Abstract
As a result of widespread antimalarial drug resistance, all African countries with endemic malaria have, in recent years, changed their malaria treatment policy. In Senegal, the health authorities changed from chloroquine (CQ) to a combination of sulfadoxine–pyrimethamine (SP) plus amodiaquine (AQ) in 2003. Since 2006, the artemisinin combination therapies (ACTs) artemether–lumefantrine (AL) and artesunate plus amodiaquine (AS/AQ) were adopted for uncomplicated malaria treatment. After several years of CQ withdrawal, the current study wished to determine the level of CQ resistance at the molecular level in selected sites in Senegal, because the scientific community is interested in using CQ again. Finger prick blood samples were collected from Plasmodium falciparum-positive children below the age of 10 years (N = 474) during cross-sectional surveys conducted in two study sites in Senegal with different malaria transmission levels. One site is in central Senegal, and the other site is in the southern part of the country. All samples were analyzed for single nucleotide polymorphisms (SNPs) in the P. falciparum CQ resistance transporter gene (Pfcrt; codons 72–76) using polymerase chain reaction (PCR) sequence-specific oligonucleotide probe (SSOP) enzyme-linked immunosorbent assay (ELISA) and real-time PCR methods. In total, the 72- to 76-codon region of Pfcrt was amplified in 449 blood samples (94.7%; 285 and 164 samples from the central and southern sites of Senegal, respectively). In both study areas, the prevalence of the Pfcrt wild-type single CVMNK haplotype was very high; in central Senegal, the prevalence was 70.5% in 2009 and 74.8% in 2010, and in southern Senegal, the prevalence was 65.4% in 2010 and 71.0% in 2011. Comparing data with older studies in Senegal, a sharp decline in the mutant type Pfcrt prevalence is evident: from 65%, 64%, and 59.5% in samples collected from various sites in 2000, 2001, and 2004 to approximately 30% in our study. A similar decrease in mutant type prevalence is noted in other neighboring countries. With the continued development of increased CQ susceptibility in many African countries, it may be possible to reintroduce CQ in the near future in a drug combination; it could possibly be given to non-vulnerable groups, but it demands close monitoring of possible reemergence of CQ resistance development.
Introduction
Chloroquine (CQ) resistance in Plasmodium falciparum is well-established in many parts of Africa, including Senegal,1 where resistance to CQ was first reported in Dakar in 1988.2 High rates of CQ treatment failures and an increased risk of childhood malaria deaths3 prompted Senegalese health authorities to abandon CQ in 2003. Sulfadoxine–pyrimethamine (SP) plus amodiaquine (AQ) was then introduced as the first-line treatment of uncomplicated P. falciparum malaria, despite the fact that low numbers of SP and AQ treatment failures were documented in the country when used alone.4–6 SP/AQ is expected to suppress or at least delay the emergence of P. falciparum malaria drug resistance, because both drugs have independent modes of action and unrelated biochemical targets. In 2006, following the World Health Organization (WHO) guidelines suggesting the use of artemisinin combination therapies (ACTs) for uncomplicated malaria treatment, which were recently re-emphasized in the second edition of its guidelines published in 2010,7 the combination SP/AQ was changed to ACTs: artemether–lumefantrine (AL) and artesunate plus AQ (AS/AQ) are first-line treatments (depending on availability) against uncomplicated malaria in Senegal. This policy decision was supported by the finding that AS/AQ was highly effective in Senegal.8 Presumably, as an effect of this decision, a large reduction of confirmed malaria cases was noted between 2005 and 2009 among children under 5 years of age.9 Furthermore, the use of ACT may have contributed to the 30% reduction in all mortality among children under 5 years of age as observed in Senegal over the same time period.10 From September of 2007, the use of malaria rapid diagnostic tests (RDTs) was incorporated by the National Malaria Control Program (NMCP) into a revised national policy for management of febrile illness. Since that time, ACT use has been restricted to confirmed malaria cases to reduce drug pressure. CQ resistance (CQR) has been linked to 15 polymorphisms in the P. falciparum CQ resistance transporter gene (Pfcrt) from different parts of the world,11–14 as well as mutations in the Plasmodium falciparum multidrug resistance (Pfmdr1) gene.15,16 However, the most important is a single codon change at position 76 (K76T) found in all natural CQR isolates to date.17 Furthermore, substitutions in the wild-type haplotype at codons 72–76 (CVMNK) led to several resistant haplotypes, the most common of which are the CVIET haplotype, which is highly prevalent in southeast Asia and Africa, and SVMNT, which has been reported in South America11 and Asia18 but rarely Africa.19 Thus, the CVIET haplotype or the single nucleotide polymorphism (SNP) at Pfcrt76T has been shown to be a suitable tool for monitoring Pfcrt resistance in Africa. As CQ is replaced by AL and AS/AQ in Senegal and there is the possibility that drugs previously compromised by resistance can regain efficacy and could be used again, it becomes important from an epidemiological point of view to monitor changes in frequency of CQR markers (CVIET) after several years withdrawal of CQ for uncomplicated malaria treatment in Senegal.
Materials and Methods
The samples for this study were collected from children under 10 years of age during cross-sectional studies conducted from 2009 to 2011 in one central site consisting of three districts (Mbour, Fatick, and Bambey) and one southern site consisting of three districts (Tambacounda, Velingara, and Saraya) in Senegal. Study sites are 400 km apart and characterized by different malaria transmission patterns. The central part has 4 months of rainy season from July to October, with an entomological inoculation rate (EIR) of 12 infectious bite per night per person, whereas the southern part has 6 months of rainy season from June to November, with a mean of EIR = 264 infected bites during the malaria transmission season.20 In both areas, the Anopheles gambiae complex is responsible for malaria transmission. Before blood sample collection, written informed consent was obtained from the parent or guardian of each child. The study was approved by the Ethics Committee of Senegal. During the study, if children presented to health huts with symptoms consistent with mild malaria, including fever, chills, headache, and a positive RDT, they were offered standard ACT first-line treatment. Finger prick blood samples were collected from each study participant and blotted onto Whatman filter paper 3MM. Samples were stored at room temperature protected with silica gel desiccant for later P. falciparum DNA isolation. Thick and thin blood films were also done for microscopical identification of Plasmodium species.
DNA extraction and genetic analysis.
P. falciparum DNA was extracted from positive finger prick blood spots by the Chelex-100 method described in the work by Wooden and others,21 with some modifications described in the work by Pearce and others.22 Different methods for CQR genotyping at codons 72–76 based on polymerase chain reaction (PCR) were used. Quantitative PCR (Q-PCR) and nested PCR were followed by a sequence-specific oligonucleotide probe–enzyme-linked immunosorbent assay (SSOP-ELISA). For samples from the central study site, the Q-PCR assay was carried out using double-labeled probes, with a different fluorophore on each probe representing the three most common Pfcrt 72–76 alleles (wild-type [CVMNK] and resistance-associated [CVIET and SVMNT] haplotypes), and previously described conditions for amplification.23 Amplification was performed in a Rotor-gene 2000 Real Time cycler machine in the presence of each of the three double-labeled probes described previously.23 Control parasite DNA was obtained directly from MR4 (ATCC, Manassas, VA). Samples were considered positive for a particular genotype if a cycle threshold (CT) value of 35 cycles or less was obtained in at least two independent PCR experiments.
For samples from the southern study site, a nested PCR described in the work by Djimde and others12 was used to amplify fragments of the Pfcrt gene. The only modification was that primers TCRD2 in the Pfcrt nested PCRs were biotinylated at the 5-end by the supplier (www.mwg-biotech.com).24 The 20-μL Pfcrt outer PCR mixture consisted of the primers P1/P2 (1 μM/primer), 1.0× TEMPase Hot Start Master Mix (3.0 mM MgCl2, 0.4 mM 2′-deoxynucleoside 5′-triphosphate [dNTP], and 0.2 units/μL TEMPase Hot Start DNA Polymerase, Ampliqon III; VWR-Bie, Berntsen, Denmark), and 1 μL extracted DNA. The reaction mixture of the nested Pfcrt PCR was identical to the mixture of the outer PCR, and the primer set TCRD1/TCRD2-biotin was used. Genomic DNA preparation of laboratory isolates 3D7, Dd2, and 7G8 were included as references for wild-type CVMNK and mutant types CVIET and SVMNT haplotypes, respectively. Amplifications were performed in 96-well PCR microplates. The nested PCR products were confirmed by running the controls by electrophoresis on a 1.5% agarose gel.
SSOP-ELISA.
This method has been described in the work by Alifrangis and others.25 Briefly, biotin-conjugated nested PCR products were fixed on streptavidin-coated ELISA plates and incubated overnight at 4°C. After washing three times in washing buffer (1× phosphate-buffered saline [PBS] with 0.05% Tween 20), digoxigenin-labeled oligonucleotide probes with specificity for the haplotypes of interest (CVMNK, CVIET, or SVMNT) were added to each plate and incubated for 1 hour at 53°C. The mixtures were washed with high stringency at 60°C two times for 10 minutes before they were incubated for 1 hour with peroxidase-conjugated antidigoxigenin antibodies (Roche Diagnostics, Mannheim, Germany) and visualized by o-phenylene-diamine (OPD; Dako, Glostrup, Denmark). The SSOP is able to detect both single and mixed haplotypes with high specificity. For each analyses, parasite samples were categorized into single or mixed infections. Infections were considered to be single haplotype when only one was present at optical density (OD) values above the threshold of positivity. Conversely, samples were considered to be mixed if OD values for both haplotypes were above the threshold of positivity. For statistical analysis purposes and adherence to Q-PCR data, parasites carrying the CVMNK haplotype only were classified as wild-type parasite infections, whereas parasite harboring both CVMNK and CVIET haplotypes were considered as resistant parasite infections.
Statistical analysis.
OD values obtained from the ELISA reader were entered in a Microsoft Excel sheet, and the haplotype of each positive sample was determined. Statistical analyses of data were performed with Epi info 6.04a (http://www.cdc.gov/epiinfo/Epi6/EI6dnjp.htm). A χ2 test was used to compare differences in proportions of parasites genotypes. Significance level of statistical tests was set at 0.05 with two sides.
Results
Overall, 9,549 (6,000 in 2009 and 3,549 in 2010) samples were collected from the central site of Senegal, and 1,903 (804 in 2010 and 1,099 in 2011) samples were collected from the southern site. Combining the data, malaria, based on RDT, was more prevalent in the southern site (12.3%; 234/1,903) compared with the central site (3.1%; 298/9,549; P < 0.001). In the same sites from year to year, malaria prevalence was not different (Table 1).
Table 1.
Baseline characteristics for malaria prevalence in the study sites
| Years | Central site | Southern site | ||||
|---|---|---|---|---|---|---|
| 2009 | 2010 | P | 2010 | 2011 | P | |
| No bloodspot collected | 6,000 | 3,549 | 804 | 1,099 | ||
| Total positive for P. falciparum by RDT | 155 | 143 | 81 | 153 | ||
| Malaria prevalence | 2.6% | 4.0% | 0.65 | 10.1% | 14.0% | 0.39 |
Overall, 532 samples were P. falciparum RDT-positive at both sites. Of these samples, 474 parasites isolates were confirmed by microscopy to be P. falciparum only, and 58 isolates were identified as mixed species (P. falciparum plus P. malariae). The 474 parasite isolates were used for the PCR analysis, whereas the mixed species infections were excluded from additional analysis.
PCR efficacy.
Of these isolates, the sequence of 94.7% (449/474) of samples was successfully determined at codons 72–76 of the Pfcrt gene using PCR-SSOP-ELISA or Q-PCR as shown in Table 2.
Table 2.
Prevalence of Pfcrt haplotype (CVMNK, CVIET, and mixed) in our study area
| Years | Central site | Southern site | ||
|---|---|---|---|---|
| 2009 | 2010 | 2010 | 2011 | |
| Number of samples analyzed | 155 | 143 | 81 | 153 |
| Total positive by PCR | 146 | 139 | 81 | 83 |
| CVMNK single infections | 103 (70.5%) | 104 (74.8%) | 53 (65.4%) | 59 (71.0%) |
| CVIET single infections | 29 (19.8%) | 24 (17.2%) | 16 (19.7%) | 15 (18.0%) |
| Mixed CVMNK/CVIET infections | 14 (9.5%) | 11 (7.9%) | 12 (14.8%) | 9 (10.8%) |
Prevalence of Pfcrt haplotypes in central and southern study sites of Senegal.
Results based on the two methodologies were comparable (data not shown), and they revealed that the CVMNK (wild type) haplotype and the CVIET mutant CQR type were present, whereas the SVMNT haplotype was not found in our samples.
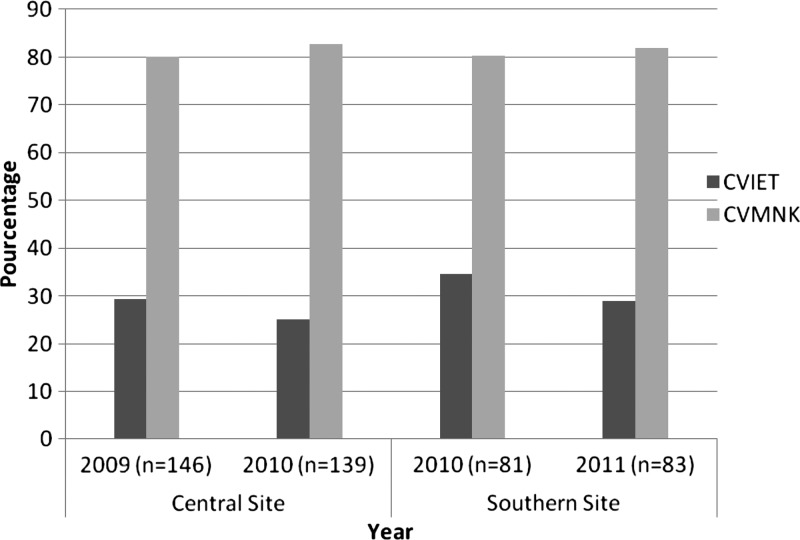
Overall, our results show a high prevalence of wild-type CVMNK haplotype in both years and by site. In the central study site, the prevalence of samples containing parasites harboring the CVMNK haplotype was 80% (117/146) in 2009 and 83% (115/139) in 2010, with no significant difference between the years (P = 0.57) (Figure 1). In the southern study site area, similar results were found; the prevalence of CVMNK was 80% (65/81) in 2010, whereas the prevalence of CVMNK was 82% (68/83) in 2011 (P = 0.78) (Figure 1). Conversely, the prevalence of the CVIET haplotype, including mixed haplotype infections, was 29% (43/146) in 2009 and 25% (35/139) in 2010, with no significant difference between the years (P = 0.49). In the southern study site, similar results were found; the prevalence of CVIET was 35% (28/81) in 2010, whereas it decreased to 29% (24/83) in 2011 (P = 0.43).
Figure 1.
Prevalence of haplotypes in codons 72–76 of the Pfcrt gene in the study areas. Central site includes Mbour, Fatick, and Bambey. Southern site includes Tambacounda, Velingara, and Saraya. Mixed CVIET and CVMNK are included in both sites. CVIET = mutant type; CVMNK = wild type.
Discussion
Drug resistance is a recurrent theme in the history of infectious disease control. In the case of malaria, resistance to previously effective drugs, such as CQ and SP, is widespread in sub-Saharan Africa.24 After resistance was identified, the WHO recommended the use of ACTs, which have now been adopted and implemented in most African countries.7 The Senegalese health authorities, through the NMCP, adopted AS/AQ and AL for uncomplicated malaria treatment in 2006 (depending on availability). With concerns about emerging ACT resistance in Asia,26,27 alternative approaches for malaria control are needed, new drugs need to be developed, and lastly, the possibility of reintroducing abandoned drugs needs to be explored. For instance, the possibility that drugs, such as CQ, previously compromised by resistance can regain efficacy has been observed in Malawi, and it should be investigated. In Malawi, the withdrawal of CQ from the health system was accompanied by a reduction in the prevalence of the molecular marker of CQR (the Pfcrt T76 allele) from 85% to 13% between 1992 and 2000.28 Recently, Kenya and Tanzania have also reported a decline in CQR after replacement of CQ by SP; however, this decline occurred at a somewhat slower rate than in Malawi.29,30
In all, this finding supports the notion that CQR parasites are less fit than wild-type parasites in the absence of CQ drug pressure.31,32 This study investigated the molecular level of CQ resistance by examining the prevalence of Pfcrt. The P. falciparum-positive samples were collected in sites in central and southern Senegal several years after official CQ withdrawal as first-line treatment of uncomplicated malaria. Our results showed that the prevalence of single Pfcrt CVMNK wild-type haplotype was high (above 70% and 60% in central and southern study areas, respectively). By comparing our data with previous studies on SNPs in the Pfcrt gene done on samples from Senegal, it seems that the prevalence of the mutant CVIET (76T) haplotype has decreased dramatically (from a prevalence of 76T, including mixed 76K/76T infections, of 65%, 64%, and 59.5% on samples collected from various sites in Senegal in 2000, 2001, and 2004, respectively,33–36 to approximately 30% in our study) (Figure 1). Compared with these older studies, it is plausible that a general trend to a dramatic decrease in Pfcrt mutant types has happened throughout the country. The low decrease of CQR haplotype observed in previous studies could be because of (1) ongoing CQ pressure despite national policy changes or (2) the fact that the samples were collected in a suburb of Dakar (Pikine), which is a malaria hypoendemic area. The low level of estimated CQ use in Senegal is associated with a declining prevalence of CQR. Thus, in all, the data suggest that the increase in prevalence of Pfcrt wild types is occurring country-wide, although with the precaution that the samples were collected at different sites and based on other sampling designs compared with the present study. High prevalence of the Pfcrt 76K wild type was observed in neighboring Guinea Bissau as early as 2001 and 2004, where the prevalence of Pfcrt K76 wild type was found at 82%, probably because of the fact that CQ has been used at high doses (76 mg/kg). The work by Ursing and others37 showed that high doses of CQ were at least two times more efficacious when treating P. falciparum with Pfcrt 76T. In neighboring Mali, studies conducted from 1996 to 2004 showed a slow decrease of Pfcrt 76T caused by a decrease in but not elimination of CQ use between 2000 and 2006.38
The increase in prevalence of the Pfcrt wild type from 2004 on shown by comparing the limited number of studies in Senegal with the current study is most likely caused by synergic factors of withdrawal of CQ since 2003 and possibly, the introduction of ACT (AL or AS/AQ) for treatment in 2006 as well, because significant selection of the Pfcrt K76 allele after AL treatment has been shown.39 In addition, in 2007, RDTs were introduced to improve malaria diagnosis before malaria treatment, which has reduced drug pressure on P. falciparum parasites, because only confirmed P. falciparum cases optimally will receive ACT treatment. Thus, our findings, in concert with similar observations in countries such as Malawi,28 Kenya,29 and Tanzania,30 suggest that CQ might once again be used combined with other antimalarials for malaria prevention or treatment in the near future. Alternative use of CQ combined with other antimalarial drugs for malaria prevention in, for example, non-vulnerable groups may be a realistic option. Intermittent preventive treatment of malaria in children (IPTc) is now a new strategy for reducing malaria morbidity among children. IPTc involves administration of antimalarial drugs at defined time intervals to individuals, regardless of whether they are known to be infected with malaria, to prevent morbidity and mortality from the infection.40 IPTc has been shown to be effective in reducing malaria burden by several studies.41,42 Indeed, to ensure a maximum protective effect, the IPTc strategy should preferably combine two long half-life drugs.43 The combination of SP/AQ is currently the most optimal regimen. However, other than this combination, few options are available for IPTc. In this context, the combination of CQ and piperaquine or SP should be investigated as an option for IPTc. One major demand for testing such a strategy would be the close and continuous monitoring of CQ resistance, where the prevalence of Pfcrt would most likely provide a sensible guideline.
ACKNOWLEDGMENTS
We would like to express our gratitude to all the study participants, particularly the study population and administrative authorities, the entire staff of the Parasitology and Mycology Department in Senegal, and the Center for Medical Parasitology in Denmark. Our sincere thanks also go to the MR4 staff (ATCC, Manassas, VA) for providing the DNA control parasite and Joy and Deirdre (National Institutes of Health/National Institute of Allergy and Infectious Disease) for correcting the manuscript. M.N. and M.A.conceived the study, designed the experiments, and carried out the molecular genetic analysis. B.F., R.H., and M.A. supervised the study and corrected the manuscript. R.T. performed the statistical analysis. A.L. and A.A. participated in samples collection. O.G. coordinated the study and provided conceptual advice. M.N., B.F., R.T., J.L.N., A.L., A.A., Y.D., D.N., R.H., M.A., and O.G. read and approved the final manuscript.
Disclaimer: None of the authors declared a conflict of interest.
Footnotes
Financial support: This work was supported by the Malaria Capacity Development Consortium, which is funded by Wellcome Trust Grant WT084289MA and Bill & Melinda Gates Foundation Grant 51941 (http://www.mcdconsortium.org).
Authors' addresses: Magatte Ndiaye, Babacar Faye, Roger Tine, Jean Louis Ndiaye, Aminata Lo, and Annie Abiola, Cheikh Anta Diop University—Parasitology and Mycology, Dakar, Senegal, E-mails: magou22000@yahoo.fr, bfaye67@yahoo.fr, rogertine@hotmail.com, jlndiaye@yahoo.fr, amlosn@yahoo.fr, and annie_abiola@yahoo.fr. Yemou Dieng and Oumar Gaye, Dakar Faculty of Medicine—Parasitology and Mycology, Cheikh Anta Diop University, Dakar, Senegal, E-mails: yemoud1@yahoo.fr and ogaye@refer.sn. Daouda Ndiaye, University Cheikh Anta Diop—Laboratory of Parasitology, Dakar, Senegal, E-mail: dndiaye@hsph.harvard.edu. Rachel Hallett, London School of Hygiene and Tropical Medicine, Faculty of Infectious and Tropical Diseases, London, United Kingdom, E-mail: rachel.hallet@lshtm.ac.uk. Michael Alifrangis, Centre for Medical Parasitology, Department of International Health, Immunology and Microbiology, University of Copenhagen, Copenhagen, Denmark; and Department of Infectious Diseases, Copenhagen University Hospital (Rigshospitalet), Copenhagen, Denmark, E-mail: micali@sund.ku.dk.
References
- 1.Trape JF. The public health impact of chloroquine resistance in Africa. Am J Trop Med Hyg. 2001;64((1–2 Suppl)):12–17. doi: 10.4269/ajtmh.2001.64.12. [DOI] [PubMed] [Google Scholar]
- 2.Trape JF, Pison G, Preziosi MP, Enel C, Desgrees DLA, Delauney V, Samb B, Lagarde E, Molez JF, Simondon F. Impact of chloroquine resistance on malaria mortality. C R Acad Sci III. 1998;321:689–697. doi: 10.1016/s0764-4469(98)80009-7. [DOI] [PubMed] [Google Scholar]
- 3.Gaye O, Soumare M, Sambou B, Faye O, Dieng Y, Diouf M, Bah IB, Dieng T, Ndir O, Diallo S. Heterogeneity of chloroquine resistant malaria in Senegal. Bull Soc Pathol Exot. 1999;92:149–152. [PubMed] [Google Scholar]
- 4.Botella DMJ, Valls FJM, Martinez PML, Espacio CA. Plasmodium falciparum resistant to sulfadoxine/pyrimethamine in Senegal. Med Interna. 1991;8:79–81. [Google Scholar]
- 5.Sokhna CS, Trape JF, Robert V. Gametocytaemia in Senegalese children with uncomplicated falciparum malaria treated with chloroquine, amodiaquine or sulfadoxine plus pyrimethamine. Parasite. 2001;8:243–250. doi: 10.1051/parasite/2001083243. [DOI] [PubMed] [Google Scholar]
- 6.Brasseur P, Diallo S, Guiguemde R, Guiyedi V, Kombila M, Ringwald P, Olliaro P. Amodiaquine remains effective for treating uncomplicated malaria in West and Central Africa. Trans R Soc Trop Med Hyg. 1999;93:645–650. doi: 10.1016/s0035-9203(99)90083-4. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . Guidelines for the Treatment of Malaria. 2nd Ed. Geneva, Switzerland: WHO Press; 2010. [Google Scholar]
- 8.Brasseur P, Agnamey P, Gaye O, Vaillant M, Taylor WR, Olliaro P. Efficacy and safety of artesunate plus amodiaquine in routine use for the treatment of uncomplicated malaria in Casamance, southern Senegal. Malar J. 2007;6:150. doi: 10.1186/1475-2875-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roll Back Malaria Partnership Roll Back Malaria. Progress and Impact Series: Focus on Senegal. 2010. http://www.rbm.who.int/ProgressImpactSeries/report4 Available at.
- 10.Ndiaye JL, Faye B, Gueye A, Tine R, Ndiaye D, Tchania C, Ndiaye I, Barry A, Cissé B, Lameyre V, Gaye O. Repeated treatment of recurrent uncomplicated Plasmodium falciparum malaria in Senegal with fixed-dose artesunate plus amodiaquine versus fixed-dose artemether plus lumefantrine: a randomized, open-label trial. Malar J. 2011;10:237. doi: 10.1186/1475-2875-10-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidock DA, Nomura T, Talley AK, Cooper RA, Dzekunov SM, Ferdig MT, Ursos LMB, Sidhu ABS, Naude B, Deitsch KW, Su X-Z, Wootton JC, Roepe PD, Wellems TE. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol Cell. 2000;6:861–871. doi: 10.1016/s1097-2765(05)00077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djimde A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourte Y, Dicko A, Su X-Z, Nomura T, Fidock D, Wellems TE, Plowe CV. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001;344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 13.Chen N, Kyle DE, Pasay C, Fowler EV, Baker J, Peters JM, Cheng Q. pfcrt allelic types with two novel amino acid mutations in chloroquineresistant Plasmodium falciparum isolates from the Philippines. Antimicrob Agents Chemother. 2003;47:3500–3505. doi: 10.1128/AAC.47.11.3500-3505.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagesha HS, Casey GJ, Rieckmann KH, Fryauff DJ, Laksana BS, Reeder JC, Maguire JD, Baird JK. New haplotypes of the Plasmodium falciparum chloroquine resistance transporter (pfcrt) gene among chloroquineresistant parasite isolates. Am J Trop Med Hyg. 2003;68:398–402. [PubMed] [Google Scholar]
- 15.Hayward R, Saliba KJ, Kirk K. Mutations in pfmdr1 modulate the sensitivity of Plasmodium falciparum to the intrinsic antiplasmodial activity of verapamil. Antimicrob Agents Chemother. 2005;49:840–842. doi: 10.1128/AAC.49.2.840-842.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foote S, Kyle D, Martin R, Oduola A, Forsyth K, Kemp D, Cowman A. Several alleles of the multidrug resistance gene are closely linked to chloroquine resistance in Plasmodium falciparum. Nature. 1990;345:255–258. doi: 10.1038/345255a0. [DOI] [PubMed] [Google Scholar]
- 17.Gadalla N, Elzaki SE, Mukhtar E, Warhurst D, Badria El-Sayed, Sutherland CJ. Dynamics of pfcrt alleles CVMNK and CVIET in chloroquine-treated Sudanese patients infected with Plasmodium falciparum. Malar J. 2010;9:74. doi: 10.1186/1475-2875-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutherland CJ, Haustein T, Gadalla N, Armstrong M, Doherty JF, Chiodini PL. Chloroquine-resistant Plasmodium falciparum infections among UK travellers returning with malaria after chloroquine prophylaxis. J Antimicrob Chemother. 2007;59:1197–1199. doi: 10.1093/jac/dkm104. [DOI] [PubMed] [Google Scholar]
- 19.Alifrangis M, Dalgaard MB, Lusingu JP, Vestergaard LS, Staalsoe T, Jensen AT, Enevold A, Ronn AM, Khalil IF, Warhurst DC, Lemnge MM, Theander TG, Bygbjerg IC. Occurrence of the southeast Asian/South American SVMNT haplotype of the chloroquine-resistance transporter gene in Plasmodium falciparum in Tanzania. J Infect Dis. 2006;193:1738–1741. doi: 10.1086/504269. [DOI] [PubMed] [Google Scholar]
- 20.Dia I, Diop T, Rakotoarivony I, Kengne P, Fontenille D. Bionomics of Anopheles gambiae Giles, An. arabiensis Patton, An. funestus Giles and An. nili (Theobald) (Diptera: Culicidae) and transmission of Plasmodium falciparum in a Sudano-Guinean zone (Ngari, Senegal) J Med Entomol. 2003;40:279–283. doi: 10.1603/0022-2585-40.3.279. [DOI] [PubMed] [Google Scholar]
- 21.Wooden J, Kyes S, Sibley CH. PCR and strain identification in Plasmodium falciparum. Parasitol Today. 1993;9:303–305. doi: 10.1016/0169-4758(93)90131-x. [DOI] [PubMed] [Google Scholar]
- 22.Pearce RJ, Drakeley C, Chandramohan D, Mosha F, Roper C. Molecular determination of point mutation haplotypes in the dihydrofolate reductase and dihydropteroate synthase of Plasmodium falciparum in three districts of northern Tanzania. Antimicrob Agents Chemother. 2003;47:1347–1354. doi: 10.1128/AAC.47.4.1347-1354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson PE, Kazadi W, Kamwendo DD, Mwapasa V, Purfield A, Meshnick SR. Prevalence of Pfcrt mutations in Congolese and Malawian Plasmodium falciparum isolates as determined by a new Taqman assay. Acta Trop. 2005;93:97–106. doi: 10.1016/j.actatropica.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Hayton K, Su XZ. Genetic and biochemical aspects of drug resistance in malaria parasites. Curr Drug Targets Infect Disord. 2004;4:1–10. doi: 10.2174/1568005043480925. [DOI] [PubMed] [Google Scholar]
- 25.Alifrangis M, Enosse S, Pearce R, Drakeley C, Roper C, Khalil IF, Nkya WMMM, Ronn AM, Theander TG, Bygjerg IBC. Simple, high-throughput method to detect Plasmodium falciparum single nucleotide polymorphisms in the dihydrofolate reductase, dihydropteroate synthase, and P. falciparum chloroquine resistance transporter genes using polymerase chain reaction- and enzyme-linked immunosorbent assay-based technology. Am J Trop Med Hyg. 2005;72:155–162. [PubMed] [Google Scholar]
- 26.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 27.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kublin JG, Cortese JF, Njunju EM, Mukadam RA, Wirima JJ, Kazembe PN, Djimdé AA, Kouriba B, Taylor TE, Plowe CV. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J Infect Dis. 2003;187:1870–1875. doi: 10.1086/375419. [DOI] [PubMed] [Google Scholar]
- 29.Mwai L, Ochong E, Abdirahman A, Kiara SM, Ward S, Kokwaro G, Sasi P, Marsh K, Borrmann S, Mackinnon M, Nzila A. Chloroquine resistance before and after its withdrawal in Kenya. Malar J. 2009;8:106. doi: 10.1186/1475-2875-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alifrangis M, Lusingu JP, Mmbando B, Dalgaard MB, Vestergaard LS, Ishengoma D, Khalil IF, Theander TG, Lemnge MM, Bygbjerg IC. Five-year surveillance of molecular markers of Plasmodium falciparum antimalarial drug resistance in Korogwe District, Tanzania: accumulation of the 581G mutation in the P. falciparum dihydropteroate synthase gene. Am J Trop Med Hyg. 2009;80:523–527. [PubMed] [Google Scholar]
- 31.Mita T, Kaneko A, Lum JK, Bwijo B, Takechi N, Zungu IL, Tsukahara T, Tanabe K, Kobayakawa T, Bjorkman A. Recovery of chloroquine sensitivity and low prevalence of the Plasmodium falciparum chloroquine resistance transporter gene mutation K76T following the discontinuance of chloroquine use in Malawi. Am J Trop Med Hyg. 2003;68:413–415. [PubMed] [Google Scholar]
- 32.Abdel-Muhsin AM, Mackinnon MJ, Ali E, Nassir el KA, Suleiman S, Ahmed S, Walliker D, Babiker HA. Evolution of drug-resistance genes in Plasmodium falciparum in an area of seasonal malaria transmission in Eastern Sudan. J Infect Dis. 2004;189:1239–1244. doi: 10.1086/382509. [DOI] [PubMed] [Google Scholar]
- 33.Henry M, Diallo I, Bordes J, Ka S, Pradines B, Diatta B, M'baye PS, Sane M, Thiam M, Gueye PM, Wade O, Touze JE, Debonne JM, Rogier C, Fusai T. Urban malaria in Dakar, Senegal: chemosusceptibility and genetic diversity of Plasmodium falciparum isolates. Am J Trop Med Hyg. 2006;75:146–151. [PubMed] [Google Scholar]
- 34.Sarr O, Myrick A, Daily J, Diop BM, Dieng T, Ndir O, Sow PS, Mboup S, Wirth DF. In vivo and in vitro analysis of chloroquine resistance in Plasmodium falciparum isolates from Senegal. Parasitol Res. 2005;97:136–140. doi: 10.1007/s00436-005-1406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarr O, Ahouidi AD, Ly O, Daily JP, Ndiaye D, Ndir O, Mboup S, Wirth DF. Mutations in PFCRT K76T do not correlate with sulfadoxine–pyrimethamine–amodiaquine failure in Pikine, Senegal. Parasitol Res. 2008;103:765–769. doi: 10.1007/s00436-008-1038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ndiaye D, Patel V, Demas A, LeRoux M, Ndir O, Mboup S, Clardy J, Lakshmanan V, Daily JP, Wirth DF. A non-radioactive DAPI-based high-throughput in vitro assay to assess Plasmodium falciparum responsiveness to antimalarials—increased sensitivity of P. falciparum to chloroquine in Senegal. Am J Trop Med Hyg. 2010;82:228–230. doi: 10.4269/ajtmh.2010.09-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ursing J, Kofoed PE, Rodrigues A, Rambo L, Gil JP. Plasmodium falciparum genotypes associated with chloroquine and amodiaquine resistance in Guinea-bissau. Am J Trop Med Hyg. 2007;76:844–848. [PubMed] [Google Scholar]
- 38.Frosch A, Venkatesan M, Laufer MK. Patterns of chloroquine use and resistance in sub-Saharan Africa: a systematic review of household survey and molecular data. Malar J. 2011;10:116. doi: 10.1186/1475-2875-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sisowath C, Petersen I, Veiga MI, Mårtensson A, Premji Z, Björkman A, Fidock DA, Gil JP. In vivo selection of Plasmodium falciparum parasites carrying the chloroquine-susceptible Pfcrt K76 allele after treatment with artemether-lumefantrine in Africa. J Infect Dis. 2009;199:750–755. doi: 10.1086/596738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenwood B. Intermittent preventive treatment–a new approach to the prevention of malaria in children in areas with seasonal malaria transmission. Trop Med Int Health. 2006;11:983–991. doi: 10.1111/j.1365-3156.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 41.Cissé B, Sokhna C, Boulanger D, Milet J, Bâ EH, Richardson K, Hallett R, Sutherland COL, Simondon K, Simondon F, Neal A, Gaye O, Targett G, Jo L, Greenwood B, Trape JF. Seasonal intermittent preventive treatment with artesunate and sulfadoxine-pyrimethamine for prevention of malaria in Senegalese children: a randomised, placebo-controlled, double-blind trial. Lancet. 2006;367:659–667. doi: 10.1016/S0140-6736(06)68264-0. [DOI] [PubMed] [Google Scholar]
- 42.Dicko A, Sagara I, Sissoko MS, Guindo O, Diallo A, Kone M, Toure OB, Sacko M, Doumbo O. Impact of intermittent preventive treatment with sulphadoxine-pyrimethamine targeting the transmission season on the incidence of clinical malaria in children in Mali. Malar J. 2008;7:123. doi: 10.1186/1475-2875-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sohna C, Cisse B, Ba EH, Milligan P, Hallett RA, Sutherland C, Gaye O, Boulanger D, Simondon K, Simondon F, Targett G, Lines J, Greenwood B, Trape JF. A trial of the efficacy, safety and impact on drug resistance of four drug regimens for seasonal intermittent preventive treatment for malaria in Senegalese children. PLoS One. 2009;3:e1471. doi: 10.1371/journal.pone.0001471. [DOI] [PMC free article] [PubMed] [Google Scholar]