Abstract
The mechanisms driving dormancy of disseminated tumor cells (DTCs) remain largely unknown. Here, we discuss experimental evidence and theoretical frameworks that support three potential scenarios contributing to tumor cell dormancy. The first scenario proposes that DTCs from invasive cancers activate stress signals in response to the dissemination process and/or a growth suppressive target organ microenvironment inducing dormancy. The second scenario asks whether therapy and/or micro-environmental stress conditions (e.g. hypoxia) acting on primary tumor cells carrying specific gene signatures prime new DTCs to enter dormancy in a matching target organ microenvironment that can also control the timing of DTC dormancy. The third and final scenario proposes that early dissemination contributes a population of DTCs that are unfit for immediate expansion and survive mostly in an arrested state well after primary tumor surgery, until genetic and/or epigenetic mechanisms activate their proliferation. We propose that DTC dormancy is ultimately a survival strategy that when targeted will eradicate dormant DTCs preventing metastasis. For these non-mutually exclusive scenarios we review experimental and clinical evidence in their support.
1 Introduction
Metastasis is responsible for the vast majority of cancer-related deaths. However, our understanding of this complex process is still vastly limited and so are our opportunities to prevent metastatic development. There are fundamental questions that remain mostly unanswered in this field: How does early dissemination start and what are the mechanisms? How does the tumor microenvironment aid this process? Are primary tumor niches responsible for programming DTCs to growth or quiesce at target organs? What role does the microenvironment of the metastatic niche play in determining the timing or extent of DTC dormancy? These questions have no or only partial answers.
The seed and soil theory of metastasis proposes that there is a match between the disseminated tumor cells (DTCs—the seeds) and the target organ (the soil) in which they can grow into overt lesions [1]. This is so because there is a relatively predictable pattern of target organ metastasis depending on the tissue origin of the primary tumor. While this is true, the timing of metastasis is difficult to predict because even in the matching sites, it can take a long time, sometimes decades, for metastases to develop [1]. It is thought that these long periods of clinical remission can be explained by minimal residual disease (i.e. DTCs) entering a non-productive or dormant state [1, 2].
In patients, DTCs that are not proliferating can be found in sites where they usually form secondary lesions or in sites where they never do [1]. Thus despite being able to disseminate these DTCs are “growth-suppressed” by certain organ microenvironments. Insight into these mechanisms might provide new pathways that if modulated could maintain DTCs dormant or eliminate them by blocking their survival pathways. This might also allow determining whether patients have dormant disease or not.
Several mechanisms are proposed to explain clinical dormancy. The lack of proliferation markers in surviving DTCs obtained from patients and experimental studies suggest that solitary DTC dormancy might be controlled by mechanisms of quiescence [1], a reversible growth arrest that can be brought about by different signals [3]. Angiogenic dormancy or immune system-mediated tumor mass dormancy might also be responsible for maintaining residual disease dormant [4, 5]. In this chapter we will review themes related to how solitary DTC fate is influenced by tumor-host interactions occurring in primary tumors and target organs. These two microenvironments are intimately interconnected by the biology of DTCs. Here, we will navigate three potential scenarios that might explain DTC dormancy. In the first, (Fig. 1) DTCs from invasive cancers activate stress signals in response to the dissemination process and/or a growth suppressive target organ microenvironment, inducing dormancy [1]. The second scenario (Fig. 2) proposes that therapy and/or microenvironmental stress conditions (e.g. hypoxia) acting on primary tumor cells carrying specific gene signatures prime new DTCs to enter dormancy [6, 7]. Here, specific primary tumor “stress microenvironments” might trigger long-term dormancy of DTCs. In the third scenario, lesions pathologically defined as noninvasive carry cells able to undergo micro-invasion and disseminate. Here, although these DTCs were able to intravasate they are unfit for expansion in secondary sites but they survive mostly arrested and perhaps with occasional cell division they progress via epigenetic and genetic pathways to a fully metastatic cell able to grow in secondary sites. We propose that DTC dormancy is ultimately a survival strategy that when blocked will eradicate dormant DTCs.
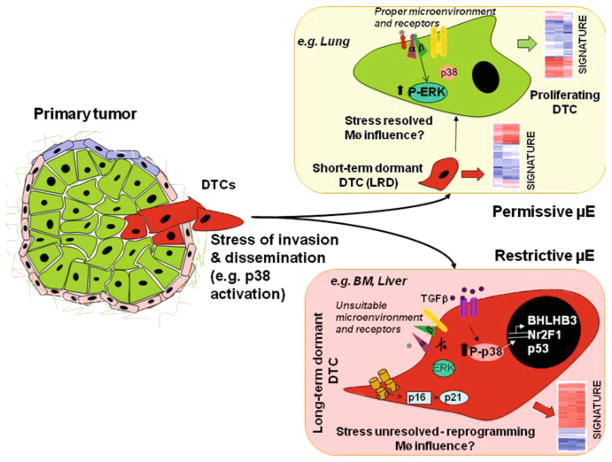
Fig. 1.
Scenario 1 envisions that the target organ microenvironment (μE) has instructive signals that determine the fate of disseminated tumor cells (DTCs) that have already been influenced by primary tumor microenvironments and stress of dissemination. Upon arrival at the secondary sites, cells can encounter two different situations: In a permissive microenvironment, (e.g. lungs), DTCs undergo a transient phase of dormancy, but interactions with the favorable microenvironment and appropriate tumor cell surface receptors will allow DTCs to adapt, integrate growth-promoting signals, such as those derived from fibronectin to transduced by the urokinase receptor (uPAR—red)–α5β1–integrin complex (green and purple) and the epidermal growth factor receptor (EGFR—yellow) which will result in activation of mitogenic signaling (activation of ERK, inactivation of p38) promoting DTCs proliferation. In the second situation DTCs will arrive to a restrictive microenvironment (e.g. bone marrow or liver), and either the loss of the surface receptors mentioned above will lead to inactivation of proliferative signals or interaction with growth-restrictive signals such as collagen-I results in stress signaling and activation of p38. This in turn leads to a prolonged dormancy. Activation of p38 could lead to the transcriptional induction of BHLHB3, NR2F1 and p53. Additionally, collagen-mediated activation of DDR2 can lead to subsequent p16- and p21-mediated tumor cell growth arrest. Furthermore, increased levels of TGFβ in the microenvironment (BM, for example) could also have a growth suppressive role. It is likely that other unidentified pathways are also involved in determining the DTC cell fate upon arrival at the secondary site. Overall, these signals might lead to specific gene expression profiles that could be derived from DTCs and used to classify patients (heat maps). Stromal cells such as machropages may also influence the choices between proliferation and dormancy, but these mechanisms have not been fully explored yet
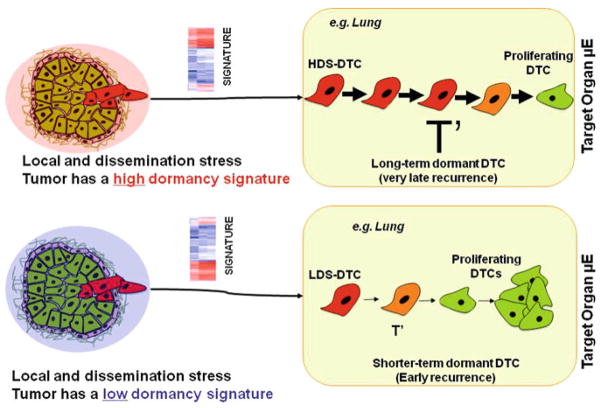
Fig. 2.
Scenario 2 envisions that the primary tumor microenvironment (μE) (e.g. hypoxic, collagen dense) can influence the fate of DTCs. The presence of a high (red shade—top) or low (blue shade—bottom) dormancy score signatures encoded in the bulk of the tumor predisposes cells to enter prolonged dormancy (large T′) or after a brief quiescence (small T′) resume proliferation, respectively. PT primary tumor. DTC disseminated tumor cell. HDS high dormancy score. LDS low dormancy score
2 Theoretical Considerations and Evidence for the Potential Scenarios of Tumor Dormancy
2.1 Scenario 1: The Target Organ Microenvironment as a Determinant of DTC Dormancy
Solitary DTCs in target organs can establish interactions with the extracellular matrix (ECM), immune cells as well as blood vessels in the stroma [8]. This and the distinct and predictable pattern of metastasis proposed by the seed and soil theory suggests that the target organ microenvironment can determine metastatic growth versus dormancy [1]. Studies on breast cancer cell lines specifically selected for vigorous growth in target organs via direct delivery to circulation identified gene expression programs that favor an organ-specific colonization [9]. On the contrary, some genes like the metastasis suppressor gene MKK4, through the activation of p38, mediates suppression of metastases [10] and this seems to respond to microenvironmental stress signals [11]. MKK4 belongs to a growing number of genes that selectively block growth at secondary sites and they include KISS1, MKK6, BHLHLB3/Sharp-1 and Nm23-H1 among others [11]. For a comprehensive review see [11]. These genes may inhibit metastasis by inducing DTC growth arrest [11]. That these genes do not suppress primary tumor growth but do suppress growth of DTCs at target organs further argues that these microenvironments provide a context where these genes now become functional.
In squamous carcinoma cells (HEp3) it was shown that reduced urokinase (uPA) receptor (uPAR) expression deactivated α5β1-integrins and this made these cells incapable of binding efficiently to fibronectin (Fig. 1) [12]. This resulted in reduced FAK and EGFR signaling but also in p38 activation. Thus tumor cells that fail to establish appropriate interactions with the ECM may perceive this microenvironment to be growth restrictive and enter a quiescence state [1]. Other investigators have reproduced these findings showing that loss of β1-integrin or FAK signaling in breast cancer models can also induce dormancy and that Src-MLKC signaling can prevent dormancy onset [1, 13]. In addition, an enriched collagen-I microenvironment in the lung can trigger intravenously delivered tumor cells to exit from dormancy as solitary cells [13]. On the other hand, environments rich in fibrillar collagen-I can induce quiescence of melanoma cells via activation of the discoidin domain receptor 2 and p15INK4b induction [14]. These results imply that stress signaling induced either by therapies or by a restrictive (i.e. fibrotic or non-fibrotic target tissues depending on the tumor type) tissue microenvironment could activate dormancy (or its interruption) in DTCs.
In the HEp3 system activation of p38α/β while inhibiting ERK1/2 signaling, activates a stress adaptive response known as the unfolded protein response (UPR) [15–17]. These signals lead to an epigenetic reprogramming and induction of survival and quiescence of dormant HEp3 (D-HEp3) cells [18]. D-HEp3 cells inoculated in vivo enter a deep G0–G1 arrest characterized by p21, p27, p18 and p15 induction [15]. At least 3 transcription factors (TFs), p53, BHLHB3/41/Sharp1, NR2F1 were regulated by p38α/β and required for dormancy of tumor cells in vivo [15]. This program is also activated in dormant DTCs recovered from the bone marrow (BM) but it is reversed when tumor cells exit from dormancy or grow persistently in lungs (our unpublished results). Bone marrow derived dormant HEp3 cells displayed a low ERK/p38 signaling ratio and induction of BHLHB3/41/Sharp-1, NR2F1 and p53. Interestingly, metastasis suppressor genes (MSGs) like MKK4 and MKK6 are upstream activators of p38 [11], BHLHB3 is a target of p38 required for quiescence induction [15] (see below) and Nm23-H1 appears to function via the downregulation of EDG2 LPA receptor a strong activator of ERK1/2 [19]. Thus, it seems that different mechanisms might converge on the regulation of the ERK/p38 signaling ratio.
Can the target organ microenvironment where DTCs lodge activate these dormancy programs? In tumors like HNSCC and breast cancer bone metastasis occurs at a frequency of 10–30% [20–22]. However, the detection of BM DTCs is much higher (>50% of patients) [23, 24]. This suggests that not all DTCs go on to form metastasis and/or that a delay takes place. In mouse models of cancer (xenografts or transgenic), BM carcinosis or metastasis are rarely observed. For example, in MMTV-Neu transgenic mice, BM DTCs are readily detected but mice never develop bone metastasis [25] (see also Scenario 3). However, if the BM microenvironment is modified, via irradiation [25] or if p38 is systemically inhibited, now DTCs expand ([25] and our unpublished data). T-HEp3 squamous carcinoma cells spontaneously disseminate from primary tumors to lungs, lymph nodes (LN) [26], liver and BM, but only in lung and LN they develop overt metastasis [26, 27]. Instead, in BM, spleen and liver DTCs remain in small numbers (<100 DTCs/106 marrow cells). Importantly, systemic p38 inhibition drastically changed this behavior and after a 3-week treatment with p38 inhibitors now DTCs, micro and macro-metastasis, were found in places where they never grow including liver and spleen (our unpublished data). Thus, in certain organs restrictive signals mediated at least by p38α/β signaling can prevent occult DTCs from expanding.
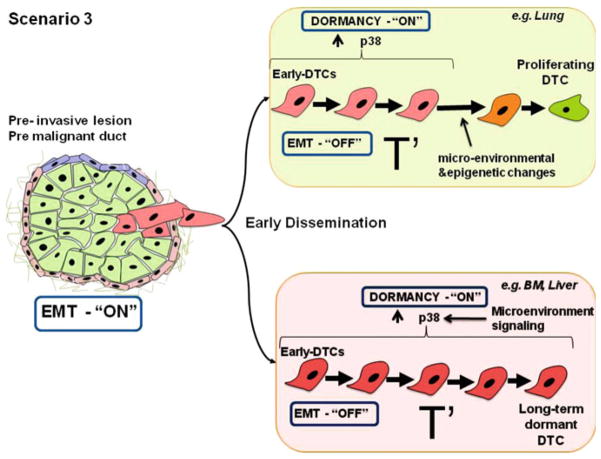
Fig. 3.
Scenario 3 envisions that in “pre-malignant” cells, from pre-invasive lesions, p38 signaling is downregulated favoring an EMT and early dissemination to target organs (e.g., lung and bone marrow). However, these early DTCs are unfit to initiate metastatic growth, thus, once these early DTCs arrive to secondary organs a dormancy program is turned “ON” possibly mediated by plastic regulation of p38 that is restored by microenvironmental signals. Therefore, early DTCs will undergo a long dormancy phase before forming metastasis. This is deduced from the long time to metastasis development after primary lesion treatment. As described in Scenario 1, DTCs that lodge in favorable microenvironments, such as lungs, will be exposed to microenvironmental and epigenetic changes that will allow early DTCs to grow and accumulate additional genetic alterations that eventually produce cells fit to initiate metastasis. In contrast, DTCs that lodge in unfavorable microenvironments, such as BM or liver, will remain dormant. Early dissemination will produce long dormancy periods at any site because cells have to accumulate additional changes. But it is possible that in certain sites microenvironmental and epigenetic changes allow cells to initiate slow proliferation that then drives genomic instability when vigorous growth is initiated
The search for signaling mediators that might induce dormancy in the BM suggest that TGFβ, which is rich in the BM microenvironment [28–31], might be important in dictating DTC dormancy. Although tumors might depend on TGFβ to metastasize [6, 7], this ligand can, depending on the degree of progression of tumors, be a potent inhibitor of epithelial tumor cell proliferation [32, 33]. TGFβ is also required to maintain the quiescence of stem cells and progenitors in the BM [28–31]. Thus, some tumors may remain sensitive to TGFβ growth inhibition in microenvironments where this factor is present (i.e. BM) [34]. It is still unknown whether during disease-free periods TGFβ maintains dormancy of DTCs. D-HEp3 cells express high levels of TGFβ2 mRNA and BHLHB3 is also induced by TGFβ2 (our unpublished results). Interestingly, BHLHB3 was also found to be upregulated by TGFβ and function as a metastasis suppressor in MDA-MB-231 breast cancer cells when the mutant p53 function present in these cells was eliminated [35]. Thus, enhanced paracrine/autocrine TGFβ signaling might contribute to dormancy in target organs if tumor cells have not subverted the pathways to stitch TGFβ signaling to be pro-growth and/or pro-invasion.
In melanoma progression a similar scenario develops. In early progressed melanoma TGFβ is anti-proliferative (tumor-suppressor), but in advanced melanoma it is pro-invasive [36–38]. How these two scenarios develop is not entirely clear [36–39]. It is possible that similar to the early dissemination in breast cancer (see Scenario 3) [40], melanoma might spread before the conversion from TGFβ-inhibitory phenotype to pro-invasive behavior is activated. Although counterintuitive, there is clinical evidence of early spread of uveal melanoma and, in a smaller proportion of patients, cutaneous melanoma thinner than 0.76 mm depth [41–43]. If true, then single cells arriving at distant sites, such as liver or BM [25] might remain in cell-cycle arrest due to high levels of TGFβ. Overall, these studies might identify therapeutic targets to induce or maintain dormancy or eradication of DTCs by targeting their survival signals or those provided by the microenvironment.
2.2 Scenario 2: Primary Tumor “Stress Microenvironments” Determine DTC Fate
In this section we review three lines of evidence on how the primary tumor might influence dormancy and progression toward metastasis. These include the gene signatures present in the primary tumors that predict the timing of metastasis development, the ability of tumor cells to return to the primary tumor to undergo further progression through self-seeding and the possibility that certain primary tumors may modulate the microenvironment of DTCs by instigating the mobilization of host cells (e.g. bone marrow derived cells) that can interact with and dictate the behavior of DTCs (Fig. 2).
Published data shows that gene signatures or even individual genes present in the primary tumors predict long-term metastatic relapse more than a decade later and in the absence of the primary tumor from which the signature derived [44, 45]. In some cases signatures from the surrounding microenvironment proximal to the tumor can also predict progression kinetics for patients [46]. In the case of hepatocellular carcinoma these signatures may inform about microenvironmental-favoring conditions for intrahepatic metastasis [47]. This suggests that a reciprocal influence of primary tumor and microenvironment in primary sites generates signatures that can dictate disease progression. Importantly, the majority of patients will undergo surgery and the deaths scored in Kaplan-Meyer curves are due to subsequent metastasis. One interpretation is that the gene signatures in the primary tumor and the microenvironment determine the fate of the DTCs. Since metastasis, once diagnosed show homogeneous progression (~2 years in breast cancer for example [48]), these data suggest that the gene signatures in the primary tumor not only inform about overt lesion biology but most likely about DTC survival, dormancy or proliferation. One additional interpretation of these data is that many of the gene signatures that predict for longer metastasis-free periods when a gene or a signature is present or absent most likely are providing information about how those individual or groups of genes influence dormancy of DTCs. We found that the dormancy signature identified in dormant D-HEp3 cells [15] when highly represented in estrogen receptor positive breast cancer invasive primary tumors (high dormancy score, HDS) predicted for longer metastasis-free periods (Kim, Aguirre-Ghiso and Segall, unpublished results). In contrast, when this signature was underrepresented (low dormancy score, LDS) patients recurred with metastasis more frequently [45, 49] (Kim, Aguirre-Ghiso and Segall, unpublished results). This strongly suggested that (1) while the signature genes do not affect primary tumor growth they might induce slower progression to metastasis possibly through dormancy of DTCs; (2) conditions in the primary tumor could influence the expression of these dormancy genes and “stress microenvironments” induced by hypoxia or therapies might induce a dormancy signature. Modeling how the genes in these signatures influence DTC survival and quiescence or subsequent recruitment of blood vessels or interaction with the immune system might reveal how they regulate dormancy and minimal residual disease biology. Importantly, determining whether signatures derived from CTCs (i.e. recently intravasated tumor cells) are more or equally informative to the primary tumor signatures might further inform about the relevance of characterizing CTCs versus DTCs (i.e. CTCs that are already lodged and reside in target organs).
As mentioned above it has been proposed that CTCs might return to the primary tumor in a self-seeding process and this helps “breed” more aggressive variants that colonize target organs [50]. These studies showed that aggressive variants of MDA-MB-231 breast cancer cells (MDA-MB-231-LM2) were highly efficient in disseminating and cross-seeding contra lateral tumors. The less aggressive variants of different cancer cell lines were less efficient in the seeding self/cross-seeding process. These data suggest that development of a more aggressive metastatic progeny requires the ability of primary tumors to attract their own CTCs back and the ability of these tumor cells to efficiently re-colonize the primary tumor. Transcriptional profiling of the isolated CTC population showed that these selected cells have gene signatures resembling those of bone, brain and lung metastatic populations, suggesting that the re-seeding might prime CTCs to acquire these gene signatures. However, the above described model did not demonstrate that DTCs from target organs can indeed seed back to the primary tumors. It is possible that these events take place when multiple metastases co-exist (for additional discussion see [51]). It will be important to determine whether patients from early and advanced lesions CTCs indeed already carry these same signatures. It will also be important to establish why the aggressiveness brought about by self- or cross-seeding takes place in patients where the primary tumors were removed and that develop metastasis years to decades after surgery of the primary tumor, which is required for the self-seeding process.
A third sub-scenario of primary tumor microenvironments influencing DTC fate includes the model of systemic tumor instigation. This hypothesis proposes that primary tumors can instigate the growth of otherwise-indolent tumor cells or micrometastases located at distant sites by mobilizing bone marrow cells (BMCs) into the stroma of the distant “indolent” lesions [52]. Although systems similar to the self-seeding model were used (MDA-MB-231 cells) the instigation model ruled out self- or cross-seeding, a discrepancy that remains unresolved. Nevertheless, McCallister and colleagues showed that instigating tumors secrete osteopontin that induces the expression of granulin by a specific subpopulation of hematopoietic cells in the host BM (Sca1+cKit–CD45+) [52]. These BMCs were activated and mobilized into the secondary sites where the responder tumors lay. There, they facilitated growth by inducing myofibroblast proliferation and thus creating a stroma supportive of tumor growth. These results suggest that growth and proliferation of poorly aggressive tumors (dormant DTCs and/or micrometastasis?) can be regulated on a systemic level by endocrine factors released by certain instigating tumors. However, like in the self-seeding model [50], it remains unclear how metastasis is instigated in the absence of a primary tumor and also the timing of these events in the experimental models does not explain why in patients that underwent surgery metastasis it take decades to develop.
Overall, these lines of evidence suggest that different mechanisms might “prime” tumor cells that exit the primary lesion to be productive and produce expanding metastasis with a predictable time lapse or non-productive [2]. The latter may be due to DTCs entering dormancy via quiescence or even if proliferative being more prone to be suppressed by the immune system or unable to recruit blood vessels (Fig. 2).
2.3 Scenario 3: Early Dissemination as a Determinant of DTC Dormancy
In this scenario we hypothesize that “pre-malignant” cells can readily undergo epithelial mesenchymal transition (EMT), making them invasive and facilitating early dissemination. However, we hypothesize that these early DTCs are not fully fit to initiate metastatic growth and thus undergo dormancy. For this to happen we propose that during these early progression stages the EMT is reversible and that upon arrival to the target organ, the stress signaling or suppressive signals from the microenvironment are reinstated to a level that prevents apoptosis but maintains quiescence of DTCs. We finally propose that micro-environmental, and epigenetic mechanisms that reverse the growth-restrictive signals and favor for example ERK1/2 activation [15] will allow early DTCs to grow and accumulate additional genetic alterations that eventually produce cells fit to initiate metastasis (Fig. 3).
Pre-malignant cells carrying specific genetic and epigenetic alterations are able to departure from early stage primary lesions and invade surrounding tissues. However, these accumulative modifications are not sufficient for these precursor cells to initiate proliferation enabling them to undergo a prolonged dormant state at secondary sites. Eventually, further subsequent genomic alterations will provide solitary dormant cells with proliferative capabilities and finally will resume metastatic growth [40]. What is the evidence supporting early dissemination? In breast cancer for example bone marrow (BM) DTCs are found in around 10–30% of breast cancer patients with noninvasive lesions (e.g., atypical ductal hyperplasia (ADH) or ductal carcinoma in situ (DCIS)) [40–53]. Their presence in BM correlates with poor prognosis [54]. Modeling these findings in mice showed that DTCs were detected in BM of MMTV-ErbB2 mice with “pre-malignant” lesions [25]. Interestingly, electron microscopy analysis revealed the presence of premalignant MECs breaching the basement membrane and this correlated with upregulation of Twist mRNA levels [25]. This suggests that pre-malignant cells can perhaps undergo an EMT and locally invade the surrounding stroma and intravasate to later become the founders of metastasis in visceral organs.
Specific gene expression signatures that predict for early dissemination have not been reported. However, some studies hint at certain genes as being (or not) modulators of this process. For instance, p53 mutations are not needed for early dissemination in breast cancer [55]. These data suggest that DTCs might still be under the control of this tumor suppressor and therefore prone to entering apoptosis, senescence or quiescence; only the latter endpoint would allow for dormancy unless senescence is reversible [56]. But it remains unknown whether early DTCs formally enter the dormancy mechanisms described above to subsequently found distant metastases.
Other data point to GATA-3 as a negative regulator of early dissemination [57]. GATA-3 loss was found to facilitate early dissemination and metastasis in a model of mammary hyperplasia [57]. In this work GATA-3 is lost in early DTCs lodged in lungs [57]. Thus, detection of GATA-3 loss in ADH or DCIS lesions may serve as a “test” to predict early dissemination.
Can stress-signaling pathways influence early dissemination and early DTC fate? There is evidence that GATA-3 nuclear translocation is stimulated by p38 [58, 59] suggesting that by up-regulating GATA-3, p38 could block dissemination in the pre-malignant epithelium. Our focus on the role of p38α/β stress signaling in dissemination and dormancy revealed that its pharmachological inhibition accelerates early breast tumor progression by inducing anoikis resistance [60] and an EMT. Our data show that systemic p38α/β inhibition strongly stimulated early dissemination and accumulation of CK8/18+/ErbB2+ cells in the BM of MMTV-ErbB2 mice. This correlated with a dramatic downregulation of E-cadherin and increased nuclear accumulation of β-catenin and ERK1/2 activation—all genes regulated during an EMT—in the MECs of MMTV-Neu pre-malignant mammary glands. Our data suggest that p38α/β might have a gatekeeper function at the dissemination steps by blocking an EMT and early dissemination of pre-malignant cells.
However, if early DTCs enter dormancy the EMT must be reversible or it may not influence the quiescence of these DTCs upon arrival to the target organ. Also, p38α/β signaling must be reinstated to a level that maintains quiescence of DTCs. Alternatively the p38 signaling threshold to prevent an EMT is lower than that required to repress proliferation. If so only transient or marginal inhibition of p38 signaling might allow for EMT and dissemination while still functioning as a growth suppressor.
Several additional questions arise from this potential dormancy scenario: Is it possible that early DTCs share the same dormancy gene signature as those DTCs released by invasive primary tumor mentioned in Scenario 2. Would this suggest that only early DTCs are responsible for recurrences? Why do larger tumors produce a similar DTC burden than smaller counterparts, but still have a worse prognosis? Are the same numbers of DTCs shed but their signatures are different? Which signals trigger the genetic switch responsible for metastatic growth? Do microenvironment (Scenario 1) and epigenetic signals precede genetic evolution of DTCs? Answers to these important questions will be critical for targeting and eliminating dormant DTCs before they can form lethal metastases.
3 Conclusions and Perspectives
Our knowledge on how the biology and genetics of DTCs influence dormancy and progression is limited. Even less understood is how these tumor cells from premalignant or invasive tumors are influenced by the primary tumor microenvironment composition, by the therapies applied to patients and by the target organ. Specifically for the topic of this book it seems that characterization of DTCs would be the most promising because they carry the aggregate information about their origin (i.e. primary tumor microenvironment), the ones that survive therapy will carry the information about how treatment influenced their adaptation and/or selection and ultimately how the target organ microenvironment also influenced their adaptation and/or selection. CTCs, because they are short-lived in circulation [61] and thus are considered only recently intravasated cells, would only carry information similar to the primary tumor and acutely influenced by therapy. Thus comparing CTC and DTC gene profiles as well as genetics will provide crucial information about whether they provide similar, complementary or different information about dormancy phases and subsequent progression to overt disease.
A final element not fully discussed here due to space limitations is how modulating host stromal cells might influence DTC dormancy. For example, the specific interaction of DTCs with macrophages at these sites might influence the decision to enter or escape dormancy. This is of importance because as recently reviewed, different populations of macrophages present in the primary tumor and lungs dictate metastatic dissemination and growth. The Condeelis and Pollard labs showed that a signaling relay is established between macrophages (CD11b+, F4/80+, CSF-1R+, Ly6G−) and breast tumor cells where the macrophage produces EGF, which in turn stimulates the tumor cell to produce CSF1, an activator of macrophages [62]. Macrophage produced EGF also drives intravasation [63]. But lung macrophages, defined by the markers indicated above and also VEGFR1+ and CCR2+ aid mammary tumor cell seeding at disseminated sites [64]. It will be important to determine whether the exit of dormancy was accompanied by a cross talk with the macrophages as this would reveal that macrophages support the exit of quiescence. These studies might reveal two additional scenarios: (1) macrophages that activate the signaling relay are only associated with DTCs actively proliferating; (2) macrophages are associated with dormant DTCs as well as with proliferative DTCs but the phenotype of these macrophages is different. These findings would perhaps identify novel “host-cell” targets to aid therapies aimed at the DTCs.
Overall the challenges presented by the problem of cancer dormancy are significant and studying DTCs and dormant disease is difficult. But the benefits of unraveling the inherent complexity of this step of metastasis biology should be of great impact for cancer patients.
Acknowledgments
This work is supported by grants from the Samuel Waxman Cancer Research Foundation Tumor Dormancy Program, NIH/National Cancer Institute (CA109182), NIEHS (ES017146) and NYSTEM to J.A.A-G.
Contributor Information
Paloma Bragado, Division of Hematology and Oncology, Department of Medicine, Department of Otolaryngology, Mount Sinai School of Medicine, Tisch Cancer Institute, Black Family Stem Cell Institute, New York, NY, USA.
Maria Soledad Sosa, Division of Hematology and Oncology, Department of Medicine, Department of Otolaryngology, Mount Sinai School of Medicine, Tisch Cancer Institute, Black Family Stem Cell Institute, New York, NY, USA.
Patricia Keely, Laboratory for Cellular and Molecular Biology, Department of Cell and Regenerative Biology, University of Wisconsin-Madison, R. M. Bock Laboratories, Room 227D 1525 Linden Drive, Madison, WI 53706, USA.
John Condeelis, Department of Anatomy and Structural Biology, Gruss Lipper Biophotonics Center, Albert Einstein Cancer Center, Tumor Microenvironment and Metastasis Program, New York, NY, USA.
Julio A. Aguirre-Ghiso, Email: julio.aguirre-ghiso@mssm.edu, Division of Hematology and Oncology, Department of Medicine, Department of Otolaryngology, Mount Sinai School of Medicine, Tisch Cancer Institute, Black Family Stem Cell Institute, New York, NY, USA. Division of Hematology and Oncology, Department of Medicine, Head and Neck Cancer Research Program, Department of Otolaryngology, Mount Sinai School of Medicine, New York, NY 10029, Box:1079, USA
References
- 1.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein CA. Framework models of tumor dormancy from patient-derived observations. Curr Opin Genet Dev. 2010;21(1):42–49. doi: 10.1016/j.gde.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Sang L, Coller HA, Roberts JM. Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science. 2008;321:1095–1100. doi: 10.1126/science.1155998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almog N, et al. Transcriptional switch of dormant tumors to fast-growing angiogenic phenotype. Cancer Res. 2009;69:836–844. doi: 10.1158/0008-5472.CAN-08-2590. [DOI] [PubMed] [Google Scholar]
- 5.Mahnke YD, Schwendemann J, Beckhove P, Schirrmacher V. Maintenance of long-term tumour-specific T-cell memory by residual dormant tumour cells. Immunology. 2005;115:325–336. doi: 10.1111/j.1365-2567.2005.02163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muraoka-Cook RS, et al. Conditional overexpression of active transforming growth factor beta1 in vivo accelerates metastases of transgenic mammary tumors. Cancer Res. 2004;64:9002–9011. doi: 10.1158/0008-5472.CAN-04-2111. [DOI] [PubMed] [Google Scholar]
- 7.Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 8.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 10.Hickson JA, et al. The p38 kinases MKK4 and MKK6 suppress metastatic colonization in human ovarian carcinoma. Cancer Res. 2006;66:2264–2270. doi: 10.1158/0008-5472.CAN-05-3676. [DOI] [PubMed] [Google Scholar]
- 11.Taylor J, Hickson J, Lotan T, Yamada DS, Rinker-Schaeffer C. Using metastasis suppressor proteins to dissect interactions among cancer cells and their microenvironment. Cancer Metastasis Rev. 2008;27:67–73. doi: 10.1007/s10555-007-9106-7. [DOI] [PubMed] [Google Scholar]
- 12.Aguirre Ghiso JA, Kovalski K, Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol. 1999;147:89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barkan D, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ossowski L, Aguirre-Ghiso JA. Dormancy of metastatic melanoma. Pigment Cell Melanoma Res. 2010;23:41–56. doi: 10.1111/j.1755-148X.2009.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adam AP, et al. Computational identification of a p38SAPK-regulated transcription factor network required for tumor cell quiescence. Cancer Res. 2009;69:5664–5672. doi: 10.1158/0008-5472.CAN-08-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranganathan AC, Ojha S, Kourtidis A, Conklin DS, Aguirre-Ghiso JA. Dual function of pancreatic endoplasmic reticulum kinase in tumor cell growth arrest and survival. Cancer Res. 2008;68:3260–3268. doi: 10.1158/0008-5472.CAN-07-6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranganathan AC, Zhang L, Adam AP, Aguirre-Ghiso JA. Functional coupling of p38-induced up-regulation of BiP and activation of RNA-dependent protein kinase-like endoplasmic reticulum kinase to drug resistance of dormant carcinoma cells. Cancer Res. 2006;66:1702–1711. doi: 10.1158/0008-5472.CAN-05-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranganathan AC, Adam AP, Aguirre-Ghiso JA. Opposing roles of mitogenic and stress signaling pathways in the induction of cancer dormancy. Cell Cycle. 2006;5(7):729–735. doi: 10.4161/cc.5.16.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taghavi P, et al. In vitro genetic screen identifies a cooperative role for LPA signaling and c-Myc in cell transformation. Oncogene. 2008;27:6806–6816. doi: 10.1038/onc.2008.294. [DOI] [PubMed] [Google Scholar]
- 20.Harrison LB, Sessions RB, Ki-Hong W. A multidisciplinary approach. Wolters Kluwer-Lippincott Williams & Wilkins; Philadelphia: 2003. Head and neck cancer; p. 960. [Google Scholar]
- 21.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 22.Gath HJ, Brakenhoff RH. Minimal residual disease in head and neck cancer. Cancer Metastasis Rev. 1999;18:109–126. doi: 10.1023/a:1006268621730. [DOI] [PubMed] [Google Scholar]
- 23.Wikman H, Vessella R, Pantel K. Cancer micrometastasis and tumour dormancy. Apmis. 2008;116:754–770. doi: 10.1111/j.1600-0463.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 24.Klein CA. The direct molecular analysis of metastatic precursor cells in breast cancer: a chance for a better understanding of metastasis and for personalised medicine. Eur J Cancer. 2008;44(18):2721–2725. doi: 10.1016/j.ejca.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 25.Husemann Y, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Ossowski L, Russo H, Gartner M, Wilson EL. Growth of a human carcinoma (HEp3) in nude mice: rapid and efficient metastasis. J Cell Physiol. 1987;133:288–296. doi: 10.1002/jcp.1041330212. [DOI] [PubMed] [Google Scholar]
- 27.Aguirre-Ghiso JA, Ossowski L, Rosenbaum SK. Green fluorescent protein tagging of extracellular signal-regulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer Res. 2004;64:7336–7345. doi: 10.1158/0008-5472.CAN-04-0113. [DOI] [PubMed] [Google Scholar]
- 28.Fan X, et al. Transient disruption of autocrine TGF-beta signaling leads to enhanced survival and proliferation potential in single primitive human hemopoietic progenitor cells. J Immunol. 2002;168:755–762. doi: 10.4049/jimmunol.168.2.755. [DOI] [PubMed] [Google Scholar]
- 29.Fortunel N, et al. Release from quiescence of primitive human hematopoietic stem/progenitor cells by blocking their cell-surface TGF-beta type II receptor in a short-term in vitro assay. Stem Cells. 2000;18:102–111. doi: 10.1634/stemcells.18-2-102. [DOI] [PubMed] [Google Scholar]
- 30.Scandura JM, Boccuni P, Massague J, Nimer SD. Transforming growth factor beta-induced cell cycle arrest of human hematopoietic cells requires p57KIP2 up-regulation. Proc Natl Acad Sci U S A. 2004;101:15231–15236. doi: 10.1073/pnas.0406771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamazaki S, et al. TGF-beta as a candidate bone marrow niche signal to induce hematopoietic stem cell hibernation. Blood. 2009;113:1250–1256. doi: 10.1182/blood-2008-04-146480. [DOI] [PubMed] [Google Scholar]
- 32.Dumont N, Arteaga CL. Targeting the TGFbeta signaling network in human neoplasia. Cancer Cell. 2003;3:531–536. doi: 10.1016/s1535-6108(03)00135-1. [DOI] [PubMed] [Google Scholar]
- 33.Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 34.Hideshima T, Podar K, Chauhan D, Anderson KC. Cytokines and signal transduction. Best Pract Res Clin Haematol. 2005;18:509–524. doi: 10.1016/j.beha.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Adorno M, et al. A Mutant-p53/Smad complex opposes p63 to empower TGF beta-induced metastasis. Cell. 2009;137:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 36.Javelaud D, Alexaki VI, Mauviel A. Transforming growth factor-beta in cutaneous melanoma. Pigment Cell Melanoma Res. 2008;21:123–132. doi: 10.1111/j.1755-148X.2008.00450.x. [DOI] [PubMed] [Google Scholar]
- 37.Hussein MR. Transforming growth factor-beta and malignant melanoma: molecular mechanisms. J Cutan Pathol. 2005;32:389–395. doi: 10.1111/j.0303-6987.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- 38.Hsu MY, Rovinsky S, Penmatcha S, Herlyn M, Muirhead D. Bone morphogenetic proteins in melanoma: angel or devil? Cancer Metastasis Rev. 2005;24:251–263. doi: 10.1007/s10555-005-1575-y. [DOI] [PubMed] [Google Scholar]
- 39.Reed JA, et al. Cytoplasmic localization of the oncogenic protein Ski in human cutaneous melanomas in vivo: functional implications for transforming growth factor beta signaling. Cancer Res. 2001;61:8074–8078. [PubMed] [Google Scholar]
- 40.Schardt JA, et al. Genomic analysis of single cytokeratin-positive cells from bone marrow reveals early mutational events in breast cancer. Cancer Cell. 2005;8:227–239. doi: 10.1016/j.ccr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Zapas JL, et al. The risk of regional lymph node metastases in patients with melanoma less than 1.0 mm thick: recommendations for sentinel lymph node biopsy. J Am Coll Surg. 2003;197:403–407. doi: 10.1016/S1072-7515(03)00432-0. [DOI] [PubMed] [Google Scholar]
- 42.Gamel JW, George SL, Edwards MJ, Seigler HF. The long-term clinical course of patients with cutaneous melanoma. Cancer. 2002;95:1286–1293. doi: 10.1002/cncr.10813. [DOI] [PubMed] [Google Scholar]
- 43.Eskelin S, Pyrhonen S, Summanen P, Hahka-Kemppinen M, Kivela T. Tumor doubling times in metastatic malignant melanoma of the uvea: tumor progression before and after treatment. Ophthalmology. 2000;107:1443–1449. doi: 10.1016/s0161-6420(00)00182-2. [DOI] [PubMed] [Google Scholar]
- 44.Villanueva A, et al. New strategies in hepatocellular carcinoma: genomic prognostic markers. Clin Cancer Res. 2010;16:4688–4694. doi: 10.1158/1078-0432.CCR-09-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 46.Troester MA, et al. Activation of host wound responses in breast cancer microenvironment. Clin Cancer Res. 2009;15:7020–7028. doi: 10.1158/1078-0432.CCR-09-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Budhu A, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 48.Holzel D, Eckel R, Emeny RT, Engel J. Distant metastases do not metastasize. Cancer Metastasis Rev. 2010;29:737–750. doi: 10.1007/s10555-010-9260-1. [DOI] [PubMed] [Google Scholar]
- 49.Pawitan Y, et al. Gene expression profiling spares early breast cancer patients from adjuvant therapy: derived and validated in two population-based cohorts. Breast Cancer Res. 2005;7:R953–R964. doi: 10.1186/bcr1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim MY, et al. Tumor self-seeding by circulating cancer cells. Cell. 2009;139:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aguirre-Ghiso JA. On the theory of tumor self-seeding: implications for metastasis progression in humans. Breast Cancer Res. 2010;12:304. doi: 10.1186/bcr2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McAllister SS, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braun S, et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med. 2000;342:525–533. doi: 10.1056/NEJM200002243420801. [DOI] [PubMed] [Google Scholar]
- 54.Braun S, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 55.Offner S, et al. p53 gene mutations are not required for early dissemination of cancer cells. Proc Natl Acad Sci U S A. 1999;96:6942–6946. doi: 10.1073/pnas.96.12.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beausejour CM, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. Embo J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kouros-Mehr H, et al. GATA-3 links tumor differentiation and dissemination in a luminal breast cancer model. Cancer Cell. 2008;13:141–152. doi: 10.1016/j.ccr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maneechotesuwan K, et al. Suppression of GATA-3 nuclear import and phosphorylation: a novel mechanism of corticosteroid action in allergic disease. PLoS Med. 2009;6:e1000076. doi: 10.1371/journal.pmed.1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maneechotesuwan K, et al. Regulation of Th2 cytokine genes by p38 MAPK-mediated phosphorylation of GATA-3. J Immunol. 2007;178:2491–2498. doi: 10.4049/jimmunol.178.4.2491. [DOI] [PubMed] [Google Scholar]
- 60.Wen H-C, et al. p38alpha Signaling induces anoikis and lumen formation during mammary morphogenesis. Sci Signal. 2011;4:ra34. doi: 10.1126/scisignal.2001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stott SL, et al. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med. 2010;2:25ra23. doi: 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ojalvo LS, Whittaker CA, Condeelis JS, Pollard JW. Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. J Immunol. 2010;184:702–712. doi: 10.4049/jimmunol.0902360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wyckoff J, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–7029. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 64.Qian B, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]