, Farag C, Richmond L, Weinstein J, Moore P, Coslett HB, Chatterjee A, Aguirre G, Grossman M. Neurocognitive contributions to verbal fluency deficits in Frontotemporal Lobar Degeneration. Neurology. 2009;73:535–542. doi: 10.1212/WNL.0b013e3181b2a4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomen-Hoerth C. Characterization of Amyotrophic Lateral Sclerosis and Frontotemporal Dementia. Dementia and Geriatric Cognitive Disorders. 2004;17:337–341. doi: 10.1159/000077167. [DOI] [PubMed] [Google Scholar]
- Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK, Miller BL. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology. 2003;60:1094–1097. doi: 10.1212/01.wnl.0000055861.95202.8d. [DOI] [PubMed] [Google Scholar]
- Mantovan MC, Baggio L, Dalla Barba G. Memory deficits and retrieval processes in ALS. European Journal of Neurology. 2003;10:221–227. doi: 10.1046/j.1468-1331.2003.00607.x. [DOI] [PubMed] [Google Scholar]
- Milton FN, Longmore CA, Wills AJ. Processes of overall similarity sorting in free classification. Journal of Experimental Psychology: Human Perception and Performance. 2008;34:676–692. doi: 10.1037/0096-1523.34.3.676. [DOI] [PubMed] [Google Scholar]
- Milton FN, Wills AJ. The influences of stimulus properties on category construction. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30:407–415. doi: 10.1037/0278-7393.30.2.407. [DOI] [PubMed] [Google Scholar]
- Milton FN, Wills AJ, Hodgson TL. The neural basis of overall similarity and single-dimension sorting. Neuroimage. 2009;46:319–326. doi: 10.1016/j.neuroimage.2009.01.043. [DOI] [PubMed] [Google Scholar]
- Minnerop M, Specht K, Ruhlmann J, Grothe C, Wüllner U, Klockgether T. In vivo voxel-based relaxometry in amyotrophic lateral sclerosis. Journal of Neurology. 2009;256:28–34. doi: 10.1007/s00415-009-0947-6. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Trojanowski JQ, Grossman M, Miller B, Dickson D, Albert M. Clinical and pathological diagnosis of frontotemporal dementia: Report of work group on frontotemporal dementia and Pick’s disease. Archives of Neurology. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzchmar HA, Trojanowski JQ, Lee VMY. Ubiquinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurology. 2007;11:994–1003. doi: 10.1016/S1474-4422(07)70265-X. [DOI] [PubMed] [Google Scholar]
- Ricker JH, Axelrod BN. Analysis of an oral paradigm for the Trail Making Test. Assessment. 1994;1:47–52. doi: 10.1177/1073191194001001007. [DOI] [PubMed] [Google Scholar]
- Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology. 2005;65:586–590. doi: 10.1212/01.wnl.0000172911.39167.b6. [DOI] [PubMed] [Google Scholar]
- Spreen O, Struss E. A Compendium of Neuropsychological Tests. New York: Oxford University Press; 1990. [Google Scholar]
- Stepniewska I, Friedman RM, Gharbawie OA, Cerkevich CM, Roe AW, Kaas JH. Optical imaging in galagos reveals parietal-frontal circuits underlying motor behavior. Proceedings of the National Academy of Science. 2011;108:E725–732. doi: 10.1073/pnas.1109925108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MJ, Grace GM, Orange JB, Leeper HA, Menon RS, Aere C. A prospective study of cognitive impairment in ALS. Neurology. 1999;53:1665–1670. doi: 10.1212/wnl.53.8.1665. [DOI] [PubMed] [Google Scholar]
- Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, Murphy J, Shoesmith C, Rosenfeld J, Leigh PN, Bruijn L, Ince P, Figlewicz D. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis. 2009;10:131–146. doi: 10.1080/17482960802654364. [DOI] [PubMed] [Google Scholar]
- Talbot PR, Goulding PJ, Lloyd JJ, Snowden JS, Neary D, Testa HJ. Interrelation between “classic” motor neuron disease and frontotemporal dementia: Neuropsychological and single photon emission computed tomography study. Journal of Neurology, Neurosurgery, and Psychiatry. 1995;58:541–547. doi: 10.1136/jnnp.58.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpato C, Piccione F, Silvoni S, Cavinato M, Palmieri A, Meneghello F, Birbaumer N. Working memory in amyotrophic lateral sclerosis: auditory event-related potentials and neuropsychological evidence. Journal of Neurophysiology. 2010;27:198–206. doi: 10.1097/WNP.0b013e3181e0aa14. [DOI] [PubMed] [Google Scholar]
- Yan C, He Y. Driving and driven architectures of directed small-world human brain functional networks. PLoS One. 2011;6:e23460. doi: 10.1371/journal.pone.0023460. Epub 2011 Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]


