Abstract
We reported on a highly sensitive and specific enzyme-linked immunosorbent assay (ELISA) that detects immunoglobulin G (IgG) in urine using rKRP42 antigen for the diagnosis of visceral leishmaniasis (VL). The ELISA was applied to study chronological change in antibody titers in five study areas in Rajshahi district, Bangladesh. A total of 585 subjects without a past VL history were examined at least three times in the 30-month follow-up period; of these subjects, 137 (23.4%) subjects became ELISA-positive at least one time during the study. Among the positive cases, 40 (29.2%) subjects developed clinical VL, and 31 (77.5%) of these subjects showed IgG titers of ≥ 1,000 U more than one time in the study period. Considering only the first ELISA results, 22 subjects with IgG titers of ≥ 1,000 U could be found, and 21 (95.5%) of these subjects turned out to be clinical cases. The high urinary IgG titers (≥ 1,000 U) will help predict possible clinical VL cases and thus, identify an outbreak in its earlier stage.
Introduction
Visceral leishmaniasis (VL; also known as kala-azar) caused by an intracellular protozoan parasite of the Leishmania donovani complex is considered as one of the most neglected diseases.1 Approximately 90% of the 500,000 estimated world annual cases occur in rural areas of Bangladesh, India, Nepal, Sudan, and Brazil, some of the world's poorest regions. The infection could be asymptomatic or cause a progressive illness characterized by prolonged fever, hepatosplenomegaly, weight loss, and even death if left untreated.2 Some 75,000 deaths were reported annually in the world.3 In Bangladesh, the total annual kala-azar cases ranged from 3,965 in 1994 to 8,920 in 2004, with a trend of rising incidence.4 However, the number is substantially underestimated, because kala-azar patients were diagnosed and counted only when they visited government health complexes at the subdistrict level (Thana health complexes). Most VL cases in peripheral health facilities are still treated on the basis of clinical diagnosis and/or the result of an inadequately sensitive and specific formol gel test (aldehyde test).5 It is also well-known that the classical clinical features of VL are shared by several other diseases like malaria, disseminated tuberculosis, and enteric fever, which are also common in many of the endemic areas.6
Demonstration of the causative parasites in aspirates from spleen, bone marrow, and lymph nodes is the most specific diagnostic method, but its application in the field is limited because of technical difficulty, invasiveness, and relatively low sensitivity (except for spleen aspiration).7 Meanwhile, observation of high antibody levels in VL facilitated the development of immunodiagnoses,8 and enzyme-linked immunosorbent assays (ELISAs) with crude or recombinant antigens9–11 and direct agglutinin tests (DATs)12,13 have been providing good diagnostic results. Among others, the recombinant antigen rK39, a part of L. chagasi kinesin-related protein, has been used successfully with ELISA or in a dipstick format.14,15 We recently developed an ELISA to detect immunoglobulin G (IgG) in urine for the diagnosis of VL using a recombinant kinesin-related protein of L. donovani (rKRP42) as antigen (rKRP42 urine ELISA).16
The incubation period of VL generally varies from 2 to 6 months, but it may have a much wider range.17 Because delays in diagnosis and treatment increase the risk of complications and death, an early diagnosis is essential.18,19 Moreover, early diagnosis and treatment may reduce the chance of disease transmission. A study conducted in Bihar state, India, reported that 69% of asymptomatic seropositives by rK39 ELISA and dipstick developed kala-azar within 1 year,20 suggesting that many of the asymptomatic seropositives were in a pre-clinical state.
Recently, urine was considered for samples for the diagnosis of VL, and it was found to have similar sensitivity and specificity to those tests with serum samples when tested with confirmed VL and non-VL samples.16,21–23 In this study, we applied rKRP42 urine ELISA to Bangladeshi subjects (N = 1,384 at registration) and studied the occurrence of clinical cases in a follow-up survey for up to 30 months.
Materials and Methods
Sample collection.
Three different areas, designated as centers 1, 2, and 3, in Godagari Thana, Rajshahi district, were used for this study. Center 1 included subjects from Nobai Bottola and its adjacent villages (Pathorghata, Dohorlongi, Nimghut, Essowripur, and Dainpara). The people were registered in March of 2005 (group A; 302 individuals), July of 2005 (group B; 236 individuals), or March of 2006 (group C; 203 individuals) and followed up at our two times per year visits (Table 1 shows the schedule of visits and ELISA-positive rates). New subjects were also registered at our follow-up visits. Center 2 included 370 subjects from Modhumath and Biroil villages (registered in March of 2006), and in center 3, a total of 273 subjects from Zhinafulbari and Shahpur villages were registered in February of 2007. A brief history was taken from each subject to determine if he/she had received treatment of kala-azar or was suffering from any symptoms of kala-azar at the time of registration. Suspected individuals were checked by project doctors and if necessary, referred to a hospital, and their treatment records were checked later in our next visit. The criteria for diagnosis of VL and initiation of treatment practiced in the hospital are described elsewhere.16 All subjects were asked to collect 5 mL urine in a plastic tube for rKRP42 urine ELISA (below). Urine samples were mixed with preservative, NaN3, at a concentration of 0.1% (wt/vol), transported to Japan at ambient temperature (usually 5–10 days for transportation), and after arrival at Aichi Medical University, kept at 4°C until IgG measurement. These procedures do not affect ELISA results. Our study with filarial antibody showed that NaN3-added urine samples could be kept at 37°C for 4 weeks without deterioration.24
Table 1.
Schedule of urine sample collection and ELISA-positive rates according to study site
| Study site | Number of positive/number examined (percent positive) | ||||||
|---|---|---|---|---|---|---|---|
| March 2005 | July 2005 | March 2006 | August 2006 | February 2007 | July 2007 | March 2008* | |
| Center 1 | |||||||
| Group A | 40/302 (13.2) | 40/218 (18.3) | 30/148 (20.3) | 32/163 (19.6) | 23/125 (18.4) | 16/126 (12.7) | 14/25 (56.0) |
| Group B | 31/236 (13.1) | 14/115 (12.2) | 12/116 (10.3) | 12/95 (12.6) | 15/85 (17.6) | 8/17 (47.1) | |
| Group C | 19/203 (9.4) | 7/113 (6.2) | 4/74 (5.4) | 4/74 (5.4) | 1/3 (33.3) | ||
| Center 2 | 65/370 (17.6) | 18/262 (6.9) | 19/183 (10.4) | 6/105 (5.7) | 5/14 (35.7) | ||
| Center 3 | 17/273 (6.2) | 7/172 (4.1) | 5/15 (33.3) | ||||
Clinical cases and ELISA-positive cases were followed up with more emphasis, resulting in higher rates.
The study was reviewed and approved by the Ethics Committee of Aichi Medical University School of Medicine, Japan, and the Ethical Review Committee of the Bangladesh Medical Research Council.
Categorizing subjects.
The study subjects were categorized into four groups. (1) Clinical VL cases included those subjects who experienced clinical VL during or after registration in our study and were treated at the hospital with sodium stibogluconate or miltefosine. (2) The ELISA-positive cases included those subjects who were asymptomatic but ELISA-positive at least one time during the study period. (3) The ELISA-negative cases included those subjects who were asymptomatic and remained ELISA-negative all through the study period. All subjects in groups 1–3 had no history of kala-azar and were tested at least three times, except for three deceased cases in group 1. (4) Old VL cases included those subjects who had VL before registration in our study. VL was confirmed either by (1) hospital documents indicating treatment for VL or without such documents, (2) a reliable history of fever for more than 2 weeks, hospital admission, and treatment of VL with injection for 20–30 days. None of our old VL cases were treated with miltefosine.
ELISA to detect urinary anti-rKRP42 IgG.
The procedure for producing the antigen and the method of ELISA were described elsewhere.16,25 Briefly, flat-bottomed 96-well microtiter plates (MaxiSorp, Nunc, Denmark) were coated with 1 μg/mL rKRP42 antigen (100 μL/well) overnight at 4°C. After blocking with casein buffer (1% casein in 0.05 M Tris-HCl buffer with 0.15 M NaCl, pH 7.6) for 2 hours at room temperature, the wells were loaded with 100 μL urine and incubated at 37°C for 1 hour. After four washes with phosphate-buffered saline (PBS; pH 7.4) containing 0.05% Tween 20, peroxidase-conjugated goat anti-human IgG (Tago, Camarillo, CA) diluted 1:4,000 with casein buffer was added and incubated at 37°C for 1 hour. After washing four times, 2, 2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS)substrate (KPL Inc., Gaithersburg, MD) was reacted for coloration for 1 hour at room temperature. The optical density (OD) was measured at 415 nm, and OD of 492 nm was used as a reference. Each sample was assayed in duplicate; if the absorbance values of the duplicates differed > 40% from their average, the sample was retested. Antibody levels were expressed arbitrarily as unit, which was estimated from the standard curve constructed for each plate with serially diluted positive sera. The cutoff value was 57.9 U.
Results
Prevalence of urinary anti-rKRP42 IgG and occurrence of clinical cases analyzed by study site, sex, and age group.
The field activities and results are shown in Table 1 chronologically. In the first visit to the group A site, 302 subjects were examined by ELISA, and 40 positives (13.2%) were obtained. In groups B and C and centers 2 and 3, the positive rates at the first visit were 13.1%, 9.4%, 17.6%, and 6.2%, respectively. The rates were not different among the groups in center 1 (χ2 test, 2 d.f. [degrees of freedom], P = 0.36), and center 2 had a significantly higher rate than group C (P = 0.008) and center 3 (P < 0.001). The positive rates in March of 2008 were unusually high, because clinical cases and past ELISA-positives were examined with more emphasis.
In Table 2, the ELISA-positive rates in the first test (N = 1,320) and the occurrence of clinical cases in 585 subjects tested at least three times during the study (three deceased clinical VL cases tested one or two times were included) were analyzed by site of study. In this case, old VL cases (N = 64) were excluded. The ELISA-positive rate in center 2 was significantly higher than rates of group B, group C, and center 3 (χ2 test, P < 0.002–0.021). The mean IgG titer among the positive cases was higher in group A and center 3 than in groups B and C and center 2 (analysis of variance [ANOVA], P < 0.001; Tukey–Kramer, P < 0.05), and accordingly, the proportion of high titer-positive values (≥ 1,000 U) was higher in the former two sites than in the latter three sites (Fisher exact test, P < 0.001–0.039). A total of 19 clinical VL cases were confirmed within 3 months of the first ELISA test: 14 cases in group A and 5 cases in center 3; 11 of 14 and 4 of 5 cases had high titers of ≥ 1,000 U at the first test. All these findings would indicate that an outbreak was beginning in group A and center 3 just when our first ELISA test was conducted.
Table 2.
ELISA-positive rates and IgG titers in the first examination according to study site and the occurrence of clinical cases detected during the study period
| Study site | Number of ELISA-positive cases/number examined* (%) | Geometric mean titer (U) among the positives | Number of positives with ≥ 1,000 U (%) | Number of clinical cases confirmed within (months) | Total clinical cases/number examined† (%) | |||
|---|---|---|---|---|---|---|---|---|
| < 3 | 3 to < 6 | 6 to < 12 | ≥ 12 | |||||
| Center 1 | ||||||||
| Group A | 28/288 (9.7) | 1,257.8 | 19 (67.9) | 14 | 4 | 0 | 8 | 26/205 (12.7) |
| Group B | 17/220 (7.7) | 146.1 | 1 (5.9) | 0 | 0 | 1 | 5 | 6/116 (5.2) |
| Group C | 14/196 (7.1) | 130.5 | 0 (0.0) | 0 | 0 | 1 | 0 | 1/80 (1.3) |
| Center 2 | 49/348 (14.1) | 174.5 | 2 (4.1) | 0 | 1 | 0 | 0 | 1/172 (0.6) |
| Center 3 | 15/268 (5.6) | 515.8 | 6 (40.0) | 5 | 0 | 0 | 1 | 6/12 (50.0) |
| Total | 123/1,320 (9.3) | 19 | 5 | 2 | 14 | 40/585 (6.8) | ||
Data from the first test (excluding old VL cases).
All cases were tested at least three times during the study period except for three deceased VL cases.
The clinical rate was analyzed by sex and age group for the 585 subjects (Table 3). The rates for males and females were 8.1% (N = 223) and 6.1% (N = 362), respectively, and there was no difference between them (χ2 test, P = 0.353). The rates by age group ranged from 3.7% to 14.5%, and there were no differences among them (χ2 test, 4 d.f., P = 0.060).
Table 3.
Clinical rate analyzed by sex and age group
| Age group | Male | Female | Total (%) |
|---|---|---|---|
| 0–9 | 5/90 | 9/121 | 14/211 (6.6) |
| 10–19 | 2/53 | 2/56 | 4/109 (3.7) |
| 20–29 | 5/21 | 6/55 | 11/76 (14.5) |
| 30–39 | 2/19 | 2/56 | 4/75 (5.3) |
| ≥ 40 | 4/40 | 3/74 | 7/114 (6.1) |
| Total (%) | 18/223 (8.1) | 22/362 (6.1) | 40/585 (6.8) |
Occurrence of clinical VL cases according to the level of IgG titer.
The 585 subjects were classified according to the highest titer obtained during the study period (Table 4). There were 40 (6.8%) clinical VL cases, 97 (16.6%) ELISA-positives, and 448 (76.6%) ELISA-negatives. All of the clinical cases were ELISA-positive, and 77.5% (31 of 40) had IgG titers of ≥ 1,000 U, whereas among 42 positive cases with ≥ 1,000 U, 31 (73.8%) cases were clinical. The occurrence rates of clinical VL were 0%, 9.5%, 44.4%, and 81.8%, respectively, in the < 57.9, ≥ 57.9 to < 1,000, ≥ 1,000 to < 3,000, and ≥ 3,000 U levels. The difference was significant in three pairs of adjacent titer levels: < 57.9 versus ≥ 57.9 to < 1,000 U (Fisher exact test, P < 0.001), ≥ 57.9 to < 1,000 versus ≥ 1,000 to < 3,000 U (P = 0.011), and ≥ 1,000 to < 3,000 versus ≥ 3,000 U (P = 0.038). The lengths of the follow-up periods were very similar (28.8–32.4 months) among the different titer groups, and they did not seem to influence the occurrence rates, although the ≥ 3,000 U level had a statistically longer period than the ≥ 57.9 to < 1,000 U (ANOVA, P = 0.028; Scheffé test, P < 0.05). Most of the ELISA-positives without disease showed low IgG titers, with 88.7% having < 1,000 U (average = 60.8 U), and they were not constantly positive. Only 24 of 97 (24.7%) became positive cases more than one time.
Table 4.
Clinical VL cases, ELISA-positive cases, and ELISA-negative cases classified by individual's highest ELISA titer obtained during the study period
| Highest IgG titer in the study period (U) | Clinical VL (%) | ELISA positive (%) | ELISA negative (%) | % Clinical rate | Follow-up period in months (SD) |
|---|---|---|---|---|---|
| < 57.9 | 0 (0.0) | – | 448 (100) | 0.0 (0/448) | 29.5 (5.5) |
| ≥ 57.9 to < 1,000 | 9 (22.5) | 86 (88.7) | – | 9.5 (9/95) | 28.8 (6.8) |
| ≥ 1,000 to < 3,000 | 4 (10.0) | 5 (5.2) | – | 44.4 (4/9) | 29.8 (8.3) |
| ≥ 3,000 | 27 (67.5) | 6 (6.2) | – | 81.8 (27/33) | 32.4 (8.0) |
| Total | 40 (100) | 97 (100) | 448 (100) | 6.8 (40/585) | 29.5 (6.0) |
All cases did not have a history of VL before the present study. They were examined three times or more during the study period. The ELISA-positive and –negative cases were without VL symptoms throughout the study.
The clinical, ELISA-positive, and ELISA-negative subjects were also analyzed according to the IgG titer determined at the first ELISA test (Table 5); 21 of 40 clinical cases (52.5%) had IgG titers of ≥ 1,000 U, and 21 of 22 subjects (95.5%) who had IgG titers of ≥ 1,000 U at the first test developed clinical VL. The occurrence rates of clinical VL were 1.8%, 14.9%, 85.7%, and 100%, respectively, in the < 57.9, ≥ 57.9 to < 1,000, ≥ 1,000 to < 3,000, and ≥ 3,000 U titer levels. The rates are different between the < 57.9 and ≥ 57.9 to < 1,000 U levels (Fisher exact test, P < 0.001) and the ≥ 57.9 to < 1,000 and ≥ 1,000 to < 3,000 U levels (P < 0.001) but not between the highest two levels (P = 0.318). The incubation period, defined as the time in months from the first urine test to the hospital admission, was shortest in the ≥ 3,000 U level (4.8 months), which was different statistically from period of the < 57.9 U level (14.6 months; ANOVA, P = 0.039, Tukey–Kramer, P < 0.05).
Table 5.
Clinical VL cases, ELISA-positive cases, and ELISA-negative cases classified by individual ELISA titer observed at the first examination
| IgG titer of the first test (U) | Clinical VL (%) | ELISA positive (%) | ELISA negative (%) | % Clinical rate | Incubation period in months* (SD) |
|---|---|---|---|---|---|
| < 57.9 | 9 (22.5) | 39 (40.2) | 448 (100) | 1.8 (9/496) | 14.6 (7.5) |
| ≥ 57.9 to < 1,000 | 10 (25.0) | 57 (58.8) | – | 14.9 (10/67) | 8.8 (8.2) |
| ≥ 1,000 to < 3,000 | 6 (15.0) | 1 (1.0) | – | 85.7 (6/7) | 8.5 (8.6) |
| ≥ 3,000 | 15 (37.5) | 0 (0.0) | – | 100 (15/15) | 4.8 (6.9) |
| Total | 40 (100) | 97 (100) | 448 (100) | 6.8 (40/585) | 8.6 (8.2) |
The definitions for subject categories are the same as in Table 4.
Average from the first ELISA test to hospital admission.
Reduction of IgG titers after recovery from VL.
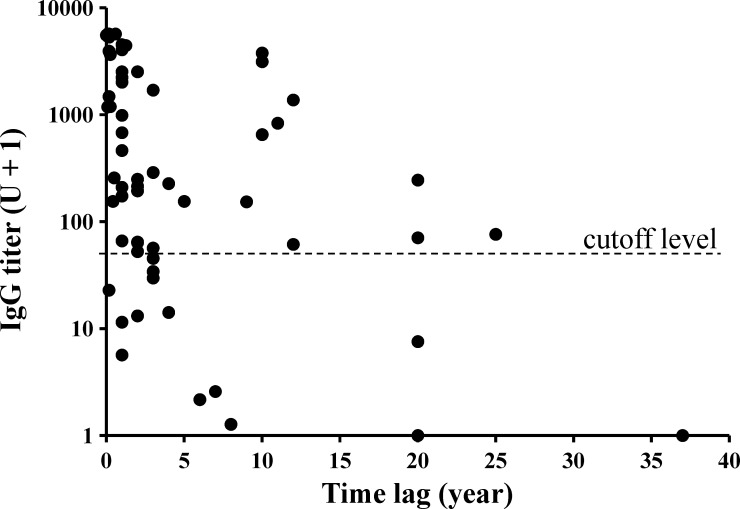
Old VL cases were used to analyze the change in titers after the latest recovery. There were 64 such cases registered, but 3 of these cases were excluded from this analysis, because they developed clinical VL during the present study (recurrence rate = 4.7%). Their IgG titers obtained at our first urine test and time lags in years between the test and the last VL episode are plotted in Figure 1. When special attention was paid to high titers of ≥ 1,000 U, there seemed to be two separate peaks: one peak just before the present study and the other one around 10 years ago. In the more recent peak, titers decreased relatively rapidly to the cutoff level in 3.5 years (r = 0.69, P < 0.001; log [unit + 1] = −0.392 [years] + 3.158), and the computation was made with 48 data points found within the time lag of ≤ 8 years.
Figure 1.
Anti-rKRP42 IgG titers in urine observed among the old VL cases after different time lags between the first urine test and the latest recovery.
Discussion
Prediction of possible clinical VL cases will be valuable for not only early treatment of patients but taking measures to interrupt transmission. For the prediction, antibody detection will be beneficial because of its high sensitivity. We reported rKRP42 recombinant antigen-based ELISA, which uses urine samples,16 and the ELISA was evaluated in the present study for the effectiveness in predicting the occurrence of clinical cases. The study started in March of 2005 and continued until March of 2008. Five study sites (groups A, B, and C and centers 2 and 3) were selected, and a total of 1,384 people were examined in the first examination. Just after the study, we noticed many high-titer ELISA-positive people in group A. Later, we concluded that the outbreak was actually starting in group A, and also, it was starting in center 3 after February of 2007 (Tables 1 and 2), because the two sites had significantly higher rates of positive cases with ≥ 1,000 U than the other three sites; a majority of them turned out to be clinical VL within 3 months. This temporal coincidence provided an opportunity to analyze antibody titers in the course of an outbreak. In 2004, the Institute of Epidemilogy, Disease Control and Research (IEDCR), Bangladesh, reported a kala-azar outbreak in Godagari Thana, the same subdistrict where the present study was carried out.26
A total of 585 individuals, who did not have a past history of kala-azar and were examined three times or more, was followed up for an average period of 29.5 months (Table 4). Of these subjects, 137 (23.4%) subjects were found to be ELISA-positive at least one time. Among the positive cases, 40 (29.2%) subjects developed clinical VL. Of 42 subjects with IgG titers of ≥ 1,000 U, 31 (73.8%) subjects became clinical cases in the study period, indicating that 1,000 U can be a critical level for predicting clinical cases. In addition, it seemed important for the high titers to be maintained; 11 subjects with a titer ≥ 1,000 U did not develop clinical VL (Table 4), of whom 8 subjects had an IgG titer ≥ 1,000 U at a single point of measurement and 3 subjects exceeded ≥ 1,000 U more than one time. Sex and age did not influence clinical rate. When only the first ELISA test was taken into account, which is a more realistic situation in a field survey, 22 positive cases with ≥ 1,000 U were detected, and 21 (95.5%) of these subjects developed clinical VL (Table 5). Thus, a single urine test could have predicted 52.5% (21 of 40) of the total clinical cases. The inclusion of group A and center 3, which were in the early stage of outbreak, probably contributed to the high prediction rate. A similar relationship between the high occurrence of clinical VL and high antibody titers was reported in the works by Singh and others.20,27 The same works also noted that anti-rK39 IgG titer in serum correlated with the parasite load and that persistent increase of antibody titers related to clinical unresponsiveness to antileishmanial treatment. The prevalence by rKRP42 urine ELISA alone does not seem to be useful, because in the first test, the highest rate was recorded in center 2 (Tables 1 and 2); however, the clinical rate there turned out to be the lowest, with only one sporadic VL case. A quantitative test is definitely needed for the prediction.
By observing the change in IgG titers among old VL subjects, with particular attention to ≥ 1,000 U titers, two possible peaks were recognizable (Figure 1). It was speculated that there could have been an outbreak about 10 years ago. Using the data from a recent outbreak, IgG titers were found to decrease relatively rapidly and become negative in 3.5 years after cure. However, high titers can persist even after 10 years because of occasional antigenic stimuli or continued active infections. In the present study, there were two ELISA-positive subjects who had persistently high IgG levels > 1,000 U for more than 2.5 years without developing clinical symptoms. These subjects are an interesting group of people; their possible roles as reservoir hosts may need to be clarified.
A major limitation of our study was that the follow-up surveys were done only two times per year. There were nine clinical cases with < 1,000 U (Table 4), and these titers could have become higher if examined several more times. Also, most clinical cases were confirmed retrospectively by questioning and/or checking medical records, if available. Information obtained from memory of years ago might include inaccuracies. Furthermore, the study was biased to including more clinical and ELISA-positive subjects than ELISA-negative subjects. Despite these weaknesses, our study has shown that, when an outbreak of VL is suspected, mass screening by urine ELISA and follow-up of the high titer-positive cases is an effective strategy for the early diagnosis and containment of transmission. The use of urine samples has definite advantages in the field because of non-invasive collection. In the village, where many people had experienced blood sampling, urine collection was accepted well or even welcomed.28 However, a combined use of antigen tests and/or molecular diagnoses will be required to confirm the active infection.
ACKNOWLEDGMENTS
We would like to thank Mr. Md. Rajaul Karim, Mr. Md. Rafiqul Islam, Mr. Md. Bazlur Rahman, Mr. Md. Tareq Ibn Morshed, Mr. Md. Yunus Ali, and Mr. Md. Kayum Ali for their generous help in sample collection. We would also like to thank Mr. Fumiaki Nagaoka for his technical support.
Footnotes
Financial support: This study was supported by Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research for JSPS Postdoctoral Fellowship for Foreign Researchers 1604227, Grant-in-Aid for Scientific Research (B) 15406018, and Grant-in-Aid for Scientific Research (B) 18406013. This work was also supported by the Special Coordination Funds for Promoting Science and Technology of Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (1200015).
Authors' addresses: Mohammad Zahidul Islam, Makoto Itoh, Hidekazu Takagi, Atsuhide Takesue, and Eisaku Kimura, Department of Parasitology, Aichi Medical University School of Medicine, Nagakute, Aichi-ken, Japan, E-mails: islammz@yahoo.com, macitoh@aichi-med-u.ac.jp, htakagi@aichi-med-u.ac.jp, atakesue@aichi-med-u.ac.jp, and kimura@aichi-med-u.ac.jp. Present address of Mohammad Zahidul Islam, Department of Biology, The Catholic University of America, Washington, DC. Md. Anwar Ul Islam and Md. Ajijur Rahman, Department of Pharmacy, University of Rajshahi, Rajshahi, Bangladesh, E-mails: profanwarulislam@yahoo.com and shamim_ru2003@yahoo.com. A. R. M. Saifuddin Ekram, Department of Medicine, Rajshahi Medical College, Rajshahi, Bangladesh, E-mail: armsekram@yahoo.com. Yoshihisa Hashiguchi, Prometeo Project, Centro de Biomedicina, Universidad Central del Ecuador, Quito, Ecuador and Department of Parasitology, Kochi Medical School, Kochi University, Nankoku City, Kochi, Japan, E-mail: hasiguti@kochi-u.ac.jp.
Reprint requests: Makoto Itoh, Department of Parasitology, Aichi Medical University School of Medicine, Nagakute, Aichi-ken 480-1195, Japan, E-mail: macitoh@aichi-med-u.ac.jp.
References
- 1.Trouiller P, Olliaro P, Torreele E, Orbinski J, Laing R, Ford N. Drug development for neglected diseases: a deficient market and a public health policy failure. Lancet. 2002;359:2188–2194. doi: 10.1016/S0140-6736(02)09096-7. [DOI] [PubMed] [Google Scholar]
- 2.Pearson R, Sausa QA. Clinical spectrum of leishmaniasis. Clin Infect Dis. 1996;22:1–13. doi: 10.1093/clinids/22.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Bern C, Chowdhury R. The epidemiology of visceral leishmaniasis in Bangladesh: prospects for improved control. Indian J Med Res. 2006;123:275–288. [PubMed] [Google Scholar]
- 5.Chowdhur MA, Rafiqueuddin AK, Hossain A. Aldehyde test (formol-gel test) in the diagnosis of kala-azar (visceral leishmaniasis) Trop Doct. 1992;22:185–186. doi: 10.1177/004947559202200432. [DOI] [PubMed] [Google Scholar]
- 6.Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol. 2002;9:951–958. doi: 10.1128/CDLI.9.5.951-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zijlstra EE, Ali MS, el-Hassan AM, el-Toum IA, Satti M, Ghalib HW, Kager PA. Kala-azar: a comparative study of parasitological methods and the direct agglutination test in diagnosis. Trans R Soc Trop Med Hyg. 1992;86:505–507. doi: 10.1016/0035-9203(92)90086-r. [DOI] [PubMed] [Google Scholar]
- 8.Ghose AC, Haldar JP, Pal SC, Mishra BP, Mishra KK. Serological investigations on Indian kala-azar. Clin Exp Immunol. 1980;40:318–326. [PMC free article] [PubMed] [Google Scholar]
- 9.Kaul P, Malla N, Kaur S, Mahajan RC, Ganguly NK. Evaluation of a 200-kDa amastigote-specific antigen of L. donovani by enzyme-linked immunosorbent assay (ELISA) for the diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2000;94:173–175. doi: 10.1016/s0035-9203(00)90264-5. [DOI] [PubMed] [Google Scholar]
- 10.Raj VS, Ghosh A, Dole VS, Madhubala R, Myler PJ, Stuart KD. Serodiagnosis of leishmaniasis with recombinant ORFF antigen. Am J Trop Med Hyg. 1999;61:482–487. doi: 10.4269/ajtmh.1999.61.482. [DOI] [PubMed] [Google Scholar]
- 11.Zijlstra EE, Daifalla NS, Kager PA, Khalil EAG, Hassan AME, Reed SG, Ghalib HW. rK39 enzyme-linked immunosorbent assay for diagnosis of Leishmania donovani infection. Clin Diagn Lab Immunol. 1998;5:717–720. doi: 10.1128/cdli.5.5.717-720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harith AE, Kolk AHJ, Leeuwenburg J, Muigai R, Kiugu S, Kiugu S, Laarman JJ. A simple and economical direct agglutination test for serodiagnosis and sero-epidemiological studies of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1986;80:583–587. doi: 10.1016/0035-9203(86)90149-5. [DOI] [PubMed] [Google Scholar]
- 13.Harith AE, Kolk AHJ, Leeuwenburg J, Muigai R, Huigen E, Jelsma T, Kager PA. Improvement of a direct agglutination test for field studies of visceral leishmaniasis. J Clin Microbiol. 1988;26:1321–1325. doi: 10.1128/jcm.26.7.1321-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns JM, Shreffler WG, Benson DR, Ghalib HW, Badaro R, Reed SG. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African American visceral leishmaniasis. Proc Natl Acad Sci USA. 1993;90:775–779. doi: 10.1073/pnas.90.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maalej IA, Chenik M, Louzir H, Salah AB, Bahloul C, Amri F, Dellagi K. Comparative evaluation of ELISAs based on ten recombinant or purified leishmania antigens for the serodiagnosis of Mediterranean visceral leishmaniasis. Am J Trop Med Hyg. 2003;68:312–320. [PubMed] [Google Scholar]
- 16.Islam MZ, Itoh M, Takagi H, Islam AU, Ekram ARMS, Rahman A, Atsuhide T, Hashiguchi Y, Kimura E. Enzyme-linked immunosorbent assay to detect urinary antibody against recombinant rKRP42 antigen made from Leishmania donovani for the diagnosis of visceral leishmaniasis. Am J Trop Med Hyg. 2008;79:599–604. [PubMed] [Google Scholar]
- 17.CDC CDC—Parasites—Leishmaniasis. Resources for Health Professionals. 2010. http://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html Available at. Accessed December 28, 2011.
- 18.Ahluwalia IB, Bern C, Costa C, Akter T, Chowdhury R, Ali M, Alam D, Kenah E, Amann J, Islam M, Wagatsuma Y, Haque R, Breiman RF, Maguire JH. Visceral leishmaniasis: consequences of a neglected disease in a Bangladeshi community. Am J Trop Med Hyg. 2003;69:624–628. [PubMed] [Google Scholar]
- 19.Collin S, Davidson R, Ritmeijer K, Keus K, Melaku Y, Kipngetich S, Davies C. Conflict and kala-azar: determinants of adverse outcome of kala-azar among patients in Southern Sudan. Clin Infect Dis. 2004;38:612–619. doi: 10.1086/381203. [DOI] [PubMed] [Google Scholar]
- 20.Singh S, Kumari V, Singh N. Predicting kala-azar disease manifestations in asymptomatic patients with latent Leishmania donovani infection by detection of antibody against recombinant rK39 antigen. Clin Diagn Lab Immunol. 2002;9:568–572. doi: 10.1128/CDLI.9.3.568-572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Islam MZ, Itoh M, Shamsuzzaman SM, Mirza R, Matin F, Ahmed I, Choudhury AKMS, Hossain MA, Qiu XG, Begam N, Furuya M, Leafasia JL, Hashiguchi Y, Kimura E. Diagnosis of visceral leishmaniasis by ELISA using urine samples. Clin Diagn Lab Immunol. 2002;9:789–794. doi: 10.1128/CDLI.9.4.789-794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Islam MZ, Itoh M, Mirza R, Ahmed I, Ekram ARMS, Sarder AH, Shamsuzzaman SM, Hashiguchi Y, Kimura E. Direct agglutination test with urine samples for the diagnosis of visceral leishmaniasis. Am J Trop Med Hyg. 2004;70:78–82. [PubMed] [Google Scholar]
- 23.Attar ZJ, Chance ML, el-Safi S, Carney J, Azazy A, El-Hadi M, Dourado C, Hommel M. Latex agglutination test for the detection of urinary antigens in visceral leishmaniasis. Acta Trop. 2001;78:11–16. doi: 10.1016/s0001-706x(00)00155-8. [DOI] [PubMed] [Google Scholar]
- 24.Itoh M, Weerasooriya MV, Qui X-G, Gunawardena NK, Anantaphruti MT, Tesana S, Rattanaxay P, Fujimaki Y, Kimura E. Sensitive and specific enzyme-linked immunosorbent assay for the diagnosis of Wuchereria bancrofti infection in urine samples. Am J Trop Med Hyg. 2001;65:362–365. doi: 10.4269/ajtmh.2001.65.362. [DOI] [PubMed] [Google Scholar]
- 25.Takagi H, Islam MZ, Itoh M, Islam MAU, Ekram ARMS, Hussain HM, Hashiguchi Y, Kimura E. Production of recombinant kinesin-related protein of Leishmania donovani and its application in the serodiagnosis of visceral leishmaniasis. Am J Trop Med Hyg. 2007;76:902–905. [PubMed] [Google Scholar]
- 26.Ahmed BN. Report on Outbreak Investigation, Kala azar in Godagari. IEDCR in Action. Dhaka, Bangladesh: Institute of Epidemiology, Disease Control & Research; 2004. Series 2. [Google Scholar]
- 27.Singh S, Reed SG, Sacks AG, Chang KP. Diagnostic and prognostic value of rK39 antigen in Indian leishmaniasis. J Parasitol. 1995;81:1000–1003. [PubMed] [Google Scholar]
- 28.Weerasooriya MV, Itoh M, Islam MZ, Qiu XG, Fujimaki Y, Kimura E. Prevalence and levels of filaria-specific urinary IgG4 among children less than five years of age and the association of the antibody positivity between the children and their mothers. Am J Trop Med Hyg. 2003;68:465–468. [PubMed] [Google Scholar]