Abstract
The burden of dengue in Nicaragua has been steadily rising during the last three decades; however, there have been few efforts to quantify the burden (measured in disability-adjusted life years [DALYs]) and cost to society. Using primary data from the Nicaraguan Ministry of Health (MINSA), the total cost and burden of dengue were calculated from 1996 to 2010. Total costs included both direct costs from medical expenditures and prevention activities and indirect costs from lost productivity. The annual disease burden ranged from 99 to 805 DALYs per million, with a majority associated with classic dengue fever. The total cost was estimated to be US$13.5 million/year (range: US$5.1–27.6 million). This analysis can help improve allocation of dengue control resources in Nicaragua and the region. As one of the most comprehensive analyses of its type to date in Nicaragua and Latin America, this study can serve as a model to determine the burden and cost of dengue.
Introduction
Dengue fever (DF) is a flavivirus infection transmitted by the Aedes aegypti mosquito vector and is considered the most important arthropod-borne viral infection in humans.1,2 Worldwide, over 2.5 billion people are at risk for dengue infection; the number of cases is estimated between 50 and 100 million annually, resulting in up to 500,000 hospitalizations and ∼24,000 deaths, mostly in children.3–5 To date, there is no vaccine available to prevent dengue infection; as a result, efforts to reduce the burden of infection are focused on vector control and surveillance activities, as well as educational interventions.6
In Latin America, DF ranks as the fifth most important neglected tropical disease (NTD) according to the disability-adjusted life year (DALY) metric.7 In Nicaragua, dengue has emerged as one of the most important public health challenges since the first epidemic was reported in 1985.8 Since then, efforts have been made to characterize the epidemiology and burden of disease in Nicaragua, but few efforts have quantified the overall cost to society.8–10
Previously, Ferrando10 described the total cost of the 1994 epidemic in Nicaragua to be US$2.7 million, with an estimated 60,916 cases. Likewise, Shepard and others11 estimated the average cost of dengue to be US$144/ambulatory case and US$306/hospitalized case during 2000–2007. However, neither of these studies accounted for the costs associated with dengue prevention, such as vector control and surveillance activities. Previous studies have shown that these activities contribute significantly to the overall costs associated with dengue; such activities consisted of 43% of the overall cost in Panama,12 49% in Puerto Rico,13 and 39% in Thailand.14
In this analysis, we focused on dengue illness in Nicaragua from 1996 to 2010, a period in which the burden of dengue was rapidly on the rise, particularly in Latin America.15 We report the burden of disease using the DALY index, as described by Murray and others.16,17 The total cost of dengue was also determined, which we have defined in this analysis to include the direct medical and indirect costs associated with illness, and the costs associated with prevention and vector control efforts; not included are transportation costs and other out-of-pocket payments. This analysis represents one of the first efforts for a comprehensive examination of total costs of dengue to a specific country and this approach can be applied toward other nations in Latin America and throughout the world. Our aim was to expand upon previous assessments of the economic impact of dengue illness in Nicaragua using primary data and an increased investigation period. The results of this analysis could be valuable for allocating scarce resources toward vector control and for determining cost-effectiveness of novel vaccine candidates and other therapeutics.
Materials and Methods
Dengue data collection.
Data on the annual number of cases in Nicaragua were obtained from the Pan American Health Organization (PAHO), as reported by the Nicaraguan Ministry of Health (MINSA).18 In Nicaragua, MINSA is the primary provider of health care, and over 90% of health facilities are directly funded by the government.19 Dengue incidence data are collected by the regional health authorities from health posts and routine active surveillance, which are compiled by the national MINSA offices and subsequently reported to PAHO.
The MINSA definition of DF is a febrile illness characterized by headache, retro-orbital pain, myalgias, arthralgias, cutaneous eruptions, diarrhea, and a positive tourniquet test.8,20 The definition of dengue hemorrhagic fever (DHF) further includes hemorrhagic manifestations such as petechiae, epistaxis, metrorrhagia, and gingivorrhagia with evidence of pleural effusions, ascites, hemoconcentration 20% above normal, and thrombocytopenia with platelet count < 100,000/mm3.8,20 This MINSA definition is consistent with the PAHO definition for DF.
The PAHO data were classified by age group and category of illness (DF, DHF, and deaths); although laboratory-confirmed case data were available from 1996 to 2010, suspected case data were only available from 2004 to 2010. Suspected cases included those reported by practitioners at various clinics and hospitals throughout Nicaragua according to the MINSA case definition described previously, but where laboratory confirmation was not available. Confirmed cases are a subset of cases in which antibody detection using immunoglobulin M (IgM) enzyme-linked immunosorbent assay (ELISA) confirmation was performed on originally collected serum samples and were serologically positive.
Age-specific incidence data were available in the MINSA data set during the period of 2000–2008 and were used in the DALY calculation. An average age-specific case distribution was determined from these data and applied to the years in which no age-specific incidence data were available. Because of the completeness of the PAHO data and its standardized use in the literature, the PAHO data were used as the basis of this analysis. The MINSA data served to establish these annual age-specific case distributions, to determine the expansion factors used in this analysis to account for the large portions of underreported and asymptomatic cases, and to inform the length of stay for hospitalized cases, which were also compared with the published literature.9,21,22 The MINSA dataset also yielded information about the ratio of total number of suspected dengue cases to confirmed cases.
The expansion factors were first based upon the MINSA data, and were later revised to account for additional cases not captured in these statistics using previously published values from the region.9,11,21,23 Separate expansion factors were chosen for the different syndromes of dengue (DF and DHF). Luz and others,23 Suaya and others,24 and Gubler,25 have shown that the burden of dengue is underreported between epidemic periods, although it is more appropriately reported during epidemic years. We therefore used two different ranges of expansion factors depending on the year, because of the endemic-epidemic cycle of dengue in Nicaragua.8 A given year was classified as an epidemic or endemic year based on the reported confirmed caseload: any year with over 5,000 reported cases was considered an epidemic year. The years 1998–2000, 2009, and 2010 were considered epidemic years, whereas the remaining years were endemic; Table 1 lists the expansion factors used in this analysis. These expansion factor estimates were in concordance with the published literature values previously described in Nicaragua9 and Puerto Rico,21 as these were used to inform the ultimate expansion factor ranges used in this analysis.
Table 1.
Variables and ranges used for calculating disability-adjusted life years (DALYs) caused by dengue illness in Nicaragua, 1996–2010*
| Variable | Value | Range | Distribution |
|---|---|---|---|
| Age-corrected constant, C26 | 0.16243 | N/A | N/A |
| Age-weighting parameter, b26 | 0.04 | N/A | N/A |
| Discount rate, r26 | 0.03 | N/A | N/A |
| Disability weight, D for DF and DHF21,26 | 0.81 | 0.6–0.92 | Triangular |
| Disability weight, D for mostly asymptomatic16 | 0 | 0–0.22 | Triangular |
| Duration of illness (DF)26 | 7 days | 4–10 days | Triangular |
| Duration of illness (DHF)26 | 15 days | 10–20 days | Triangular |
| Expansion factor of DF (epidemic)9,27,28 | 10 | 8–14 | Triangular |
| Expansion factor of DF (endemic)9,27,28 | 20 | 18–25 | Triangular |
| Expansion factor of DHF9,27,28 | 6 | 5–10 | Triangular |
| Asymptomatic to symptomatic ratio29 | 10 | 8–12 | Triangular |
| Life expectancy at birth30 | 72.3 years | N/A | N/A |
DF = dengue fever; DHF = dengue hemorrhagic fever; N/A = not available.
Economic data collection.
Data on expenditures associated with dengue control and prevention were obtained directly from MINSA's national budget for dengue control and averaged US$1.5 million/year; half of this budget was attributed to personnel salaries, whereas the remaining amount went toward insecticides, other material costs, operational costs, and education campaigns. These costs represent a fixed budgetary expense specifically allocated toward dengue vector control and surveillance and did not vary over the study period. Direct medical costs for treatment of individual dengue cases were estimated based upon patient health records and figures provided by MINSA and the regional health authority in Bluefields, Nicaragua, Sistema Local de Atención Integral en Salúd (SILAIS), regarding average daily costs of hospitalization, average cost of patient examination, and materials relating to diagnostic tests performed for infection confirmation. The costs of US$4.77 per ambulatory clinic visit (US$3.00 for wages, and US$1.77 for consumable laboratory test materials) are all incurred by the government.22 Each suspected dengue case was assumed to have one ambulatory clinic visit, and the duration of hospitalization was estimated from local MINSA data and compared with other studies from the region.11,21
The indirect costs associated with lost productivity caused by dengue illness were determined from the incidence data and the average number of days lost to illness for the different age groups and syndromes of dengue, as described elsewhere.12,27,28,31–34 For individuals 15 years of age and older, average wage data in Nicaragua were used to estimate the economic loss in productivity caused by illness. We assumed children < 15 years of age would not be used in the workforce; therefore, the loss in productivity would be for a caregiver. We estimated the lost caregiver productivity by multiplying the duration of illness by the average caregiver wage, estimated to be half of the average wage. The mean wage in Nicaragua was used as the basis for the lost productivity calculations; it was estimated at C$5,000/month, which corresponded to US$1.74/hour at an exchange rate of 20 Córdobas per dollar and 160 working hours per month based on data from the US State Department.30 The loss of caregiver productivity was assumed to range from 2 to 4 hours per day per case.
DALY calculations.
We calculated the burden of disease using the DALY model first described by Murray and others16,17 to account for multiple factors that contribute to the time lost to disease, including the age of onset and severity of disease. DALYs lost by each case of dengue were calculated using the following formula:
where D is the disability weight (D = 1 for premature death, D = 0 for perfect health); a is the age of onset; L is the duration of disability or years of life lost to premature death; r is the social discount rate that reflects the net present value of health in the future; C and b are parameters from an age-weighing function, which serves to capture the value of life at different ages, reflecting the dependence of the young and elderly on adults in the population.16,17 We used values for C, b, and r from the World Health Organization (WHO) Global Burden of Disease (GBD) studies for dengue to compare disability associated with dengue in Nicaragua with results from similar studies around the world.35 Instead of using the disability weight D of 0.21, as described in the GBD, we used 0.81 for both DF and DHF, as has been described by Meltzer and used throughout the literature as a more appropriate assessment of the disability of a DF or DHF infection, as even mild dengue cases can impede an individual from conducting their normal daily activities for some time.11,21,23,27,32,33
According to the PAHO data used in this analysis, overall mortality rates of dengue in Nicaragua varied and were calculated to be between 0.5% and 1% of the total reported cases of dengue. Productivity losses vary depending on age at death, with children losing a greater amount of productive years than adults; this fact was incorporated into the model through an age-group dependent productivity loss factor. Dengue mortality is higher among children than among adults in Nicaragua, with a majority of deaths occurring in individuals under-five years of age, a finding also described elsewhere.36–38
Simulations and sensitivity analysis.
Tables 1 and 2 list the parameters and their ranges for both the disease burden and cost assessment and the sources used in their determination. Simulations were conducted using Microsoft Excel 2010 (Microsoft Corporation, Seattle, WA) with a Crystal Ball 11.1.1.3 (Oracle USA, Inc., Redwood City, CA) add-in; 5,000 iterations were conducted for each simulation. Probabilistic sensitivity analyses (i.e., a Monte-Carlo simulation) simultaneously varied each parameter throughout the ranges listed in Tables 1 and 2. One-way sensitivity analyses were performed to determine the relative contributions of key parameters on the resulting disease burden and cost calculations. These one-way sensitivity analyses varied the asymptomatic to symptomatic case ratio, the expansion factor for DF (for epidemic and endemic years), the disability weight D in the DALY calculation, and the duration of illness. These parameters were changed one by one, whereas all other parameter ranges were held at their median values to determine their relative impact. Results are presented as mean values with 95% confidence intervals.
Table 2.
Variables and ranges used in calculating the cost of dengue illness in Nicaragua, 1996–2010*
| Variable | Value | Range | Distribution |
|---|---|---|---|
| Average wage30 | US$1.74 | US$0.87–2.61 | Triangular |
| Cost per ambulatory clinic visit (including wages and laboratory test supplies) (MINSA, unpublished data) | US$4.77 | US$4–8 | Triangular |
| Daily hospitalization cost (MINSA, unpublished data) | US$250 | US$200–300 | Triangular |
| Percent of suspected cases of DF requiring hospitalization31,33 | 5% | 1–8% | Triangular |
| Percent of DHF cases requiring hospitalization31,33 | 67% | 50–90% | Triangular |
| Duration of DF hospitalization22 | 4 days | 2–6 days | Triangular |
| Duration of DHF hospitalization22 | 7 days | 4–12 days | Triangular |
MINSA = Nicaraguan Ministry of Health; DF = dengue fever; DHF = dengue hemorrhagic fever.
Results
Dengue burden of disease.
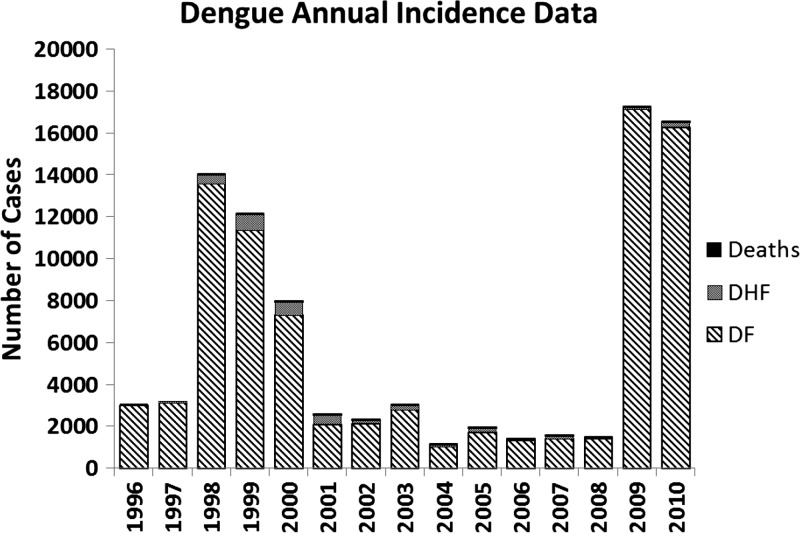
As shown in Figure 1, the total number of dengue cases in Nicaragua varied from 1996 to 2010, peaking in 1998–2000, and again in 2009–2010, with a relatively low case burden during the rest of the study period. This variation in the number of cases is the result of the endemic-epidemic cycle of dengue in Nicaragua, and the development of natural immunity in significant proportions of the population following epidemics.8 The vast majority of cases over the study period were caused by DF (81–99%), whereas DHF and deaths contributed 0–18% and 0–1% of the overall caseload of dengue, respectively.
Figure 1.
Stacked bar graph of the incident cases of dengue illness reported to the Nicaraguan Ministry of Health (MINSA) from 1996 to 2010, broken down by type of dengue illness. Classic dengue (DF) represents the majority of cases (81–99%), with dengue hemorrhagic fever (DHF) representing 0–18% of the total cases. Deaths directly attributable to dengue were uncommon and ranged from 0 to 23 (0–1% of total cases) annually in this study period.
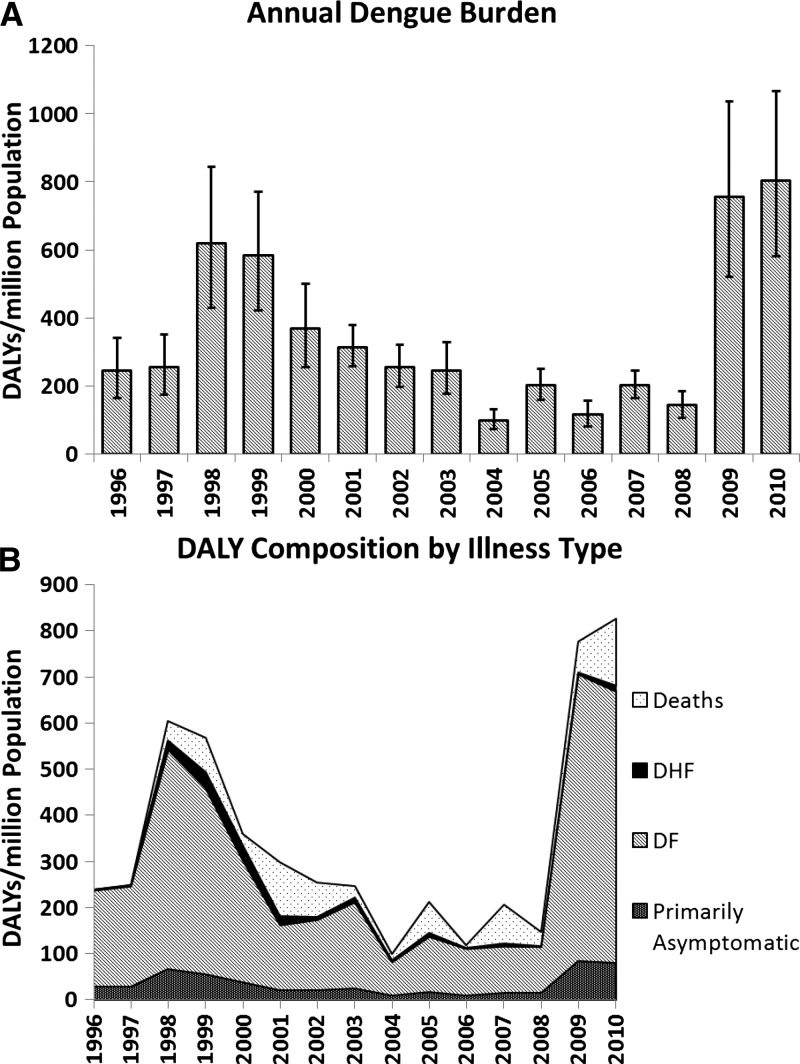
Summary data on the disease burden over the study period can be found in Figure 2 and Table 3. The DALY burden caused by dengue varied during this period as a result of the difference in the number of cases, ranging from 99 DALYs per million in 2004 to 805 DALYs per million in 2010, with a mean of 347 DALYs per million over the entire period (1996–2010). The greatest contributor to the disease burden was DF, which constituted 48–87% of the DALYs attributable to dengue; DHF and deaths contributed a smaller portion of the disease burden at 1–9% and 0–40%, respectively.
Figure 2.
(A) Bar graph of the disease burden associated with dengue illness in Nicaragua from 1996 to 2010, measured in disability-adjusted life years (DALYs) per million population, with 95% confidence intervals displayed. (B) Breakdown of total DALYs per million population by dengue outcome (primarily asymptomatic, classic dengue [DF], dengue hemorrhagic fever [DHF], or death). The majority contribution to the total DALY burden was caused by DF; deaths contributed a variable amount.
Table 3.
DALYs (95% confidence interval) lost in Nicaragua from 1996 to 2010 by type of dengue illness*
| Year | DALYs for DF (95% CI) | DALYs for DHF (95% CI) | DALYs for mostly asymptomatic cases (95% CI) | DALYs for deaths |
|---|---|---|---|---|
| 1996 | 1088 (736–1467) | 13 (9–17) | 145 (5–456) | 0 |
| 1997 | 1132 (771–1531) | 17 (12–22) | 148 (5–458) | 0 |
| 1998 | 2483 (1662–3410) | 106 (75–142) | 339 (10–1054) | 220 |
| 1999 | 2106 (1417–2884) | 187 (133–250) | 281 (8–884) | 385 |
| 2000 | 1384 (915–1924) | 172 (121–231) | 189 (5–604) | 125 |
| 2001 | 779 (529–1058) | 112 (79–150) | 106 (3–331) | 637 |
| 2002 | 792 (542–1065) | 39 (28–52) | 109 (3–335) | 386 |
| 2003 | 970 (663–1310) | 58 (41–78) | 129 (4–408) | 126 |
| 2004 | 373 (252–503) | 22 (16–30) | 50 (1–153) | 70 |
| 2005 | 627 (425–845) | 42 (30–57) | 84 (3–262) | 356 |
| 2006 | 492 (338–665) | 13 (9–17) | 67 (2–207) | 31 |
| 2007 | 517 (353–696) | 36 (25–48) | 70 (2–222) | 422 |
| 2008 | 512 (345–692) | 7 (5–10) | 69 (2–215) | 157 |
| 2009 | 3157 (2086–4353) | 20 (14–26) | 421 (12–1315) | 346 |
| 2010 | 2987 (1989–4090) | 67 (47–89) | 404 (12–1288) | 736 |
The years 1998–2000, 2009, and 2010 were considered epidemic years, whereas the remaining years were considered endemic years.
DALYs = disability-adjusted life years; DF = dengue fever; DHF = dengue hemorrhagic fever.
Costs associated with dengue illness.
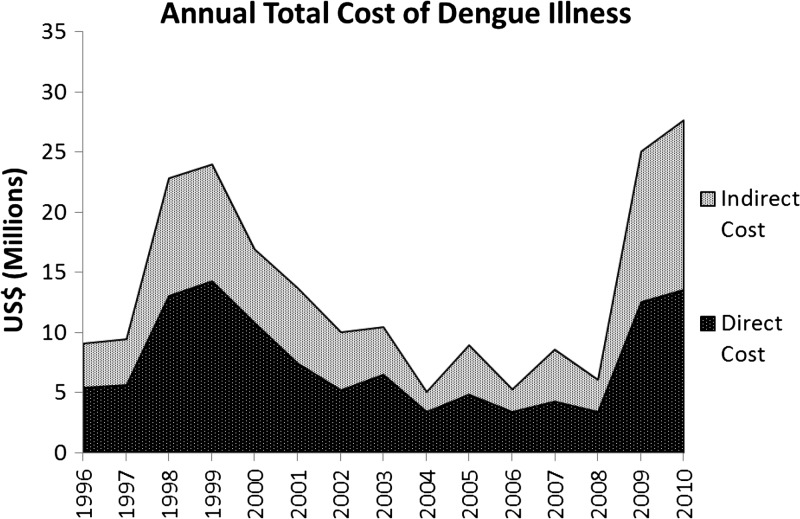
Figure 3 and Table 4 show the total annual costs associated with dengue from 1996 to 2010, which ranged from US$5.1 million in 2004 to US$27.6 million in 2010, with a mean cost of US$13.5 million. The cost per case of dengue ranged from US$125 to US$273, and the average per capita cost of dengue varied from US$0.97 to US$5.44.
Figure 3.
Stacked area plot of the total costs (includes direct and indirect costs) associated with dengue illness in Nicaragua from 1996 to 2010. For both direct and indirect costs, the factors that had the greatest impact on costs were length of illness and expansion factor.
Table 4.
Economic burden of dengue illness in Nicaragua from 1996 to 2010 by cost type*
| Year | Direct costs in US$ millions (95% CI) | Indirect costs in US$ millions (95% CI) | Total costs in US$ millions (95% CI) |
|---|---|---|---|
| 1996 | 5.4 (3.5–7.6) | 3.7 (2.1–5.5) | 9.0 (6.6–11.8) |
| 1997 | 5.6 (3.7–7.8) | 3.8 (2.2–5.7) | 9.5 (6.9–12.3) |
| 1998 | 13.0 (8.2–18.5) | 9.8 (5.9–14.5) | 22.8 (16.4–30.1) |
| 1999 | 14.2 (9.3–20.0) | 9.7 (6.0–14.2) | 23.8 (17.5–31.0) |
| 2000 | 10.8 (7.3–14.9) | 6.1 (3.7–9.1) | 16.9 (12.4–22.2) |
| 2001 | 7.4 (5.2–10.0) | 6.3 (4.0–8.6) | 13.7 (10.5–17.3) |
| 2002 | 5.2 (3.7–6.9) | 4.8 (3.1–6.8) | 10.0 (7.6–12.6) |
| 2003 | 6.5 (4.6–8.7) | 3.9 (2.4–5.7) | 10.4 (7.8–13.2) |
| 2004 | 3.4 (2.7–4.3) | 1.7 (1.0–2.4) | 5.0 (4.0–6.2) |
| 2005 | 4.8 (3.6–6.4) | 4.1 (2.6–5.6) | 8.9 (6.9–11.1) |
| 2006 | 3.4 (2.6–4.5) | 1.8 (1.1–2.6) | 5.3 (4.1–6.6) |
| 2007 | 4.3 (3.2–5.5) | 4.3 (2.8–5.9) | 8.6 (6.7–10.6) |
| 2008 | 3.4 (2.5–4.4) | 2.6 (1.6–3.7) | 6.0 (4.6–7.5) |
| 2009 | 12.5 (6.8–19.2) | 12.5 (7.4–18.6) | 24.9 (17.0–34.2) |
| 2010 | 13.5 (8.2–19.9) | 14.1 (8.6–20.6) | 27.4 (19.3–36.7) |
The years 1998–2000, 2009, and 2010 were considered epidemic years, whereas the remaining years were considered endemic years.
CI = confidence interval.
The direct costs associated with dengue during this period resulted mostly from hospitalization and inpatient treatment, which ranged from US$3.4 to US$14.2 million per year, with the greatest costs during epidemic years: 1998–2000 and 2009–2010. These direct costs constituted a majority of the overall costs associated with dengue illness, ranging from 49% to 67% of the annual costs during the study period. At a cost of US$250/day, the greatest proportion of hospitalization costs was caused by classic DF, even though only a small fraction of DF cases require hospitalization (1–8%), whereas nearly all DHF cases are hospitalized (50–90%); this is the result of the higher proportion of DF cases to DHF and deaths (Table 2). Medical costs as a result of ambulatory clinic visits and laboratory tests constituted only a small portion of the total direct costs. Another direct cost associated with dengue was for prevention and mosquito control activities, which was a fixed amount determined annually by MINSA. The indirect costs associated with dengue illness during this period ranged from US$1.7 to US$14.1 million, representing 33–51% of the total costs, with much of those costs attributable each year to premature death (Figure 3 and Table 4).
Sensitivity analysis.
From the one-way analysis, the parameters found to have the greatest impact on the DALY burden in epidemic and endemic years, in order of importance, were the expansion factors followed by the disability weight value. The duration of illness and asymptomatic/symptomatic case ratio had less of an impact on the total DALY burden. The expansion factors had the greatest impact on the overall cost burden (direct plus indirect costs) for epidemic and endemic years, followed by the duration of illness. The disability weight and asymptomatic/symptomatic ratio did not have much of an effect on the overall cost.
Discussion
This study represents the first effort to quantify both the burden of disease and the costs associated with dengue illness in Nicaragua, and is one of the most comprehensive analyses conducted in the region to date. The burden of DF is substantial in terms of the DALYs lost and the economic costs associated with illness. Our study quantified these burdens over the period of 1996–2010, illustrating the range in the burden of disease during both endemic and epidemic years. Previous analyses have investigated the burden and cost of disease during epidemic and endemic years, but none have examined this broad a time period.11,21 Additionally, previous attempts to characterize the burden of disease in Nicaragua did not account for the costs associated with dengue prevention, which have been shown to contribute significantly to the overall costs associated with dengue.12–14 Furthermore, in this analysis, mostly asymptomatic, DF, DHF, and death were all considered separately and included in the DALY burden determination.
The annual DALY burden of disease varied with the incidence of cases, ranging from 516 to 4,194 DALYs, which corresponds to 99–805 DALYs per million population. Classic DF cases constituted the majority of the burden of disease at 373–3,157 DALYs, which corresponded to 48–87% of the total burden. The number of DALYs caused by DHF varied from 7 to 187 (1–9% of the total burden), and deaths, although rare, spiked during particular years; thus, 0–736 (0–40%) of the total DALYs were attributable to deaths, mostly in children. We found that over the period studied, there was a range in the mortality rate of dengue, which subsequently had a great impact on the calculated DALY burden of disease. In 2001, there were 21 dengue reported deaths; as a result, this year had the highest percentage of the total DALY burden attributable to death from dengue, although the overall disease burden of dengue was lower than that of other years in which the DF case load was higher.
The average annual costs of dengue for Nicaragua ranged from US$5.1 to US$27.6 million, which did not include any special one-time costs for epidemic response or other similar special campaigns. Each case of dengue was estimated to cost the government between US$0.97 and US$2.49 per capita during endemic years and up to US$5.44 in epidemic years. In a country where the average gross domestic product (GDP) per capita is US$3000, this cost represents a substantial burden, as well as resources that could be directed toward other efforts if an effective dengue vaccine were available. The costs of dengue in Nicaragua are similar to those described for dengue and chikungunya in India in 2006 and dengue in Panama in 2005.12,39
In determining the direct costs of dengue in Nicaragua, each case brought to medical attention was found to cost the government ∼US$5/ambulatory clinic visit and roughly US$250/day if hospitalized. Additionally, the annual budget for dengue prevention and vector control activities was ∼US$1.5 million, as reported by MINSA. Including both the direct and indirect costs of illness, the average cost per confirmed dengue case ranged from US$125 to US$273 (peak cost occurring during the 2001 season). This average cost per case was greater than the previous estimates made by Ferrando of US$44/case in the 1994 epidemic and is in accordance with the estimates made by Shepard and others for the period of 2000–2007 of US$144/ambulatory case and US$306/hospitalized case.10,11 The differences between the Ferrando estimates and those presented here likely resulted from the inclusion of direct, indirect, and vector control costs.
The first challenge in this study was accounting for the underreporting of dengue cases and estimating the total number of asymptomatic and symptomatic dengue cases during the study period. The cases reported to PAHO by MINSA represent only the laboratory-confirmed cases of disease, whereas a large portion of cases are never confirmed because of delays in testing or lack of diagnostic supplies. MINSA data have indicated that there were at least nine times as many suspected cases as confirmed ones from 2004 to 2006 for DF, and at least five times as many suspected DHF cases compared with confirmed.18 There are additional cases that may not come to the attention of medical authorities; in Nicaragua, Standish calculated that their cohort study captured on average 21.3 times the number of cases reported to MINSA.9 Thus, we used a range of expansion factors to account for the underreporting and rate of mostly asymptomatic illness, depending on the epidemic-endemic cycle of dengue in Nicaragua, as shown in Table 1. Those with DHF were assumed to be hospitalized at a much higher rate than those with DF; therefore, the underreporting factors for DHF were significantly lower than those for DF, as previously described. We also assumed that there was no underreporting of deaths caused by dengue to MINSA; if any were underreported, however, they would significantly increase the burden and cost of dengue. This use of expansion factors and the values used were consistent with similar studies conducted in the region.11,21
Estimating the disease burden caused by dengue in Nicaragua was another challenge, and the DALY metric was used because it has been well established in the literature for similar analyses.11,21,23 A sensitivity analysis was conducted using a range of values for the disability weight to yield various estimates for the total disability associated with dengue infections, and the results were consistent with other estimates from the region. In Puerto Rico, Meltzer reported the average DALY burden caused by dengue from 1984 to 1994 (epidemic period) to be 658 DALYs per million population, and Shepard reported an average 144 DALYs per million per year in Nicaragua during the period of 2000–2007 (endemic period).11,21 The disease burden estimations produced in this analysis ranged from 99 to 805 DALYs per million population, with an average of 347; these figures are consistent with the magnitude of these previously published DALY burdens.11,21 Because the time period studied in this analysis included both endemic and epidemic years, the estimates fell in between these two previously published values. When compared with other diseases in the region from the WHO, the DALY burden caused by dengue was consistent in magnitude with that of other infectious agents, such as meningitis (785 per million), Hepatitis B (839 per million), sexually transmitted infections excluding human immunodeficiency virus (HIV) (1,050/million), and non-infectious diseases such as cervical and colon cancers (846/million and 723/million, respectively).35
Finally, the medical and hospitalization costs, as well as the indirect costs, were estimated to calculate the economic costs associated with dengue illness; the average wage estimations for Nicaragua were used as the basis for these calculations.30 The MINSA budget data were used to determine the vector control and prevention costs, which were assumed to be allocated specifically for dengue prevention, although the personnel salaries and prevention efforts may also benefit other programs. This could result in a slight overestimation of these expenses to the total cost of dengue illness; however, the prevention and control expenses only contribute a small portion of the overall direct and total costs associated with disease. Although other efforts have included out-of-pocket payments covered by the population, as a result of transportation, food, indebtedness, or other costs, these were not included here because of the variability in costs and the lack of reliable data for determining these contributions to the total costs.40,41 Our definition of total costs, therefore, necessarily excludes these; future in-depth analyses could include these other costs that we have not included.
Despite the comprehensive nature of this assessment, no model can account for every possible event, outcome, or expenditure associated with dengue illness. The model could not account for all heterogeneities that exist within the population, nor take into account co-morbidities. Our model contains some assumptions, caused by data limitations, and inputs came from studies of varying quality; therefore, the results may change as more data become available. Our results are mere estimations of the burden of disease and the cost associated with illness over the time period studied.
The usefulness of cost of illness studies has been debated in the literature.42–45 Although there are limitations to these analyses, a dengue expert panel has compiled previous economic dengue efforts and recently called for additional cost of illness and DALY burden assessments over greater time periods to inform policy and decision-making, a decision echoed by another recent publication by the Scientific Working Group on Dengue Research.24,46
In this study, we have quantified the economic cost and burden of disease associated with dengue illness in Nicaragua from 1996 to 2010. These findings allow for a better estimate of the burden of disease in the region, and can be used presently to assess the allocation of resources for dengue on the national and international levels. The costs associated with dengue infection in Nicaragua are not insignificant; these data illustrate the potential resources that could be reallocated for other pressing health concerns such as an efficacious DF vaccine intervention to be implemented in Nicaragua or the region. Previous efforts have determined the cost-effectiveness of potential dengue vaccine interventions, but few in Latin America47–49; because there are several dengue vaccine candidates currently under development, this analysis could prove useful for key stakeholders in informing policy and decision-making. In the meantime, the incorporation of the costs associated with control and surveillance activities will prove useful in the assessment of current vector control programs, as these results show a discrepancy in terms of the funds budgeted for dengue prevention and control, and the total cost of disease in Nicaragua. Furthermore, decision makers could use these updated DALY and cost assessments to compare the burden with other prevalent diseases in the region and allocate funds accordingly.
Although this study was conducted in the context of Nicaragua, the methodologies, analysis, and results are applicable to the greater context of dengue in the Latin American region. Such a comprehensive analysis can be used as a model for determining the burden and total cost of disease in other settings to inform policy and decision making.
ACKNOWLEDGMENTS
This work was supported by the partnership of the Dengue Relief Foundation and the Nicaraguan Ministry of Health. From the Nicaraguan Ministry of Health, we thank Francisco Acevedo, Gina Hodgson, and Luisa Amanda Campos for their assistance with data collection.
Disclaimer: The funders had no role in the design and conduct of this study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript.
Footnotes
Financial support: This research was supported in part by a grant from the Doris Duke Foundation to AYC. This study was supported by the National Institute of General Medical Sciences Models of Infectious Disease Agent Study (MIDAS) grant 1U54GM088491-0109 and the Vaccine Modeling Initiative.
Authors' addresses: Zachary S. Wettstein, Michael Fleming, Aileen Y. Chang, David J. Copenhaver, and Rajan P. Kulkarni, Dengue Relief Foundation, Managua, Nicaragua, E-mails: zwettstein@gmail.com, ayc2113@columbia.edu, michael.fleming@gmail.com, dcopenhaver@gmail.com, and rkulkarni2@gmail.com. Angela R. Wateska, Sarah M. Bartsch, and Bruce Y. Lee, Public Health Computational and Operations Research (PHICOR), Pittsburgh, PA, E-mails: arw38@pitt.edu, smm168@pitt.edu, and BYL1@pitt.edu.
References
- 1.Massad E, Coutinho FA. The cost of dengue control. Lancet. 2011;377:1630–1631. doi: 10.1016/S0140-6736(11)60470-4. [DOI] [PubMed] [Google Scholar]
- 2.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martínez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. Dengue: a continuing global threat. Nat Rev Microbiol. 2010;8:S7–S16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 4.Halstead SB. Dengue. Lancet. 2007;370:1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 5.Torres JR, Castro J. The health and economic impact of dengue in Latin America. Cad Saude Publica. 2007;23((Suppl 1)):S23–S31. doi: 10.1590/s0102-311x2007001300004. [DOI] [PubMed] [Google Scholar]
- 6.WHO . Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization; 2009. [PubMed] [Google Scholar]
- 7.Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2:e300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris E, Videa E, Perez L, Sandoval E, Tellez Y, Perez ML, Cuadra R, Rocha J, Idiaquez W, Alonso RE, Delgado MA, Campo LA, Acevedo F, Gonzalez A, Amador JJ, Balmaseda A. Clinical, epidemiologic, and virologic features of dengue in the 1998 epidemic in Nicaragua. Am J Trop Med Hyg. 2000;63:5–11. doi: 10.4269/ajtmh.2000.63.5. [DOI] [PubMed] [Google Scholar]
- 9.Standish K, Kuan G, Aviles W, Balmaseda A, Harris E. High dengue case capture rate in four years of a cohort study in Nicaragua compared to national surveillance data. PLoS Negl Trop Dis. 2010;4:e633. doi: 10.1371/journal.pntd.0000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrando J. Estimate of the Costs of the Dengue Epidemic in 1994 in Nicaragua. Washington, DC: Pan American Health Organization; 1995. [Google Scholar]
- 11.Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH. Economic impact of dengue illness in the Americas. Am J Trop Med Hyg. 2011;84:200–207. doi: 10.4269/ajtmh.2011.10-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armien B, Suaya JA, Quiroz E, Sah BK, Bayard V, Marchena L, Campos C, Shepard DS. Clinical characteristics and national economic cost of the 2005 dengue epidemic in Panama. Am J Trop Med Hyg. 2008;79:364–371. [PubMed] [Google Scholar]
- 13.Shepard DS. Aggregate economic cost of dengue in Puerto Rico. Proceedings of the 58th Annual Meeting of the American Society of Tropical Medicine and Hygiene; Washington, DC. November 18–22.2009. [Google Scholar]
- 14.Kongsin S, Jiamton S, Suaya JA, Vasanawathana S, Sirisuvan P, Shepard DS. Cost of Dengue in Thailand. Bangkok, Thailand: Mahidol University School of Public Health; 2010. [Google Scholar]
- 15.Tapia-Conyer R, Méndez-Galván JF, Gallardo-Rincón H. The growing burden of dengue in Latin America. J Clin Virol. 2009;46:S3–S6. doi: 10.1016/S1386-6532(09)70286-0. [DOI] [PubMed] [Google Scholar]
- 16.Murray CJ. Quantifying the burden of disease: the technical basis for disability-adjusted life years. Bull World Health Organ. 1994;72:429–445. [PMC free article] [PubMed] [Google Scholar]
- 17.Murray CJ, Lopez AD. Quantifying disability: data, methods and results. Bull World 0048ealth Organ. 1994;72:481–494. [PMC free article] [PubMed] [Google Scholar]
- 18.PAHO Dengue Regional Information: Number of Cases. 2012. http://new.paho.org/hq/index.php?option=com_content&task=view&id=264&Itemid=363& Available at. Accessed January 12, 2012.
- 19.Muiser J, Sáenz M, Bermúdez J. Sistema de salud de Nicaragua. Salud Publica Mex. 2011;53:S233–S242. [PubMed] [Google Scholar]
- 20.PAHO . Norms and Standards in Epidemiology: Case Definitions for Dengue Fever and Leptospirosis. Epidemiol Bull 21. 2000. http://www.paho.org/english/sha/eb_v21n2-cov.htm Available at. Accessed January 12, 2012. [Google Scholar]
- 21.Meltzer MI, Rigau-Perez JG, Clark GG, Reiter P, Gubler DJ. Using disability-adjusted life years to assess the economic impact of dengue in Puerto Rico: 1984–1994. Am J Trop Med Hyg. 1998;59:265–271. doi: 10.4269/ajtmh.1998.59.265. [DOI] [PubMed] [Google Scholar]
- 22.Salvatierra-González R. Washington DC: OPS; 2003. (Costo de la infección nosocomial en nueve países de América Latina). [Google Scholar]
- 23.Luz PM, Grinsztejn B, Galvani AP. Disability adjusted life years lost to dengue in Brazil. Trop Med Int Health. 2009;14:237–246. doi: 10.1111/j.1365-3156.2008.02203.x. [DOI] [PubMed] [Google Scholar]
- 24.Suaya J, Shepard D, Beatty M. Dengue Burden of Disease and Cost of Illness. Geneva: World Health Organization on behalf of the Special Programme for Research and Training in Tropical Diseases; 2007. pp. 35–49. [Google Scholar]
- 25.Gubler DJ. How effectively is epidemiological surveillance used for dengue programme planning and epidemic response? Dengue Bull. 2002;26:96–106. [Google Scholar]
- 26.Murray CJ, López AD, Bank W. The Global Burden of Disease: a Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and projected to 2020. Published by the Harvard School of Public Health on behalf of the World Health Organization and the World Bank; 1996. [Google Scholar]
- 27.Clark DV, Mammen MP, Nisalak A, Puthimethee V, Endy TP. Economic impact of dengue fever/dengue hemorrhagic fever in Thailand at the family and population levels. Am J Trop Med Hyg. 2005;72:786–791. [PubMed] [Google Scholar]
- 28.Suaya JA, Shepard DS, Siqueira JB, Martelli CT, Lum LC, Tan LH, Kongsin S, Jiamton S, Garrido F, Montoya R, Armien B, Huy R, Castillo L, Caram M, Sah BK, Sughayyar R, Tyo KR, Halstead SB. Cost of dengue cases in eight countries in the Americas and Asia: a prospective study. Am J Trop Med Hyg. 2009;80:846–855. [PubMed] [Google Scholar]
- 29.Balmaseda A, Hammond SN, Tellez Y, Imhoff L, Rodriguez Y, Saborio SI, Mercado JC, Perez L, Videa E, Almanza E, Kuan G, Reyes M, Saenz L, Amador JJ, Harris E. High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua. Trop Med Int Health. 2006;11:935–942. doi: 10.1111/j.1365-3156.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 30.U.S. State Department 2010 Human Rights Report: Nicaragua. 2011. http://www.state.gov/g/drl/rls/hrrpt/2010/wha/154513.htm Available at. Accessed January 12, 2012.
- 31.Anderson KB, Chunsuttiwat S, Nisalak A, Mammen MP, Libraty DH, Rothman AL, Green S, Vaughn DW, Ennis FA, Endy TP. Burden of symptomatic dengue infection in children at primary school in Thailand: a prospective study. Lancet. 2007;369:1452–1459. doi: 10.1016/S0140-6736(07)60671-0. [DOI] [PubMed] [Google Scholar]
- 32.Beaute J, Vong S. Cost and disease burden of dengue in Cambodia. BMC Public Health. 2010;10:521. doi: 10.1186/1471-2458-10-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garg P, Nagpal J, Khairnar P, Seneviratne SL. Economic burden of dengue infections in India. Trans R Soc Trop Med Hyg. 2008;102:570–577. doi: 10.1016/j.trstmh.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Huy R, Wichmann O, Beatty M, Ngan C, Duong S, Margolis HS, Vong S. Cost of dengue and other febrile illnesses to households in rural Cambodia: a prospective community-based case-control study. BMC Public Health. 2009;9:155. doi: 10.1186/1471-2458-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathers C, Fat DM, Boerma JT. The Global Burden of Disease: 2004 Update. Geneva: World Health Organization; 2008. [Google Scholar]
- 36.Guzman MG, Kouri G, Bravo J, Valdes L, Vazquez S, Halstead SB. Effect of age on outcome of secondary dengue 2 infections. Int J Infect Dis. 2002;6:118–124. doi: 10.1016/s1201-9712(02)90072-x. [DOI] [PubMed] [Google Scholar]
- 37.Malavige GN, Fernando S, Fernando DJ, Seneviratne SL. Dengue viral infections. Postgrad Med J. 2004;80:588–601. doi: 10.1136/pgmj.2004.019638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinheiro FP, Corber SJ. Global situation of dengue and dengue hemorrhagic fever, and its emergence in the Americas. World Health Stat Q. 1997;50:161–169. [PubMed] [Google Scholar]
- 39.Seyler T, Hutin Y, Ramanchandran V, Ramakrishnan R, Manickam P, Murhekar M. Estimating the burden of disease and the economic cost attributable to chikungunya, Andhra Pradesh, India, 2005–2006. Trans R Soc Trop Med Hyg. 2010;104:133–138. doi: 10.1016/j.trstmh.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 40.Valdes LG, Mizhrahi JV, Guzman MG. Economic impact of dengue 2 epidemic in Santiago de Cuba, 1997. Rev Cubana Med Trop. 2002;54:220–227. [PubMed] [Google Scholar]
- 41.Von Allmen SD, Lopez-Correa RH, Woodall JP, Morens DM, Chiriboga J, Casta-Velez A. Epidemic dengue fever in Puerto Rico, 1977: a cost analysis. Am J Trop Med Hyg. 1979;28:1040–1044. doi: 10.4269/ajtmh.1979.28.1040. [DOI] [PubMed] [Google Scholar]
- 42.Tarricone R. Cost-of-illness analysis: what room in health economics? Health Policy. 2006;77:51–63. doi: 10.1016/j.healthpol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 43.Byford S, Torgerson DJ, Raftery J. Cost of illness studies. BMJ. 2000;320:1335. doi: 10.1136/bmj.320.7245.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiell A, Gerard K, Donaldson C. Cost of illness studies: an aid to decision-making? Health Policy. 1987;8:317–323. [Google Scholar]
- 45.Hodgson TA. Cost of illness studies: no aid to decision making? Comments on the second opinion by Shiell et al. (Health Policy 8(1987)317–323) Health Policy. 1989;11:57–60. doi: 10.1016/0168-8510(89)90055-9. [DOI] [PubMed] [Google Scholar]
- 46.Beatty ME, Beutels P, Meltzer MI, Shepard DS, Hombach J, Hutubessy R, Dessis D, Coudeville L, Dervaux B, Wichmann O, Margolis HS, Kuritsky JN. Health economics of dengue: a systematic literature review and expert panel's assessment. Am J Trop Med Hyg. 2011;84:473–488. doi: 10.4269/ajtmh.2011.10-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee BY, Connor DL, Kitchen SB, Bacon KM, Shah M, Brown ST, Bailey RR, Laosiritaworn Y, Burke DS, Cummings DA. Economic value of dengue vaccine in Thailand. Am J Trop Med Hyg. 2011;84:764–772. doi: 10.4269/ajtmh.2011.10-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shepard DS, Suaya JA, Halstead SB, Nathan MB, Gubler DJ, Mahoney RT, Wang DNC, Meltzer MI. Cost-effectiveness of a pediatric dengue vaccine. Vaccine. 2004;22:1275–1280. doi: 10.1016/j.vaccine.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 49.Shepard DS, Suaya JA. Cost-effectiveness of a dengue vaccine in Southeast Asia and Panama: preliminary estimates. In: Preedy VR, Watson RR, editors. Handbook of Disease Burdens and Quality of Life Measures. New York: Springer; 2010. pp. 1281–1296. Chapter 73. [Google Scholar]