Abstract
Little data are available regarding the association of ancylostomiasis with overt gastrointestinal bleeding. This 6-year retrospective study describes the clinical and biological profiles of unexpectedly identified ancylostomiasis in a 4-month-old baby and four adults; they presented with melena and were referred for urgent diagnostic gastrointestinal endoscopy, which confirmed numerous small intestine injuries with surrounding blood pools caused by Ancylostoma duodenale worms. Gastric erosions were also encountered in one patient. Uniquely, worm biological activities were recorded live in vivo, including mucosal invasion through a vigorous, rapid piercing process, repeated bloodsucking habits, and gut appearance during the stages of feeding, digestion, and excretion in male and female worms. In conclusion, ancylostomiasis-induced melena may occur in all ages from infants to the elderly. Worm bloodfeeding occurs after quick mucosal piercing, with blood loss being aggravated by a repeated feeding behavior. After treatment is started, bleeding stops rapidly in response to anthelmintic therapy.
Introduction
Hookworm infections have a worldwide distribution, with approximately 600 million people being affected globally according to the latest estimates; it is ranked one of the most common parasitic infections of humans. The highest prevalence is reported in southeast Asia, sub-Saharan Africa, and the Indian subcontinent, especially in rural areas with low socioeconomic status, poor sanitary facilities, and indiscriminate defecation habits that allow larvae to develop in the soil.1,2
As a soil-transmitted disease, the infection is acquired primarily through the penetration of human skin (usually the bare foot) by the infective filariform larvae. They migrate into the circulation, reach the pulmonary alveoli and trachea, are expelled to the esophagus, are swallowed with bronchial secretions, and molt two times into adult mature bloodfeeding worms that attach to the upper part of the small intestinal mucosa. One cycle takes 5–8 weeks. Each adult female worm produces thousands of eggs daily that exit in the feces. When deposited in the soil, under adequate warmth, shade, and moisture, the eggs hatch within 24–48 hours, producing first-stage larvae that molt two times to develop into third-stage infective larvae.3
Human hookworm infections are caused primarily by two species, Ancylostoma duodenale and Necator americanus. A. duodenale is the only hookworm of Egypt,4,5 North Africa, and the European Mediterranean littoral, the west coast of South America, and parts of India and China.6 Compared with N. americanus, A. duodenale is slightly larger (adult males are 8–11 mm in length; adult females are 10–13 mm in length), with a higher consumption of blood, averaging approximately 0.26 mL/day per adult worm; this high blood consumption results in iron-deficiency anemia, which develops insidiously when the chronic blood loss exceeds the iron intake and reserves of the patient.7 The diagnosis depends primarily on finding the characteristic eggs by fecal examination of patients with microcytic hypochromic anemia and eosinophilia. Although the eggs of the two species cannot be differentiated by basic light microscopy, the adult worms have characteristic differences.7
Thus, it is unusual for hookworm infection to be a cause of overt gastrointestinal bleeding and diagnosed by an endoscopic examination. The aim of this 6-year retrospective study was to describe the gathered clinical and biological profiles of endoscopically identified Ancylostoma worms in an infant and four adult patients with melena and severe anemia.
Materials and Methods
This retrospective study included five patients that presented with melena and severe pallor: a 4-month-old baby girl admitted to the Gastroenterology and Hepatology Unit, Department of Pediatrics, Assiut University Hospital, and four adult male patients between the ages of 19 and 87 years admitted to the Department of Tropical Medicine and Gastroenterology, Assiut University Hospital, from July of 2004 to September of 2010.
Full clinical assessments were performed, including detailed histories and thorough clinical examinations. None of the patients had any other serious illnesses (e.g., cardiac, hepatic, renal, or neurologic illnesses) or a history of hospitalization or blood transfusion before admission.
In all cases, an upper gastrointestinal source of bleeding was suspected, and all patients had notably low hemoglobin levels ranging from 1.9 to 7.1 g/dL. According to the guidelines applied in the emergency laboratory (with a huge burden in our central hospital), only the total blood cell counts were provided using electronic automated counters within 1 hour of admission (together with the hemoglobin level and red blood cells indices). A detailed complete blood count (CBC) with a differential white cell count could be obtained 5–24 hours later through the main laboratory, which was confirmed by a blood smear.
Apart from the marked anemia, no abnormalities were revealed through other routine urgent investigations, including abdominal ultrasound examinations and laboratory profiles of serum glucose, sodium, potassium, blood urea nitrogen, creatinine, bilirubin, and liver aminotransferases (alanine and aspartate transferases).
All patients were referred for urgent endoscopy within 4–10 hours of admission (after receiving a packed red cell transfusion) for diagnostic and therapeutic purposes if necessary (because the possibility of helminth infection was not raised at that time). Endoscopic examinations were performed using a standard video esophagogastroduodenoscope. A pediatric-size videoendoscope (Pentax EC-3440F; Asahi Optical Co. Limited, Tokyo, Japan) with a distal diameter of 11.5 mm was used for the examination of the baby girl after fasting with no breastfeeding for 3 hours under midazolam intravenous light sedation at a dose of 0.05 mg/kg body weight (a written informed consent was obtained from the parents, the procedure was explained, and the mother was allowed to attend). Push enteroscopy (Pentax VSB-2900; Asahi Optical Co. Limited, Tokyo, Japan) for the examination of the small intestine was performed in one patient after reporting negative findings with both esophagogastroduodenoscopy and colonoscopy. All examinations were performed by the same endoscopist (M.B.) with video image recording. When hookworms were identified, one of the worms was grasped by standard biopsy forceps, extracted, and examined under light microscopy; worms from all patients were identified as A. duodenale.
Data analysis was performed. All of the data were descriptive; numerical data were expressed as direct numbers, and endoscopic imaging findings were detailed.
Results
In all patients in this study (with their case notes briefly outlined below), the etiology of bleeding was revealed on endoscopic examination, which generally showed various findings (Figures 1 and 2). Findings included:
-
(1)
the presence of hookworms in large numbers along the duodenum and/or jejunum;
-
(2)
oozing blood spots from the duodenal and jejunal mucosa at the attachment sites of the worms during bloodsucking, including hemorrhagic pools in severe cases;
-
(3)
several duodenal erosions of previously bitten sites;
-
(4)
several erosions in the gastric antrum in one of the patients (case 3);
-
(5)
the appearance of the gut during the various stages of feeding (partially and fully fed gut), digestion, and excretion in both male and female worms; and
-
(6)
unique, live, in vivo demonstration of hookworm biological behavior.
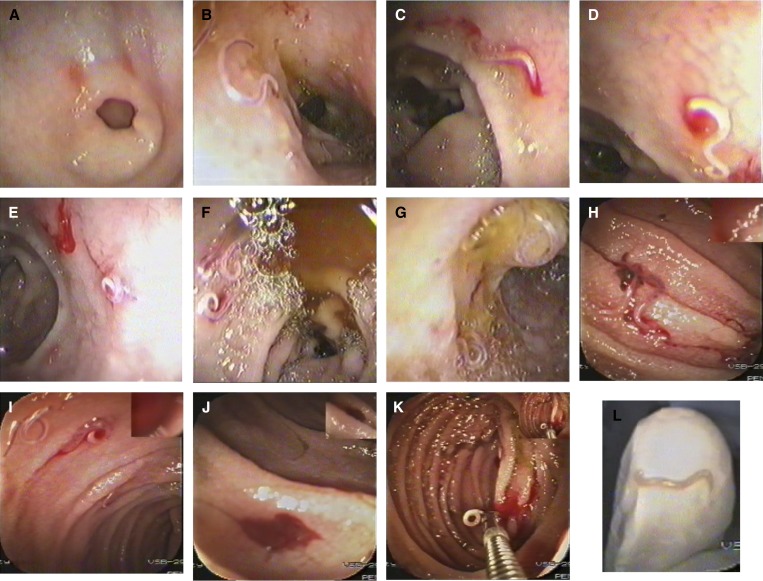
Figure 1.
Serial endoscopic imaging of ancylostomiasis-induced gastrointestinal bleeding. (A) Gastric erosions around the pyloric ring. (B) Worms in the duodenal bulb with nearby red spots of previous bites. (C) A duodenal bulb erosion at the worm's attachment site. (D) A close-up view on a bleeding spot at the attachment site. (E) A coiled worm at the end of the duodenal first part with a nearby fresh blood. (F) Several worms in the duodenal second part with bile around. (G) A worm coiled over the ampulla of Vater. (H) Worms in the jejunum with surrounding clotted blood. (I) Jejunal worms with surrounding fresh blood. (J) A blood pool in a jejunal segment of previous bites. (K) A worm caught by the biopsy forceps with dark and fresh blood spots in the jejunum. (L) A close-up view of the extracted worm on the endoscopist's gloved index finger tip (imaged by the videoendoscope itself).
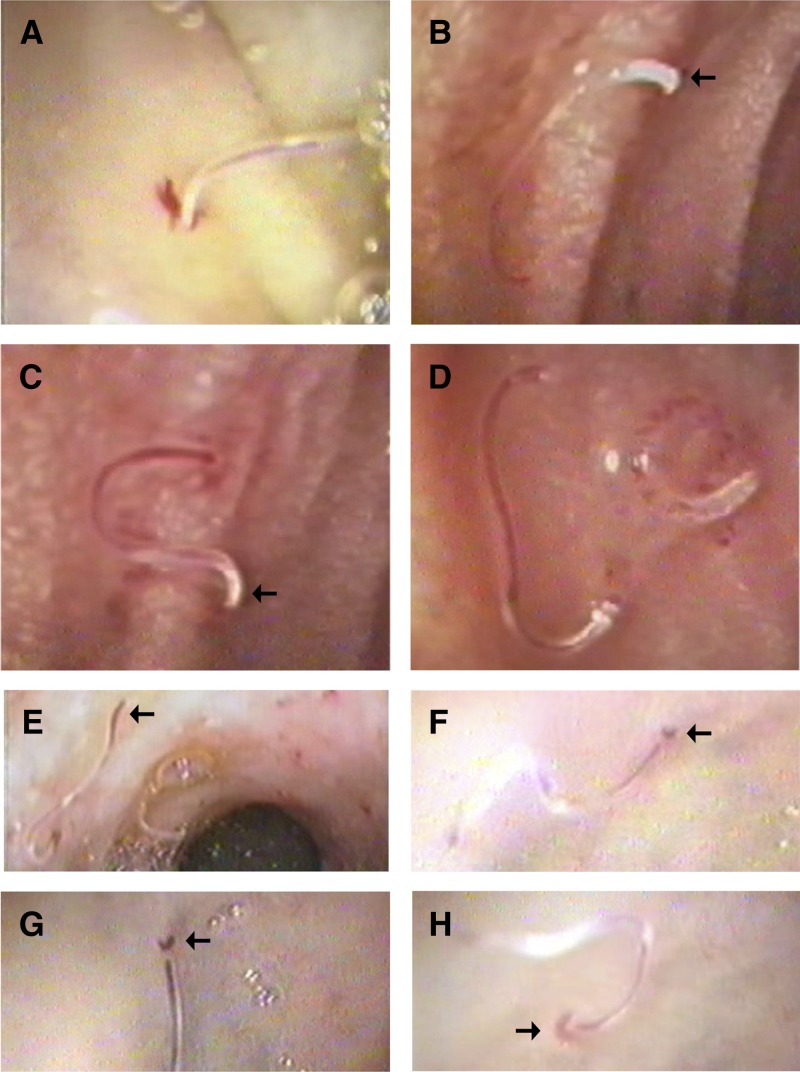
Figure 2.
Biologic behavior of hookworms on close-up gastrointestinal endoscopic views. (A) Mucosal piercing with blood oozing. (B) Feeding stage with a half-full gut (thin red blood line) of a male worm identified by its broad posterior end (arrow). (C) Half-full gut in a female worm identified by its tapering posterior end (arrow). (D) Full gut with a red line extending along the whole worm (left worm) and digestion phase with specks of blood inside the worm gut (right worm). (E) Excretion stage. Blood reached the posterior end in a female worm (arrow). (F) Blood reached the posterior end in a male worm (arrow). (G) A closer-up view on a male worm just before excretion (arrow). (H) Crescent-shaped red fluid film at a male worm posterior end just on excretion (arrow).
Four specific behaviors were apparent from the demonstration (Supplemental Videos 1–3):
-
(1)
the mucosal piercing process, which is a mechanical, quickly spinning, body-pushing movement that caused piercing within a few seconds (simulating electric piercing devices and appearing on magnification as a horrifying piercing);
-
(2)
repeated feeding by the same worm within a short time (few seconds; although the mucosa was bleeding from a previous invasion, a second nearby piercing was performed by the same worm, leading to several injuries of the mucosa per worm);
-
(3)
graceful smooth movement after fixation of the worm to the mucosa, which was maintained during the rest of the meal, together with the excretion process; and
-
(4)
struggling motility after being grasped from the jejunum by the biopsy forceps.
Case 1.
Case 1 was a 4-month-old baby girl referred to the hospital after vomiting for 4 days and having melena (black fluid tarry stool) for 2 days. There were no remarkable findings on examination, except for severe pallor. The main abnormalities of the emergency CBC were normocytic normochromic anemia and leukocytosis: hemoglobin (Hb) = 3.8 g/dL (normal [N] Hb = 11–14 g/dL), mean corpuscular volume (MCV) = 84.8 fL (N MCV = 70–86 fL), mean corpuscular hemoglobin concentration (MCHC) = 30.9 g/dL (N MCHC = 30–36 g/dL), and white blood cell (WBC) count = 49,200/mL (N WBC count = 6,000–18,000/mL). An upper endoscopy revealed numerous actively feeding hookworms in the duodenum and jejunum, with several surrounding fresh, bright red hemorrhagic pools; 1 day later (through the main laboratory), the WBC differential count revealed an absolute eosinophilia of 30% (N = 0.1–3.0%).
Case 2.
Case 2 was a 87-year-old male patient presenting with anorexia, dull aching epigastric pain, and a sensation of general weakness over the past month, with melena 1 day before admission. His examination revealed severe pallor with no abdominal tenderness or masses. An emergency CBC showed microcytic hypochromic anemia (Hb = 2.3 g/dL, MCV = 55.9 fL, MCHC = 23.3 g/dL) with normal total WBC and platelet counts. Upper endoscopy revealed several hookworms along the duodenum, with several hemorrhagic spots and blood ooze at the attachment sites. The subsequent differential WBC count revealed an eosinophilia of 11%.
Case 3.
Case 3 was a 25-year-old male patient complaining of fatigue for 2 weeks, with a transient fainting attack and melena 3 hours before admission. Marked pallor was detected on examination. An urgent CBC showed microcytic hypochromic anemia (Hb = 1.9 g/dL, MCV = 69 fL, MCHC = 25.8 g/dL) with no other abnormalities. An upper endoscopy revealed hookworms with several erosions in the duodenum. Erosions were also observed in the gastric antrum, but there were no worms in the stomach. Additional laboratory results showed characteristic hookworm ova by stool analysis and an eosinophilia of 18% from the differential WBC count.
Case 4.
Case 4 was a 49-year-old male patient presenting with epigastric pain, vomiting, and the recurrent passage of black stool for 3 weeks, with marked pallor on examination. His urgent CBC result showed microcytic hypochromic anemia (Hb = 7.1 g/dL, MCV = 60 fL, MCHC = 28.5 g/dL). After negative upper endoscopy and colonoscopy examinations with no abnormal findings, push enteroscopy was performed for the examination of the small intestine, where hookworms were observed in the jejunum scattered through fresh blood pools. The differential WBC count revealed an eosinophilia of 20%.
Case 5.
Case 5 was a 19-year-old male patient presenting with recurrent palpitations and weakness for 10 days, with upper abdominal pain and black stool in the last 5 days before admission. His clinical examination revealed marked pallor and mild ankle edema. An emergency CBC result showed microcytic hypochromic anemia (Hb = 2.6 g/dL, MCV = 67 fL, MCHC = 28 g/dL). An upper endoscopy revealed several hemorrhagic spots and hookworms along the duodenum. The detailed CBC revealed an eosinophilia of 15% by a differential WBC count.
For all patients, anthelmintic treatment was started just after endoscopic examination using mebendazole at an oral dose of 100 mg two times daily for 3 days. Supplementary oral iron therapy was maintained throughout the entire follow-up period. The patients were discharged within 8–11 days after clinical follow-up, and CBC was performed every 2 weeks (and every 1 week for case 1). The hemoglobin level reached normal values in all patients within 6–10 weeks.
Discussion
This retrospective study gathers the most varying cases in the literature to address the clinical, endoscopic, and biological profiles of hookworm infection as a cause of gastrointestinal bleeding.
Clinically, hookworm infection was not initially considered as a possible etiology in these cases of gastrointestinal bleeding before endoscopy. For example, in the elderly patient (case 2; 87 years), gastrointestinal malignancy or peptic ulcer disease was suspected. Similarly, in the 4-month-old infant (case 1), visualization of the worms with surrounding blood pools was astonishing, because hookworm infection is acquired primarily while walking on contaminated soil; the baby had not even started crawling. However, on the completion of the upper endoscopy procedure, retaking the patient's history revealed that the mother used to lay the baby on nearby soil while working in the village field, raising the possibility that the infection had occurred because of the exposure of the baby's naked skin to larvae in the soil at the age of 2 months (because it takes up to 8 weeks to develop into adult worms).
Interestingly, the clinical examination and CBC data of the mother were normal, and stool analysis was negative for hookworm ova, excluding the possibility of vertical transmission from the mother. Lactogenic transmission can occur with A. duodenale-infective larvae (in contrast to N. americanus), which can arrest development, enter the mammary gland during pregnancy, and pass through the milk to the baby.8 Furthermore, another interesting finding regarding the infant is that the injuries induced by the large number of worms gave a CBC picture of acute hemorrhage (i.e., normocytic normochromic anemia), not the characteristic Ancylostoma microcytic hypochromic anemia. In contrast, the CBCs in the four adult patients showed chronic iron deficiency anemia (i.e., microcytic hypochromic anemia) caused by chronic slow blood loss (unnoticed by the patients for a period of time); then, melena occurred as a late, occasional incident, when the injuries from the worms became exceptionally extensive.
Despite the fact that hookworm infection can be heavy with high worm loads, overt gastrointestinal bleeding has been reported only occasionally.9 This lack of reporting produces a diagnostic difficulty when facing such cases, including resorting to various endoscopic techniques, such as double balloon enteroscopy,10 after negative upper and lower gastrointestinal endoscopies; it can even result in performing an exploratory laparotomy to identify the etiology.11 The occurrence of overt bleeding might be related to a more pronounced host response to the pharmacologically active peptides secreted by the worms, including anticoagulants and platelet inhibitors, that facilitate bloodfeeding and maintain blood oozing,12,13 leading to massive bleeding.9 However, in most cases, the chronic, slowly progressive blood loss allows for compensatory mechanisms, which delay the patient's complaints until low hemoglobin levels are reached.14 Surprisingly, gastrointestinal bleeding can be induced by not only live hookworms but occasionally, dead ones. In a patient from Hungary, a mummified hookworm was observed on endoscopic examination as a speculum-like foreign body embedded in a mucosal protuberance with an ulcerated edge that caused bleeding from the duodenum.15
Endoscopically, the visible blood pools and oozing around the mouthparts of the worms confirmed the blood loss both through bloodsucking and mucosal injury. Interestingly, no mucosal erythema or signs of inflammation were observed at the sites of bites and attachment; this finding may be attributed to the action of various molecules produced by the worms (including the neutrophil inhibitory factor), which have an immunomodulating or down-regulating effect on the host inflammatory response.16,17 However, a severe small intestinal inflammation that simulates Crohn's disease has been reported.18
Although the upper part of the small intestine represents the usual habitat of hookworms, gastric erosions were observed in one of the patients (case 3) who had no history of intake of drugs that may induce such lesions (e.g., non-steroidal anti-inflammatory drugs). Thus, the question of whether these gastric erosions were directly induced by bites from previous hookworms or coincidental lesions was raised. In support of the first possibility are the occasional reports confirming visualization of the worms hooked into the gastric antrum as an ectopic site.19,20 Furthermore, a heavy infection may spread hookworms along the whole gastrointestinal tract, including the stomach, duodenum, jejunum, proximal ileum, and down to the ascending and sigmoid colonic segments, which was reported in a patient examined both by capsule endoscopy and colonoscopy.21 However, in support of the second possibility (i.e., that the gastric erosions were coincidental), several lesions were reported in a study from Thailand, including gastric ulcers, duodenal ulcers, and even gastric and colonic cancers, in some patients with hookworm infections and a mean age of 62 years.22 This latter situation is probably related to the high prevalence of hookworm infection in southeast Asia,1,2 such that there is not a causal relationship between hookworms and lesions. Thus, in case 3, regardless of whether the gastric erosions were induced by the Ancylostoma worms, they were another source of blood loss and aggravated his anemia; a proton pump inhibitor was prescribed to that patient in addition to the usual anthelmintic therapy.
Biologically, gastrointestinal endoscopy provided a unique chance to capture, for the first time, a live demonstration of the bloodfeeding activity of the adult worms together with a series of events that shed light on the biological behavior of hookworms. First, it is already known that the attachment of hookworms to the intestinal mucosa and submucosa is achieved by the buccal capsule and facilitated by the release of several connective tissue hydrolases.23 However, we observed that the invasion is also aided by a mechanical, quick-spinning, body-pushing movement as a third factor that enforced the piercing process within a few seconds, simulating electric piercing devices. Second, the feeding process could be repeated within a short time (a few seconds), leading to several mucosal injuries for each worm and blood losses greater than what was ingested by the worm. Multiplying this behavior by the total number of worms can explain the resulting severe anemia and the blood pools that may produce the melena encountered in these cases. Third, after the fixation of the worm to the mucosa, a graceful smooth movement was maintained during the rest of the meal intake, likely to maintain its position on the host intestinal peristalsis. Fourth, the close-up views enabled the distinction between male and female worms in addition to imaging of the worm gut, highlighting the ingested blood during the feeding stage and the specks of blood lysed during the digestion stage by the proteolytic enzymes that degrade hemoglobin in an orchestrated cascade.24 Fifth, the excretion stage was observed, showing fresh blood passing out of the posterior end of the worm. This observation solves the controversial debate concerning the extent of blood lysis and digestion in the worm canal and supports the view that some red cells are passed intact out of the worm.23
In conclusion, ancylostomiasis can induce melena in all ages from infancy to the elderly, particularly in tropical areas. Endoscopically, varying small intestinal injuries resulting in luminal blood pools can be seen. Biologically, worm mucosal piercing occurs through a quick spinning movement, and the repeated feeding behavior aggravates blood loss. After treatment is started, bleeding stops with a rapid response to anthelmintic therapy plus supplementary iron.
Supplementary Material
Footnotes
Authors' addresses: Maha Barakat and Ahmed Nasr, Department of Tropical Medicine and Gastroenterology, Assiut University Hospital, Assiut, Egypt, E-mails: mahabarakat2001@yahoo.com and amnasr98@yahoo.com. Naglaa Ibrahim, Department of Pediatrics, Assiut University Hospital, Assiut, Egypt, E-mail: n_ibrahim_pediat@yahoo.com.
References
- 1.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 2.Hotez PJ, Bethony J, Bottazzi ME, Brooker S, Diemert D, Loukas A. New technologies for control of hookworm infections. Trends Parasitol. 2006;22:327–331. doi: 10.1016/j.pt.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med. 2004;351:799–805. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- 4.El-Kholy SI, Abdel-Magied S, El-Ganayni GA. Some epidemiological studies on Ancylostoma duodenale infection. J Egypt Soc Parasitol. 1983;13:447–453. [PubMed] [Google Scholar]
- 5.Bakr IM, Arafa NA, Ahmed MA, Mostafa Mel H, Mohamed MK. Prevalence of intestinal parasitosis in a rural population in Egypt, and its relation to socio-demographic characteristics. J Egypt Soc Parasitol. 2009;39((Suppl)):371–381. [PubMed] [Google Scholar]
- 6.John DT, Petri WA. Markell and Voge's Medical Parasitology. 9th ed. Philadelphia, PA: Saunders Elsevier; 2006. The intestinal nematodes; pp. 239–273. [Google Scholar]
- 7.Roberts LS, Janovy J. Nematodes: strongyloidea, bursate rhabditidans. In: Schmidt GD, Roberts LS, editors. Foundations of Parasitology. 8th ed. New York, NY: McGraw-Hill; 2010. pp. 419–432. [Google Scholar]
- 8.Yu SH, Jiang ZX, Xu LQ. Infantile hookworm disease in China. A review. Acta Trop. 1995;59:265–270. doi: 10.1016/0001-706x(95)00089-w. [DOI] [PubMed] [Google Scholar]
- 9.Bhargava DK, Dasarathy S, Chowdhry GC, Anand AC, Saraswat V. Upper gastrointestinal bleeding due to hookworms (Ancylostoma duodenale): a case report. Endoscopy. 1993;25:548–549. doi: 10.1055/s-2007-1010399. [DOI] [PubMed] [Google Scholar]
- 10.Kshaunish D, Kausik D, Dasgupta J, Dhali GK, Chowdhury A. Hookworm infection presenting as overt gastrointestinal bleeding: lessons from double-balloon enteroscopy in the tropics. Am J Gastroenterol. 2008;103:1310–1311. doi: 10.1111/j.1572-0241.2007.01782_5.x. [DOI] [PubMed] [Google Scholar]
- 11.Linterman JP. Severe intestinal bleeding leading to exploratory laparotomy in an infant with hookworm infection. Clin Pediatr. 1976;15:1073–1074. doi: 10.1177/000992287601501117. [DOI] [PubMed] [Google Scholar]
- 12.Harrison LM, Nerlinger A, Bungiro RD, Cordova JL, Kusmic P, Cappello M. Molecular characterization of Ancylostoma inhibitors of coagulation factor Xa. Hookworm anticoagulant activity in vitro predicts parasite blood feeding in vivo. J Biol Chem. 2002;277:6223–6229. doi: 10.1074/jbc.M109908200. [DOI] [PubMed] [Google Scholar]
- 13.Lee AY, Vlasuk GP. Recombinant nematode anticoagulant protein c2 and other inhibitors targeting blood coagulation factor VIIa/tissue factor. J Intern Med. 2003;254:313–321. doi: 10.1046/j.1365-2796.2003.01224.x. [DOI] [PubMed] [Google Scholar]
- 14.Zuckerman KS. Approach to the anemias. In: Goldman L, Ausiello D, editors. Cecil Medicine. 23rd ed. Philadelphia, PA: Saunders Elsevier; 2008. pp. 1179–1187. [Google Scholar]
- 15.Rácz I, Szabó A, Goda M, Magyar É. Embedded mummified hookworm as a cause of bleeding from the duodenal bulb. Endoscopy. 2007;39((Suppl 1)):E159. doi: 10.1055/s-2006-944652. [DOI] [PubMed] [Google Scholar]
- 16.Moyle M, Foster DL, McGrath DE, Brown SM, Laroche Y, De Meutter J, Stanssens P, Bogowitz CA, Fried VA, Ely JA. A hookworm glycoprotein that inhibits neutrophil function is a ligand of the integrin CD11b/CD18. J Biol Chem. 1994;269:1008–1015. [PubMed] [Google Scholar]
- 17.Zhan B, Badamchian M, Bo MH, Ashcom J, Feng JJ, Hawdon J, Xiao SH, Hotez PG. Molecular cloning and purification of Ac-TMP a developmentally regulated putative tissue inhibitor of metalloprotease released in relative abundance by adult Ancylostoma hookworms. Am J Trop Med Hyg. 2002;66:238–244. doi: 10.4269/ajtmh.2002.66.238. [DOI] [PubMed] [Google Scholar]
- 18.Gill KRS, Stark ME. Hookworm infestation masquerading as Crohn's disease: diagnosis by double balloon enteroscopy. Am J Gastroenterol. 2009;104:795–796. doi: 10.1038/ajg.2008.121. [DOI] [PubMed] [Google Scholar]
- 19.Dumont A, Seferian V, Barbier P. Endoscopic discovery and capture of Necator americanus in the stomach. Endoscopy. 1983;15:65–66. doi: 10.1055/s-2007-1021467. [DOI] [PubMed] [Google Scholar]
- 20.Rana SS, Bhasin DK, Sinha SK. Endoscopic diagnosis of chronic severe upper GI bleeding due to helminthic infection. Gastrointest Endosc. 2008;68:1023. doi: 10.1016/j.gie.2008.03.1061. [DOI] [PubMed] [Google Scholar]
- 21.Wu IC, Lu CY, Wu DC. Acute hookworm infection revealed by capsule endoscopy. Endoscopy. 2007;39((Suppl 1)):E306. doi: 10.1055/s-2007-967015. [DOI] [PubMed] [Google Scholar]
- 22.Ovartlarnporn B, Chamroonkul N, Sirithanaratanakul N, Kosolbharn P. Gastrointestinal lesions in patients over 40 years of age with iron deficiency anemia and hookworm infection. Southeast Asian J Trop Med Public Health. 1990;21:594–597. [PubMed] [Google Scholar]
- 23.Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol. 2004;58:197–288. doi: 10.1016/S0065-308X(04)58004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williamson AL, Brindley PJ, Knox DP, Hotez PJ, Loukas A. Digestive proteases of blood-feeding nematodes. Trends Parasitol. 2003;19:417–423. doi: 10.1016/s1471-4922(03)00189-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.