Abstract
We evaluated the inhibitory effects of pepstatin A and mefloquine on the in vitro and in vivo growths of Babesia parasites. The in vitro growth of Babesia bovis, B. bigemina, B. caballi, and B. equi was significantly inhibited (P < 0.05) by micromolar concentrations of pepstatin A (50% inhibitory concentrations = 38.5, 36.5, 17.6, and 18.1 μM, respectively) and mefloquine (50% inhibitory concentrations = 59.7, 56.7, 20.7, and 4 μM, respectively). Furthermore, both reagents either alone at a concentration of 5 mg/kg or in combinations (2.5/2.5 and 5/5 mg/kg) for 10 days significantly inhibited the in vivo growth of B. microti in mice. Mefloquine treatment was highly effective and the combination treatments were less effective than other treatments. Therefore, mefloquine may antagonize the actions of pepstatin A against babesiosis and aspartic proteases may play an important role in the asexual growth cycle of Babesia parasites.
Introduction
Babesiosis is an infectious disease caused by an intraerythrocytic protozoon of the genus Babesia; the parasites are transmitted by the bite of ticks of family Ixodidae such as Boophilus, Dermacentor, Rhipicephalus, and Haemaphysalis.1 The main clinical symptoms of babesiosis in infected animals include fever, hemolytic anemia, jaundice, hemoglobinuria, and edema.2 A well-recognized disease of veterinary importance in cattle, horses, and dogs, babesiosis has also received increased attention as a globally distributed zoonosis in humans. In addition, serious economic losses have been caused by Babesia infections in the livestock and other industries.3–5
Although some anti-babesial agents have been used to control the disease, continuous searches for the development of new drugs against Babesia are caused by toxic side effects, repeated relapse of parasite infections, and the possibility of emerging drug-resistant parasites.6 Several novel anti-babesial drugs, such as triclosan,6 artesunate, pyrimethamine, pamaquine,7 heparin,8 imidazole derivatives, staurosporine,9 and cysteine protease inhibitors,10 have been successfully studied by using in vitro and in vivo models. However, these drugs have not been evaluated for field application. Therefore, development of new compounds that have chemotherapeutic effects against babesiosis with high specificity for the parasite and no side effects in the host is desired.
Aspartic proteases (APs) are a widely distributed family of enzymes among protozoan parasites, and several APs, including those of Plasmodium falciparum (plasmepsin),11 Eimeria tenella (eimepsin),12,13 Cryptosporidium parvum (cryptomepsin), and Trypanosoma cruzi (cruzipsin I and II),14 have been characterized. Among them, the P. falciparum (plasmepsin)11 enzyme of this class initiates the hemoglobin breakdown pathway that provides intraerythrocytic malaria parasites with nutritional resources. Inhibition of their activity results in the death of malaria parasites.15–17 Pepstatin A, a potent inhibitor of AP, binds to the active site of plasmepsins in food vacuoles of P. falciparum,18 prevents the degradation of hemoglobin, and kills malaria parasites. In in vitro studies, pepstatin A had a potent effect against cultured P. falciparum.19,20 In an in vivo study using a murine malaria model, pepstatin A cured P. vinckei-infected mice.20 Moreover, P. vivax AP is inhibited by pepstatin.21
Mefloquine is currently one of the recommended chemoprophylactic regimens for travelers visiting malaria-endemic areas.22 Recently, mefloquine has been used for treatment23,24 and prophylaxis22,25 against P. falciparum. Mefloquine works by attacking parasites once they have entered erythrocytes by killing the parasites and preventing them from further multiplications. However, its exact mechanism is unknown. A possible explanation is that mefloquine, which is similar to chloroquine and quinine, appears to interfere with the ability of the parasite to metabolize and use erythrocyte hemoglobin.26 Mefloquine might bind free heme, thus inhibiting the polymerization of heme or the swelling of food vacuoles. Conversely, mefloquine is believed to act by forming toxic heme complexes that damage parasitic food vacuoles. In addition, Mungthin and others27 reported that combination of plasmepsin I, the Ro40-3488 inhibitor, with a drug such as chloroquine, amodiaquine, quinine, or mefloquine, resulted in antagonism between plasmepsin I inhibitor and chloroquine and mefloquine.
The class of enzymes known as APs has not yet been characterized in Babesia parasites. Additionally, Babesia parasites have similarities to malaria parasites and the AP target genes present in the Babesia genome sequence database.28 Thus, the present study was conducted to evaluate possible inhibitory effects of pepstatin A and mefloquine, alone or combined, on the growth of bovine and equine Babesia parasites in in vitro and B. microti) in in vivo experiments.
Materials And Methods
Parasites.
The Texas strain of B. bovis, the Argentina strain of B. bigemina, the U.S. Department of Agriculture strains of B. caballi and B. equi, and the Munich strain of B. microti were used in this study. Parasites were grown in bovine and equine erythrocytes by using a continuous micro-aerophilous stationary phase culture system.8 Medium M199 (for bovine Babesia and B. equi) and RPMI 1640 (for B. caballi) (both from Sigma-Aldrich, Tokyo, Japan) supplemented with 40% bovine serum (for bovine Babesia) or equine serum (for equine Babesia) were used in the culture media. Penicillin G (60 U/mL), streptomycin (60 μg/mL), and amphotericin B (0.15 μg/mL) were added to the culture media. Hypoxanthine (13.6 μg/mL) was added to B. equi culture.29,30 N-Tris (hydroxymethyl) methyl-2-ainomethanesulfonic acid hemisodium salt (229 mg/mL) was added to bovine Babesia parasite cultures as a pH stabilizer (pH 7.2).29,31,32 Culture plates for parasites were incubated in atmospheres of 5% CO2 and 5% O2 at 37°C.29
Mice.
The Munich strain of B. microti was maintained by passage in blood of BALB/c mice. Twenty-four female BALB/c mice (8 weeks old) were obtained from CLEA Japan (Tokyo, Japan) and were used for in vivo studies.
Chemical reagents.
Pepstatin A (Isovaleryl-L-Val-L-Val-AHMHA-L-ALa-AHMHA (AHMHA= (3S, 4S)-4-amino-3-hydroxy-6-methyl-heptanoic acid) was obtained from Peptide Institute, Inc. (Osaka, Japan). Mefloquine (AS)-rel-a-(2R)-2-piperidinyl-2, 8-bis(trifluoromethyl)-4-quinolinemethanol monohydrochloride was obtained from Sigma-Aldrich. A working stock solution of 10 mM pepstatin A and mefloquine dissolved in dimethyl sulfoxide (DMSO) (Wako Pure Chemical Industrial, Ltd., Osaka, Japan) was prepared and stored at −20°C until use. Diminiazine aceturate was obtained from Ciba-Geigy Japan, Ltd. (Tokyo, Japan) and used as a comparator drug. A stock solution of 10 mM was prepared in distilled water and stored at –30°C until use. Tetracycline hydrochloride was obtained from Sigma-Aldrich (St. Louis, MO) and used as a comparator drug. A stock solution of 20 mM tetracycline hydrochloride was prepared in distilled water and stored at –30°C until use.
In vitro growth inhibition assay and drug combination test.
The inhibitory effects of AP inhibitors upon Babesia growth were examined as described.8 Parasite-infected erythrocytes were obtained from cultures with parasitemias of approximately 6–8%. Twenty microliters of erythrocytes with a parasitemia of 1% was dispensed into a 96-well microtiter plate (Nunc, Roskilde, Denmark) with 200 μL of the culture medium containing the indicated concentration of pepstatin A (5, 25, 50, 100, 250, and 500 μM) and mefloquine (1, 5, 25, 50, 100, and 500 μM) and then incubated at 37°C in a humidified multi-gas water-jacketed incubator. For the experimental control, cultures without the drug and cultures containing only 0.1% DMSO (for pepstatin A and mefloquine) were prepared. In addition, a solution of 2 μM diminazene aceturate and 100 μM tetracycline hydrochloride dissolved in distilled water, which is known to cause complete suppression of growth,29 was used as a control. Three separate trials, consisting of triplicate experiments for individual drug concentrations, were performed over a period of four days. During the incubation period, the overlying culture medium was replaced daily with 200 μL of fresh medium containing the indicated concentration of pepstatin A and mefloquine. Combination therapies of pepstatin A/mefloquine were tested in in vitro cultures of B. bovis, B. bigemina, B. caballi, and B. equi as models for bovine and equine Babesia parasites. Pepstatin A/mefloquine combinations (C1, C2, C3, and C4) were prepared as described29,33 with some modifications. The concentration of each drug used in the combination was not destructive to the parasites.
Concentrations of pepstatin A/mefloquine applied simultaneously to the cultures were as follows: B. bovis (C1, C2, C3, and C4) = B. bovis (3/1.9, 3/3.9, 6/1.9, and 6/3.9 μM), B. bigemina (2.9/1.9, 2.9/3.8, 5.8/1.9, and 5.8/3.8 μM), B. caballi (2.6/2.2, 2.6/4.4, 5.2/2.2, and 5.2/4.4 μM), and B. equi (0.5/2.3, 0.5/4.6, 1/2.3, and 1/4.6 μM), respectively. Both reagents alone were tested in the in vitro cultures of all parasites by using the high and low concentrations of pepstatin A/mefloquine combination of each parasite as mentioned below. Concentrations of pepstatin A applied simultaneously to the cultures were as follows: B. bovis (Pa and Pb) = B. bovis (6 and 3 μM), B. bigemina (5.8 and 2.9 μM), B. caballi (5.2 and 2.6 μM), and B. equi (1 and 0.5 μM), respectively. The concentrations of mefloquine applied simultaneously to the cultures were as follows: B. bovis (Ma and Mb) = B. bovis (3.9 and 1.9 μM), B. bigemina (3.8 and 1.9 μM), B. caballi (4.4 and 2.2 μM), and B. equi (4.6 and 2.3 μM), respectively. Parasitemia were monitored daily by counting parasitized erythrocytes to approximately 1,000 erythrocytes in Giemsa-stained thin blood smears. The 50% inhibitory concentration (IC50) values for the drugs upon growth of all parasites tested were calculated on the basis of the maximum growth of the DMSO control in the in vitro cell culture system by using interpolation after curve-fitting.
Viability test.
After four days of treatment, 6 μL each of the control and drug-treated (at the various indicated concentrations) erythrocytes were mixed with 14 μL of parasite-free erythrocytes and suspended in fresh growth medium without drug supplementation. The plates were then incubated for five days. Culture medium was replaced daily, and parasite recrudescence was determined by using light microscopy to assess parasite viability.8
In vivo growth inhibition assay.
The in vivo growth inhibition assay for pepstatin A, mefloquine, and pepstatin A/mefloquine combinations (2.5 mg/kg/2.5 mg/kg and 5 mg/kg/5 mg/kg) was performed in BALB/c mice as described.8,34 Twenty-eight week-old female BALB/c mice were divided into five groups of four mice and intraperitoneally inoculated with 1 × 106 B. microti-infected erythrocytes. In the first group, mefloquine dissolved in DMSO was administered at a dose rate of 5 mg/kg. In the second group, pepstatin A dissolved in DMSO was administered at a dose rate of 5 mg/kg. In the third group, a combination of pepstatin A and mefloquine (2.5 mg/kg of each) dissolved in DMSO was administered. In the fourth group, a combination of pepstatin A and mefloquine (5 mg/kg of each) dissolved in DMSO was administered. Phosphate-buffered saline containing 0.02% DMSO was administered to the fifth group as a placebo control.
When infected mice showed parasitemias of approximately 1%, mice in all experimental groups were administered daily intraperitoneal injections from days 3 to 8 post-infection. Levels of parasitemia in all mice were monitored daily until 30 days post-infection by examination of Giemsa-stained thin blood smears prepared from venous tail blood. All animal experiments were conducted in accordance with the Standard Relating to the Care and Management of Experimental Animals set by the National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine (Hokkaido, Japan).
Statistical analysis.
Differences in percentages of parasitemia for in vitro cultures and among groups of in vivo studies were analyzed by using the independent Student's t-test.8,33 P values < 0.05 was considered statistically significant for all tests.
Results
In vitro inhibitory effect of pepstatin A on Babesia parasites.
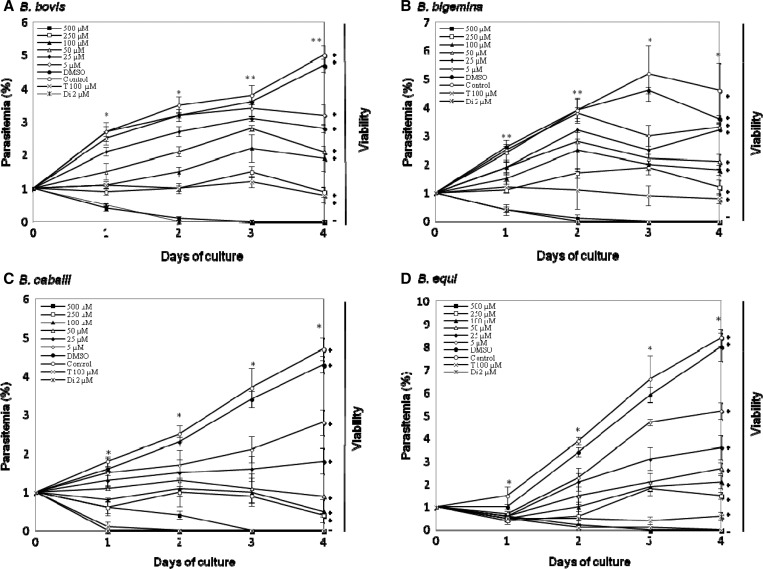
The in vitro growth of B. bovis (Figure 1A) and B. bigemina (Figure 1B) was significantly inhibited (P < 0.05) by 50 μM and 25 μM pepstatin A, respectively, and 5 μM pepstatin A significantly inhibited the growth of B. caballi (Figure 1C) and B. equi (Figure 1D). In the presence of 500 μM pepstatin A, growth of all parasites was completely suppressed. Complete suppression of parasites was observed with 2 μM diminazene aceturate (Figure 1A–C), and 100 μM tetracycline completely suppressed parasites (Figure 1C and D). Complete elimination of the growth of four species (B. bovis, B. bigemina, B. caballi, and B. equi) from pepstatin A-treated cultures was observed on day 3 (Figure 1A–D). Complete elimination of the three parasites from diminazene aceturate–treated cultures was observed on day 3 of the treatment for B. bovis, B. bigemina, and B. caballi (Figure 1A–C).
Figure 1.
Inhibitory effect of different concentrations of pepstatin A on the in vitro growth of Babesia bovis (A), B. bigemina (B), B. caballi (C), and B. equi (D). Diminazene aceturate (Di) and tetracycline hydrochloride (T) were used as positive controls. Each value represents the mean ± SD for experiments performed in triplicate. Curves represent results of one representative experiment of three separate replicates. Asterisks, (*) and (**), indicate statistically significant differences (P < 0.05, by Student's t-test) between 50 and 25 μM drug-treated cultures and the control cultures, respectively. DMSO = dimethylsulfoxide.
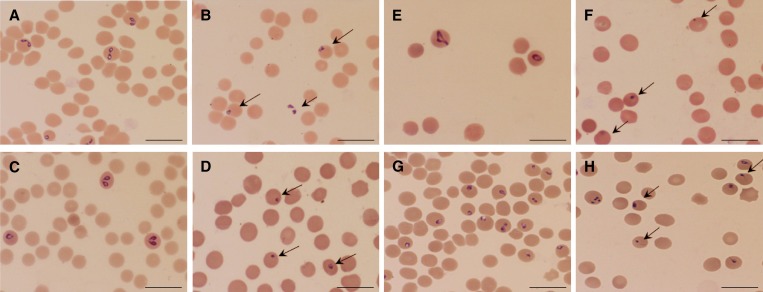
The IC50 values of pepstatin A for growth inhibition of B. bovis, B. bigemina, B. caballi, and B. equi were 38.5, 36.5, 17.6, and 18.1 μM, respectively (Table 1). Subsequent viability tests showed that there was no re-growth of the four species with pepstatin A at a concentration of 500 μM. Moreover, pepstatin A affected the morphology of B. bovis (Figure 2B), B. bigemina (Figure 2D), B. caballi (Figure 2F), and B. equi (Figure 2H) parasites in treated cultures. The pepstatin-treated cultures showed a high number of degenerated parasites, which appeared to be dot-shaped when compared with those in control cultures. Some parasites appeared as abnormally multidividing forms.
Table 1.
IC50 values of mefloquine and pepstatin A for growth inhibition of different Babesia species
| Organism | IC50 (μM)* | References | |
|---|---|---|---|
| Mefloquine | Pepstatin A | ||
| B. bovis | 59.7 | 38.5 | Present study |
| B. bigemina | 56.9 | 36.5 | |
| B. caballi | 20.7 | 17.6 | |
| B. equi | 4 | 18.1 | |
| Plasmodium falciparum | 0.028–0.13 | 4 | 19, 27, 34, and 35 |
| Dog (neuron) | 272 | ND | 36 |
50% inhibitory concentration (IC50) values are expressed as mefloquine and pepstatin A concentrations and are in micromolars of the growth medium and were determined on the day of maximum growth of the dimethylsulfoxide control in vitro culture by using a curve-fitting technique from three separate experiments. ND = not determined.
Figure 2.
Light micrographs of pepstatin A-treated bovine and equine Babesia parasites in an in vitro culture. Micrographs were taken on day 3 of the experiment. B. bovis: control (A) and 25 μM pepstatin A (B). B. bigemina: control (C) and 50 μM pepstatin A (D). B. caballi: control (E) and 5 μM pepstatin A (F). B. equi: control (G) and 5 μM pepstatin A (H). Drug-treated cultures showed a higher number of degenerated parasites than control cultures. Scale bars = 10 μm.
In vitro inhibitory effect of mefloquine on Babesia parasites.
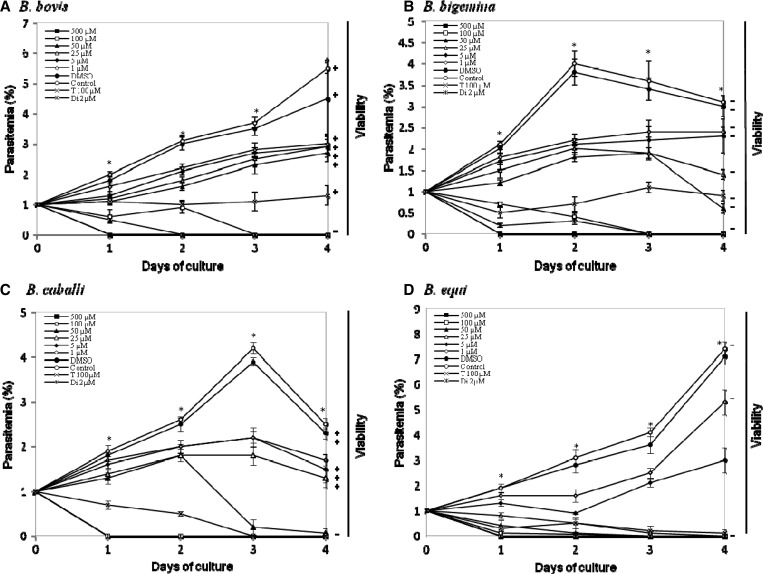
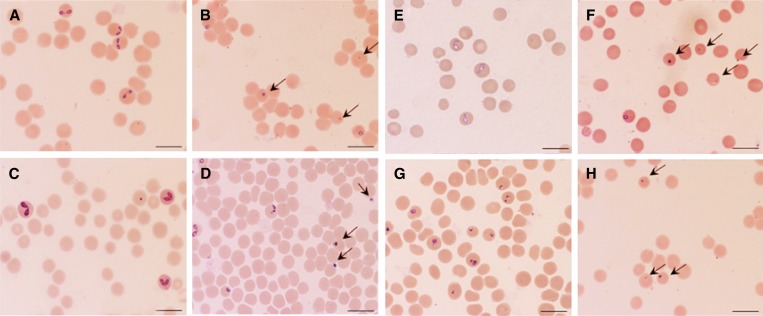
The growth of B. bovis (Figure 3A), B. bigemina (Figure 3B), B. caballi (Figure 3C), and B. equi (Figure 3D) was significantly inhibited (P < 0.05) by 1 μM mefloquine. In the presence of 100 μM of mefloquine, growth of all parasites was completely suppressed. In addition, B. caballi and B. equi were completely suppressed at concentrations of 50 and 25 μM, respectively. Complete suppression of diminazene aceturate–treated parasites was observed at a concentration of 2 μM (Figure 3A–C), and 100 μM tetracycline completely suppressed parasites (Figure 3C). After the mefloquine-treatment regimen, Babesia parasites were completely eliminated as early as day 1 (B. caballi and B. equi) and day 3 (B. bovis and B. bigemina) (Figure 3A–D). Complete elimination of the three parasites from diminazene aceturate–treated cultures was observed on day 1 (for B. bovis, B. bigemina, B. caballi, and B. equi) of treatment (Figure 3A–D), and tetracycline hydrochloride eliminated equine Babesia parasites on days 3 and 4 of treatment (Figure 3C and D). The IC50 values of mefloquine were 59.7, 56.9, 20.7, and 4 μM for B. bovis, B. bigemina, B. caballi, and B. equi, respectively (Table 1). Notably, B. equi was the most susceptible to mefloquine. Subsequent viability tests showed that there was no re-growth of the four species at a concentration of 100 μM mefloquine. Moreover, mefloquine affected the morphology of B. bovis (Figure 4B), B. bigemina (Figure 4D), B. caballi (Figure 4F), and B. equi (Figure 4H) parasites in treated cultures. The mefloquine-treated cultures showed a high number of degenerated parasites, which appeared dot-shaped when compared with those in control cultures. Abnormal multidividing forms were also observed.
Figure 3.
Inhibitory effects of different concentrations of mefloquine on the in vitro growth of Babesia bovis (A), B. bigemina (B), B. caballi (C), and B. equi (D). Diminazene aceturate (Di) and tetracycline hydrochloride (T) were used as positive controls. Each value represents the mean ± SD for experiments performed in triplicate. Curves represent the results of one representative experiment of three separate replicates. *Statistically significant differences (P < 0.05, by Student's t-test) between the drug-treated cultures and control cultures. DMSO = dimethylsulfoxide.
Figure 4.
Light micrographs of mefloquine-treated bovine Babesia parasites in an in vitro culture. Micrographs were taken on day 3 of the experiment. B. bovis: control (A) and 1 μM mefloquine (B). B. bigemina: control (C) and 1 μM mefloquine (D). B. caballi: control (E) and 1 μM mefloquine (F). B. equi: control (G) and 1 μM mefloquine (H). Drug-treated cultures showed a higher number of degenerated parasites than control cultures. Scale bars = 10 μm.
In vitro inhibitory effect of combined compounds on Babesia parasites.
Combination therapies of pepstatin A and mefloquine were assessed in in vitro cultures of bovine (B. bovis and B. bigemina) and equine (B. caballi and B. equi) Babesia parasites. Drug combination experiments were performed to evaluate potential synergistic or antagonistic effects. Pepstatin A/mefloquine combinations were prepared and applied simultaneously to cultures of B. bovis, B. bigemina, B. caballi, and B. equi. Although no complete elimination was observed in in vitro cultures of all parasites from any of combination-treated cultures during treatment, combination-treated cultures of all parasites showed significant inhibition compared with control cultures (Table 2). There was no significant different between both reagents alone and combinations treatment for B. bovis, B. bigemina, B. caballi, and B. equi. Subsequent viability tests showed that there was re-growth of bovine and equine Babesia parasites for all drug combinations used (Table 2).
Table 2.
Percentages of actual parasitemia and growth inhibition of combined applications of pepstatin A and mefloquine for Babesia bovis, B. bigemina, B. caballi, and B. equi
| Pepstatin A/mefloquine (μM) | B. bovis | B. bigemina | B. caballi | B. equi | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P %‡ | I %§ | V¶ | P %ׇ | I %§ | V¶ | P %‡ | I %§ | V¶ | P %‡ | I %§ | V¶ | |
| A (medium) | 6 ± 2.8 | 0 | + | 5.6 ± 1.7 | 0 | + | 4.5 ± 1.6 | 0 | + | 6 ± 2.5 | 0 | + |
| B (0.0002% DMSO) | 5.2 ± 1.9 | 0 | + | 4.7 ± 1.6 | 0 | + | 4.2 ± 1.2 | 0 | + | 5.7 ± 1.9 | 0 | + |
| P (high) | 1.7 ± 1.1 | 67.3# | + | 2.2 ± 1.2 | 53.2# | + | 1.6 ± 0.9 | 61.9# | + | 1.3 ± 0.9 | 77.2# | + |
| P (low) | 2.3 ± 1.5 | 55.8# | + | 2.7 ± 1.8 | 42.6# | + | 2.4 ± 1.1 | 42.9# | + | 2.2 ± 1.3 | 61.4# | + |
| M (high) | 1.4 ± 0.8 | 73.1# | + | 2 ± 0.7 | 57.7# | + | 0.6 ± 0.2 | 85.7# | + | 0.3 ± 0.1 | 94.7# | + |
| M (low) | 2.1 ± 0.7 | 59.6# | + | 2.5 ± 1.3 | 46.8# | + | 0.8 ± 0.5 | 81# | + | 0.5 ± 0.1 | 91.2# | + |
| C1 | 2.8 ± 1.1 | 46.2# | + | 3 ± 1.5 | 36.2# | + | 2.2 ± 0.8 | 47.4# | + | 2.4 ± 1.2 | 57.9# | + |
| C2 | 2.2 ± 0.9 | 57.7# | + | 2.3 ± 1.1 | 51.1# | + | 1.5 ± 0.6 | 62.5# | + | 0.9 ± 0.6 | 84.2# | + |
| C3 | 1.9 ± 0.8 | 63.5# | + | 2 ± 0.9 | 57.5# | + | 0.9 ± 0.5 | 78.6# | + | 0.7 ± 0.4 | 87.7# | + |
| C4 | 1.3 ± 0.5 | 75# | + | 1.9 ± 0.5 | 59.6# | + | 0.6 ± 0.1 | 85.7# | + | 0.3 ± 0.03 | 94.7# | + |
P% = percent actual parasitemia; I% = percent growth inhibition; V, viability testing; DMSO = dimethylsulfoxide.
A = each medium. and, B = DMSO (0.0002%). + indicated each medium (0.03%). P = pepstatin A for each parasite: (P high and P low) = B. bovis (6 and 3 μM), B. bigemina (5.8 and 2.9 μM), B. caballi (5.2 and 2.6 μM), and B. equi (1 and 0.5 μM); M = mefloquine for each parasite (M high and M low) = B. bovis (3.9 and 1.95 μM), B. bigemina (3.8 and 1.9 μM), B. caballi (4.4 and 2.2 μM), and B. equi (4.6 and 2.3μM). Pepstatin A/mefloquine combinations for each parasite: (C1, C2, C3, and C4) = B. bovis (3/1.9, 3/3.9, 6/1.9, and 6/3.9 μM), B. bigemina (2.9/1.9, 2.9/3.8, 5.8/1.9, and 5.8/3.8 μM), B. caballi (2.6/2.2, 2.6/4.4, 5.2/2.2, and 5.2/4.4 μM), and B. equi (0.5/2.3, 0.5/4.6, 1/2.3, and 1/4.6).
Percentage actual parasitemia is mean ± SD at day 3 of culture.
Percentage growth inhibition was determined at day 3 of culture by compared with the control parasitemia.
Viability test indicates viability after 10 days of subsequent drug-free culture. + = viable.
Significant difference (P < 0.05) between treated and control groups.
In vivo effects of pepstatin A and mefloquine on B. microti infection.
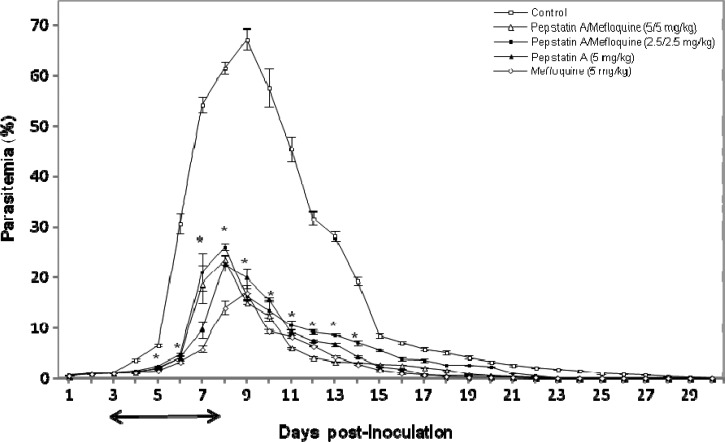
To examine the effects of pepstatin A and mefloquine on rodent Babesia, infected mice were treated with pepstatin A and mefloquine either alone or in combinations. In the pepstatin A-, mefloquine-, and combination-treated groups, levels of parasitemia were significantly lower than those in the control group (P < 0.05). Peak parasitemia reached an mean of 23.5% and 26.1% when treated with pepstatin A/mefloquine combinations of 5 mg/kg/5 mg/kg and 2.5 mg/kg/2.5 mg/kg, respectively, and 22.8% in treatment with 5 mg/kg pepstatin A 8 days after inoculation (Figure 5). The mean for the mefloquine-treated (5 mg/kg) group was 17.1%, and the mean for the control group was 67.3% (DMSO) 9 days after inoculation (Figure 5). Parasites were completely eliminated on day 23 post-infection in the treated groups. Conversely, parasites were completely cleared on day 30 post-infection in the control group. There were significant differences (P < 0.05) between the control and treatment groups on days 5–14 post-infection (Figure 5).
Figure 5.
Inhibitory effect of combinations of pepstatin A and mefloquine (2.5 mg/kg of each and 5 mg/kg of each), pepstatin A (5 mg/kg), and mefloquine (5 mg/kg) on the in vivo growth of Babesia based on observations obtained for four mice per experimental group. Each value represents the mean ± SD. * = statistically significant differences (P < 0.05, by Student's t-test) between the treated groups and the control group. ↔ = time of inoculation or control reagent (dimethlysulfoxide) application of intraperitoneal pepstatin A, mefloquine, or a combination of pepstatin A and mefloquine.
Discussion
Babesia, an intraerythrocytic protozoan parasite, is similar to the malarial parasite P. falciparum. Plasmodium spp. use hemoglobin for reproduction in infected erythrocytes. Hemoglobin degradation takes place in an acidic food vacuole of the parasite, and many current antimalarial drugs appear to disrupt important vacuolar functions.16
Aspartic protease inhibitors prevent degradation of hemoglobin and kill malaria parasites in vitro and in vivo.18–21 However, in the Babesia parasite, these processes require further investigations. In the current study, AP inhibitor and anti-malarial drug were demonstrated to significantly inhibit in vitro and in vivo growth of Babesia parasites. Exposure of parasites to higher concentrations of both reagents completely suppressed the growth of bovine and equine Babesia parasites tested. Because treatment with DMSO only had no effect on parasitic growth, it this growth inhibition was likely caused by effects of both reagents.
The IC50 values of pepstatin A against bovine Babesia parasites (B. bovis and B. bigemina) were 38.5 μM and 36.5 μM, respectively, and those of equine Babesia parasites (B. caballi and B. equi) were 17.6 μM and 18.1 μM, respectively. In malaria parasites, 4 μM pepstatin A (IC50) killed P. falciparum before trophozoite development and had a major effect on schizont maturation.19
The IC50 values of mefloquine against bovine Babesia parasites (B. bovis and B. bigemina) were 23 μM and 33.3 μM, respectively, and the values of mefloquine against equine Babesia parasites (B. caballi and B. equi) were 7.6 μM and 15 μM, respectively. Earlier studies showed that mefloquine was effective against P. falciparum in vitro, and showed low IC50 values of 28.7–130 nM.27,34,35 Mefloquine showed high IC50 values (272 μM) for mammalian cells (dog neurons).36
Doses of pepstatin A and mefloquine for bovine babesiosis most effectively suppressed parasite growth compared with other drugs that have been tested for the treatment of babesiosis.6,8,37 Conversely, effective doses of pepstatin A and mefloquine for bovine Babesia parasites were higher than those of other drugs tested in previous studies.10,29,38–41 For equine Babesia parasites, the IC50 values of pepstatin A and mefloquine were similar to those reported in previous studies6,7,16,42 but significantly lower than the IC50 values reported for equine Babesia.8
Moreover, the combination of pepstatin A and mefloquine produced antagonistic effects on in vitro-cultured parasites. It was previously reported that the plasmepsin inhibitor Ro40-4388 and the antimalarial drug chloroquine interacted antagonistically against P. falciparum.17 Similar antagonistic actions between Ro40-4388 and mefloquine, quinine, amodiaquine, and halofantrine were found.27 Conversely, AP inhibitors reduce hemoglobin degradation and subsequent release of heme, thus antagonizing the antimalarial activity of these drugs.
Furthermore, pepstatin A and mefloquine alone or in combination had inhibitory effects against B. microti infection in mice given single doses of 5 mg/kg, 5 mg/kg, or combinations of 2.5 mg/kg of each and 5 mg/kg of each during 6-day treatments. In the treated group, the parasitemia increased more slowly and achieved lower peaks compared with the parasitemia dynamics of the control group. In contrast, DMSO alone did not affect the growth of the parasites. Mefloquine was more effective than pepstatin A for treatment of infected mice, and pepstatin A/mefloquine combinations (2.5/2.5 mg/kg and 5/5 mg/kg) were less effective than each single treatment. The treated mice showed no signs of toxicity. As described in a previous study, P. vinckei-infected mice treated with 50 mg/kg of pepstatin A showed no toxic signs, and the in vivo effect was improved by combinations of AP and peptidyl cysteine inhibitors.20 Mefloquine was used for treatment of mice infected with Schistosoma mansoni and S. japonicum43 and of hamsters infected with Opisthorchis viverrini44 at concentrations of 100–400 mg/kg for 2–4 weeks. There were no signs of toxicity observed in the murine model. Thus, pepstatin A (5 mg/kg) and mefloquine (5 mg/kg) did not induce any signs of toxicity. These results are consistent with those of Seminov and others,20 who showed that 50 mg/kg of pepstatin A was used without any side effects. Therefore, mefloquine at this dose is not toxic to mice and might be used for treatment of babesiosis.
Treatment with pepstatin A at 5 mg/kg, mefloquine at 5 mg/kg, and the combinations of 2.5/2.5 mg/kg and 5/5 mg/kg resulted in 66.1, 74.6, 61.2, and 65.1% inhibition, respectively, of the growth of B. microti in infected mice on days 9–10 post-infection, and parasites were completely eliminated on day 22 post-infection in the treated groups. Conversely, parasites were completely cleared on day 30 post-infection in the control group. In the mouse model, pepstatin A and mefloquine were more effective than other drugs tested in previous studies.8,41 In addition, in combination treatment, the antiparasitic activity of pepstatin A and mefloquine was reduced to that of each single treatment in in vitro and in vivo studies, suggesting that mefloquine might antagonize actions of the inhibitor of AP, pepstatin A, against Babesia parasites.
The gene sequences of APs can be found in the B. bovis genome sequence database.28 Therefore, in vitro and in vivo inhibition of these enzymes indicates an important role of APs in the growth cycle of Babesia parasites.
In conclusion, results of the present study showed that pepstatin A and mefloquine potently inhibit Babesia parasites in vitro and in vivo. Furthermore, pepstatin A might antagonize actions of mefloquine on the inhibitory effects of in vitro and in vivo combinations. This study also shows that AP has an important role in the growth cycle of Babesia parasites. Therefore, characterization, enzymatic activity, and effect of APs on hemoglobin degradation are needed.
Footnotes
Financial support: This study was supported by Grants-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science, the Global Center of Excellence Program, Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Authors' addresses: Tserendorj Munkhjargal, Mahmoud AbouLaila, Mohamad Alaa Terkawi, Thillaiampalam Sivakumar, Madoka Ichikawa, Batdorj Davaasuren, Tserendorj Nyamjargal, Naoaki Yokoyama, and Ikuo Igarashi, National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, Inada-cho, Obihiro, Hokkaido 080-8555, Japan, E-mails: muugii_f@yahoo.com, hethet2004@yahoo.com, alaaterkawi@hotmail.com, sivavets@gmail.com, ichikawa@obihiro.ac.jp, davlag_mgl@yahoo.com, nyama18@yahoo.com, yokoyama@obihiro.ac.jp, and igarcpmi@obihiro.ac.jp. Mahmoud AbouLaila, Department of Parasitology, Faculty of Veterinary Medicine, Minoufiya University, Sadat City, Minoufiya, Egypt, E-mail: hethet2004@yahoo.com. Batdorj Davaasuren, Laboratory of Molecular Genetic, Institute of Veterinary Medicine, Zaisan 210153, Ulaanbaatar, Mongolia, E-mails: davlag_mgl@yahoo.com.
Reprint requests: Ikuo Igarashi, National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, Inada-cho, Obihiro, Hokkaido 080-8555, Japan, E-mail: igarcpmi@obihiro.ac.jp.
References
- 1.Kuttler KL. World-wide impact of babesiosis. In: Ristic M, editor. Babesiosis of Domestic Animals and Man. Boca Raton, FL: CRC Press; 1988. pp. 1–22. [Google Scholar]
- 2.Wright IG, Goodger BV. Pathogenesis of babesiosis. In: Ristic M, editor. Babesiosis of Domestic Animals and Man. Boca Raton, FL: CRC Press; 1988. pp. 99–118. [Google Scholar]
- 3.Homer MJ, Aguilar-Delfin I, Telford III, Sr., Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000;13:451–469. doi: 10.1128/cmr.13.3.451-469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kjemtrup AM, Conrad PA. Human babesiosis: an emerging tick-borne disease. Int J Parasitol. 2000;30:1323–1337. doi: 10.1016/s0020-7519(00)00137-5. [DOI] [PubMed] [Google Scholar]
- 5.Vial HJ, Gorenflot A. Chemotherapy against babesiosis. Vet Parasitol. 2006;138:147–160. doi: 10.1016/j.vetpar.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 6.Bork S, Yokoyama N, Matsuo T, Claveria FG, Fujisaki K, Igarashi I. Growth inhibitory effect of triclosan on equine and bovine Babesia parasites. Am J Trop Med Hyg. 2003a;68:334–340. [PubMed] [Google Scholar]
- 7.Nagai A, Yokoyama N, Matsuo T, Bork S, Hirata H, Xuan X. Growth-inhibitory effects of artesunate, pyrimethamine, and pamaquine against Babesia equi and Babesia caballi in in vitro cultures. Antimicrob Agents Chemother. 2003;47:800–803. doi: 10.1128/AAC.47.2.800-803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bork S, Yokoyama N, Ikehara Y, Kumar S, Sugimoto C, Igarashi I. Growth-inhibitory effect of heparin on Babesia parasites. Antimicrob Agents Chemother. 2004;48:236–241. doi: 10.1128/AAC.48.1.236-241.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bork S, Das S, Okubo K, Yokoyama N, Igarashi I. Effect of protein kinase inhibitors on the in vitro growth of Babesia bovis. Parasitology. 2006;132:775–779. doi: 10.1017/S0031182006009917. [DOI] [PubMed] [Google Scholar]
- 10.Okubo K, Yokoyama N, Govind Y, Alhassan A, Igarashi I. Babesia bovis: effects of cysteine protease inhibitors on in vitro growth. Exp Parasitol. 2007;117:214–217. doi: 10.1016/j.exppara.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg DE, Slater AFG, Beavis R, Chait B, Cerami A, Henderson GB. Hemoglobin degradation in the human malaria pathogen Plasmodium falciparum: a catabolic pathway initiated by a specific aspartic protease. JEM. 1991;173:961–969. doi: 10.1084/jem.173.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurent F, Bourdieu C, Kaga M, Chilmonczyk S, Zgrzebski G, Yvore P, Pery P. Cloning and characterization of an Eimeria acervulina sporozoite gene homologous to aspartyl proteinases. Mol Biochem Parasitol. 1993;62:303–312. doi: 10.1016/0166-6851(93)90119-i. [DOI] [PubMed] [Google Scholar]
- 13.Jean L, Grosclaude J, Labbe M, Tomley F, Pery P. Differential localization of an Eimeria tenella aspartyl proteinase during the infection process. Int J Parasitol. 2000;30:1099–1107. doi: 10.1016/s0020-7519(00)00099-0. [DOI] [PubMed] [Google Scholar]
- 14.Pinho R, Beltramini L, Alves C, De-Simone S. Trypanosoma cruzi: isolation and characterization of aspartyl proteases. Exp Parasitol. 2009;122:128–133. doi: 10.1016/j.exppara.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Francis SE, Gluzman IY, Oksman A, Knickerbocker A, Mueller R, Bryant ML, Sherman DR, Russell DG, Goldberg DE. Molecular characterization and inhibition of a Plasmodium falciparum aspartic hemoglobinase. EMBO J. 1994;13:306–317. doi: 10.1002/j.1460-2075.1994.tb06263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva AM, Lee AY, Gulnik SV, Majer P, Collins J, Bhat TN. Structure and inhibition of plasmepsin II, a hemoglobin-degrading enzyme from Plasmodium falciparum. Proc Natl Acad Sci USA. 1996;93:10034–10039. doi: 10.1073/pnas.93.19.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon RP, Tyas L, Certa U, Rupp K, Bur D, Jacquet C, Matile H, Loetscher H, Grueninger-Leitch F, Kay J, Dunn BM, Berry C, Ridley RG. Expression and characterisation of plasmepsin I from Plasmodium falciparum. Eur J Biochem. 1997;244:552–560. doi: 10.1111/j.1432-1033.1997.00552.x. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee R, Liu J, Beatty W, Pelosof L, Klemba M, Goldberg DE. Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine. Proc Natl Acad Sci USA. 2002;99:990–995. doi: 10.1073/pnas.022630099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailly E, Jambou R, Savel J, Jaureguiberry G. Plasmodium falciparum: differential sensitivity in vitro to E-64 (cysteine protease inhibitor) and pepstatin A (aspartyl protease inhibitor) J Protozool. 1992;39:593–599. doi: 10.1111/j.1550-7408.1992.tb04856.x. [DOI] [PubMed] [Google Scholar]
- 20.Semenov A, Olson JE, Rosenthal PJ. Antimalarial synergy of cysteine and aspartic protease inhibitors. Antimicrob Agents Chemother. 1998;42:2254–2258. doi: 10.1128/aac.42.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma A, Eapen A, Subbararao SK. Purification and characterization of a hemoglobin degrading aspartic protease from the malarial parasite Plasmodium vivax. J Biochem. 2005;138:71–78. doi: 10.1093/jb/mvi105. [DOI] [PubMed] [Google Scholar]
- 22.Palmer KJ, Holliday SM, Brogden RN. Mefloquine. A review of its antimalarial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1993;45:430–475. doi: 10.2165/00003495-199345030-00009. [DOI] [PubMed] [Google Scholar]
- 23.Mockenhaupt FP. Mefloquine resistance in Plasmodium falciparum. Parasitol Today. 1995;11:248–253. doi: 10.1016/0169-4758(95)80201-0. [DOI] [PubMed] [Google Scholar]
- 24.Schlagenhauf P. Mefloquine for malaria chemoprophylaxis 1992–1998: a review. J Travel Med. 1999;6:122–133. doi: 10.1111/j.1708-8305.1999.tb00843.x. [DOI] [PubMed] [Google Scholar]
- 25.Nevin RL. Epileptogenic potential of mefloquine chemoprophylaxis: a pathogenic hypothesis. Malar J. 2009;8:188. doi: 10.1186/1475-2875-8-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorn A, Vippagunta SR, Matile H, Jaquet C, Vennerstrom JL, Ridley RG. An assessment of drug-haematin binding as a mechanism for inhibition of haematin polymerisation by quinoline antimalarials. Biochem Pharmacol. 1998;55:727–736. doi: 10.1016/s0006-2952(97)00510-8. [DOI] [PubMed] [Google Scholar]
- 27.Mungthin M, Bray PG, Ridley RG, Ward SA. Central role of hemoglobin degradation in mechanisms of action of 4-aminoquinolines, quinoline methanols, and phenanthrene methanols. Antimicrob Agents Chemother. 1998;42:2973–2977. doi: 10.1128/aac.42.11.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brayton KA, Lau AO, Herndon DR, Hannick L, Kappmeyer LS, Berens SJ. Genome sequence of Babesia bovis and comparative analysis of apicomplexan hemoprotozoa. PLoS Pathog. 2007;3:1401–1413. doi: 10.1371/journal.ppat.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AbouLaila M, Nakamura K, Govind Y, Yokoyama N, Igarashi I. Evaluation of the in vitro growth-inhibitory effect of epoxomicin on Babesia parasites. Vet Parasitol. 2010a;167:19–27. doi: 10.1016/j.vetpar.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 30.Zweygarth E, Just MC, de Waal DT. Continuous in vitro cultivation of erythrocytic stages of Babesia equi. Parasitol Res. 1995;81:355–358. doi: 10.1007/BF00931544. [DOI] [PubMed] [Google Scholar]
- 31.Erp EE, Smith RD, Ristic M, Osorno BM. Optimization of the suspension culture method for in vitro cultivation of Babesia bovis. Am J Vet Res. 1980;41:2059–2062. [PubMed] [Google Scholar]
- 32.AbouLaila M, Munkhjargal T, Sivakumar T, Ueno A, Nakano Y, Yokoyama M, Yoshinari T, Nagano D, Katayama K, El-Bahy N, Yokoyama N, Igarashi I. Apicoplast-targeting antibacterials inhibit the growth of Babesia parasites. Antimicrob Agents Chemother. 2012;56:3196–3206. doi: 10.1128/AAC.05488-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bork S, Yokoyama N, Matsuo T, Claveria FG, Fujisaki K, Igarashi I. Clotrimazole, ketoconazole, and clodinafop-propargyl inhibit the in vitro growth of Babesia bigemina and Babesia bovis (Phylum Apicomplexa) Parasitology. 2003c;127:311–315. doi: 10.1017/s0031182003003895. [DOI] [PubMed] [Google Scholar]
- 34.Rogers WO, Sem R, Tero T, Chim P, Lim P, Muth S, Socheat D, Ariey F, Wongsrichanalai C. Failure of artesunate-mefloquine combination therapy for uncomplicated Plasmodium falciparum malaria in southern Cambodia. Malar J. 2009;8:10. doi: 10.1186/1475-2875-8-10. doi:10.1186/1475-2875-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wurtz N, Briolant S, Gil M, Parquet V, Henry M, Baret E, Amalvict R, Almeras L, Rogier C, Pradines B. Synergy of mefloquine activity with atorvastatin, but not chloroquine and monodesethylamodiaquine, and association with the pfmdr1 gene. J Antimicrob Chemother. 2010;65:1387–1394. doi: 10.1093/jac/dkq173. [DOI] [PubMed] [Google Scholar]
- 36.Lee HS, Go ML. Effects of mefloquine on Ca2+ uptake and release by dog brain microsomes. Arch Int Pharmacodyn Ther. 1996;331:221–231. [PubMed] [Google Scholar]
- 37.Takabatake N, Hashiba S, Bork S, Okamura M, Yokoyama N, Igarashi I. Fucoidan inhibits the in vitro growth of Babesia bovis. J Protozool Res. 2004;14:55–60. [Google Scholar]
- 38.Igarashi I, Njonge F, Kaneko Y, Nakamura Y. Babesia bigemina: in vitro and in vivo effects of curdlan sulfate on the growth of parasites. Exp Parasitol. 1998;90:290–293. doi: 10.1006/expr.1998.4331. [DOI] [PubMed] [Google Scholar]
- 39.Munkhjargal T, Aboulaila M, Sivakumar T, Yokoyama N, Igarashi I. Inhibitory effect of apicidin on in vitro and in vivo growth of Babesia parasites. J Protozool Res. 2009;19:42–49. [Google Scholar]
- 40.Bork S, Yokoyama N, Matsuo T, Claveria FG, Fujisaki K, Igarashi I. Clotrimazole, ketoconazole, and clodinafop-propargyl inhibit the in vitro growth of Babesia bigemina and Babesia bovis (Phylum Apicomplexa) Parasitology. 2003b;127:311–315. doi: 10.1017/s0031182003003895. [DOI] [PubMed] [Google Scholar]
- 41.AbouLaila M, Yokoyama N, Igarashi I. Inhibitory effect of (-)-Epigallocatechin-3-gallate from green tea on the growth of Babesia parasites. Parasitology. 2010b;137:7858–7791. doi: 10.1017/S0031182009991594. [DOI] [PubMed] [Google Scholar]
- 42.AbouLaila M, Sivakumar T, Yokoyama N, Igarashi I. Inhibitory effect of terpene nerolidol on the growth of Babesia parasites. Parasitol Int. 2010d;59:278–282. doi: 10.1016/j.parint.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 43.Keiser J, Chollet J, Xiao SH, Mei JY, Jiao PY, Utzinger J, Tanner M. Mefloquine – an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl Trop Dis. 2009;3:e350. doi: 10.1371/journal.pntd.0000350. doi:10.1371/journal.pntd.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keiser J, Odermatt P, Tesana S. Dose-response relationships and tegumental surface alterations in Opisthorchis viverrini following treatment with mefloquine in vivo and in vitro. Parasitol Res. 2009;105:261–266. doi: 10.1007/s00436-009-1395-z. [DOI] [PubMed] [Google Scholar]