Abstract
This work describes the efficacy of a solar-thermal powered autoclave used for the wet sterilization of medical instruments in off-grid settings where electrical power is not readily available. Twenty-seven trials of the solar-thermal powered system were run using an unmodified non-electric autoclave loaded with a simulated bundle of medical instruments and biological test agents. Results showed that in 100% of the trials the autoclave achieved temperatures in excess of 121°C for 30 minutes, indicator tape displayed visible reactions to steam sterilization, and biological tests showed that microbial agents had been eliminated, in compliance with the Centers for Disease Control and Prevention requirements for efficacious wet sterilization.
Introduction
Many global health problems are directly related to the lack of access to reliable electricity. Experts estimate that about 1.4 billion people do not have access to electricity,1 and another 1 billion have intermittent and oftentimes unaffordable access to electricity.2 Certain regions are particularly negatively impacted. For example, the International Energy Agency estimates that about 585 million people, or about 70% of those living in sub-Saharan Africa, do not have electricity—an especially acute problem in rural areas.3 South Asia has 493 million people living off-grid.3 The United Nations has identified the lack of energy services, including electricity for activities such as lighting, cooking, and motive power, as a major deterrent to socio-economic development and has made energy part of its Millennium Development Goals.4
Of particular concern is that the lack of access to electricity is associated with negative public health outcomes. Without electricity, there are significant hindrances to the effective provision of many health services5 such as the ability to properly refrigerate vaccines, perform diagnostics,6 provide indoor lighting, and—the focus of this project—to sterilize medical instruments. Without electricity, medical instrument sterilization using autoclaves, called wet sterilization, is compromised. The World Health Organization (WHO) reports that nosocomial (within the health facility) infections of patients and health care workers are linked to contaminated equipment and poor infection control practices,7 conditions that are especially prevalent in developing countries.8 In the Democratic Republic of Congo, the reuse of medical equipment without adequate sterilization at a prenatal women's clinic was linked to the transmission of filovirus infections.9 A study in Egypt found that the hepatitis C virus was passed through inadequately sterilized medical equipment.10 A study of three private clinics in Pakistan with a high prevalence of hepatitis C virus reported contaminated instruments and no facilities for heat sterilizing medical equipment.11
This work presents a solar-thermal powered autoclave system that is suitable for off-grid wet sterilization of medical instruments using unmodified and commercially available nonelectric autoclaves. Results show that such system is able to produce enough steam to sustain pressure and temperatures inside the autoclave at levels required by the Centers for Disease Control and Prevention (CDC) to ensure efficacious wet sterilization of medical instruments.12 This system offers a compelling solution for remote and off-grid communities, particularly rural areas of developing countries, which may not have reliable access to consumables such as gas or may have only very expensive gas, the current method used to power such autoclaves. The solar-thermal powered autoclave system bypasses the need for gas, using instead energy from sunlight. As a result, this solar-thermal system stretches the reach of wet sterilization by thermal autoclaves, a technology that has been proven to significantly reduce or eliminate contamination from unsterilized medical instruments, which in turn should lead to significant improvements in the quality of medical services provided to the people living in such settings.
Materials and Methods
Solar-thermal powered autoclave system.
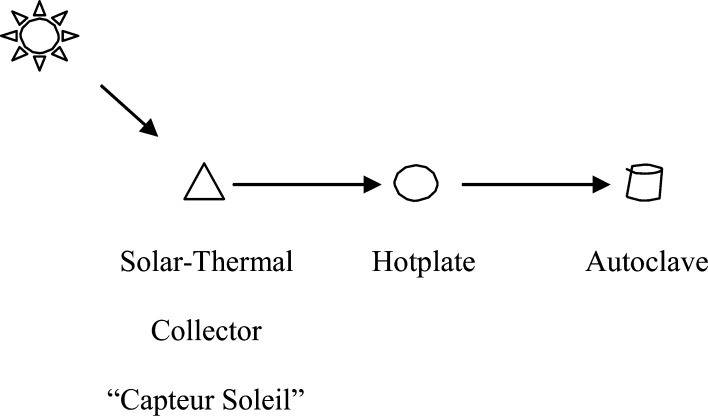
The solar-thermal powered autoclave system is composed of three main components, namely 1) the “Capteur Soleil” used to produce steam, 2) the “hotplate” used to harness the steam for productive work, and 3) the commercially available thermal autoclave used to sterilize medical instruments. Figure 1 shows a simple schematic of the system.
Figure 1.
Simple schematic of solar-thermal powered autoclave system.
Capteur Soleil.
Capteur Soleil is a solar-thermal collector that produces steam from solar energy. It was invented by Jean Boubour and his associates in the early 1990s and subsequently was granted a patent by the French government (FR2754592). The Capteur Soleil (Figure 2) consists of semi-parabolic mirrors of 2 m2 made of aluminum sheets that sit on a steel frame. A steel tube, called the boiler, sits at focal point, about 1.5 m above the mirrors. The steel frame is adjusted vertically with a hand operated jack to capture the maximum amount of sunlight (solar irradiance) on the mirrors, as indicated by a visual marker on the boiler. The boiler is filled with water by a small, hand operated pump that is then heated from the sunlight radiating onto the boiler. The boiler transfers that heat into the water by conduction, generating steam (thermal energy). The boiler commonly runs at a temperature of 150°C (maximum temperature is ∼164°C/6 bar), producing sufficient quantities of steam to heat a hotplate (more below) that in turn powers an autoclave for several hours at a time. The Capteur Soleil is normally affixed to the ground but could be placed on a terrace or a roof if sufficient sunlight is present and users can safely access it.
Figure 2.

Solar-thermal collector, Capteur Soleil.
Generally speaking, the best condition to operate the Capteur Soleil is when the solar flux is equal to or > 800 W/m2. This power is most likely available in places where cumulative annual solar irradiance is > 1,600 kWh/m2. Solar flux is affected by many factors, such as the distance of a given place is from the equator (latitude 0°) because sunlight becomes diffuse as it travels through the earth's atmosphere and local weather conditions including clouds and humidity.13
Hotplate.
The second component is a hotplate, shown in Figure 3. The hotplate extracts thermal energy from the steam produced by the Capteur Soleil to heat (by convection) a flat, aluminum plate. The hotplate consists of two sections and a gasket. The top section is an aluminum plate with a 5-mm-wide pathway etched into it. Steam produced by the Capteur Soleil circulates through this pathway and maximizes the surface area over which thermal energy may be transferred to the aluminum plate through forced conduction. The bottom section is another aluminum plate, to which brass fittings are affixed bringing steam into and condensed water out of the plate, controlled by a valve. A gasket between the plates provides an effective seal to prevent undesired steam leakage. The hotplate sits near the rear (non-sunny side) of the Capteur Soleil, either affixed to a shelf or sitting upon a small table, permitting easy access for the user. The hotplate serves two important functions. First, it maximizes heat transfer from the steam to the hotplate surface. Second, it is easy and safe for the user to operate. The autoclave (more below) is simply placed on top of the hotplate, similar to how an autoclave might be placed upon a gas-powered burner.
Figure 3.
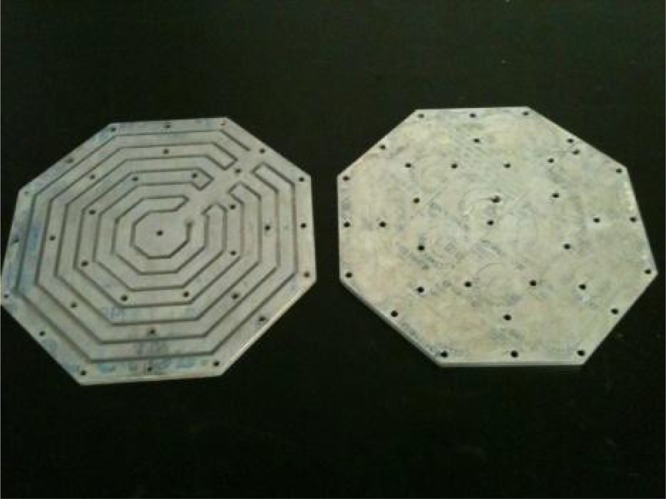
Hotplate. Top section (left) and bottom section (right).
Autoclave.
The autoclave used in this study is an unmodified thermal autoclave called All-American 1915X (non-electric sterilizer) produced by WAFCO of Manitowoc, Wisconsin. The gross capacity of the autoclave is 14 L. The hotplate can also power a 24 L autoclave such as All-American 1925X. Many health service providers currently use such autoclaves in off-grid locations of developing countries for wet sterilization; however, they are generally powered by gas (propane, butane, kerosene, etc.). In our arrangement, the autoclave rests on top of the hotplate with a teaspoon of cooking oil between the top of the plate and the bottom of the autoclave to ensure efficient energy transfer from the hotplate to the bottom of the autoclave. To minimize thermal loss, the autoclave is placed in an insulated box14† designed to permit easy access to the user, as shown in Figure 4.
Figure 4.

Autoclave housed within insulated box.
It is important to note that the water poured into the Capteur Soleil and the steam that flows from it into the hotplate are never in direct contact with the medical instruments inside of the autoclave.
Testing the system.
We sought to simulate the sterilization of a sample load of medical instruments in the field. For each run, we loaded the autoclave with several items: two aluminum rods (total weight is 3.2 kg) onto which autoclave indicator tape (Propper tape from Fisher HealthCare, Waltham, MA) was affixed, one dental instrument pouch containing 0.02 kg aluminum plate, and four biological agents test kits (Getinge Biosign SSI Test Pack or SGM Biotech EZ Test Self Contained Biological Indicators, Bozeman, MT).
Indicator tests.
We assessed the efficacy of the sterilization process for each run using three indicators. First, after venting the autoclave per the requirements of the manufacturer, we verified that the autoclave maintained a temperature of 121°C for 30 minutes. Second, after the sterilization process was complete and the pressure within the autoclave was reduced sufficiently for it to be safely opened, we observed whether the autoclave indicator tape had changed colors, thus indicating it had been exposed to steam at the appropriate temperature and for the appropriate length of time. Third, after the sterilization process was complete and the autoclave was opened, we used the biological agent kits to test for the presence or absence of microbial contamination.
Results
Between June 13 and August 3, 2011, 27 independent trials of the solar-thermal powered device coupled to the unmodified and commercially available autoclave were run. The All-American 1915X autoclave was used in all but two trials, where the larger capacity All-American 1925X autoclave was used. The run times to achieve proper sterilization varied in length depending on whether it was the first run of the day (the slowest because the entire system needs to heat up), the time of day (solar mid-day has the most solar radiation), and the climatic conditions for that day (presence of clouds and direct light intensity). The average time necessary for the initial run was 183 minutes, the second run was 101 minutes, and the third run was 93 minutes.
Table 1 reports the results of these tests for each of the trials. Data show that in all cases the solar-thermal powered autoclave system successfully led to sterilization as demonstrated by the temperature-time, autoclave indictor tape, and the biological agents test kit results. In no trial using indicators did the solar-thermal powered device fail to perform effective sterilization. To the best of our knowledge, this is the first report of sterilization tests using a solar powered autoclave that meet the sterilization requirements of the CDC.15
Table 1.
Results of the trials of the solar-thermal powered system with autoclave*
| Trial no. | Temperature > 121°C for 30 min† | Indicator tape‡ | Biological control test§ |
|---|---|---|---|
| 1 | Pass | Pass | Pass |
| 2 | Pass | Pass | Pass |
| 3 | Pass | Pass | Pass |
| 4 | Pass | ∥ | ∥ |
| 5 | Pass | Pass | Pass |
| 6 | Pass | Pass | Pass |
| 7 | Pass | Pass | Pass |
| 8 | Pass | Pass | Pass |
| 9 | Pass | Pass | Pass |
| 10 | Pass | ∥ | ∥ |
| 11 | Pass | Pass | Pass |
| 12 | Pass | Pass | Pass |
| 13 | Pass | Pass | Pass |
| 14 | Pass | Pass | Pass |
| 15 | Pass | Pass | Pass |
| 16 | Pass | Pass | Pass |
| 17 | Pass | Pass | Pass |
| 18 | Pass | Pass | Pass |
| 19 | Pass | Pass | Pass |
| 20 | Pass | Pass | Pass |
| 21 | Pass | Pass | Pass |
| 22 | Pass | Pass | Pass |
| 23 | Pass | ∥ | Pass |
| 24 | Pass | Pass | Pass |
| 25 | Pass | Pass | Pass |
| 26 | Pass | Pass | Pass |
| 27 | Pass | Pass | Pass |
Trials occurred between June 13 and August 3, 2011 in Houston, TX. The autoclave used was the All-American 1915X, except for trials 25 and 27 when the larger capacity 1925X autoclave was used.
The CDC recommended standard for effective sterilization in a wet sterilizer autoclave. “Pass” indicates that the reading on the autoclave's gauge was at or in excess of 121°C for 30 consecutive minutes.
Indicator tape (Propper brand from Fisher HealthCare) is designed to change colors when exposed to saturated steam at 121°C. “Pass” indicates that the tape changed colors.
Biological samples used were Getinge Biosign SSI Test Pack and SGM Biotech EZ Test Self Contained Biological Indicators. “Pass” indicates that the biological samples, once incubated, showed no bacterial growth after the autoclave sterilization run.
The testing materials were inadvertently left out.
Discussion
Comparison to current methods of sterilization in off-grid settings.
Although reported data are sparse, fieldwork, including that of our own,‡ highlights some of the most prevalent methods of sterilization in use today. Common methods used for sterilization in off-grid settings include boiling water, chemicals (predominantly liquid chlorine such as household bleach), and wet sterilization by autoclaves powered by gas burners. We note that many off-grid clinics do not regularly perform sterilization of medical instruments, oftentimes related to the lack of electricity or other sources of power.16
In terms of the efficacy of eliminating biological contaminants, wet sterilization using steam is superior to other sterilization techniques such as the use of soap, boiling water, and solar-produced dry heat.12 The CDC states that of all available methods for sterilization, moist heat in the form of saturated steam under pressure such as through an autoclave is the most dependable for killing microorganisms,17 which is the method of choice for this study. Chemicals can destroy a range of biological contaminants, but are less desirable as they require access to a supply chain because they are a consumable, may be hazardous to the operator, and after use must be disposed of in a safe fashion.18§ Other methods such as solar dry heat suffer from the slow rate of heat penetration and microbial killing and its lack of suitability for most materials.19
To the best of our knowledge, currently, there is only one proven option to successfully power a non-electric autoclave for wet sterilization off-grid: the use of a gas burner. To this short list, we now add our option: the solar-thermal powered system described in this article. Gas burners use tanks of propane, butane, kerosene, and other gases and are widely distributed globally. The initial costs are modest—ranging from about US$20 for a low quality burner to US$200 for a high quality burner. The cost of tanks of gas varies widely based upon the region. Additional costs of running a burner may include regulators, hoses, seals, and valves. Assuming the use of a good quality burner at US$88, consumption of 0.6L of kerosene per hour at an approximate cost of US$1.25/L,20,21 operation of the unit for 1,000 hours/year, and no additional expenses for the burners (such as a regulator that might break during use), the 5-year total cost of a gas burner powered system is ∼US$3,838. Our solar option has a higher up-front capital cost, ∼US$2,100 (to construct the prototype of the Capteur Soleil, hotplate, and insulated box). As the Capteur Soleil does not rely, however, upon the purchase of consumables, but instead relies upon sunlight, which is free, the total estimated cost of operation of the solar-thermal powered system over 5 years is about US$2,100, lower than that of gas burners.
In our opinion, the total operating cost is not the most important factor of comparison between the two modes of powering non-electric autoclaves. The most important factor is the lack of the availability of gas at many remote hospitals and clinics. Because tanks of gas are a consumable good, they require access to a reliable distribution network for replenishment, something that does not exist in many regions of the world, oftentimes because of poor transportation infrastructure.22 Furthermore, the cost of liquid fuels such as gas is generally expensive in these remote regions, especially true in sub-Sahara Africa.23¶ For the medical facilities sitting outside of a reliable supply chain and with poor-quality logistical infrastructure, the use of gas to operate autoclaves is not a feasible option—these facilities oftentimes simply do not have gas or, if available, it is far too expensive. The solar-powered system is most valuable in such settings because it side-steps entirely the need to procure consumable fuels. Under these conditions, the solar-thermal powered autoclave system becomes a desirable solution to provide effective wet sterilization using autoclaves.
We note, here, that our device can complement a gas-powered burner sterilization system. We envision certain settings with infrequent and unreliable access to gas or with expensive gas that would use both systems: using the solar-thermal system when sun is available and gas at other times. This might represent an ideal configuration for many off-grid health care facilities.
Potential sites for the solar-thermal powered autoclave system.
Although we have determined the site of the initial field test of our solar-thermal powered autoclave system (more below), we list here five criteria for ideal locations for the system, and briefly present several countries that might most benefit from the system. We believe that target characteristics of the system's location include: 1) large rural population to support small hospitals and clinics performing basic surgical procedures (i.e., maternal care, infant and early childhood care) where it might be expected to have basic surgical instruments and may have developed a cleaning and sterilization protocol; 2) lack of reliable connection to an electricity grid; 3) poor-quality logistical infrastructure such as unpaved roads; 4) a population that suffers from bad health outcomes; and 5) cumulative annual solar irradiance at a minimum of 1,600 k Wh/m2 to increase the likelihood of having many hours of efficient production of steam using solar energy.
Using the United Nation's 2011 Human Development Index (HDI), we created a table listing how the 10 lowest ranked countries fared on measures related to these five criteria. Table 2 suggests that there are opportunities to use our system within each of these countries. Across these 10 countries, we can identify over 100 million persons living in rural communities who live outside of power grids and have poor logistical connections to other places such as through dirt roads, and experiencing health outcomes in maternal and early childhood health lagging the averages for developing countries. Each of these countries is located in areas of the world with high levels of solar irradiance, suggesting that solar power could be used for many days of the year. We anticipate working with governments and global health organizations to locate specific sites—which we believe are plentiful—within this set of countries.
Table 2.
Characteristics of the 10 lowest ranked HDI countries as targets for off-grid wet sterilization using solar power
| Country | HDI rank* | Rural population (millions)† | Access to electricity %‡ | Use of solid fuels, rural areas %§ | Paved roads % of total roads¶ | Under-5 infant mortality rate (per 1,000 live births)∥ | Maternal mortality ratio (per 100,000 live births)** | Solar irradiance (annual average) |
|---|---|---|---|---|---|---|---|---|
| Congo | 187 | 42.7 | 11.1 | > 95 | 1.8 | 199 | 670 | 1,700–2,000 |
| Niger | 186 | 12.9 | n.a. | > 95 | 20.7 | 160 | 820 | 2,100–2,300 |
| Burundi | 185 | 7.5 | n.a. | > 95 | 10.4 | 166 | 970 | 1,700–1,900 |
| Mozambique | 184 | 14.4 | 11.7 | > 95 | 20.8 | 142 | 550 | 1,900–2,100 |
| Chad | 183 | 8.1 | n.a. | 94 | 0.8 | 209 | 1200 | 2,100–2,400 |
| Liberia | 182 | 1.5 | n.a. | > 95 | 6.2 | 112 | 990 | 1,800–2,000 |
| Burkina Faso | 181 | 13.1 | 14.6 | > 95 | 4.2 | 166 | 560 | 2,100–2,300 |
| Sierra Leone | 180 | 3.6 | n.a. | > 95 | 8.0 | 192 | 970 | 1,800–2,000 |
| Central African Republic | 179 | 2.7 | n.a. | > 95 | 2.7 | 171 | 850 | 2,000–2,200 |
| Guinea | 178 | 6.4 | n.a. | > 95 | 9.8 | 142 | 680 | 1,900–2,000 |
2011 rank. United Nations Development Program. International Human Development Indicators. http://hdr.undp.org/en/statistics/. Listed in descending order; 187 countries are ranked.
2010 figures. Estimated from data available from The World Bank. Population, Total: http://data.worldbank.org/indicator/SP.POP.TOTL; and Urban Population: http://data.worldbank.org/indicator/SP.URB.TOTL.IN.ZS.
2009 figures. The World Bank. Access to Electricity (% of population) http://data.worldbank.org/indicator/EG.ELC.ACCS.ZS/countries. n.a. = data are not available.
2010 figures. World Health Organization. World Health Observatory Data Repository: http://apps.who.int/ghodata/.
Various years (post-2000, except for 1998 for the CAR). United Nations. UNData. Roads, paved (% of total roads) http://data.un.org/Data.aspx?q=road&d=WDI&f=Indicator_Code%3aIS.ROD.PAVE.ZS.
2009 figures. World Health Organization. WHO Statistical Information System. World Health Statistics 2011, p. 24, Table 2. http://www.who.int/whosis/whostat/2011/en/whs2011_Full.pdf. Africa average is 127.
2008 figures. World Health Organization. WHO Statistical Information System. World Health Statistics 2011, Part I: Health-related Millennium Development Goals. P. 26, 4. Maternal mortality ratio (per 100,000 live births). http://www.who.int/whosis/whostat/2011/en/whs2011_Full.pdf. Africa average is 620. Average annual sum (kWh/m2), 2004–2010. GeoModel.Solar. 2011.
HDI = Human Development Index.
We are currently in the final phases of planning for our initial field test in Sierra Leone, one of these low ranked HDI countries. In partnership with the government of Sierra Leone, and two health services non-governmental organizations, Wellbody Alliance USA and World Missions Possible, our solar-thermal powered autoclave system will be installed at the Koidu Governmental Hospital in the eastern region of Sierra Leone. The hospital has a small operating room, employs a nurse technician for cleaning and sterilization using a gas-powered autoclave, and performs ∼10 to 20 procedures per week. However, their autoclave system is oftentimes broken. We plan to implement our system and train the users in late 2013. We have been informed that there are 13 similar district-level hospitals in Sierra Leone that might use our solar-thermal powered autoclave system pending the performance of the roll-out.
Limitations.
The solar-thermal powered autoclave system outlined here uses a relatively small solar collector. Although this sized system is able to successfully produce energy to operate the All American 1915X (14 L) and the All American 1925X (24 L) autoclaves, it may be undersized for larger autoclaves. We note that an identical but larger solar-thermal collector has been built at Université de Pau in France24 with a much greater output of thermal energy that might be used to run two or more autoclaves in parallel. As with other solar devices, the solar-thermal powered autoclave system works best in geographic areas enjoying high levels of solar irradiance. The system is limited to 6 hours of work per suitable sunny day, limiting the daily quantity of medical instruments that could be sterilized.
Conclusions
There is a felt need for providing proper medical instrument sterilization to underserved, off-grid communities in developing nations. The lack of access to reliable electricity contributes to poor health services outcomes for a very large number of people. In many rural areas of sub-Saharan Africa and South Asia, hundreds of millions of persons do not have access to electricity. As a result, medical services that require electricity—such as operating an autoclave for medical instrument wet sterilization—are compromised. Existing technologies for off-grid wet sterilization using autoclaves rely upon ready access to gas, a consumable, that is oftentimes unavailable or expensive in remote, off-grid communities because of poor-quality and unreliable logistical infrastructure. Our solar-thermal powered autoclave system, in contrast, is able to power such autoclaves using the sun, avoiding the reliance upon fuels that must travel through these inefficient and expensive distribution systems.
The results of the testing of the solar-thermal powered autoclave system presented here show it to be completely effective, per the CDC guidelines on wet sterilization, including against biological test agents. On the basis of our testing, we conclude that the solar-thermal powered autoclave system is well suited to apply an efficacious wet sterilization technology to an off-grid setting that generally lacks this capability. Our hope is that the presentation of cleaner and safer medical instruments to doctors and other health practitioners should in turn be associated with better health outcomes for the many patients living in these remote and off-grid communities.
ACKNOWLEDGMENTS
We thank Marcia O'Malley, Pauline Rosenau, Lauren Vestewig, Vladimir Novak, Joe Gesenhues, Claire Krebs, Sam Major, William Dunk, David Luker, Daniel Rist, Alexandra Ernst, Michael Heisel, Sabha Momin, and Kristen Hogan for their guidance and assistance. We also thank Maria Oden for permission to use the facilities of the Oshman Engineering Design Kitchen at Rice University for our testing. We are grateful to Rodney Gray of Getinge USA, Inc. for donating testing equipment and materials, Benjamin Jacobs of the Wisconsin Aluminum Foundry, Inc. for donating autoclaves, and Brian Payne and Will Pendleton of Houston Center Valve and Fitting (Swagelok) for donating valves, fittings, and other materials.
Footnotes
Financial support: This study was funded in part by grants from The Shell Center for Sustainability at Rice University, Jim and Linda Hargrove, and The Shell Oil Company Foundation.
Authors' addresses: Tremayne Kaseman and Jean Boubour, Rice University, Brown School of Engineering Houston, TX, E-mails: tremayne.kaseman@gmail.com and jean.boubour@orange.fr. Douglas A. Schuler, Jones Graduate School of Business, Rice University, Houston, TX, E-mail: schuler@rice.edu.
The box is constructed with thin plywood and polystyrene insulation. There is virtually no chance of a fire because the system does not use a flame (unlike a gas burner). The auto ignition temperature for pine wood is 427°C and for polystyrene is 490°C, significantly below the maximum temperature inside the box of ∼150°C.
Three Rice University engineering students, under our direction, conducted assessments of medical instrument sterilization protocols within three health facilities in rural Malawi during the summer of 2011.
Occupational diseases among cleaning and sterilization personnel are associated with the use of several chemical disinfectants, including chlorine (p. 38). The use of chlorine may produce ocular irritation and oropharyngeal, esophageal, and gastric burns (p. 40). Use and disposal of chlorine pose risks such as the release of toxic gases if chlorine is mixed with other chemicals such as ammonia or acids.
By region, sub-Saharan Africa has the highest gasoline and diesel prices in the developing world, a consequence of the landlocked nature of some of its countries, inadequate economies of scale in small markets, inadequate infrastructure for transporting fuels, rising demand for diesel to offset power shortages, and relatively high rates of taxation.
References
- 1.International Energy Agency World Energy Outlook 2010. 2010. http://www.worldenergyoutlook.org/ Available at. Accessed May 19, 2012.
- 2.United Nations Development Programme United Nations Development Programme, Energy for a Sustainable Future: The Secretary-General's Advisory Group on Energy and Climate Change Summary Report and Recommendations. 2010. http://www.un.org/wcm/webdav/site/climatechange/shared/Documents/AGECC%20summary%20report%5B1%5D.pdf Available at. Accessed July 11, 2012.
- 3.International Energy Agency World Energy Outlook 2011. 2011. http://www.worldenergyoutlook.org/ Available at. Accessed May 19, 2012.
- 4.UN-Energy The Energy Challenge for Achieving the Millennium Development Goals. 2005. http://www.unhabitat.org/downloads/docs/920_88725_The%20Energy%20challenge%20for%20achieving%20the%20millenium%20development%20goals.pdf Available at. Accessed July 11, 2012.
- 5.Guruswamy L. Energy poverty. Annu Rev Environ Resour. 2011;36:139–161. [Google Scholar]
- 6.Brown J, Theis L, Kerr L, Zakhidova N, O'Connor K, Uthman M, Oden ZM, Richards-Kortum R. A hand-powered, portable, low-cost centrifuge for diagnosing anemia in low-resource settings. Am J Trop Med Hyg. 2011;85:327–332. doi: 10.4269/ajtmh.2011.10-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization, Department of Communicable Disease, Surveillance and Response . Prevention of Hospital-Acquired Infections: A Practical Guide. Second edition. 2002. WHO/CDS/CSR/EPH/2002.12.http://www.who.int/csr/resources/publications/drugresist/WHO_CDS_CSR_EPH_2002_12/en/ Available at. Accessed July 11, 2012. [Google Scholar]
- 8.Rosenthal VD, Maki D, Salomao R, Alvarez-Moreno C, Mehta Y, Higuera F, Cuellar LE, Arikan OA, Abouqal R, Leblebicioglu H. International Nosocomial Infection Control Consortium. Device-associated nosocomial infections in 55 intensive care units of 8 developing countries. Ann Intern Med. 2006;145:582–591. doi: 10.7326/0003-4819-145-8-200610170-00007. [DOI] [PubMed] [Google Scholar]
- 9.Fisher-Hoch SP. Lessons from nosocomial viral hemorrhagic fever outbreaks. British Med Bull. 2005;73 and 74:123–137. doi: 10.1093/bmb/ldh054. [DOI] [PubMed] [Google Scholar]
- 10.Frank C, Geogr D, Mohamed MK, Strickland GT, Lavanchy D, Arthur RR, Magder LS, El Khoby T, Abdel-Wahab Y, Ohn ES, Anwar W, Sallam I. The role of parenteral antischistosomal therapy in the spread of hepatitis C virus in Egypt. Lancet. 2000;355:887–891. doi: 10.1016/s0140-6736(99)06527-7. [DOI] [PubMed] [Google Scholar]
- 11.Luby SP, Qumruddin K, Shah A, Omair A, Pahsa O, Khan AJ, McCormick JB, Hoodbhouy F, Fisher-Hoch S. The relationship between therapeutic injections and high prevalence of hepatitis C infection in Hafizabad, Pakistan. Epidemiol Infect. 1997;119:349–356. doi: 10.1017/s0950268897007899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Centers for Disease Control and Prevention Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. http://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf Available at. Accessed July 11, 2012.
- 13.Stoffel T, Renne D, Myers D, Wilcox S, Sengupta M, George R, Turchi C. Concentrating Solar Power: Best Practices Handbook. Washington, DC: U.S. Department of Energy; Golden, CO: National Renewable Energy Laboratories; 2010. Technical Report NREL/TP 550-47465. [Google Scholar]
- 14.The Engineering ToolBox, Fuels and Chemicals – Auto Ignition Temperatures 2012. http://www.engineeringtoolbox.com/fuels-ignition-temperatures-d_171.html Available at. Accessed May 16, 2012.
- 15. We have located three other solar-powered autoclaves: Sydney University (2003), MIT/Solarclave (2010), and University of Kansas (2011). To our knowledge, there are no published data about the efficacy of these devices to achieve wet sterilization per the CDC's guidelines.
- 16.The Forum of Energy Ministers of Africa Energy and the Millennium Development Goals in Africa. 2006. http://siteresources.worldbank.org/EXTAFRREGTOPENERGY/Resources/Energy_and_MilleniumFEMA_Report.pdf April. Available at. Accessed July 11, 2012.
- 17.U.S. Centers for Disease Control and Prevention Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. p. 58.http://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf Available at. Accessed July 11, 2012.
- 18.U.S. Centers for Disease Control and Prevention Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. Accessed July 11, 2012. http://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf Available at.
- 19.U.S. Centers for Disease Control and Prevention Guideline for Disinfection and Sterilization in Healthcare Facilities. 2008. pp. 68–69.http://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf Available at. Accessed July 11, 2012.
- 20.http://procurement.ifrc.org/catalogue/detail.aspx?itemcode=XSTEAUTO39BK%20%20%20&from=kit We identified the Prahbat Nr2 as an appropriate burner for an autoclave as it is listed for such a purpose on the web-site of the International Federation of the Red Cross and Red Crescent Societies. For prices and specifications about fuel consumption, see.
- 21.Fuel price of kerosene is estimated from the World Bank, Changes in End-User Petroleum Product Prices. Extractive Industries and Development Series #2. February 2009. Figure 3. Retail Prices of Kerosene, August 2008. Mozambique. p. 9. http://siteresources.worldbank.org/INTOGMC/Resources/ei_for_development_2.pdf Available at.
- 22.Ramachandra V. Power and Roads for Africa: What the United States Can Do. In: Birdsall N, editor. The White House and the World. Washington, DC: Center for Global Development; 2008. pp. 91–119. [Google Scholar]
- 23.World Bank Changes in End-User Petroleum Product Prices. 2009. http://siteresources.worldbank.org/INTOGMC/Resources/ei_for_development_2.pdf Extractive Industries and Development Series #2. February, 1. Available at. Accessed July 11, 2012.
- 24.Alaphilippe M, Bonnet S, Stouffs P. Low power thermodynamic solar energy conversion: coupling of a parabolic trough concentrator and an Ericsson engine. Int J Thermodynamics. 2007;10:37–45. [Google Scholar]