Abstract
Japanese encephalitis virus (JEV) is an emerging arbovirus, and inter-continental spread is an impending threat. The virus is maintained in a transmission cycle between mosquito vectors and vertebrate hosts, including birds. We detected variation in interspecies responses among North American birds to infection with strains of two different JEV genotypes (I and III). Several native North American passerine species and ring-billed gulls had the highest average peak viremia titers after inoculation with a Vietnamese (genotype I) JEV strain. Oral JEV shedding was minimal and cloacal shedding was rarely detected. The majority of birds, both viremic (72 of 74; 97.3%) and non-viremic (31 of 37; 83.8%), seroconverted by 14 days post-inoculation and West Nile virus-immune individuals had cross-protection against JEV viremia. Reservoir competence and serologic data for a variety of avian taxa are important for development of JEV surveillance and control strategies and will aid in understanding transmission ecology in the event of JEV expansion to North America.
Introduction
Japanese encephalitis virus (JEV, family Flaviviridae, genus Flavivirus) is in the Japanese encephalitis antigenic serocomplex, which includes West Nile and St. Louis encephalitis viruses (WNV and SLEV, respectively). Japanese encephalitis virus is an emerging zoonotic arbovirus that is transmitted to vertebrate amplifying hosts (i.e., birds and pigs) by mosquito vectors. Infection with JEV leads to thousands of cases of fatal childhood encephalitis annually, thereby posing a serious public health threat.1,2 The present geographic range of JEV includes eastern, southern, and southeastern Asia, India, and the Middle East, with relatively recent expansion to Papua New Guinea and the Torres Strait of northern Australia. Similar to the unanticipated spread of WNV to North America in 1999, the geographic range of JEV has expanded within the past decade. The spread of arboviruses such as WNV and JEV can occur by wind-blown mosquitoes, migrating viremic birds, or anthropogenic activities.2,3 The inter-continental spread of JEV and other arboviruses to non-endemic regions is a continual, impending threat.4
Birds play an important role in the maintenance and transmission of many arboviruses. Furthermore, they are ubiquitous and often share urban and suburban habitats with humans and mosquitoes. Members of the avian family Ardeidae (i.e., herons, egrets, black-crowned night herons, and bitterns) have been established as important JEV amplifying hosts, although this notion is based on relatively limited data that are difficult to collectively analyze. Several avian species have exhibited JEV viremia upon experimental and natural infections5–9 and naturally acquired anti-JEV antibodies have been detected in a variety of free-ranging birds in India, Japan, Korea, and West Bengal.5,10–16 However, comparisons of infection outcomes for different JEV strains among a diversity of avian species are needed to better define the potential role of birds in the epidemiology of the virus. Furthermore, pre-emptive knowledge of the responses of North American birds to JEV infection would help guide surveillance efforts and management strategies if this virus is introduced to the western hemisphere.
We compared the pathogenesis of experimental JEV infection among North American bird species using virus strains of two genotypes. Our goals were to delineate baseline responses of North American birds to JEV infection while also determining avian-derived samples that may be useful for detection of virus activity. Through controlled infections of individuals from a variety of avian taxa, we determined the following for each species: 1) Viremia profiles; 2) Oral and cloacal shedding profiles; 3) Susceptibility to clinical disease; 4) Seroconversion; 5) Inter-species variation among avian responses to JEV infection; and 6) Comparative responses of birds to infection with JEV strains of different genetic lineages. We also assessed whether there was variation in response to differing doses of JEV in a single avian species, and if anti-WNV antibodies were cross-protective against JEV infection in several species.
Materials and Methods
Bird collection and husbandry.
When possible captive birds were purchased from breeders (e.g., chickens [Gallus domesticus], mallards [Anas platyrhynchos], and ring-necked pheasants [Phasianus colchicus]). Otherwise, free-ranging birds were trapped by mist nets, walk-in traps, or cannon nets at various locations throughout northern Colorado, except for double-crested cormorants (Phalacrocorax auritus; Minnesota), American white pelicans (Pelecanus erythrorhynchos; North Dakota), great egrets (Ardea alba; Mississippi), cattle egrets (Bubulcus ibis; Oklahoma), and fish crows (Corvus ossifragus; Oklahoma). Birds of 16 species from eight taxonomic orders were collected (N = 2–10 individuals per species, except for house sparrows [Passer domesticus; N = 20]). Birds were banded and bled upon arrival, and then caged or allowed free-flight in rooms with clean water and food ad libitum. Diet varied by species, and consisted of seed mix (millet, milo, cracked corn, cracked sunflower seed, and oats, in equal parts), mealworms, cat food, dog food, poultry feed, fish, and/or ground beef. Aquatic birds were provided with pools of fresh water. Birds were acclimated to captive conditions for ≤ 1 week before inoculation.
Virus strains.
Japanese encephalitis virus strain 826309 (hereafter JE-IN) was isolated in 1982 in Goa, India from the brain of a human and passaged three times in cell culture, twice in suckling mice, and twice in Vero cells. The second JEV strain (hereafter JE-VN) was isolated from a Culex tritaeniorhynchus mosquito in Vietnam in 2003 and passaged once in suckling mice and twice in Vero cells. The final JEV strain (hereafter JE-SA) was isolated in Sagiyama, Japan from a Cx. tritaeniorhynchus mosquito in 1957 and passaged eight times in cell culture, once in a suckling mouse, and once in Vero cells. Molecular sequencing of the prM region indicated that JE-IN and JE-SA are genotype III viruses and JE-VN is a genotype I virus (Bosco-Lauth A and Bowen R, unpublished data).17
Experimental inoculation and sampling scheme.
Birds were separated into rooms and/or cages according to species and inoculating strain. Inoculation was by subcutaneous injection (0.1 mL) over the breast muscle and the inoculum dose was between 101.9 and 105.8 plaque-forming units (PFU). The number of WNV-seronegative birds of each species inoculated for each JEV strain is presented in Table 1; for several species, a limited number of birds allowed for assessment of only a single JEV strain. After inoculation, all birds were bled (0.1 mL by the jugular or ulnar vein) and swabbed (oropharyngeal and cloacal) once daily for 7 days and again on 14 days post-inoculation (DPI). After sampling, swabs were placed in BA-1 medium (M199-Hank's salts, 1% bovine serum albumin, 350 mg/L sodium bicarbonate, 100 U/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL amphotericin B in 0.05 M Tris, pH 7.6). Blood was centrifuged for 3 minutes at 12,000 × g for serum separation. All samples were stored at −80°C until testing.
Table 1.
Summary of viremia and oral shedding of birds experimentally inoculated with Vietnamese or Indian strains of Japanese encephalitis virus
| Species‡ | JEV strain | Total N | Viremia* | Oral shedding* | Seroconversion† | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Range peak (PFU/mL) | DPI | N | Range peak (PFU/mL) | DPI | No. sero(+)/No. viremic | No. sero(+)/No. non-viremic | |||
| Fish crow (Corvus ossifragus) | VN | 4 | 0 | < 101.7 | – | 0 | < 100.7 | – | – | – |
| IN | 4 | 0 | < 101.7 | – | 0 | < 100.7 | – | – | – | |
| Ring-necked pheasant (Phasianus colchicus) | VN | 5 | 0 | < 101.7 | – | 0 | < 100.7 | – | – | 5/5 |
| IN | 5 | 0 | < 101.7 | – | 0 | < 100.7 | – | – | 3/5 | |
| Mallard (Anas platyrhynchos) | VN | 4 | 3 | 102.0–3.3 | 2–5 | 1 | 101.8 | 4–5 | 3/3 | 1/1 |
| IN | 4 | 2 | 101.7 | 1–4 | 0 | < 100.7 | – | 2/2 | 2/2 | |
| House sparrow (Passer domesticus) | VN | 15 | 11 | 101.7–3.7 | 1–7 | 0 | < 100.7 | – | 9/11 | 4/4 |
| IN | 3 | 3 | 101.7–2.3 | 2–7 | 0 | < 100.7 | – | 3/3 | – | |
| Red-winged blackbird (Agelaius phoeniceus)§ | VN | 5 | 5 | 102.3–4.0 | 2–4 | 1 | 100.7 | 5 | 5/5 | – |
| IN | 5 | 4 | 101.7–2.6 | 1 | 0 | < 100.7 | – | 3/3 | 1/1 | |
| Rock pigeon (Columba livia) | VN | 5 | 5 | 102.7–4.3 | 1–6 | 0 | < 100.7 | – | 5/5 | – |
| IN | 5 | 4 | 101.7–3.8 | 4–6 | 0 | < 100.7 | – | 4/4 | 1/1 | |
| European starling (Sturnus vulgaris) | VN | 5 | 4 | 102.5–3.6 | 2–4 | 1 | 100.7 | 7 | 4/4 | 1/1 |
| IN | 4 | 3 | 102.3–3.1 | 2–6 | 0 | < 100.7 | – | 3/3 | 1/1 | |
| House finch (Carpodacus mexicanus)¶ | VN | 3 | 3 | 103.8–4.9 | 1–4 | 1 | 102.2 | 4–6 | 3/3 | – |
| IN | 2 | 2 | 102.2–3.2 | 1–4 | 0 | < 100.7 | – | 1/1 | – | |
| Common grackle (Quiscalus quiscula) | VN | 5 | 5 | 103.3–4.4 | 2–5 | 5 | 100.7–1.7 | 4–7 | 5/5 | – |
| IN | 4 | 1 | 103.5 | 3 | 0 | < 100.7 | – | 1/1 | 3/3 | |
| Ring-billed gull (Larus delawarensis) | VN | 4 | 4 | 103.5–5.4 | 1–5 | 2 | 101.3–2.0 | 4–6 | 4/4 | – |
| IN | 4 | 4 | 102.7–3.8 | 1–5 | 3 | 100.7–2.2 | 4–5 | 4/4 | – | |
| Cattle egret (Bubulcus ibis) | VN | 5 | 5 | 102.0–3.1 | 2–5 | 0 | < 100.7 | – | 5/5 | – |
| IN | 5 | 5 | 102.0–3.7 | 1–5 | 0 | < 100.7 | – | 5/5 | – | |
| American crow (Corvus brachyrhynchos) | IN | 5 | 0 | < 101.7 | – | 0 | < 100.7 | – | – | – |
| SA | 5 | 0 | < 101.7 | – | 0 | < 100.7 | – | – | 1/5 | |
| American white pelican (Pelecanus erythrorhynchos) | VN | 1 | 0 | < 100.7 | – | 0 | < 100.7 | – | – | 1/1 |
| Double-crested cormorant (Phalacrocorax auritus)∥ | VN | 4 | 0 | < 100.7 | – | 0 | < 100.7 | – | – | 4/4 |
| Chicken (Gallus domesticus) | IN | 4 | 1 | 101.7 | 1 | 0 | < 100.7 | – | 1/1 | 3/3 |
| Great egret (Ardea alba) | IN | 2 | 2 | 103.4–4.2 | 1–4 | 2 | 102.0–3.1 | 2–7 | 2/2 | – |
The minimum threshold of JEV detection was 101.7 PFU/mL serum and 100.7 PFU/swab.
Sera collected on 14 DPI with ≥ 75% JEV neutralization at a 1:10 serum dilution or reciprocal endpoint 80% JEV neutralization titer of ≥ 10 were considered positive. The number of individuals that seroconverted over the total number of viremic and non-viremic individuals, respectively, is provided.
Great blue herons are not included because both inoculated individuals had pre-existing WNV immunity and no detectable JEV viremia.
One JE-IN-inoculated red-winged blackbird died on 7 DPI before serology.
House finches were bled every other day and one JE-IN-inoculated individual died on 2 DPI before serology.
Oral and cloacal swabs collected from cormorants on 4–6 DPI were not tested.
JEV = Japanese encephalitis virus; PFU = plaque-forming units; DPI = days post-inoculation.
Dose comparison.
House sparrows were inoculated as described previously with a high dose of JE-VN (105.1–5.8 PFU) or a low dose (101.9 PFU) to compare subsequent viremia profiles for birds in each dose group (N = 5 birds/group).
Seroconversion and cross-protection.
The majority of birds were negative for anti-WNV antibodies upon capture; however, some individuals had naturally acquired WNV immunity before JEV inoculation and were used to assess cross-protection. Eleven WNV-seropositive birds (i.e., three American white pelicans, two great blue herons [Ardea herodias], two great egrets, two double-crested cormorants, and two house sparrows) were inoculated concurrently with WNV-seronegative conspecifics to assess the effects of pre-existing WNV infection on JEV infection. All birds were negative for anti-SLEV antibodies before JEV inoculation.
Serial PRNT80 titers (14, 21, and 28 DPI) were determined for a subset of birds inoculated with either JE-VN or JE-IN (two rock pigeons [Columba livia], four common grackles [Quiscalus quiscula], four red-winged blackbirds [Agelaius phoeniceus], two house finches [Carpodacus mexicanus], and four European starlings [Sturnus vulgaris]) to assess short-term variations in antibody titers following infection.
Necropsy and tissue processing.
Birds were euthanized on 14 or 28 DPI by intravenous sodium pentobarbital overdose. Birds euthanized on 14 DPI were necropsied within 2 hours and the following tissues were collected: heart, liver, lung, spleen, kidney, skeletal (pectoral) muscle, gonad, small intestine, brain, and eye. Tissues were weighed and added to BA-1 medium containing 20% fetal bovine serum for an approximate 10% tissue suspension. Tissues were homogenized using a mixer mill (Retsch GmbH, Haan, Germany; 5 min at 25 cycles/second), clarified by centrifugation (12,000 × g for 3 min), and supernatants stored at −80°C until testing. Tissues from birds with absent or low viremia titers of some species were not tested, including cattle egrets, double-crested cormorants, and American white pelicans. Tissues from birds euthanized on 28 DPI were not tested.
Laboratory methods.
Virus isolation and titration was performed by Vero cell plaque assay18 for sera, oral and cloacal swabs, and tissue homogenates. Briefly, Vero cell monolayers in 6-well plates were inoculated in duplicate with 0.1 mL of sample per well. Serum samples were subjected to serial 10-fold dilutions beginning with a 1:10 serum: BA-1 dilution to determine viremia titers, and undiluted swab samples were initially tested and titrations performed if necessary to determine viral titers. The plates were incubated for 1 hour at 37°C after which the cells were overlaid with 3 mL/well of 0.5% agarose in MEM medium containing amphotericin B and antibiotics. Two days later, cells were overlaid with a second 3-mL overlay containing 0.004% neutral red dye. Viral plaques were counted on the third and fourth days of incubation. The minimum thresholds of JEV detection were 101.7 PFU/mL serum, 100.7 PFU/swab, and 100.7 PFU/mL 10% tissue homogenate. Based on the range of peak viremia titers observed among the species tested, peak viremia titers were subjectively classified as low (< 103.0 PFU/mL serum), moderate (103.0–5.0 PFU/mL serum), and high (> 105.0 PFU/mL serum).
Neutralizing antibody titers were determined by a plaque reduction neutralization test (PRNT) on Vero cells in 6-well plates.18 Before PRNT, sera were heat-inactivated (56°C for 30 min). A challenge dose of ∼100 PFU of the respective virus was used to screen for antibodies at a 1:10 serum: BA-1 dilution; strains used included WNV strain NY99-4132, SLEV strain TBH-28, and the same respective JEV strains as used for inoculation. Birds with ≥ 75% serum neutralization of WNV at 1:10 serum dilution were considered WNV-seropositive. Serial, same-bird sera were titrated on the same neutralization assay to avoid inter-assay variation. The PRNT titers are expressed as the reciprocal of the highest serum dilution with ≥ 80% reduction (PRNT80) of viral PFU compared with control wells. Sera with ≥ 75% neutralization of JEV or PRNT80 ≥ 10 were considered positive, and those with < 50% were considered negative (all samples tested fell within these ranges).
Statistical analyses.
Dose responses as determined by viremia profiles in house sparrows inoculated with JE-VN with one of two doses (101.9 or 105.1–5.8) were analyzed by a one-way analysis of variance (SAS Institute, version 9.2). P values of < 0.05 were considered significant.
The MIXED procedure (SAS Institute, version 9.2, Cary, NC) was used to examine the factors that affected viremia profiles following experimental JEV inoculation. The data set for this analysis included species with both JE-VN and JE-IN-inoculated individuals. Nineteen models were constructed to determine the effects of species, viral strain, and DPI on viremia. Akaike information criteria with small sample size correction (AICc) was used for model selection and multimodel inference.19 Bird species, viral strain (JE-VN or JE-IN) and DPI were considered fixed effects and viremia was the dependent variable. Individual AIC weights were calculated for each model. The model set included a model with no effects, three single-effect models for DPI, species or viral strain, and numerous additive and interactive models (Table 2).
Table 2.
Model set testing the relationship between factors (i.e., bird species, virus strain, and DPI) and viremia profiles after JEV inoculation of North American birds
| Model | K | −2logL | AICc | ΔAICc | AICc weight |
|---|---|---|---|---|---|
| Intercept-only | 2 | 2369 | 2373 | 308 | 0.00 |
| DPI | 3 | 2745 | 2751 | 686 | 0.00 |
| Species | 3 | 2703 | 2709 | 644 | 0.00 |
| Viral strain | 3 | 2818 | 2824 | 759 | 0.00 |
| DPI+Sp | 4 | 2605 | 2613 | 548 | 0.00 |
| DPI*Sp | 5 | 2317 | 2327 | 262 | 0.00 |
| DPI+ST | 4 | 2732 | 2740 | 675 | 0.00 |
| DPI*ST | 5 | 2715 | 2725 | 660 | 0.00 |
| Sp+ST | 4 | 2688 | 2696 | 631 | 0.00 |
| Sp*ST | 5 | 2673 | 2683 | 618 | 0.00 |
| DPI+Sp+ST | 6 | 2588 | 2600 | 535 | 0.00 |
| DPI+Sp+ST DPI*Sp | 7 | 2295 | 2310 | 244 | 0.00 |
| DPI+Sp+ST DPI*ST | 7 | 2567 | 2581 | 516 | 0.00 |
| DPI+Sp+ST Sp*ST | 7 | 2572 | 2586 | 521 | 0.00 |
| DPI+Sp+ST DPI*Sp DPI*ST | 8 | 2250 | 2266 | 201 | 0.00 |
| DPI+Sp+ST DPI*Sp Sp*ST | 8 | 2276 | 2292 | 227 | 0.00 |
| DPI+Sp+ST DPI*ST Sp*ST | 8 | 2551 | 2567 | 502 | 0.00 |
| DPI+Sp+ST DPI*Sp DPI*ST Sp*ST | 9 | 2229 | 2248 | 182 | 0.00 |
| DPI+Sp+ST DPI*Sp DPI*ST Sp*ST DPI*Sp*ST | 10 | 2045 | 2065 | 0 | 1.00 |
K = number of parameters in each model; −2logL = 2 × log likelihood; AIC = Akaike's information criteria; AICc = AIC with a small sample size correction factor; ΔAICc = standardized AICc values (most supported model = 0); Akaike weight = the weight of evidence.
Results
Viremia.
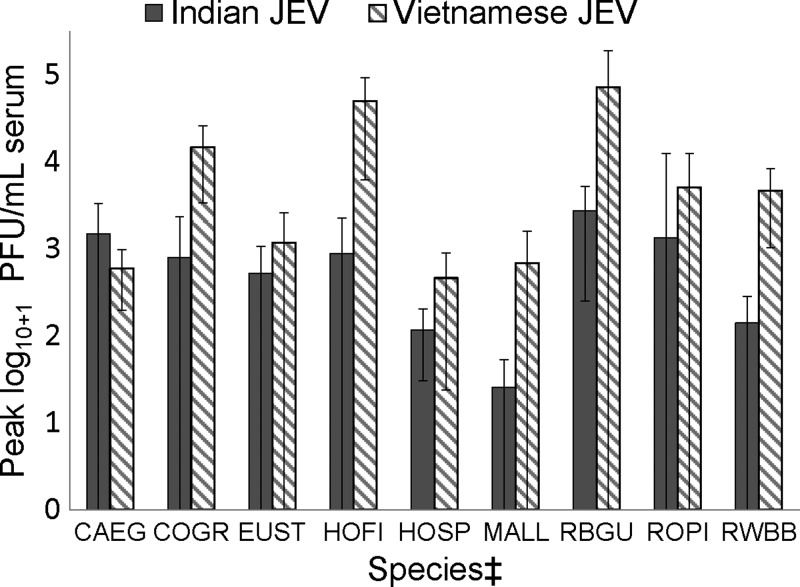
The majority of individuals of all species inoculated with JE-VN had detectable viremia except for ring-necked pheasants, fish crows, American white pelicans, and double-crested cormorants; no individuals of these species had detectable viremia. In addition, fish crows, American crows (Corvus brachyrhynchos), and ring-necked pheasants failed to become viremic after inoculation with JE-IN (no American white pelicans or double-crested cormorants were inoculated with JE-IN). Variable proportions of other species had detectable JE-IN viremia titers, with the highest proportions of viremic individuals occurring in red-winged blackbirds, rock pigeons, European starlings, house finches, ring-billed gulls (Larus delawarensis), and cattle egrets. Average peak viremia titers were higher for JE-VN versus JE-IN in all species except for cattle egrets (Table 1, Figures 1 and 2). Five American crows inoculated with JE-SA had no detectable viremia.
Figure 1.
Peak viremia titers with 95% confidence intervals* for nine avian species† inoculated with both Indian and Vietnamese strains of Japanese encephalitis virus. *Confidence interval less than zero were truncated at 0. †Peak viremia titers for fish crows and ring-necked pheasants were zero and therefore not included in the figure. ‡CAEG = cattle egret; COGR = common grackle; EUST = European starling; HOFI = house finch; HOSP = house sparrow; MALL = mallard; RBGU = ring-billed gull; ROPI = rock pigeon; RWBB = red-winged blackbird.
Figure 2.
Viremia profiles for nine North American bird species inoculated with both Indian and Vietnamese strains of Japanese encephalitis virus.
Shedding.
Japanese encephalitis virus-inoculated individuals of seven species shed transient, low titers of infectious JEV by the oropharyngeal cavity. Oropharyngeal shedding of JE-IN (≤ 103.1 PFU/swab) was detected only in great egrets and ring-billed gulls. Oropharyngeal JE-VN shedding (≤ 102.2 PFU/swab) was detected in all common grackles and less commonly in ring-billed gulls, red-winged blackbird, house finches, European starlings, and mallards (Table 1).
Viral shedding by the cloaca was rarely detected, and positive detections were low-titered. Cloacal viral shedding was detected in 2 of 4 mallards inoculated with JE-VN (peak 101.7–2.6 PFU/swab on 3–5 DPI), 1 of 5 rock pigeons with JE-VN (peak 101.7 PFU/swab on 5 and 7 DPI), and 1 of 5 with JE-IN (peak 100.7 PFU/swab on 7 DPI), and 1 of 4 ring-billed gulls inoculated with JE-VN (peak 102.0 PFU/swab on 3–5 DPI), and 1 of 4 with JE-IN (peak 101.4 PFU/swab on 3–5 DPI). No oropharyngeal or cloacal shedding was detected in any American crows, fish crows, house sparrows, chickens, ring-necked pheasants, American white pelicans, double-crested cormorants, or cattle egrets inoculated with JE-VN or JE-IN. In addition, five American crows inoculated with JE-SA had no detectable oral or cloacal shedding.
Tissue tropism.
No JEV was detected in any tissue homogenates collected on 14 DPI.
Serologic responses.
All individuals with detectable JEV viremia seroconverted by 14 DPI except for two house sparrows inoculated with JE-VN (72 of 74; 97.3%; a red-winged blackbird and house finch that died before assessment of seroconversion are not included). The majority of non-viremic individuals also seroconverted (31 of 37; 83.8%). Two of five ring-necked pheasants and all inoculated crows had no detectable viremia and failed to seroconvert by 14 DPI. One of five American crows inoculated with JE-SA seroconverted by 14 DPI (Table 1). The PRNT80 titers ranged from 10 to 2,560 among individuals inoculated with JE-VN or JE-IN.
For the majority of individuals for which serial (14, 21, and 28 DPI) PRNT80 titers were assessed, titers did not vary > 2-fold over time. However, in two JE-VN-inoculated house finches and three JEV-inoculated grackles (two with JE-VN and one with JE-IN), titers increased 4- or 8-fold between 14 and 28 DPI. Titers of one JE-VN-inoculated blackbird and one JE-VN-inoculated starling decreased 4-fold during the same time period.
WNV-JEV cross-protection.
Two house sparrows with naturally acquired anti-WNV antibodies before JE-IN inoculation had no detectable JEV viremia, whereas all three of their WNV-naive counterparts had low-level viremia titers between 2 and 7 DPI. Two WNV-seropositive great egrets had no detectable JE-IN viremia or shedding from 1 to 7 DPI, whereas their WNV-naive counterparts had moderate viremia titers from 1 to 4 DPI and low oral shedding titers from 2 to 7 DPI (Table 1). One of two great blue herons immune to WNV before inoculation with JE-IN had minimal (≤ 101.3 PFU/swab) oral and cloacal shedding for 1 day each (4–5 DPI), and neither bird had detectable viremia.
For both American white pelicans and double-crested cormorants inoculated with JE-VN, viremia and shedding were undetectable in all WNV-seropositive and WNV-seronegative individuals before JEV-inoculation; therefore, cross-protection was not evident.
Clinical responses.
No clinical signs attributable to JEV infection were observed in any birds during the study. One house finch and one red-winged blackbird (both inoculated with JE-IN strain) died or were euthanized on 2 and 7 DPI, respectively, due to cage-related injuries.
Statistical analyses.
There was no difference in the viremic responses of house sparrows experimentally inoculated with one of two different JE-VN doses (P = 0.403). Therefore, viral inoculum dose was not factored into further analyses. The comparison of peak viremia titers among bird species for which there were JE-VN and JE-IN inoculated individuals indicated that peak viremia titers differed by viral strain, and by bird species (Figure 1). In addition, DPI, bird species, and viral strain influenced viremia profiles. The top model was the fully interactive model, which held an AICc weight of 1.00, indicating that all three variables and the interactions affected the resulting viremia (Table 2).
Discussion
The continuous emergence of zoonotic pathogens can result in significant public health risks.20 Preemptive knowledge of the virulence and pathogenicity of such pathogens within natural hosts allows for more efficient assessment and intervention in the case of novel pathogen introductions and epidemics. The geographic range of some arboviruses, including JEV and WNV, can expand by movements of reservoir hosts or vectors.2,3 Phylogenetically similar JEV strains have been isolated thousands of miles apart, whereas less related strains have been isolated within close proximity, suggesting rapid and frequent virus dispersal.21,22 The presence of high numbers of JEV-competent vectors that ecologically overlap and preferentially feed on available, amplifying hosts in non-endemic areas would facilitate virus establishment and spread.2,23 Laboratory studies suggest that some aspects of North American mosquito (Culex spp., Aedes spp., and Culiseta spp.) biology could support JEV transmission.24,25
Species within the avian family Ardeidae (e.g., herons, bitterns, and egrets) were initially targeted for JEV research because as colonial nesters, they are seasonally abundant and available, and are relatively easy to sample because of their large size. Subsequent high seroprevalence and JEV isolations from ardeids in Japan and India led to the notion that they may be important JEV reservoir hosts.5,26 However, exposure to competent ornithophilic mosquito vectors is also an important determinant of the host's role in transmission, and many ardeids coexist with high densities of mosquitoes in marsh and wetland habitats.5,13,15,27 Characterization of avian host species responses to JEV infection is important in consideration of other variables that affect transmission dynamics, such as vector feeding preferences, vector and host densities, host behaviors, and ecological factors such as habitat and climate.23
Differences in JEV viremia profiles among a variety of avian species, including numerous ardeids, were observed in the present and past studies, suggesting that reservoir competence varies among taxa6,7,27; previous studies most often quantified viremia titers by the lethal viral dose in ≥ 50% of intracerebrally inoculated mice (LD50). The majority of experimentally inoculated (Japanese JEV strain) rufous night herons (Nycticorax caledonicus) and plumed egrets (Egretta intermedia) had low level, sometimes intermittent viremia titers between 1 and 5 DPI, whereas smaller proportions of black-crowned night herons (N. nycticorax) and little egrets (Egretta garzetta) became viremic.6,28 Viremia was short-lived (≤ 1–2 days) in Chinese little bitterns (Ixobrychus sinensis sinensis) inoculated with a Japanese JEV strain; however, night herons (N. nycticorax nycticorax) and non-ardeid aquatic birds such as Indian moorhens (Gallinala chloropus parisfrons) and mallards (Anas platyrhynchos platyrhynchos) became viremic and died between 4 and 10 DPI.7 No cattle egrets (N = 8) or Indian pond herons (N = 13; Ardeola grayii) inoculated with an Indian JEV strain had clinical signs but were viremic between 2 and 6 DPI, whereas no little egrets (N = 9) or cormorants (N = 2; Phalacrocorax niger) became viremic as determined by TCID50 in suckling mice.9 Ardeids inoculated with JEV in the present study included cattle egrets, great egrets, and WNV-immune great blue herons. Numerous species of ardeids (i.e., cattle egret and great egret) and non-ardeids (e.g., American white pelican, double-crested cormorant, ring-billed gull, and mallard) that occupy aquatic habitats in the United States were also included. Among these species, all cattle egrets, great egrets, and ring-billed gulls, as well as most mallards, had low to moderate peak viremia titers of varying duration from 1 to 5 DPI.
Passerines are a diverse avian order, and species within this order occupy a wide variety of habitats and have proven to be important amplifying hosts of WNV.29,30 Past research has examined the potential of numerous passerine species to serve as JEV amplifying hosts. All of three house finches and four tricolored blackbirds (Agelaius tricolor) subcutaneously inoculated with JEV (Okinawa strain) had relatively high viremia titers from ∼2 to 4 DPI, whereas 1 of 5 house sparrows had lower titers on 2 and 6 DPI.27 Viremia was of moderate titers and short duration in 8 of 8 JEV-inoculated Japanese tree sparrows (Passer montanus saturatus Stejneger) from 1 to 3 DPI and 2 of 6 gray starlings (Spodiopsar cineracae) from 3 to 5 DPI.6,10 In the present study, most passerine species had moderate viremia titers (i.e., 103.0–5.0 PFU/mL serum) except for two Corvus spp. for which all individuals had undetectable viremia titers. This result is in stark contrast to WNV infection in corvids, including American and fish crows, which often attain viremia titers as high as 1010 PFU/mL serum, as well as high titers of WNV in tissues, followed rapidly by death.30 Low to undetectable JEV viremia titers in gallinaceous birds (i.e., chickens and ring-necked pheasants) in the present study are similar to previous results in adult chickens.7,27
Unlike with numerous birds infected with closely-related WNV,30 JEV did not cause clinical disease in birds in the present study. Additionally, previous documentation of overt clinical disease among JEV experimentally inoculated birds is infrequent. However, one study documented death in JEV-inoculated birds from 3 to 10 DPI, including individuals of the following species: Indian moorhen, mallard, shoveller (Spatula clypeata), and black-crowned night heron. These birds were viremic at the time of death, and positive tissues included brain, spinal cord, spleen, and liver. One of these birds, a mallard that died on 8 DPI, had neurologic signs (i.e., head tilt, ataxia, and torticollis) and mild encephalitis.7 Additional instances of JEV detection in bird tissues include spleen and kidney from experimentally inoculated tree sparrows killed on 3 and 7 DPI,10 and liver and kidney of a pigeon on 39 DPI.31 In the present study, no infectious JEV was detected in tissues from individuals of 17 avian species on 14 DPI, suggesting that bird carcass testing for some species would not prove as useful for JEV as it has for WNV surveillance in the United States.32
Because oropharyngeal and cloacal shedding, as well as orally acquired infection and bird-to-bird transmission, have been documented for numerous North American avian species infected with WNV,30 shedding was assessed for JEV-inoculated birds in this study. Oropharyngeal and cloacal shedding was infrequently detected and was rarely above 102.0 PFU/swab for all species. Great egrets were the only species with more than intermittent or 1–2 day duration of oropharyngeal shedding. Shedding in these birds lasted 4–6 days, and titers reached 103.1 PFU/swab in one bird. Cloacal shedding was detected in only ∼5% of 137 individuals tested between 1 and 7 DPI. These data suggest that JEV oral-oral and fecal-oral transmission is relatively unlikely, and contamination of the environment with feces or oral secretions is limited.
The JEV Vietnamese strain (genotype I) used in the present study was apparently more virulent than the Indian strain for most species. Cattle egrets were an exception to this pattern, as the average peak viremia titer was higher among individuals infected with JE-IN versus JE-VN. In contrast, no qualitative differences were observed in the ability of six different JEV strains to infect and induce viremia in 3–10 day old chicken chicks.6 However, the effects of the varied number and types of passages for the viral strains used in the present study are unknown and may account for differences in past results among experimentally or naturally JEV-infected birds. Recently, in vitro and in vivo experiments with a JEV swine isolate from China revealed that multiple amino acid substitutions in the nonstructural proteins altered viral virulence in mice.33 Perhaps minor virus alterations in the JEV genome have led to past and possibly future changes in avian virulence, as has been demonstrated for WNV.34 Historically, human JEV outbreaks have most often been associated with genotype III viruses; however, in the past two decades, substantially more human infections have been caused by genotype I viruses, and the distribution of these two epidemic genotypes overlaps. There is no evidence to date that either genotype is more virulent in humans or other vertebrates, but minor genetic mutations may have increased the host or vector fitness of the genotype I viruses without altering clinical presentation.22,35,36
To address possible dose differences in JEV viremia, house sparrows were inoculated with various JEV doses. Resulting viremia profiles in these birds were not statistically different. Previously, researchers concluded that JEV doses did not affect the duration or peak titers of viremia among chicken chicks.6 However, another study concluded that the degree and duration of viremia in mallards and little egrets varied according to the delivered dose.7 Results from the present study revealed that relatively small JEV doses injected subcutaneously into birds resulted in infection, implying that relatively low viral quantities injected into birds by mosquitoes could result in infection. Several species of Culex mosquitoes have become infected in the laboratory at relatively low viral titers (i.e., as low as 10 PFU).37 Varied inoculum doses in the present study were unintentional, and were likely in part caused by the effects of a single freeze-thaw cycle. In addition to differing viral doses, previous arbovirus experimental studies in birds compared the inoculation method (needle versus mosquito), with no differences observed in magnitude or duration of viremia in chickens or house finches.38,39
Characterization of seroconversion patterns among abundant and common, free-ranging viral hosts can aid in determining the use of serology as a surveillance tool. Serologic responses in birds after JEV infection were inconsistent both in previous and present studies. For example, anti-JEV antibodies in some Japanese tree sparrows were undetectable by 30 DPI, but were detected for up to 390 DPI in other individuals.10 Neutralizing antibodies in some ardeids and chickens were detected between 3 and 12 weeks PI, whereas other individuals failed to seroconvert by 12 weeks PI.6 Some JEV-inoculated (Okinawa strain) house finches and tricolored blackbirds became viremic but failed to seroconvert by 26–29 DPI.27 Alternately, some chickens and black-crowned night herons had no detectable viremia after subcutaneous JEV inoculation but seroconverted by 2–3 weeks PI, after which antibodies persisted for up to 6–8 weeks in some birds.6 Nearly all birds with detectable viremia in the present study seroconverted by 14 DPI, along with most non-viremic birds. Although JEV in the latter group of birds was apparently of low virulence, it was often immunogenic. Birds that failed to seroconvert may have experienced delayed or prolonged immune recognition of JEV. Neutralizing antibodies were detectable for ≥ 28 days in rock pigeons and four passerine species in this study, and antibody titers remained constant from 14 to 28 DPI. In a previous study, anti-JEV antibody titers in some black-crowned night herons were detected at 1–2 weeks PI, and declined over the subsequent 7 months.6 Great egrets and house sparrows had evidence of cross-protective WNV immunity to JEV infection, a finding that supports those of a previous study in red-winged blackbirds.40 Similar cross-protection has also been observed in several mammalian species, including pigs.41,42 The interpretation of avian JEV serosurveillance in North America would undoubtedly be complicated by existing, circulating flaviviruses (i.e., WNV and SLEV).
Although additional data regarding North American mosquito JEV vector competence (e.g., threshold of infection) are needed to better assess the potential roles of avian reservoir hosts, the present data suggest that birds would play a role in shaping JEV epidemiology and ecology in North America. However, responses and susceptibility to JEV infection were variable among North American bird species and by JEV genotype; therefore, the ability of JEV to be maintained and to spread in North America will depend on numerous factors, including amplifying host availability, abundance and location, contact rates among competent hosts and mosquito vectors, viral genotype and strain, and climatic and environmental parameters. As previous research within JEV-endemic regions suggest,6,9,28,43 some ardeid (e.g., black-crowned night herons) and passerine species are potentially competent amplifying hosts in North America. Because of the relatively higher densities and variety of habitats occupied by passerines, this large avian order could contribute to the maintenance and spread of JEV in North America. In addition, pigs are recognized as important JEV-amplifying hosts44 and the expanding feral swine population in the United States45 would likely contribute to the risk of JEV spread following introduction. Furthermore, other mammalian species could serve as potential hosts, as evidenced by recent evaluation in bats and mongooses.46–49 Because JEV is an emerging arbovirus with the capacity for intercontinental spread, it should be included in global surveillance efforts, and future research should focus on determining vector and host reservoir susceptibility and competence among a variety of avian and mammalian species.
ACKNOWLEDGMENTS
We are grateful to Todd Felix, Jim Carlson, Christi Yoder, Kerri Pedersen, Seth Swafford, Paul Wolf, Ryan Powers, Pat Whitley, Scott Barras, Scott Woodruff, Katie Hanson-Dorr, Shelagh Tupper, Jennifer Marlow (USDA Wildlife Services), and Aaron Brault (CDC) for providing birds, and Brad Neuschwanger of the Bellevue Watson Fish Hatchery for supplying fish. Paul Gordy and Airn Tolnay (CSU) provided technical support. Sherry Henry, Barbara Johnson, and Barry Miller (CDC) supplied JEV isolates.
Footnotes
Financial support: This research was funded by National Institutes of Health, contract N01-AI25489.
Authors' addresses: Nicole Nemeth, Southeastern Cooperative Wildlife Disease Study, Department of Pathology, University of Georgia, Athens, GA, E-mail: nmnemeth@uga.edu. Angela Bosco-Lauth, Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Fort Collins, CO, E-mail: mopargal@rams.colostate.edu. Paul Oesterle, Southeastern Cooperative Wildlife Disease Study, Department of Population Health, University of Georgia, Athens, GA, E-mail: poester@uga.edu. Dennis Kohler, USDA/APHIS/Wildlife Services, National Wildlife Disease Program, Fort Collins, CO, E-mail: dennis.kohler@aphis.usda.gov. Richard Bowen, Department of Biomedical Sciences, Colorado State University, Fort Collins, CO, E-mail: Richard.Bowen@colostate.edu.
References
- 1.Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. 2009;15:1–7. doi: 10.3201/eid1501.080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackenzie JS, Gubler DJ, Petersen LR. Emerging Flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 3.van den Hurk AF, Ritchie SA, Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- 4.Nett RJ, Campbell GL, Reisen WK. Potential for the emergence of Japanese encephalitis virus in California. Vector Borne Zoonotic Dis. 2009;9:511–517. doi: 10.1089/vbz.2008.0052. [DOI] [PubMed] [Google Scholar]
- 5.Buescher EL, Scherer WF, Rosenberg MZ, Kutner LJ, McClure HE. Ecologic studies of Japanese encephalitis virus in Japan. IV. Avian infection. J Immunol. 1959;83:614–619. [PubMed] [Google Scholar]
- 6.Buescher EL, Scherer WF, Rosenberg MZ, McClure HE. Immunologic studies of Japanese encephalitis virus in Japan. III. Infection and antibody responses of birds. J Immunol. 1959;83:605–613. [PubMed] [Google Scholar]
- 7.Kitaoka M, Okubo K, Miura T, Nakamura Y. Relationship between Japanese B and Russian spring-summer encephalitis and birds. Jpn Med J (Natl Inst Health Jpn) 1953;6:247–259. doi: 10.7883/yoken1952.6.247. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi R, Ando K, Toyama Y, Kuratsuka K, Arima S, Saito K, Takayama Y, Hironaka N, Ishii K, Honda Y, Kondo K. On susceptibility of Japanese wild birds for Japanese B encephalitis virus. Jpn Med J. 1948;1:282–288. [Google Scholar]
- 9.Soman RS, Rodrigues FM, Guttikar SN, Guru PY. Experimental viremia and transmission of Japanese encephalitis virus by mosquitoes in ardeid birds. Indian J Med Res. 1977;66:709–718. [PubMed] [Google Scholar]
- 10.Hasegawa T, Takehara Y, Takahashi K. Natural and experimental infections of Japanese tree sparrows with Japanese encephalitis virus. Arch Virol. 1975;49:373–376. doi: 10.1007/BF01318247. [DOI] [PubMed] [Google Scholar]
- 11.Jamgaonkar AV, Yergolkar PN, Geevarghese G, Joshi GD, Joshi MV, Mishra AC. Serological evidence for Japanese encephalitis virus and West Nile virus infections in water frequenting and terrestrial wild birds in Kolar District, Karnataka State, India. A retrospective study. Acta Virol. 2003;47:185–188. [PubMed] [Google Scholar]
- 12.Kaul HN, Venkateshan CN, Mishra AC, Modi GB, Ghosh SN. Serological evidence of arbovirus activity in birds and small mammals in Japanese encephalitis affected areas of Bankura District, West Bengal. Indian J Med Res. 1976;64:1735–1739. [PubMed] [Google Scholar]
- 13.Khan FU, Banerjee K. Mosquito collection in heronries and antibodies to Japanese encephalitis virus in birds in Asansol-Dhanbad region. Indian J Med Res. 1980;71:1–5. [PubMed] [Google Scholar]
- 14.Loach TR, Narayan KG, Choudhary SP. Serological evidence of persistence of Japanese encephalitis virus activity in Bihar, India. Int J Zoonoses. 1983;10:7–14. [PubMed] [Google Scholar]
- 15.Rodrigues FM, Guttikar SN, Pinto BD. Prevalence of antibodies to Japanese encephalitis and West Nile viruses among wild birds in the Krishna-Godavari Delta, Andhra Pradesh, India. Trans R Soc Trop Med Hyg. 1981;75:258–262. doi: 10.1016/0035-9203(81)90330-8. [DOI] [PubMed] [Google Scholar]
- 16.Yang DK, Oh YI, Kim HR, Lee YJ, Moon OK, Yoon H, Kim B, Lee KW, Song JY. Serosurveillance for Japanese encephalitis virus in wild birds captured in Korea. J Vet Sci. 2011;12:373–377. doi: 10.4142/jvs.2011.12.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen WR, Tesh RB, Ricohesse R. Genetic variation of Japanese encephalitis virus in nature. J Gen Virol. 1990;71:2915–2922. doi: 10.1099/0022-1317-71-12-2915. [DOI] [PubMed] [Google Scholar]
- 18.Beaty BJ, Calisher CH, Shope RE. Diagnostic procedures for viral, rickettsial, and chlamydial infections. In: Lennette EH, Lennette DA, Lennette ET, editors. Arboviruses. Washington, DC: American Public Health Association; 1995. pp. 189–212. [Google Scholar]
- 19.Burnham KP, Anderson DR. Model Selection and Multi–Model Inference: A Practical Information–Theoretic Approach. New York: Springer-Verlag; 2002. [Google Scholar]
- 20.Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P. Global trends in emerging infectious diseases. Nature. 2008;451:990–994. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomon T, Ni H, Beasley DWC, Ekkelenkamp M, Cardosa MJ, Barrett ADT. Origin and evolution of Japanese encephalitis virus in southeast Asia. J Virol. 2003;77:3091–3098. doi: 10.1128/JVI.77.5.3091-3098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchil PD, Satchidanandam V. Phylogenetic analysis of Japanese encephalitis virus: envelope gene based analysis reveals a fifth genotype, geographic clustering, and multiple introductions of the virus into the Indian subcontinent. Am J Trop Med Hyg. 2001;65:242–251. doi: 10.4269/ajtmh.2001.65.242. [DOI] [PubMed] [Google Scholar]
- 23.van den Hurk AF, Johansen CA, Zborowski P, Paru R, Foley PN, Beebe NW, Mackenzie JS, Ritchie SA. Mosquito host-feeding patterns and implications for Japanese encephalitis virus transmission in northern Australia and Papua New Guinea. Med Vet Entomol. 2003;17:403–411. doi: 10.1111/j.1365-2915.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- 24.Reeves WC, Hammon WM. Laboratory transmission of Japanese encephalitis virus by seven species (three Genera) of North American mosquitoes. J Exp Med. 1946;83:184–194. [PubMed] [Google Scholar]
- 25.Rosen L, Lien JC, Shroyer DA, Baker RH, Lu LC. Experimental vertical transmission of Japanese encephalitis virus by Culex tritaeniorhynchus and other mosquitoes. Am J Trop Med Hyg. 1989;40:548–556. doi: 10.4269/ajtmh.1989.40.548. [DOI] [PubMed] [Google Scholar]
- 26.Carey DE, Reuben R, Myers RM, George S. Japanese encephalitis studies in Vellore, South India. Part IV. Search for virological and serological evidence of infection in animals other than man. Indian J Med Res. 1968;56:1340–1352. [PubMed] [Google Scholar]
- 27.Hammon WM, Reeves WC, Sather GE. Japanese B encephalitis virus in the blood of experimentally inoculated birds. Am J Hyg. 1951;53:249–261. doi: 10.1093/oxfordjournals.aje.a119452. [DOI] [PubMed] [Google Scholar]
- 28.Boyle DB, Dickerman RW, Marshall ID. Primary viremia responses of herons to experimental infection with Murray Valley encephalitis, Kunjin and Japanese encephalitis viruses. Aust J Exp Biol Med Sci. 1983;61:655–664. doi: 10.1038/icb.1983.62. [DOI] [PubMed] [Google Scholar]
- 29.Kilpatrick AM, LaDeau SL, Marra PP. Ecology of West Nile virus transmission and its impact on birds in the western hemisphere. Auk. 2007;124:1121–1136. [Google Scholar]
- 30.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chunikhin SP, Takahashi M. An attempt to establish the chronic infection of pigeons with Japanese encephalitis virus. Jap J Sanit Zool. 1971;22:155–160. [Google Scholar]
- 32.Eidson M, Kramer L, Stone W, Hagiwara Y, Schmit K. New York State West Nile Virus Avian Surveillance Team Dead bird surveillance as an early warning system for West Nile virus. Emerg Infect Dis. 2001;7:631–635. doi: 10.3201/eid0704.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu R, Tian YX, Deng JH, Yang KL, Liang WW, Guo R, Duan ZY, Liu ZW, Zhou DN, Xu DP. Multiple amino acid variations in the nonstructural proteins of swine Japanese encephalitis virus alter its virulence in mice. Arch Virol. 2011;156:685–688. doi: 10.1007/s00705-010-0871-1. [DOI] [PubMed] [Google Scholar]
- 34.Brault AC, Huang CY, Langevin SA, Kinney RM, Bowen RA, Ramey WN, Panella NA, Holmes EC, Powers AM, Miller BR. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nat Genet. 2007;39:1162–1166. doi: 10.1038/ng2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nga PT, Parquet MD, Cuong VD, Ma SP, Hasebe F, Inoue S, Makino Y, Takagi M, Nam VS, Morita K. Shift in Japanese encephalitis virus (JEV) genotype circulating in northern Vietnam: implications for frequent introductions of JEV from Southeast Asia to East Asia. J Gen Virol. 2004;85:1625–1631. doi: 10.1099/vir.0.79797-0. [DOI] [PubMed] [Google Scholar]
- 36.Saito M, Taira K, Itokazu K, Mori N. Recent change of the antigenicity and genotype of Japanese encephalitis viruses distributed on Okinawa Island, Japan. Am J Trop Med Hyg. 2007;77:737–746. [PubMed] [Google Scholar]
- 37.Weng MH, Lien JC, Lin CC, Yao CW. Vector competence of Culex pipiens molestus (Diptera: Culicidae) from Taiwan for a sympatric strain of Japanese encephalitis virus. J Med Entomol. 2000;37:780–783. doi: 10.1603/0022-2585-37.5.780. [DOI] [PubMed] [Google Scholar]
- 38.Reisen WK, Chiles RE, Kramer LD, Martinez VM, Eldridge BF. Method of infection does not alter response of chicks and house finches to western equine encephalomyelitis and St. Louis encephalitis viruses. J Med Entomol. 2000;37:250–258. doi: 10.1603/0022-2585-37.2.250. [DOI] [PubMed] [Google Scholar]
- 39.Styer LM, Bernard KA, Kramer LD. Enhanced early West Nile virus infection in young chickens infected by mosquito bite: effect of viral dose. Am J Trop Med Hyg. 2006;75:337–345. [PubMed] [Google Scholar]
- 40.Nemeth NM, Bosco-Lauth AM, Bowen RA. Cross-protection between West Nile and Japanese encephalitis viruses in red-winged blackbirds (Agelaius phoeniceus) Avian Dis. 2009;53:421–425. doi: 10.1637/8574-010109-Reg.1. [DOI] [PubMed] [Google Scholar]
- 41.Ilkal MA, Prasanna Y, Jacob PG, Geevarghese G, Banerjee K. Experimental studies on the susceptibility of domestic pigs to West Nile virus followed by Japanese encephalitis virus infection and vice versa. Acta Virol. 1994;38:157–161. [PubMed] [Google Scholar]
- 42.Bosco-Lauth A, Mason G, Bowen R. Pathogenesis of Japanese encephalitis virus infection in a golden hamster model and evaluation of flavivirus cross-protective immunity. Am J Trop Med Hyg. 2011;84:727–732. doi: 10.4269/ajtmh.2011.11-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Banerjee K, Deshmukh PK. Transmission of Japanese encephalitis virus to chicks by individual Culex bitaeniorhynchus mosquitoes. Indian J Med Res. 1987;86:726–727. [PubMed] [Google Scholar]
- 44.Carey DE, Reuben R, Myers RM. Japanese encephalitis studies in Vellore, South India. Part V. Experimental infection and transmission. Indian J Med Res. 1969;57:282–289. [PubMed] [Google Scholar]
- 45.Ditchkoff SS, West BC. Ecology and management of feral hogs. Hum Wildl Confl. 2007;1:149–151. [Google Scholar]
- 46.Saito M, Nakata K, Nishijima T, Yamashita K, Saito A, Ogura G. Proposal for Japanese encephalitis surveillance using captured invasive mongooses under an eradication project on Okinawa Island, Japan. Vector Borne Zoonotic Dis. 2009;9:259–266. doi: 10.1089/vbz.2008.0099. [DOI] [PubMed] [Google Scholar]
- 47.van den Hurk AF, Smith CS, Field HE, Smith IL, Northill JA, Taylor CT, Jansen CC, Smith GA, Mackenzie JS. Transmission of Japanese encephalitis virus from the black flying fox, Pteropus alecto, to Culex annulirostris mosquitoes, despite the absence of detectable viremia. Am J Trop Med Hyg. 2009;81:457–462. [PubMed] [Google Scholar]
- 48.Wang JL, Pan XL, Zhang HL, Fu SH, Wang HY, Tang Q, Wang LF, Liang GD. Japanese encephalitis viruses from bats in Yunnan, China. Emerg Infect Dis. 2009;15:939–942. doi: 10.3201/eid1506.081525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sulkin SE, Allen R, Sims R. Studies of arthropod-borne virus infections in Chiroptera I. Susceptibility of insectivorous species to experimental infection with Japanese B and St. Louis encephalitis viruses. Am J Trop Med Hyg. 1963;12:800–814. [PubMed] [Google Scholar]