Abstract
We surveyed the genetic ancestry and recorded the occurrence of autogeny, the developmental times, and survival rates in families of Culex pipiens in Santa Clara County, CA, at 37°N latitude. Females in 95% of the families produced fertile egg rafts without access to blood (= autogeny) after mating in stenogamous conditions. Developmental time, survival, and egg raft production were closely correlated to temperature. Male DV/D ratios overwhelmingly matched Cx. pipiens but a microsatellite analysis revealed these were Cx. pipiens form molestus hybridized with Culex quinquefasciatus and to a lesser extent to Cx. pipiens form pipiens, a genetic mix heretofore not recorded elsewhere. Greater DV/D ratios and larger proportions of genetic ancestry from Cx. quinquefasciatus were negatively correlated to autogeny. The combination of multiple overwintering strategies and widespread autogeny in females arising from aboveground larval sites supports the hypothesis that some North American populations of Cx. pipiens complex mosquitoes express unusual phenologies.
Introduction
Members of the Culex pipiens L. complex are broadly considered critical urban vectors in North America of the West Nile and Saint Louis encephalitis flaviviruses.1 The complex includes populations with distinct behaviors and physiologies that greatly influence their capabilities as vectors. The ability of adult females to survive freezing winters (by undergoing dormancy or true diapause), the willingness of males to mate in small confined spaces (stenogamy), the ability of females to lay a first batch of eggs without requiring a blood meal (autogeny), and the female choice of both bird and mammalian blood, all traits whose expression varies widely across the complex, have obvious implications for distribution, abundance, and consequent vectorial capacity.2
The current taxonomy of the Cx. pipiens complex in the Catalog of the Mosquitoes of the World3 maintained by the Walter Reed Biosystematics Unit at the Smithsonian Institution (wrbu.si.edu), recognizes Culex quinquefasciatus Say and Cx. pipiens as separate species. These species are reliably recognizable by the shape of the male genitalia,4 a standard source of taxonomic characters in mosquitoes, and by DNA-based rapid assays.5 Culex quinquefasciatus (the southern house mosquito) and Cx. pipiens (the northern house mosquito) have been widely distributed across the world by humans,6,7 with whom they are closely associated. In overlapping areas, the species have hybridized extensively.8–13 The worldwide movement of these two species continues to this day as revealed by rapid expansion of insecticide resistance14 and the recent invasion of remote locations such as the Galapagos Islands.15
To complicate matters further, Cx. pipiens has two recognized ecological forms: Cx. pipiens form pipiens, the original type of mosquito described by Linnaeus in 1758 as Cx. pipiens, a diapausing, mostly bird-biting form,16 and Cx. pipiens form molestus, the Egyptian mosquito described by Forskål in 1775 as Culex molestus, a non-diapausing, stenogamous, human-biting mosquito that, however, often lays its first egg mass autogenously.17,18 The existence of Cx. molestus as a separate species was terminated because of a lack of morphologically distinguishing characters and evidence for sympatric interfertility.18 Recently, however, microsatellite markers have shown genetic isolation between populations of these two forms in northern Europe, as well as extensive hybridization and introgression in the United States9 and some hybridization in southern Europe.9,19 Furthermore, Bahnck and Fonseca20 have identified fixed genetic differences between the two forms and developed a rapid assay that distinguishes them. The distinctiveness and relatively low genetic diversity of underground populations of Cx. pipiens form molestus has been remarked upon repeatedly.11,19,21
In the Palearctic region, autogeny is commonly reported in populations of Cx. pipiens that inhabit sheltered locations that remain warm during the winter, usually underground.22,23 In contrast, in northern Africa,24 Eastern Asia,25 and Australia,26,27 autogeny was found in all populations of Cx. pipiens collected both above and below ground. Aboveground populations of autogenous Cx. pipiens in northern California have been described, but only in a dissertation completed in 1966 by W. G. Iltis.28 We observed that the overwhelming majority of Cx. pipiens females reared from egg rafts collected out of a decorative pond at a garden store in San José, California, deposited eggs without a blood meal. The objective of this study was to examine in detail the genetics and phenology of Californian autogenous Cx. pipiens populations.
Materials and Methods
Sources and handling of specimens.
The Santa Clara County Vector Control District intensified surveillance for the Cx. pipiens group and Culex tarsalis Coquillett mosquitoes in 2003 in anticipation of the introduction of West Nile virus into the county. Working with the California Department of Health Services and the Santa Clara County Department of Public Health, the spread of the virus was carefully documented by the appearance of dead, infected crows29 and, eventually, infected mosquitoes. These efforts resulted in the opportunity to work more closely with Cx. pipiens populations in the region.
We first observed the occurrence of autogenous oviposition by Cx. pipiens group mosquitoes in a colony started from egg rafts collected from a decorative pond (60 L capacity containing clear water and aquatic plants) at a garden store in central San José (37.32°N, 121.90°W) on August 26, 2003. Females reared locally from eggs started laying eggs without access to blood. Next, we saw the same phenomenon in another colony established from first-instar Cx. pipiens group mosquitoes collected on June 10, 2004 from an outdoor funerary urn at a cemetery in San José (37.36°N, 121.84°W).
In response to the discovery of autogeny in mosquitoes from aboveground sites, in 2004 we collected 48 egg rafts (= families) using Reiter traps30 from 13 sites between July 16 and September 24, 2004 (Table 1). An additional 22 families were collected from a bucket (11 L capacity) containing aged alfalfa-pellet infusion at Arzino Ranch (37.43°N, 121.96°W), Alviso neighborhood, San José, between September 24 and October 14, 2004 (Table 2).
Table 1.
Number of males and mean DV/D ratio of males from autogenous egg rafts deposited by the progeny of Culex pipiens collected from gravid traps located in Santa Clara County, July 16 through September 24, 2004
| Location* | No. of families | Rafts/female† | No. of males | Mean (range) DV/D‡ |
|---|---|---|---|---|
| 800 N. Emory St., SJ | 25 | 0.52 | 117 | 0.056a (0–0.21) |
| 3410 Ross Ave., SJ | 1 | 0.10 | 5 | 0.071ab (0.036–0.12) |
| 2840 The Villages Pkwy, SJ | 3 | 0.26 | 13 | 0.095ab (0.012–0.18) |
| 160 Cuesta Dr., MV | 1 | 0 | 6 | 0.022a (0–0.071) |
| 2195 Shoreline Blvd., MV | 1 | 1.0 | 5 | 0.034a (0–0.11) |
| 22620 Stevens Creek Blvd., CO | 4 | 0.19 | 20 | 0.092ab (0–0.19) |
| 485 W. Sunnyoaks, CL | 1 | 0.22 | 7 | 0.095ab (0–0.18) |
| 1912 Bowers, SC | 1 | 0.19 | 5 | 0.14b (0.071–0.18) |
| 3495 Benton, SC | 4 | 0.49 | 20 | 0.076ab (0–0.17) |
| 3011 Corvin Dr., SC | 1 | 0.75 | 4 | 0.068ab (0.067–0.071) |
| 910 Ticonderoga Dr., SE | 3 | 0.47 | 15 | 0.040a (0–0.12) |
| 19800 Cox Ave., SA | 5 | 0.15 | 24 | 0.11ab (0–0.21) |
| 711 Serra St., PA | 2 | 0.26 | 10 | 0.04a (0–0.10) |
| Total | 52 | 0.42 | 367 | 0.070 (0–0.21) |
SJ = San José; MV = Mountain View; CO = Cupertino; SC = Santa Clara; SE = Sunnyvale; SA = Saratoga; PA = Palo Alto.
Forty-eight of 52 families (92%) deposited eggs autogenously.
Locations not followed by the same superscripted letter were significantly different (one-way ANOVA followed by Student-Newman-Keuls test: df = 12/250; F = 4.169; P < 0.001).
Table 2.
Mean DV/D ratios and number of autogenous egg rafts deposited by progeny of Culex pipiens collected as rafts in a bucket of foul water at Arzino Ranch, San José, California, September 24 through October 14, 2004
| Family* | No. of males | Mean (SD) of DV/D | Autogenous rafts per family |
|---|---|---|---|
| 16a | 5 | 0.001 (0.037) | 2 |
| 5ab | 5 | 0.003 (0.038) | 12 |
| 6abc | 5 | 0.018 (0.018) | 4 |
| 13abc | 5 | 0.027 (0.084) | 14 |
| 15abc | 5 | 0.030 (0.059) | 15 |
| 19abc | 5 | 0.033 (0.047) | 10 |
| 7abc | 5 | 0.039 (0.016) | 7 |
| 1abc | 5 | 0.052 (0.019) | 19 |
| 22abc | 5 | 0.054 (0.068) | 11 |
| 17abc | 4 | 0.055 (0.039) | 13 |
| 11abc | 5 | 0.060 (0.005) | 13 |
| 2abc | 5 | 0.066 (0.040) | 16 |
| 30abc | 5 | 0.066 (0.077) | 0 |
| 18abc | 5 | 0.072 (0.045) | 5 |
| 14abc | 5 | 0.079 (0.071) | 11 |
| 20abc | 5 | 0.088 (0.044) | 7 |
| 8abc | 5 | 0.092 (0.046) | 17 |
| 12abc | 5 | 0.102 (0.081) | 13 |
| 10abc | 5 | 0.113 (0.074) | 26 |
| 3abc | 5 | 0.119 (0.040) | 9 |
| 4bc | 5 | 0.123 (0.054) | 6 |
| 23c | 5 | 0.134 (0.053) | 9 |
| Total | 109 | 0.065 (0.061) | 10.9 |
Families not followed by the same superscripted letter had significantly different DV/D ratios (one-way analysis of variance followed by Student-Newman-Keuls test: degrees of freedom (df) = 21/108, F = 2.693, P = 0.001).
Each of the 70 egg rafts was held individually and watched carefully to collect first instars within 12 hours of hatching. Exactly 50 larvae from each egg raft were transferred to clear plastic 3.8 L bottles (originally containing drinking water) with a screen top, containing 1 L of dechlorinated tap water and 25 mg of food (ground TetraMin Tropical Fish Food, Tetra Werke, Melle, Germany). These bottles were kept in a covered area that experienced ambient temperature and light, although no direct sunlight. Larvae were fed 50 mg of food in slurry every third day until the first pupa appeared, in an effort to provide optimum nutrition. Adults were allowed to emerge directly into the space above the water (2.8 L volume) and had constant access to a cut raisin on the screen top. These adults were considered to be the F0 generation. The females did not have access to blood; therefore, any egg rafts deposited by these females developed autogenously. We removed the egg rafts carefully by inserting a long stick through the top of the bottle and snagging each raft individually. The rafts were allowed to hatch in a small amount of water and 50 first instars from each family placed in individual bottles as above to form the F1 generation. The number of females in each bottle for the families originating from Reiter traps was assessed by examination of the pupal skins at the end of 2 weeks so the relative occurrence of autogeny was expressed as number of (autogenous) egg rafts per female. However, in families originating from the Arzino Ranch the number of females was not counted; therefore, autogeny was expressed as number of egg rafts per family.
Morphological measurement.
The DV/D ratio4 was measured on ∼5 males in each F0 collection and on three males from the original June 10, 2003 collection. The DV/D ratio divides the relative distance between DV (the distance between the tip of one ventral arm of the male genitalia and the lateral side of the dorsal arm at the point where the ventral arm intersects when viewed from the dorsal aspect) by the distance between the points where dorsal and ventral arms intersect on each side (D). A ratio < 0.2 is considered to be typical of Cx. pipiens, a ratio greater than 0.5 is considered to be typical of Cx. quinquefasciatus, and a ratio between 0.2 and 0.5 is considered to be typical of a hybrid.31 Specimens were prepared according to Barr's4 original method (gentle clearing in potassium hydroxide), except that they were mounted in Euparal (Bioquip, El Segundo, CA).
Temperature and development.
The ambient temperature was recorded hourly using an underwater electronic recording device (Model H20-001, Onset Computer Corporation, Bourne, MA; same as HOBO Pro v2 Underwater Data Logger, MicroDaq.com, Ltd., Contoocook, NH) placed in one of the bottles where larvae were reared. The maximum and minimum temperatures for each day were averaged to produce a mean daily temperature. The daily rate of mortality for females was calculated from the survivorship equation, Nt = N0(ert); where Nt is the number of individuals alive at time t (days), N0 is the number of individuals at the beginning (assuming a 50% gender ratio for each group of 50 larvae, resulting in an estimate of 25 females), e is the natural logarithm, and r is the daily mortality rate.32 Analyses of various relationships between egg raft production, developmental time, and mortality were performed in Microsoft Excel (Microsoft Corporation, Redmond, WA).
The mean-minus-base method was used to calculate a base temperature for a day-degree model,33 and was calculated by finding the base temperature (intentionally varied for the calculation) that produced the lowest standard deviation of DD for all families in the following equation:
, where:
TD = total developmental time from hatching to first emergence of an adult in days;
DD = observed total day-degrees (from average of maximum and minimum daily temperatures) during development of each family;
MT = mean water temperature during entire developmental period; and
BT = base temperature (i.e., maximum temperature at which there is no development).
The final model used the base temperature as calculated previously and the mean value of DD to estimate total developmental time.
Analysis of overwintering.
The F0 and F1 families were held at ambient light and temperature conditions as described previously until March 13, 2005. On January 7 and 10, 45 F0 females from 17 families were dissected to determine their gonotrophic status34 and to look for signs of the accumulation of fat body. Fat body as an indication of the mosquito being in a state of diapause35 or an intermediate state of dormancy36 was considered to be in excess of the normal condition when it filled one or more segments of the abdomen, often forming firm lobes. No attempt was made to distinguish the kinds of fat cells in the fat body. Cessation of dormancy was recorded in a sample of females from F0 families by offering females blood meals on two dates in late winter (February 21 and March 13). Five females from a single family were aspirated without anesthesia in a plastic capsule (45 mm diameter, 25 mm high) with a screen bottom. The capsule was held against the lower arm of the author (D. S.) in a darkened room during the morning for 20 minutes and then the number of blood fed mosquitoes counted. Those mosquitoes that took blood were held for observation of oviposition, which was taken to be a sign of the cessation of dormancy.37
Field observations.
A large storm drain with pooled water located under Willow Glen Way, Willow Glen neighborhood, San José (37.30°N, 121.89°W) provided an example of an overwintering site for Cx. pipiens group mosquitoes in this part of California. City engineers described how a 60-inch storm drain running down the center of the street emptied into the nearby Guadalupe River through an outlet elevated above the bottom of the pipe. As a result, the system held water constantly, with the depth of the water gradually decreasing as the pipe sloped upward. The water was shallow enough in 480 m of the pipe to expose the bottoms of the manhole tubes that were located about every 48 m along the street, so that a larval habitat ∼731 m2 in extent was formed. Water temperature was recorded with a data logger operated from January 27 through February 3. The site was treated with a Bacillus sphaericus preparation (Vectolex WDG, Valent BioSciences Corp., Libertyville, IL) on January 27. Sampling was accomplished with four carbon-dioxide baited encephalitis virus surveillance (EVS) traps that were hung in manholes 0.3 m below the street along the length of the larval habitat on January 20 (before treatment) and on 3, 10, and 24 February (after treatment).
Genetic analysis.
We used a panel of eight microsatellite loci that amplify consistently across all United States Cx. pipiens complex populations (CQ11, CQ26, CxqGT4, CxqGT6b, CxpGT4, CxpGT9, CxpGT12, CxpGT46),38 These same loci have been used in multiple studies of populations of the Cx. pipiens complex in Western Europe, northern Africa, the Middle East, Asia, and Australia,9,10 Portugal,19 and across north-south transects in the United States (Fonseca DM and others, unpublished data), and therefore allow for broad comparisons. We examined the genetic ancestry of four mosquitoes, both males and females, from each of the 47 families (Table 1). Analyses of mosquito families have shown that all the microsatellite loci used in this study are inherited in a Mendelian fashion and are not sex-linked.39,40 Microsatellite loci were amplified and sized as described by Smith and colleagues.38 We assigned individuals to clusters (taxa) based on their multilocus genotypes with a maximum likelihood algorithm implemented in the program Structure 2.0.41 We used 100,000 burn-in steps and 1,000,000 runs with a model of uncorrelated allele frequencies allowing admixture (gamma = 0.34, calculated at K = 1).42 In this analysis, the origin of each specimen is not disclosed but the number of clusters (K) is decided a priori for each run. To assess the consistency of the analysis we performed an exhaustive comparison of 10 runs scoring the similarity coefficient described in Rosenberg and others.43 We chose the most appropriate putative number of clusters (K = 3), by choosing the K with the highest associated ΔK.44 To examine the ancestry of the California populations, we included in the analysis several other populations: 1) specimens collected as larvae in midwinter in Jersey City, NJ and in Philadelphia, PA (specimens from these populations were brought to the laboratory and all adult females were autogenous); 2) specimens of Cx. pipiens form pipiens and Cx. pipiens form molestus collected in Germany and the United Kingdom,9 and 3) specimens identified morphologically as Cx. quinquefasciatus from Chino, CA and New Orleans, LA.6
Results
Morphological measurements.
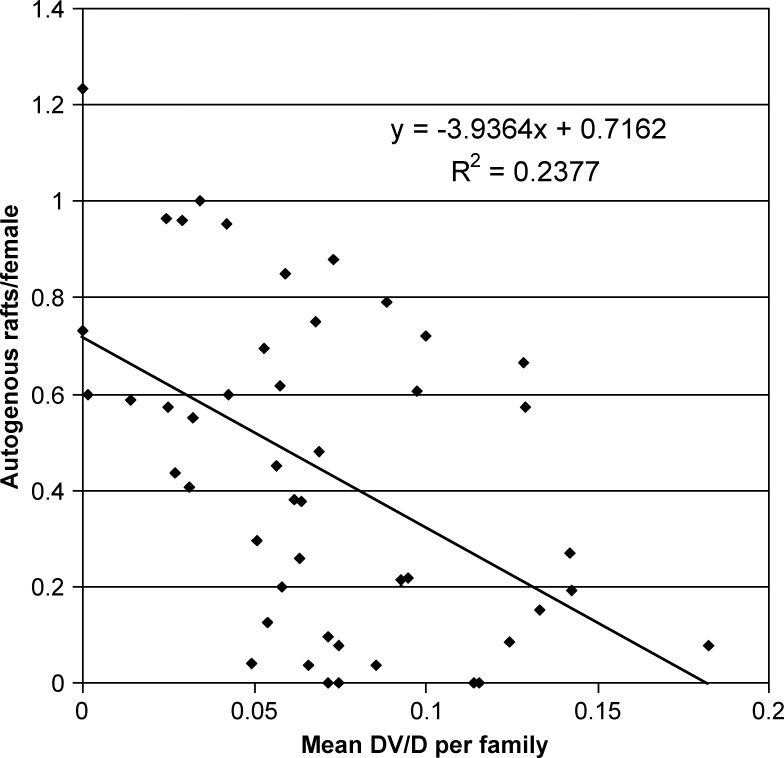
The DV/D ratios measured were well within the range generally associated with male Cx. pipiens, rather than with either Cx. quinquefasciatus or with a hybrid of the two species. Three F0 males from the autogenous colony that originated on June 10 had a mean DV/D ratio of 0.075 (0.033–0.125). Mosquitoes reared from egg rafts deposited by females from a wide area of northern Santa Clara County had a mean DV/D ratio of 0.070, with only a single male above 0.20 (Table 1). Twenty-two of the families collected from Arzino Ranch between September 24 and October 14 had a mean DV/D ratio of 0.065 (−0.001–0.13) (Table 2). There was a strong inverse relationship between the DV/D ratio and autogenous egg raft production among the families collected from across northern Santa Clara County (Figure 1).
Figure 1.
Inbred families of F0 Cx. pipiens group mosquitoes reared from egg rafts deposited by females collected from aboveground Reiter traps in Santa Clara County, CA, July 16–October 14, 2004. Average number of autogenous egg rafts deposited per female negatively correlated to DV/D ratio of males from corresponding inbred families.
Temperature and development.
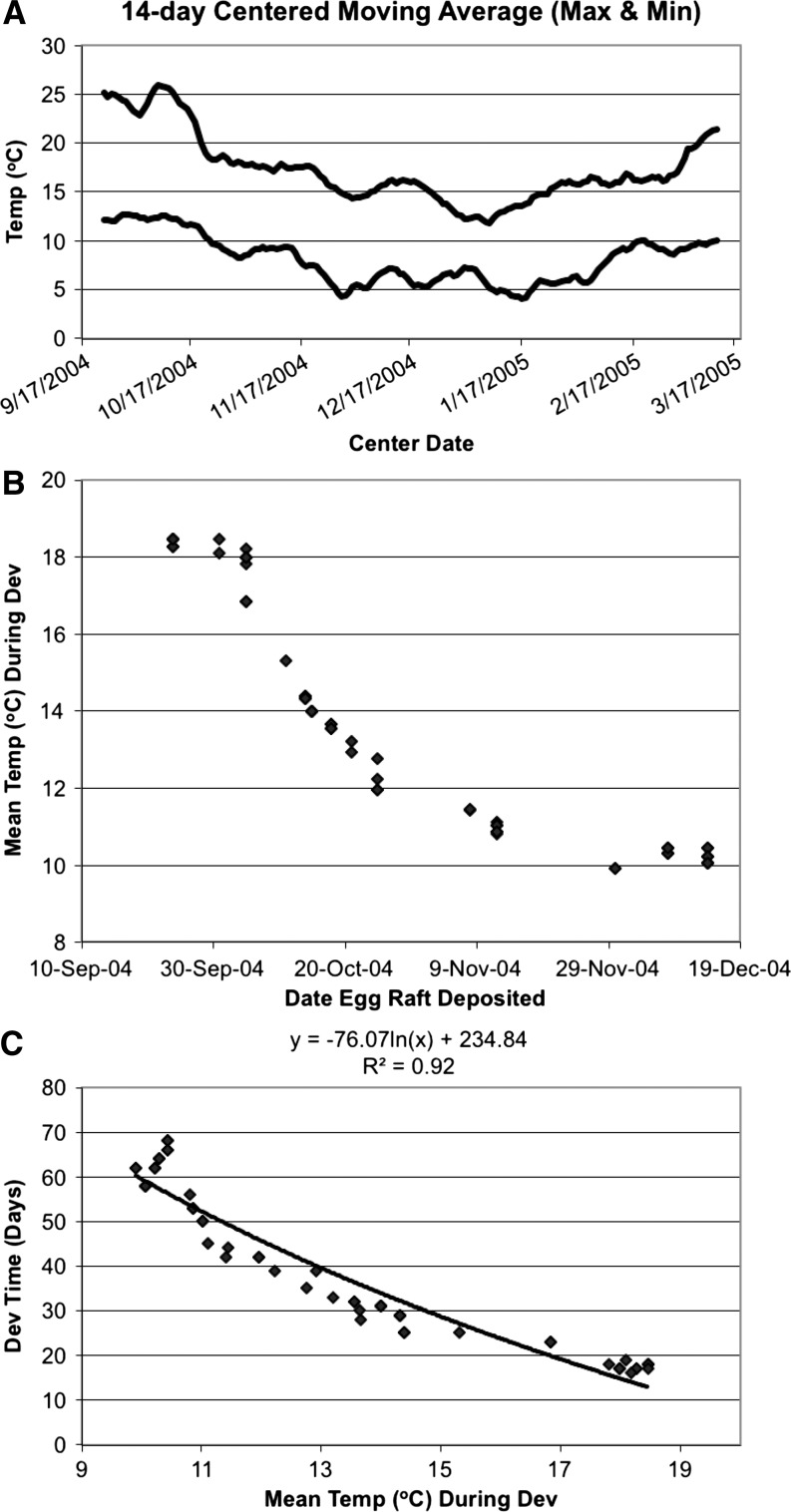
The 74 families of mosquitoes reared from egg rafts deposited at various times in the fall provided the opportunity to measure the relationship between naturally occurring temperature patterns and developmental time. Autogenous oviposition occurred through December, resulting in development of larvae under low temperature conditions (Figure 2A and B). Not surprisingly, there was a very close relationship between the natural log of the daily mean temperature and the time required from deposition of the egg raft to emergence of the first adult (Figure 2C), with a determination coefficient (R2) of 0.92.
Figure 2.
Effects of temperature on the development rates of fall and winter populations of Culex pipiens. (A) Moving average (14-day, centered) of maximum and minimum temperatures during the period of the fall and winter study. (B) Mean temperature (°C) during development (hatching to first emergence) of Cx. pipiens group egg rafts deposited during the fall and winter. (C) Relationship between development time (days) and mean water temperature (°C) for 74 F0 and F1 autogenously produced families of Cx. pipiens group mosquitoes collected in Santa Clara County, CA, and reared under ambient outdoor conditions from September 24 through February 14.
The data supported development of a day-degree model based on mean daily temperatures. The optimum (i.e., lowest standard deviation among day-degree totals) base temperature was 7.1°C (data not shown), producing a mean of 199.6 days × °C for development and standard deviation of 15.23 days (N = 74). Using this model, the developmental time in the Willow Glen Way underground site, based on a water temperature of 13.9°C, would have been 29.4 days (199.6 days × °C/[13.9°C −7.1°C]). The generation of mosquitoes reared from eggs deposited in the field (F0) developed faster and experienced higher temperatures than their offspring (F1). The difference in temperature almost certainly accounted for the difference in developmental time. Application of the day-degree model to the mean temperatures yielded estimates of developmental times very close to those that were observed (Table 3).
Table 3.
Differences in developmental time and temperature experienced by families of Cx. pipiens reared from eggs rafts deposited in the field (F0) and those reared from progeny egg rafts (F1)
| Generation | n | Dates of deposition | Mean ± SD, days | Mean ± SD | Calculated days to dev. |
|---|---|---|---|---|---|
| Days to emergence | Temp. (°C) | ||||
| F0 | 43 | 9/24–10/21 | 24.6* ± 6.28 | 15.80† ± 2.06 | 22.9 |
| F1 | 32 | 10/25–12/14 | 55.7* ± 9.40 | 10.72† ± 0.72 | 55.1 |
Means significantly different, t = −16.2, P < 0.001.
Means significantly different, t = 15.0, P < 0.001.
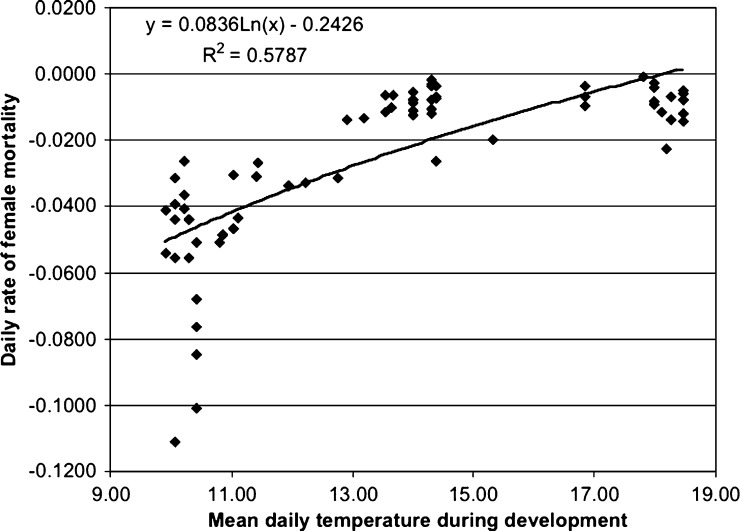
The daily mortality rate of female mosquitoes was generally very low during the experiment, with mean −0.0261 and range −0.0009 to −0.1110. As shown in Figure 3, a significant component of the mortality rate was correlated to the mean temperature during larval and pupal development. The low mortality rate was reflected by the long survival of some individuals during the experiment (Table 4). Some individuals among the F0 families (that were in the laboratory much longer than the F1 families) were still alive when the experiment was terminated on March 11, nearly 5 months after emergence.
Figure 3.
Rate of daily female mortality (negative values, the absolute values of which are correlated to the rate of mortality) of F0 Culex pipiens group mosquitoes correlated to mean daily temperature (°C) from hatching to first emergence within the family held at ambient outdoor conditions in families of mosquitoes reared from egg rafts deposited by field-caught mosquitoes or egg rafts deposited in the field July 16–October 14, 2004, Santa Clara County, CA.
Table 4.
Survival of individual female Cx. pipiens group mosquitoes from the F0 generation reared from field-collected egg rafts and held under ambient outdoor conditions*
| Days from emergence to 3/11/2004 | No. of families | Percentage of females survived | |
|---|---|---|---|
| Mean | Range | ||
| 101–110 | 3 | 13 | 0–20 |
| 111–120 | 17 | 38 | 20–76 |
| 121–130 | 5 | 28 | 0–60 |
| 131–140 | 8 | 46 | 24–84 |
| 141–151† | 11 | 20 | 0–44 |
The experiment was terminated on March 11, 2004.
Two families survived 151 days, one with 8% of individuals surviving and the other with 32%.
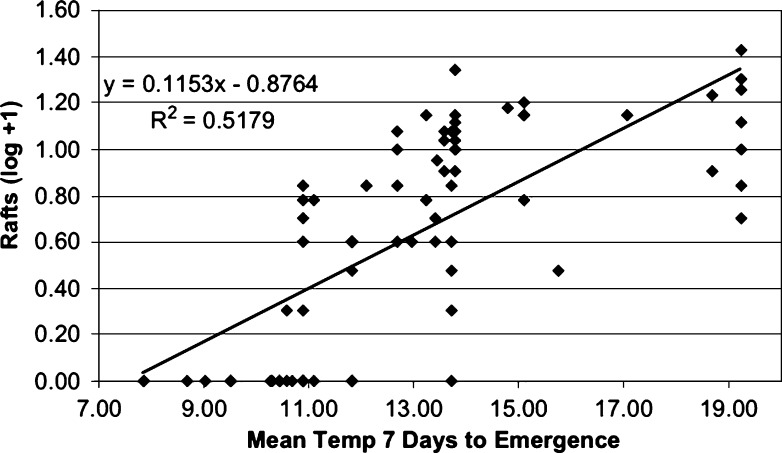
Autogenous egg raft production was related to the temperature experienced by the larvae and pupae for the 7 days before emergence (Figure 4). The F0 generation deposited a mean of 9.4 egg rafts per family (range 0–26) and the F1 generation deposited a mean of 1.2 egg rafts per family (range 0–6), probably corresponding to the difference in temperature experienced by the two generations. Surprisingly, 22 of the 43 F0 families deposited a total of 38 autogenous egg rafts in March, apparently having held the oocytes for several months despite the constant presence of water in which to oviposit.
Figure 4.
Number of egg rafts deposited by F0 and F1 families of Culex pipiens group mosquitoes correlated to the mean water temperature during the 7 days before first emergence. Santa Clara County, CA, July 16, 2004–March 11, 2005.
Analysis of overwintering behavior.
An accumulation of fat body was present in 38 (84%) of the 45 F0 females dissected on January 7 and 10. Eleven of the 38 females (29%) with accumulations of fat body were nulliparous with undeveloped (Christopher's stage I–II) ovaries, 25 (66%) were parous with undeveloped ovaries, and 2 (5%) could not be determined because of the advanced state of oocyte development (Christopher's stage IV). The 12 females without an accumulation of fat body were for the most part (N = 11, 92%) parous with undeveloped ovaries. One individual without an accumulation of fat body had Christopher's stage V ovaries.
Of the 20 F0 females (from 5 families) offered a blood meal on February 21, two (10%) took a blood meal. The two blooded females were 132 days old when they fed and they went on to deposit egg rafts 20 days later. Of the 40 females from the same 5 families offered a blood meal on March 13, 8 (20%) took blood (they were 114–125 days old).
Field observations.
The presence of winter-active, underground populations of Cx. pipiens was confirmed in the field. The site on Willow Glen Way came to our attention originally because residents in the area had complained for several years of mosquito bites in the winter. Four carbon-dioxide baited EVS traps hung in manholes 0.3 m below the street captured 202 female and 197 male Cx. pipiens group mosquitoes on January 20. Treatment with a B. sphaericus preparation reduced the total number of female mosquitoes from 50.5/trap on January 20 to 32.5 females/trap on February 3, and 8.4 females/trap on February 10. During the same period, the number of male mosquitoes was reduced from 49.2 males/trap to 2.0 males/trap. Technicians did not need to treat the site again until May. Water temperature in the underground site was remarkably constant (average 13.9°C from January 27 to February 5), except during a period of rainfall on February 5.
Genetic analyses.
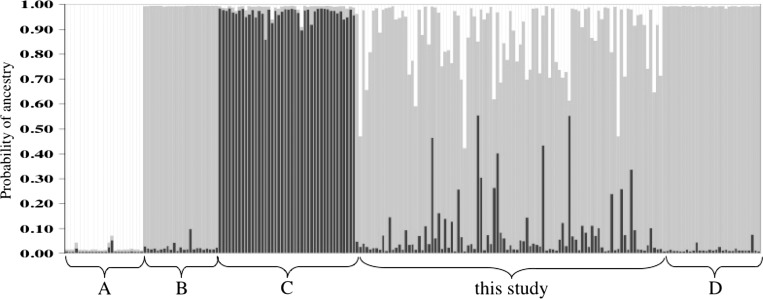
The microsatellite analysis revealed that these populations have an overwhelmingly Cx. pipiens form molestus predicted genetic ancestry obtained from the multilocus Bayesian analysis (average Cx. pipiens form molestus ancestry = 0.79 ± 0.02, mean ± SE, Figure 5) consistent with the expression of the autogeny trait. There is also strong evidence of hybridization with Cx. quinquefasciatus (average Cx. quinquefasciatus ancestry = 0.13 ± 0.01), and to a lesser extent to Cx. pipiens form pipiens (average Cx. pipiens form pipiens ancestry = 0.08 ± 0.01). There was a significant positive correlation (slope = 0.55, P = 0.04) across families between the average number of autogenous egg rafts per female and average genetic ancestry from Cx. pipiens form molestus in each family. In contrast, there was a significant negative relationship between ancestry from Cx. quinquefasciatus (slope = −0.7, P = 0.04) and number of autogenous eggs rafts per female. Average familiar ancestry from Cx. pipiens form pipiens was not correlated with number of autogenous eggs (P = 0.66). As expected, specimens with increasing Cx. pipiens form molestus ancestry were from families with significantly lower average DV/D ratios, although the relationship was not very strong (slope = 0.07, P = 0.002).
Figure 5.
Multilocus genotype analysis of San Jose autogenous families. A = Culex quinquefasciatus from Chino, CA and New Orleans, LA; B = Culex pipiens form molestus from Germany and Scotland; C = Cx. pipiens from pipiens from Germany and England; D = Cx. pipiens form molestus from Philadelphia, PA and Jersey City, NJ. Each of the individuals included in the analysis is represented by a thin vertical line, partitioned into colored segments that represent the individual's probability of belonging to one of each of the genetic clusters (genetic ancestry). Although the origin of each specimen is not used in the analysis, in this figure specimens were grouped by location. To simplify the figure we included only half of the specimens from California genotyped for this study (94 = 188/2).
Discussion
The observed low DV/D ratios were well within the Cx. pipiens range and provided no indication of the complex genetic mix of both Cx. pipiens forms and Cx. quinquefasciatus. On the other hand, the genetic complexity paralleled the complex set of phenotypes observed in these populations. The overwhelming prevalence of autogeny in these California populations agreed with Iltis' prior detailed findings.28 However, in addition we found an inverse relationship between the DV/D ratio and autogenous egg rafts per female (i.e., autogenous egg raft production), indicating that females from families with higher DV/D ratios, even though still within the range identified as Cx. pipiens, were less likely to lay autogenous eggs. There was also a significant correlation between genetic ancestry and autogenous capacity: families with increasing Cx. pipiens molestus ancestry or conversely decreasing Cx. quinquefasciatus ancestry had a higher number of autogenous egg masses per female. The genetic ancestry from Cx. pipiens form pipiens in these populations is very low (around 8%) and predictably exhibited no significant correlations. All together, these associations indicate that despite extensive hybridization, morphological, physiological, and genetic traits were still linked. Even though the expression of autogeny characteristically differs between the two forms of Cx. pipiens, both exhibit equally low DV/D ratios likely leading to the lack of strong correlation between the DV/D ratio and genetic ancestry. In summary, and in the United States at least, microsatellite signature correlates strongly with the phenotype of populations of the Cx. pipiens complex with respect to autogeny (this study) and host preference.45,46
Although temperatures in coastal Santa Clara County are mild in the winter, cool temperatures may persist for many weeks (Figure 2A). Egg rafts deposited very early in the fall may experience relatively warm temperatures during development, but the temperature decreases rapidly in September and October, exposing the larvae hatching from later egg rafts to cold developmental temperatures. As others before us, we found that temperature was an important driver of development and survivorship, as populations took longer to develop but survived longer at lower temperatures.47,48 For this population, fall and winter development required about 200 day-degrees (°C) above a base of 7°C, which was different from the day-degree model calculated for Cx. pipiens from experimental data by Madder and others49 in southern Ontario. That study found that development from hatching to emergence required 132 day-degrees with a base temperature of 9.4°C. The difference in day-degree models could be the result of genetic differences between the populations, with the California population able to develop successfully under suboptimally cool temperature conditions that would not be experienced by diapausing populations in Ontario.
In areas where temperatures do not become lethally low, females can lay eggs all winter using a variety of strategies for dormancy followed or preceded by autogenous or anautogenous oviposition. A significant proportion (66%) of the females with fat bodies was parous, likely from autogenous egg deposition. Reisen and others36 reported a variety of gonotrophic states for female Cx. pipiens from populations in Sacramento and Davis, CA that survived through January. We do not therefore know if they would have sought a blood meal had they had a chance before dormancy. It was clear that a significant proportion of the F1 females were capable of taking a blood meal and producing anautogenous egg rafts by February. More detailed studies would be required to determine whether there had been a true hormonal cessation of ovarian development usually associated with diapausing females50; interestingly, autogenous egg raft production was inversely related to temperature, possibly caused by less efficient larval feeding at lower temperatures under the conditions in which the females were reared.
These findings show that under the climatic conditions of coastal central California, the Cx. pipiens group includes populations that have an unusual mixture of genotypes and phenotypes. The genetic results clearly showed that the populations examined were overwhelmingly Cx. pipiens form molestus, with clear signs of admixture of Cx. quinquefasciatus, and Cx. pipiens form pipiens. Most notable in this population was the abundance of autogeny, present in over 90% of individuals identified as Cx. pipiens morphologically. Autogeny was not only expressed in underground non-diapausing populations, as is considered typical of the molestus form, but also in individuals that oviposited in aboveground sites and in individuals that entered a dormant state with accumulation of fat body. Some individuals did not accumulate fat body, but maintained slow activity through the cool winter, as is typical of subtropical populations of Cx. quinquefasciatus.51,52 Apparently, the genetic pool of traits in the Cx. pipiens complex has resulted in a population with a distinctive combination of phenotypical traits.
The epidemiological significance of this unusual population of Cx. pipiens group mosquitoes is uncertain, though we suspect that the variety of phenotypes would allow the population to adapt to many different conditions. Presumably, segments of the population in Santa Clara County and similar areas of the region could become more or less abundant depending on the severity of the winter, availability of oviposition sites, and availability of hosts. Although the predominance of autogeny would tend to delay the period in an adult female's life when it would become infectious for a particular virus, recent studies in Australia27 and experience with laboratory colonies (Strickman D and Fonseca DM, personal observation) reveal that Cx. pipiens form molestus females oviposit the small autogenous egg masses shortly after emerging and quickly start seeking a blood meal. Critically, this trait also allows many individuals to reproduce without the risk of host seeking. More quantitative studies may show that the blend of traits we observed in the California populations allow the population of Cx. pipiens group mosquitoes to become maximally abundant during the entire year.
ACKNOWLEDGMENTS
We are deeply grateful to Carolyn Bhanck, Genetics Programs, Smithsonian Institution and Academy of Natural Sciences, for processing most of the specimens for microsatellite analysis and Tapan Ganguly and the DNA Sequencing Facility, University of Pennsylvania, for technical assistance.
Footnotes
Financial support: NIH R01GM063258, CDC/NIH#U50/CCU220532, and Rutgers University startup funds to DMF.
Disclosure: This is a NJ Agricultural Experiment Station Publication number D-08-08194-02-12.
Authors' addresses: Daniel Strickman, USDA Agricultural Research Service, Office of National Programs, Beltsville, MD, E-mail: Daniel.Strickman@ars.usda.gov. Dina M. Fonseca, Center for Vector Biology, Rutgers University, New Brunswick, NJ, E-mail: dinafons@rci.rutgers.edu.
References
- 1.Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61–81. doi: 10.1146/annurev.ento.53.103106.093258. [DOI] [PubMed] [Google Scholar]
- 2.Mattingly PF. The systematics of the Culex pipiens complex. Bull World Health Organ. 1967;37:257–261. [PMC free article] [PubMed] [Google Scholar]
- 3.Knight KL. Supplement to the Catalog of the Mosquitoes of the World (Diptera: Culicidae) Lanham, MD: Entomological Society of America; 1978. [Google Scholar]
- 4.Barr AR. The distribution of Culex p. pipiens and Culex p. quinquefasciatus in North America. Am J Trop Med Hyg. 1957;6:153–165. doi: 10.4269/ajtmh.1957.6.153. [DOI] [PubMed] [Google Scholar]
- 5.Farajollahi A, Fonseca DM, Kramer LD, Marm Kilpatrick A. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect Genet Evol. 2011;11:1577–1585. doi: 10.1016/j.meegid.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fonseca DM, Smith JL, Wilkerson RC, Fleischer RC. Pathways of expansion and multiple introductions illustrated by large genetic differentiation among worldwide populations of the southern house mosquito. Am J Trop Med Hyg. 2006;74:284–289. [PubMed] [Google Scholar]
- 7.Lounibos LP. Invasions by insect vectors of human disease. Annu Rev Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- 8.Cornel AJ, Mcabee RD, Rasgon J, Stanich MA, Scott TW, Coetzee M. Differences in extent of genetic introgression between sympatric Culex pipiens and Culex quinquefasciatus (Diptera: Culicidae) in California and South Africa. J Med Entomol. 2003;40:36–51. doi: 10.1603/0022-2585-40.1.36. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca DM, Keyghobadi N, Malcolm CA, Mehmet C, Schaffner F, Mogi M, Fleischer RC, Wilkerson RC. Emerging vectors in the Culex pipiens complex. Science. 2004;303:1535–1538. doi: 10.1126/science.1094247. [DOI] [PubMed] [Google Scholar]
- 10.Fonseca DM, Smith JL, Kim HC, Mogi M. Population genetics of the mosquito Culex pipiens pallens reveals sex-linked asymmetric introgression by Culex quinquefasciatus. Infect Genet Evol. 2009;9:1197–1203. doi: 10.1016/j.meegid.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kothera L, Zimmerman EM, Richards CM, Savage HM. Microsatellite characterization of subspecies and their hybrids in Culex pipiens complex (Diptera: Culicidae) mosquitoes along a north-south transect in the central United States. J Med Entomol. 2009;46:236–248. doi: 10.1603/033.046.0208. [DOI] [PubMed] [Google Scholar]
- 12.Urbanelli S, Silvestrini F, Reisen WK, De Vito E, Bullini L. Californian hybrid zone between Culex pipiens pipiens and Cx. p. quinquefasciatus revisited (Diptera:Culicidae) J Med Entomol. 1997;34:116–127. doi: 10.1093/jmedent/34.2.116. [DOI] [PubMed] [Google Scholar]
- 13.Urbanelli S, Silvestrini F, Sabatinelli G, Raveloarifera F, Petrarca V, Bullini L. Characterization of the Culex pipiens complex (Diptera: Culicidae) in Madagascar. J Med Entomol. 1995;32:778–786. doi: 10.1093/jmedent/32.6.778. [DOI] [PubMed] [Google Scholar]
- 14.Raymond M, Callaghan A, Fort P, Pasteur N. Worldwide migration of amplified insecticide resistance genes in mosquitoes. Nature. 1991;350:151–153. doi: 10.1038/350151a0. [DOI] [PubMed] [Google Scholar]
- 15.Bataille A, Cunningham AA, Cedeno V, Cruz M, Eastwood G, Fonseca DM, Causton CE, Azuero R, Loayza J, Martinez JD, Goodman SJ. Evidence for regular ongoing introductions of mosquito disease vectors into the Galapagos Islands. Proc Biol Sci. 2009;276:3769–3775. doi: 10.1098/rspb.2009.0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harbach RE, Dahl C, White GB. Culex (Culex) pipiens Linnaeus (Diptera: Culicidae): concepts, type designations, and description. Proc Entomol Soc Wash. 1985;87:1–24. [Google Scholar]
- 17.Gad AM, Farid HA, Ramzy RR, Riad MB, Presley SM, Cope SE, Hassan MM, Hassan AN. Host feeding of mosquitoes (Diptera: Culicidae) associated with the recurrence of Rift Valley fever in Egypt. J Med Entomol. 1999;36:709–714. doi: 10.1093/jmedent/36.6.709. [DOI] [PubMed] [Google Scholar]
- 18.Harbach RE, Harrison BA, Gad AM. Culex (Culex) molestus Forskal (Diptera: Culicidae): neotype designation, description, variation, and taxonomic status. Proc Entomol Soc Wash. 1984;86:521–542. [Google Scholar]
- 19.Gomes B, Sousa CA, Novo MT, Freitas FB, Alves R, Corte-Real AR, Salgueiro P, Donnelly MJ, Almeida AP, Pinto J. Asymmetric introgression between sympatric molestus and pipiens forms of Culex pipiens (Diptera: Culicidae) in the Comporta region, Portugal. BMC Evol Biol. 2009;9:262. doi: 10.1186/1471-2148-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahnck CM, Fonseca DM. Rapid assay to identify the two genetic forms of Culex (Culex) pipiens L. (Diptera: Culicidae) and hybrid populations. Am J Trop Med Hyg. 2006;75:251–255. [PubMed] [Google Scholar]
- 21.Huang S, Molaei G, Andreadis TG. Genetic insights into the population structure of Culex pipiens (Diptera: Culicidae) in the northeastern United States by using microsatellite analysis. Am J Trop Med Hyg. 2008;79:518–527. [PubMed] [Google Scholar]
- 22.Spielman A. Studies on autogeny in natural populations of Culex pipiens. II. Seasonal abundance of autogenous and anautogenous populations. J Med Entomol. 1971;8:555–561. doi: 10.1093/jmedent/8.5.555. [DOI] [PubMed] [Google Scholar]
- 23.Spielman A. Bionomics of autogenous mosquitoes. Annu Rev Entomol. 1971;16:231–248. doi: 10.1146/annurev.en.16.010171.001311. [DOI] [PubMed] [Google Scholar]
- 24.Gad AM, Abdel Kader M, Farid HA, Hassan AN. Absence of mating barriers between autogenous and anautogenous Culex pipiens L. in Egypt. J Egypt Soc Parasitol. 1995;25:63–71. [PubMed] [Google Scholar]
- 25.Oda T, Fujita K. A short review of the ecology of Culex pipiens molestus in Japan: oviposition activity in open water. Tropical Medicine. 1986;28:73–78. [Google Scholar]
- 26.Dobrotworsky NV. The problem of the Culex pipiens complex in the South Pacific (including Australia) Bull World Health Organ. 1967;37:251–255. [PMC free article] [PubMed] [Google Scholar]
- 27.Kassim NF, Webb CE, Russell RC. Culex molestus Forskal (Diptera: Culicidae) in Australia: colonization, stenogamy, autogeny, oviposition and larval development. Aust J Entomol. 2011;51:67–77. [Google Scholar]
- 28.Iltis WG. Biosystematics of the Culex pipiens Complex in Northern California. Davis, CA: University of California; 1966. [Google Scholar]
- 29.Konrad SK, Miller SN, Reeves WK, Tietze NS. Spatially explicit West Nile virus risk modeling in Santa Clara County, California. Vector Borne Zoonotic Dis. 2009;9:267–274. doi: 10.1089/vbz.2008.0084. [DOI] [PubMed] [Google Scholar]
- 30.Cummings RF. Design and use of a modified Reiter gravid mosquito trap for mosquito-borne encephalitis surveillance in Los Angeles County, California. Proceedings of the California Mosquito and Vector Control Association. 1992;60:170–176. [Google Scholar]
- 31.Jakob WL, Francy DB. Observations on the DV/D ratio of male genitalia of Culex pipiens complex mosquitoes in the United States. Mosq Syst. 1984;16:282–288. [Google Scholar]
- 32.Service MW. Mosquito Ecology Field Sampling Methods. New York: John Wiley & Sons; 1976. [Google Scholar]
- 33.Pruess KP. Day-degree methods for pest management. Environ Entomol. 1983;12:613–619. [Google Scholar]
- 34.WHO . Manual on Practical Entomology in Malaria. Part II: Methods and Techniques. Geneva: World Health Organization; 1975. [Google Scholar]
- 35.Mitchell CJ, Briegel H. Inability of diapausing Culex pipiens (Diptera: Culicidae) to use blood for producing lipid reserves for overwinter survival. J Med Entomol. 1989;26:318–326. doi: 10.1093/jmedent/26.4.318. [DOI] [PubMed] [Google Scholar]
- 36.Reisen WK, Thiemann T, Barker CM, Lu H, Carroll B, Fang Y, Lothrop HD. Effects of warm winter temperature on the abundance and gonotrophic activity of Culex (Diptera: Culicidae) in California. J Med Entomol. 2010;47:230–237. doi: 10.1603/me09207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitchell CJ, Briegel H. Fate of the blood meal in force-fed, diapausing Culex pipiens (Diptera: Culicidae) J Med Entomol. 1989;26:332–341. doi: 10.1093/jmedent/26.4.332. [DOI] [PubMed] [Google Scholar]
- 38.Smith JL, Fonseca DM. Rapid assays for identification of members of the Culex (Culex) pipiens complex, their hybrids, and other sibling species (Diptera: Culicidae) Am J Trop Med Hyg. 2004;70:339–345. [PubMed] [Google Scholar]
- 39.Fonseca DM, Atkinson CT, Fleischer RC. Microsatellite primers for Culex pipiens quinquefasciatus, the vector of avian malaria in Hawaii. Mol Ecol. 1998;7:1617–1619. [PubMed] [Google Scholar]
- 40.Keyghobadi N, Matrone MA, Ebel GD, Kramer LD, Fonseca DM. Microsatellite loci from the northern house mosquito (Culex pipiens), a principal vector of West Nile virus in North America. Mol Ecol Notes. 2004;4:20–22. [Google Scholar]
- 41.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pritchard JK, Donnelly P. Case-control studies of association in structured or admixed populations. Theor Popul Biol. 2001;60:227–237. doi: 10.1006/tpbi.2001.1543. [DOI] [PubMed] [Google Scholar]
- 43.Rosenberg NA, Pritchard JK, Weber JL, Cann HM, Kidd KK, Zhivotovsky LA, Feldman MW. Genetic structure of human populations. Science. 2002;298:2381–2385. doi: 10.1126/science.1078311. [DOI] [PubMed] [Google Scholar]
- 44.Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- 45.Huang S, Hamer GL, Molaei G, Walker ED, Goldberg TL, Kitron UD, Andreadis TG. Genetic variation associated with mammalian feeding in Culex pipiens from a West Nile virus epidemic region in Chicago, Illinois. Vector Borne Zoonotic Dis. 2009;9:637–642. doi: 10.1089/vbz.2008.0146. [DOI] [PubMed] [Google Scholar]
- 46.Kilpatrick AM, Kramer LD, Jones MJ, Marra PP, Daszak P, Fonseca DM. Genetic influences on mosquito feeding behavior and the emergence of zoonotic pathogens. Am J Trop Med Hyg. 2007;77:667–671. [PubMed] [Google Scholar]
- 47.Mead SS, Conner GE. Proc Pap 55th Annu Conf Calif Mosq Vector Control Assoc. 1987. Temperature-related growth and mortality rates of four mosquito species; pp. 133–137. [Google Scholar]
- 48.Rueda LM, Patel KJ, Axtell RC, Stinner RE. Temperature-dependent development and survival rates of Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae) J Med Entomol. 1990;27:892–898. doi: 10.1093/jmedent/27.5.892. [DOI] [PubMed] [Google Scholar]
- 49.Madder DJ, Surgeoner GA, Helson BV. Number of generations, egg production, and developmental time of Culex pipiens and Culex restuans (Diptera: Culicidae) in southern Ontario. J Med Entomol. 1983;20:275–287. doi: 10.1093/jmedent/20.3.275. [DOI] [PubMed] [Google Scholar]
- 50.Mitchell CJ. Differentiation of host-seeking behavior from blood-feeding behavior in overwintering Culex pipiens (Diptera: Culicidae) and observations on gonotrophic dissociation. J Med Entomol. 1983;20:157–163. doi: 10.1093/jmedent/20.2.157. [DOI] [PubMed] [Google Scholar]
- 51.Strickman D. Rate of oviposition by Culex quinquefasciatus in San Antonio, Texas, during three years. J Am Mosq Control Assoc. 1988;4:339–344. [PubMed] [Google Scholar]
- 52.Strickman D, Lang JT. Activity of Culex quinquefasciatus in an underground storm drain in San Antonio, Texas. J Am Mosq Control Assoc. 1986;2:379–381. [PubMed] [Google Scholar]