Abstract
Background and Purpose
On average, systolic blood pressure (SBP) rises with age, while diastolic blood pressure (DBP) increases to age 50 and then declines. As elevated blood pressure is associated with cardiovascular disease and mortality, it also might be linked to frailty. We assessed the association between blood pressure, age, and frailty in a representative population-based cohort.
Methods
Individuals from the second clinical examination of the Canadian Study of Health and Aging (n = 2305, all 70+ years) were separated into four groups: history of hypertension ± antihypertensive medication, and no history of hypertension ± antihypertensive medication. Frailty was quantified as deficits accumulated in a frailty index (FI).
Results
SBP and DBP changed little in relation to age, except in untreated hypertension, where SBP declined in individuals >85 years. In contrast, SBP declined in all groups up to an FI of 0.55, and then rose sharply. DBP changed little in relation to FI. The slope of the line relating FI and age was highest in untreated individuals without a history of hypertension, indicating the highest physiological reserve.
Conclusions
SBP declined as frailty increased in older adults, except at the highest FI levels. SBP and age had little or no relationship.
Keywords: aging, hypertension, frailty
INTRODUCTION
The structure and function of the human heart and vasculature change with age.1–4 Structural changes in the vasculature increase arterial stiffness, which reduces arterial buffering capacity and gives rise to age-associated changes in systolic and diastolic blood pressure.4,5 On average, systolic blood pressure rises with age, while diastolic blood increases until approximately 50 years and then declines.5 The relationship between blood pressure and mortality in older adults may be more complex. Some studies suggest the relationship between diastolic blood pressure and all-cause/cardiovascular mortality is J or U shaped in older adults,6,7 while the relationship between systolic blood pressure and mortality in adults older than 85 years is U shaped.8 Elevated systolic or diastolic blood pressure is associated with an increased risk of cardiovascular disease and death.9 Indeed, isolated systolic hypertension is a major cause of morbidity and mortality in older adults.5
Average changes in blood pressure with age may obscure important interindividual variability. In some people there may be little age-related change in blood pressure, whereas in others changes may be marked or not in accord with the mean trend.10 One way to summarize interindividual variability with age is through the construct of frailty—the idea that people of similar ages have varying degrees of vulnerability to adverse outcomes. Frailty can be defined in many ways,11 including by a count of deficits, combined in a so-called frailty index (FI).12 It is felt that the FI captures the loss of physiological reserve present in an individual.13 This idea has been tested in many settings,13–20 but to date has not been related to blood pressure in older adults. Here, we evaluated a population-based cohort to assess the relationship between systolic and diastolic blood pressures and frailty in older adults.
METHODS
Patients, Setting, and Sample
We used data from the second clinical examination of the Canadian Study of Health and Aging (CSHA-2). The CSHA was a cohort study of health problems of older adults, specifically dementia. The second clinical examination was conducted in 1996–1997.21 While the CSHA sample was population based, the clinical cohort drawn from that sample was weighted towards people with cognitive impairment. However, the CSHA-2 clinical sample was enriched to allow for cognitively intact people to be added so that frailty could be evaluated separately from cognitive impairment no dementia and dementia.22 The clinical examination included a history from participants and/or knowledgeable informants, as well as access to health records where available. Participants were classified as hypertensive based on either a history of hypertension or a measured systolic blood pressure of greater than 145 mmHg. Characteristics of the participants in this study are summarized in Table 1.
TABLE 1.
Characteristics of people with and without hypertension, receiving or not receiving antihypertensive medications.
| No hypertension; no antihypertensive medication (n = 890) | No hypertension; on antihypertensive medication (n = 374) | Hypertension; no antihypertensive medication (n = 156) | Hypertension; on antihypertensive medication (n = 785) | |
|---|---|---|---|---|
| Age (years), mean ± SD | 84.0±6.9 | 86.2±7.0 | 85.6±7.3 | 84.3±6.8 |
| % Female | 58 | 54 | 72 | 69 |
| Systolic blood pressure (mmHg), mean ± SD | 138.4±22.0 | 131.7±23.6 | 151.7±27.1 | 149.0±23.9 |
| Diastolic blood pressure (mmHg), mean ± SD | 71.9±12.1 | 68.6±12.6 | 76.6±12.9 | 75.1±12.3 |
| Pulse (beats/min), mean ± SD | 73.5±10.3 | 72.8±11.7 | 73.7±10.2 | 73.0±10.6 |
| FI-CGA, mean ± SD | 0.21±0.14 | 0.27±0.13 | 0.28±0.15 | 0.25±0.13 |
FI-CGA = frailty index-comprehensive geriatric assessment.
Measures
The clinical data collection protocol was modified to include all elements of a standardized comprehensive geriatric assessment (CGA). A multidimensional assessment, using information from the nursing and physician assessments, and cognitive and performance testing was performed.23 Areas evaluated included cognition, affect and other aspects of the mental state, communication (speech, hearing, vision) mobility, balance, bowel and bladder function, activities of daily living, weight, appetite, social factors, active diagnoses, and medications. An FI based on this information was calculated as described previously.13 Each variable, selected to correspond to a standardized CGA,23 was scored such that 0 = deficit absent and 1 = deficit present. The numbers were added and divided by 51, which is the total number of deficits evaluated, to produce an FI-CGA scored from 0 (no deficits present) to a maximum of 1.0 (all 51 deficits present). Typically hypertension is considered to be a deficit, but it was not included as a deficit here. Medications were scored as though they had no protective value, and multiple medications were considered to be a deficit as described previously.24
Analysis
The sample was separated into four groups, being people with a clinical history of hypertension with or without antihypertensive medication and those with no history of hypertension with or without antihypertensive medication.
FI-CGA scores were compared across these groups. Here, we also introduce the application to a disease/treatment state of a novel measure of physiological reserve—the slope of the line that relates the FI-CGA to age.13 As the slope decreases, so does physiological reserve. This occurs because there is a limit to frailty (usually at an FI value of about 0.7) beyond which survival is not possible.25 This observation is in accordance with the reliability theory of aging.26 As that limit is approached, physiological reserve diminishes. In consequence, we tested for attenuation in slope in groups with more deficits. The explanation of why the slope attenuates is that a high initial deficit burden makes it harder to accumulate more deficits and survive, so that—seemingly paradoxically—the rate of deficit accumulation is highest in people with the lowest initial level of deficits.13
The systolic and diastolic blood pressures were averaged at each age from 70 to 95 years and compared with the averages for each 0.03 increase in the value of the FI-CGA. Averages at each 0.03 increase in the FI-CGA were compared to reduce the number of data points. The 0.03 interval was chosen, as it is both relatively small and readily divisible by 51. The FI-CGA and blood pressures were plotted to evaluate the relationship between various aspects of vascular function and frailty. The mean FI-CGA score of each group was compared by age.
Data were analyzed with MatLab software (release 7.4; MathWorks, Inc, Natick, MA, USA). Differences in proportions were calculated using a chi-square test. Differences in mean values were calculated using analysis of variance.
Ethics
The CSHA was approved by the research ethics committees of each participating institution. All participants (or their designates) signed informed-consent forms.
Results
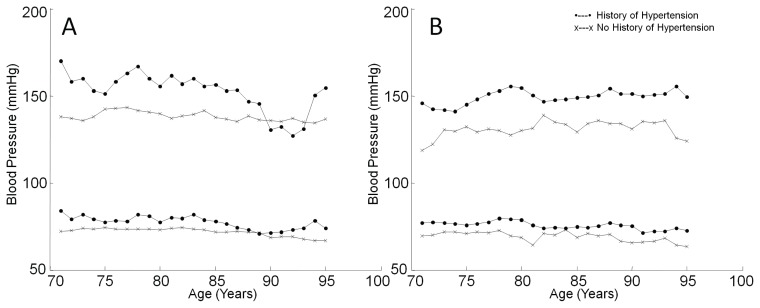
Figure 1A shows the relationship between blood pressure and age for individuals with and without a history of hypertension not taking blood pressure-lowering medications. Systolic and diastolic pressures were higher in individuals with a history of hypertension than in those without. In untreated hypertensive people, systolic blood pressure declined with age in those older than 85 years but increased again above 93 years. In contrast, systolic blood pressure showed no relationship to age in individuals without hypertension. Diastolic blood pressure was not affected by age in either group (Figure 1A). Figure 1B shows the relationship between blood pressure and age in treated participants. Systolic and diastolic blood pressures were higher in individuals with a history of hypertension than in those without, but age had virtually no impact on blood pressure in either group (Figure 1B).
FIGURE 1.
Relationship between blood pressure and age. (A) Relation between blood pressure and age in nonmedicated individuals. There was little relation between systolic blood pressure (top) and age in individuals with no history of hypertension, although systolic blood pressure declined above age 85 in hypertensives and rose again above age 93. Diastolic blood pressure (bottom) did not change as a function of age in either group. (B) Systolic and diastolic blood pressures did not vary with age in people who were treated with blood pressure-lowering medications.
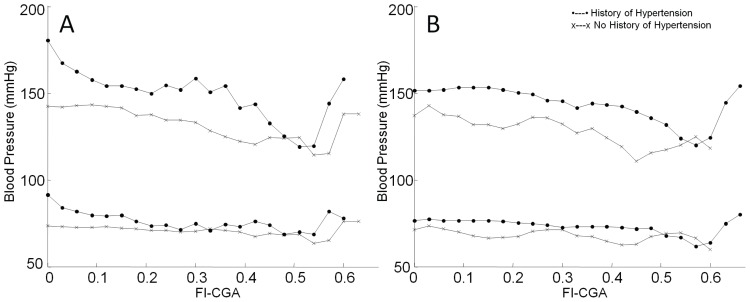
Figure 2 illustrates the relation between blood pressure and frailty. In nonmedicated individuals, regardless of a history of hypertension, systolic blood pressure declined as frailty increased to an FI-CGA of approximately 0.55, at which point it increased sharply (Figure 2A). Diastolic blood pressure also followed this trend. Figure 2B shows that a similar U-shaped relationship between systolic blood pressure and frailty held in treated hypertensive individuals. Systolic blood pressure declined with frailty in people with no history of hypertension who were on treatment. No increase was seen at the highest levels of frailty because there were no individuals in this group who did not have hypertension (Figure 2B).
FIGURE 2.
Relationship between blood pressure and frailty, as measured by a frailty index based on comprehensive geriatric assessment (FI-CGA), in (A) nonmedicated and (B) medicated individuals. Systolic blood pressure (top) declined as frailty increased up to an FI-CGA >0.55 and then increased in all groups. Diastolic blood pressure (bottom) had little relationship to frailty.
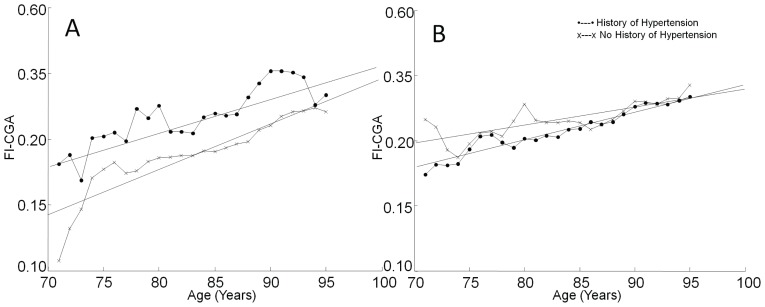
Figure 3 shows the relationship between frailty and age. In untreated participants with no history of hypertension, the slope of the line relating FI-CGA to age was 0.035 (Figure 3A). For people with untreated hypertension, the slope of the line was 0.026 (Figure 3A). Figure 3B shows the relation between FI-CGA and age for nonhypertensive and hypertensive individuals on antihypertensive medication. Slopes of the lines relating FI-CGA to age were 0.022 for participants with and 0.014 for those without a history of hypertension (Figure 3B).
FIGURE 3.
Relationship between frailty and age. A frailty index based on comprehensive geriatric assessment (FI-CGA) was calculated for each group and plotted as a function of age. (A) Relation between age and FI-CGA in nonmedicated individuals. The slope of the best-fit line for mean change was 0.035 in those with no history of hypertension and 0.026 in those with a history of hypertension. (B) The relationship between age and FI-CGA in individuals on antihypertensive medication. The slopes of the best-fit lines declined in both groups on treatment (0.022 in those with a history of hypertension and 0.014 in those with no history of hypertension).
DISCUSSION
This study evaluated a population-based cohort to assess the relationship between blood pressure, age, and frailty. Systolic and diastolic blood pressures did not change in relation to age, except in untreated hypertension, where systolic pressure declined in individuals older than 85 years and then rose above age 93. In contrast, systolic blood pressure showed a U-shaped relationship to frailty in all groups. Systolic blood pressure decreased to an FI-CGA of 0.55 and then rose sharply. Diastolic blood pressure changed little in relation to frailty. Frailty was linked to declines in systolic blood pressure in older adults, except at the highest levels of frailty, where it rose in each group studied. Of interest, the slopes of the lines relating FI-CGA and age were lower in individuals with untreated hypertension and in those on blood pressure-lowering medications than in untreated participants without a history of hypertension. Individuals without a history of hypertension who were not treated with antihypertensive medications had the lowest deficit burden, and as expected showed the highest rate of deficit accumulation and hence the highest physiological reserve.
Our data must be interpreted with caution. The CSHA-2 data are cross-sectional, so it is not possible to make strong statements about causality. In addition, the CSHA-2 clinical sample, while population based, is not representative. While the CSHA-2 clinical evaluation cohort was enriched to include more people without cognitive impairment, this group included many people who were fitter than those seen in clinical practice.
We found that blood pressure changed little in relation to age in all groups, except in individuals with untreated hypertension. In contrast, there was a clear relation between systolic blood pressure and frailty. We found that the relationship between systolic blood pressure and frailty was U shaped. As systolic blood pressure declined as FI-CGA increased to 0.55, our findings demonstrate that many frail older adults had low systolic blood pressure, rather than systolic hypertension.5 Of interest, several studies have reported that low systolic blood pressure is associated with cognitive impairment and increased mortality in older adults.10,27,28 The explanation for an association between low systolic blood pressure, frailty, and mortality is unclear. We found that systolic blood pressure declined with frailty in all groups, regardless of whether treatment with antihypertensive medications had been initiated. This suggests that the decrease in systolic blood pressure in frail older adults cannot be explained entirely by the use of blood pressure-lowering medications. It has been suggested that low systolic blood pressure is an indicator of poor overall health10 and may be associated with deaths from noncardiovascular causes.28 We also found that, at the highest levels of frailty, systolic blood pressure rose. This increase in systolic blood pressure in very frail individuals may be due to a “survivor” effect: individuals with higher systolic blood pressures may be more likely to survive to the highest levels of frailty. On the other hand, the Hypertension in the Very Elderly Trial29 data focus attention on the possible benefit of treatment in people aged 80+ years. Even so, how frailty might modify the relationship between mortality and treatment is not clear.
We found that the slope of the line relating FI-CGA and age was highest in untreated individuals with no history of hypertension when compared to the other groups, which indicates that these people had the highest rate of deficit accumulation. This relationship between the rate of deficit accumulation and age can seem counterintuitive and is only now beginning to be understood. As detailed elsewhere,13 findings such as those seen here are in keeping with the reliability theory of aging.26 Reliability theory says that as the redundancy of a system is exhausted it will have quantitatively more deficits, as seen here. The rate of accumulation will slow, however, as the ability of the organism to withstand deficits (“hits”) diminishes. In short, the slowing of deficit accumulation does not represent stability, but survival—the more deficits that are accumulated, the higher the likelihood of death. This is what accounts for the apparent slowing; people with high deficit counts appear to accumulate deficits more slowly because they are close to the limit beyond which the system will fail. In this way, the slope of the line relating mean frailty to age can serve as a measure of physiological reserve in older adults, with the first derivative being a candidate measure for individuals, a possibility requiring further investigation.
Deficit accumulation was highest in untreated individuals with no history of hypertension. The link between disease, treatment and deficit accumulation offers a novel means of addressing whether and how older adults might benefit from medical interventions, and therefore warrants further study.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
None declared.
REFERENCES
- 1.O’Rourke MF. Arterial aging: pathophysiological principles. Vasc Med. 2007;12:329–41. doi: 10.1177/1358863X07083392. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–46. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 3.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–54. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 4.Howlett SE. Effects of aging on the cardiovascular system. In: Fillit HM, Rockwood K, Woodhouse K, editors. Brocklehurst’s Textbook of Geriatric Medicine and Gerontology. 7th Edition. New York: WB Saunders; 2010. pp. 168–70. [Google Scholar]
- 5.Williams B, Lindholm LH, Sever P. Systolic pressure is all that matters. Lancet. 2008;371:2219–21. doi: 10.1016/S0140-6736(08)60804-1. [DOI] [PubMed] [Google Scholar]
- 6.Pastor-Barriuso R, Banegas JR, Damián J, et al. Systolic blood pressure, diastolic blood pressure, and pulse pressure: an evaluation of their joint effect on mortality. Ann Intern Med. 2003;139:731–9. doi: 10.7326/0003-4819-139-9-200311040-00007. [DOI] [PubMed] [Google Scholar]
- 7.Cacciatore F, Abete P, de Santis D, et al. Mortality and blood pressure in elderly people with and without cognitive impairment. Gerontology. 2005;51:53–61. doi: 10.1159/000081436. [DOI] [PubMed] [Google Scholar]
- 8.Molander L, Lövheim H, Norman T, et al. Lower systolic blood pressure is associated with greater mortality in people aged 85 and older. J Am Geriatr Soc. 2008;56:1853–9. doi: 10.1111/j.1532-5415.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 9.Lewington S, Clarke R, Qizilbash N, et al. Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 10.Rastas S, Pirttilä T, Viramo P, et al. Association between blood pressure and survival over 9 years in a general population aged 85 and older. J Am Geriatr Soc. 2006;54:912–8. doi: 10.1111/j.1532-5415.2006.00742.x. [DOI] [PubMed] [Google Scholar]
- 11.Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm—issues and controversies. J Gerontol A Biol Sci Med Sci. 2007;62:731–7. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26. doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Rockwood K, Rockwood MR, Mitnitski A. Physiological redundancy in older adults in relation to the change with age in the slope of a frailty index. J Am Geriatr Soc. 2010;58:318–23. doi: 10.1111/j.1532-5415.2009.02667.x. [DOI] [PubMed] [Google Scholar]
- 14.Kulminski AM, Ukraintseva SV, Kulminskaya IV, et al. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56:898–903. doi: 10.1111/j.1532-5415.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridda I, Macintyre CR, Lindley R, et al. Immunological responses to pneumococcal vaccine in frail older people. Vaccine. 2009;27:1628–36. doi: 10.1016/j.vaccine.2008.11.098. [DOI] [PubMed] [Google Scholar]
- 16.Hubbard RE, O’Mahony MS, Woodhouse KW. Characterising frailty in the clinical setting: a comparison of different approaches. Age Ageing. 2009;38:115–9. doi: 10.1093/ageing/afn252. [DOI] [PubMed] [Google Scholar]
- 17.García-González JJ, García-Peña C, Franco-Marina F, et al. A frailty index to predict the mortality risk in a population of senior Mexican adults. BMC Geriatr. 2009;9:47. doi: 10.1186/1471-2318-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupre ME, Gu D, Warner DF, et al. Frailty and type of death among older adults in China: prospective cohort study. BMJ. 2009;338:b1175. doi: 10.1136/bmj.b1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Lee LC. Dynamics and heterogeneity in the process of human frailty and aging: evidence from the U.S. older adult population. J Gerontol B Psychol Sci Soc Sci. 2010;65B:246–55. doi: 10.1093/geronb/gbp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo J, Chan R, Leung J, et al. Relative contributions of geographic, socioeconomic, and lifestyle factors to quality of life, frailty, and mortality in elderly. PLoS One. 2010;5:e8775. doi: 10.1371/journal.pone.0008775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Canadian Study of Health and Aging Working Group. The incidence of dementia in Canada. Neurology. 2000;55:66–73. [PubMed] [Google Scholar]
- 22.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–95. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rockwood K, Silvius JL, Fox RA. Comprehensive geriatric assessment: helping your elderly patients maintain functional well-being. Postgrad Med. 1998;103:247–9. 264. doi: 10.3810/pgm.1998.03.424. [DOI] [PubMed] [Google Scholar]
- 24.Rockwood K, Rockwood MR, Andrew MK, et al. Reliability of the hierarchical assessment of balance and mobility in frail older adults. J Am Geriatr Soc. 2008;56:1213–7. doi: 10.1111/j.1532-5415.2008.01773.x. [DOI] [PubMed] [Google Scholar]
- 25.Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev. 2006;127:494–6. doi: 10.1016/j.mad.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Gavrilov LA, Gavrilova NS. The reliability theory of aging and longevity. J Theor Biol. 2001;213:527–45. doi: 10.1006/jtbi.2001.2430. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson SE, Read S, Berg S, et al. Low systolic blood pressure is associated with impaired cognitive function in the oldest old: longitudinal observations in a population-based sample 80 years and older. Aging Clin Exp Res. 2007;19:41–7. doi: 10.1007/BF03325209. [DOI] [PubMed] [Google Scholar]
- 28.Okumiya K, Matsubayashi K, Wada T, et al. A U-shaped association between home systolic blood pressure and four-year mortality in community-dwelling older men. J Am Geriatr Soc. 1999;47:1415–21. doi: 10.1111/j.1532-5415.1999.tb01559.x. [DOI] [PubMed] [Google Scholar]
- 29.Beckett NS, Peters R, Fletcher AE, et al. HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–98. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]