Abstract
Ethnopharmacological relevance
Quercus cerris L., Fagaceae has been used in traditional Mediterranean medicine for numerous purposes, including anti-infective therapies for diarrhea and wound care.
Aim of the study
To evaluate the anti-staphylococcal activity of fractions of ethanolic extracts of Q. cerris leaf and stem/fruit samples in models for biofilm and growth inhibition.
Materials and methods
Ethanolic extracts of Q. cerris leaves and stems/fruits were prepared, resuspended in water and fractioned by successively partitioning with hexane, ethyl acetate and butanol. The ability of the fractions to inhibit Staphylococcus aureus biofilm formation was tested using static crystal violet staining methods and confocal laser scanning microscopy. Growth studies were conducted to determine if the diminished capacity to form a biofilm was related to growth inhibition.
Results
The butanol extracts of both the leaf and stem/fruit samples were the most active, and at a dose of 200 μg/ml, the capacity to form a biofilm was limited to a level equivalent to that of the sarA mutant controls. Further examination of the impact of these fractions on S. aureus growth revealed that biofilm inhibition by the leaf butanol fraction was due to its bacteriostatic activity. The stem/fruit butanol fraction, however, showed a limited impact on growth, thus demonstrating that biofilm inhibition in this case is not related to the bacteriostatic activity of the extract.
Conclusion
Our evaluation of a medicinal plant used in Mediterranean ethnotherapies for infectious disease has demonstrated significant activity in the inhibition of staphylococcal biofilm formation with a mechanism unrelated to staphylococcal growth inhibition. These results contribute towards validation of this botanical remedy and form the groundwork for future studies in the search for novel biofilm inhibiting drugs.
Keywords: biofilm, Staphylococcus aureus, Oak, Quercus cerris
1. Introduction
Staphylococcus aureus is a pathogen of great importance to human health. Besides its ability to cause large number of diseases, it has also acquired the adaptations necessary to survive hostile environments and repeated antibiotic insult. These qualities have converged to make S. aureus a significant burden on our current healthcare system.
One of the patient populations most vulnerable to S. aureus infection are those with implanted medical devices such as central venous catheters, cardiac valves and pacemakers, artificial joints and various orthopedic devices. Upon formation of a biofilm on these devices, staphylococcal cells can gain a state of metabolic dormancy and create a microenvironment that protects them from the host immune response and antibiotic exposure (Hall-Stoodley et al., 2004), making the infection nearly impossible to treat. Consequentially, novel therapies that address the issue of intrinsic biofilm-related resistance are highly desired. In this context, we have used in vitro and in vivo studies to demonstrate that mutations in the sarA gene, which limit the capacity of S. aureus to form a biofilm, also increase the susceptibility of S. aureus to antibiotics (Weiss et al., 2009a; Weiss et al., 2009b). Likewise, a recent study on an ellagic acid derivative rich plant extract, which limits S. aureus biofilm formation, also improves antibiotic efficacy in biofilm removal in a catheter model (Quave et al., 2012). These studies, among others, strongly support the concept that drugs targeting pathogenesis via biofilm inhibition could have great therapeutic value.
A report on the anti-staphylococcal activity of crude ethanolic extracts of Italian plants highlighted the anti-biofilm potential of Q. cerris extracts (Quave et al., 2008). Commonly known as the Turkey Oak, it is used in traditional medical practices for the treatment of diarrhea, leucorrhea, hemorrhoids, rheumatism, and to induce hyperhidrosis (Antonone et al., 1988; Bellomaria and Della, 1985). This plant is also used in ethnoveterinary medicine for wound care (Pieroni, 2000). Here, we expand on this prior work by using bioassay-guided fractionation techniques to isolate the fraction(s) with the greatest activity against two clinically relevant strains of S. aureus using in vitro biofilm models.
2. Materials and Methods
2.1 Acquisition of Plant Materials
Bulk samples of Quercus cerris L. (Fagaceae) leaves, fruits and stems were collected from wild populations in August 2009 in the village of Ginestra, Basilicata province, southern Italy by CQ. Procedures from the 2003 WHO Guidelines on Good Agricultural and Collection Practices for Medicinal Plants (WHO, 2003) were followed for the collection and identification of bulk and voucher specimens. Voucher specimens (CQ-228) were deposited at the Herbarium Lucanum (HLUC) at the Università della Basilicata in Potenza, Italy. The specimens were identified using the standard Italian Flora (Pignatti, 2002) and identification was confirmed at HLUC. Dried plant materials were packed into plastic bags with silica packets, vacuum sealed, and then exported to the USA under USDA permit PDEP-09-00228 for phytochemical evaluation and bioassays.
2.2 Extraction and bioassay-guided fractionation of plant materials
Plant materials were homogenized into a fine powder, creating two sets of samples for extraction and testing (sample 1: leaves; sample 2: fruit and stems). Each sample (1 kg) was extracted with 95% EtOH (2 × 10L) at room temperature for 72 hours with constant agitation. Filtered extracts were combined, concentrated at reduced pressure, and a temperature <45°C, and lyophilized prior to being resuspended in water and fractioned by partitioning in succession with hexane, ethyl-acetate, and butanol (Fisher Chemical, Certified ACS). Partitions were dried over anhydrous sodium sulfate, concentrated at reduced pressure, and lyophilized before testing in bioassays.
2.3 Quality control
Extracts and fractions were dissolved in DMSO, and then sterile filtered (0.2 μm; nylon filter) and stored in sterile vials at −20°C prior to use in all bioassays. Sterility controls were included in all assays (extract + media) and tests for sterility followed standard quality control measures (Isenberg, 2004).
2.4 Bacterial strains and growth conditions
Two clinically-relevant stains of Staphylococcus aureus were used in this study: UAMS-1, a USA200 MSSA osteomyelitis isolate; and UAMS-1782, a USA300 community-associated MRSA isolate. The isogenic sarA mutants of these strains (UAMS-929 and UAMS-1804, respectively) were used as negative controls based on the observation that mutation of sarA results in a reduced capacity to form a biofilm that can be correlated with increased antibiotic susceptibility (Beenken et al., 2003; Weiss et al., 2009b). All strains have been previously described (Beenken et al., 2010).
For biofilm assays, strains were grown in tryptic soy broth (TSB) supplemented with 3.0% NaCl (wt/vol) and 0.5% dextrose (biofilm medium, BM). For all assays, overnight cultures of the test strains were used to inoculate fresh medium at an initial cell density of 5 × 105 colony forming units (CFU) per ml (confirmed with plate counts). This cell density was achieved by taking the optical density of overnight cultures and diluting to an OD560nm of 0.05. Studies examining growth were done at 37°C in BM with constant shaking (200 rpm) with a volume-to-flask ratio of 0.4.
2.5 Assessment of biofilm formation
Biofilm assays were undertaken using a human plasma protein-coated microtiter plate assay as described (Beenken et al., 2003). Following inoculation, the plates were incubated at 37°C for 24 hours, the wells were gently washed twice with 200 μl of phosphate-buffered saline (PBS) to remove all non-adherent cells. Adherent biofilms were fixed with 200 °l of 100% ethanol prior to staining for 2 minutes with 200 °l 0.41% (wt/vol) crystal violet in 12% ethanol. Following aspiration of the stain, wells were washed several times with PBS. A quantitative assessment of biofilm formation was then taken by adding 100% ethanol and incubating at room temperature for 10 min. The eluate was then transferred to a new polystyrene microtiter plate and the absorbance (OD595nm) was determined using a plate reader.
2.6 Growth curve
The impact of the two most promising fractions (001-1-D and 002-1-D, butanol partitions of leaf and fruit/stem samples, respectively) on S. aureus growth was determined by taking aliquots for plate counts at various time points following inoculation to determine the number of CFU/ml in the growth media.
2.7 Confocal laser scanning microscopy (CLSM) of static biofilms
Confocal imaging of biofilms following treatment with extract was undertaken as previously described (Quave et al., 2012). S. aureus strains (UAMS-1 and UAMS-1782) and their isogenic sarA mutants (UAMS-929 and UAMS-1804) were grown in 96 well microtiter plates (Costar 3603, Corning Life Sciences). After 20 hours, the well contents were aspirated and the wells were gently rinsed 3 times with 0.85% (wt/vol) NaCl. The adherent biofilm was stained with LIVE/DEAD (Invitrogen) stain at room temperature in the dark for 18 minutes. Following stain removal, wells were gently washed with 0.85% NaCl before collecting CLSM images using a Zeiss LSM 510 Meta confocal scanning system and inverted microscope.
2.8 High performance liquid chromatographic (HPLC) analysis of the most active fraction
Fraction 002-1-D was subjected to HPLC analysis for the purpose of batch to batch quality control using an Agilent 1260 Infinity system with quaternary gradient pump, in-line degasser, autosampler, column heater, diode array detector and acquisition system (OpenLab CDS ChemStation, Agilent Technologies, Santa Clara, CA, USA). A 4.6 × 150 mm, 5 μm reverse-phase Zorbax octadecylsilane (ODS-C18; Agilent) column was used at a temperature of 30°C. The extract was dissolved in solvent A and a 2 μg injection was eluted at a flow rate of 1 ml/min using a gradient of 2 solvent systems: (A) 2% acetic acid in water; (B) 2% acetic acid in acetonitrile. The mobile phase was 0% B at time 0 min and 100% at time 40 minutes, followed by a hold at 100% B for 6 minutes and a hold at 0% B for 8 minutes (54 minute total run time). Optimal peak resolution was detected at 280 nm.
2.9 Statistical analysis
Pair-wise testing was performed based on t-tests as formatted with Sigma Stat® Statistical Software Version 2 (SPSS, Inc) with P values < 0.05 considered significant.
3. Results and Discussion
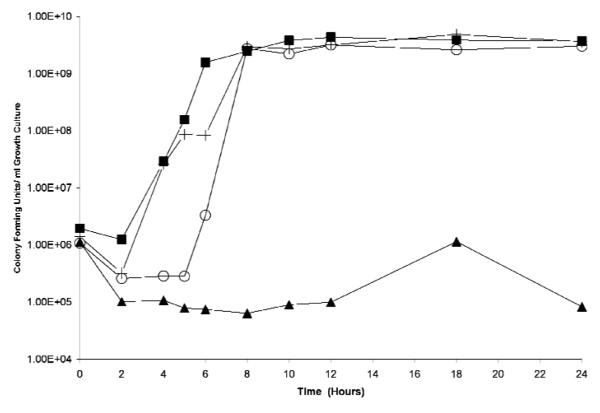
Biofilm assays on crude ethanolic extracts and their fractions revealed that the butanol fractions of both the leaf (001-1-D; 0.018 % yield) and the stem/root (002-1-D; 0.031% yield) extracts exhibited the most potent biofilm inhibitory activity, with 63±10% and 74±4% inhibition in relation to the growth control at a test dose of 200 μg/ml (P values <0.05). This approximates a similar range (85±5%) of limited biofilm formation exhibited by the isogenic sarA mutant control. Furthermore, growth studies on these two fractions demonstrated that 001-1-D inhibited growth, whereas 002-1-D did not (Figure 1), indicating that the biofilm inhibitory activity of 002-1-D is due to a mechanism unrelated to inhibition of growth.
Figure 1.
Growth study on the two most active biofilm-inhibiting fractions tested at 200 μg/ml. While the 00-1-D significantly limited growth, 00-2-1-D demonstrated only limited impact on growth, causing a ~3h delay in transition to log phase of growth and reaching stationary phase at the same time (~8h) as controls. Figure legend: ∎: wild-type growth control (UAMS-1); +: sarA mutant growth control (UAMS-929); ▲: butanol fraction of leaf extract (001-1-D); ○: butanol fraction of stem/root extract (002-1-D).
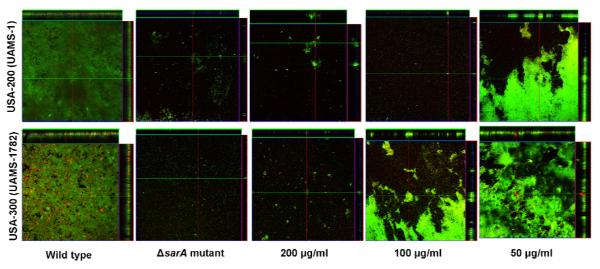
CLSM studies of 002-1-D revealed a dose-dependent response in biofilm inhibition for the two pathogenic strains (Figure 2). Importantly, the extract was effective in the treatment of a strong biofilm-former (UAMS-1) and a MRSA isolate associated with community acquired infections (UAMS-1782) and at levels equivalent to that seen in the ΔsarA controls (100 μg/ml for UAMS-1, and 200 μg/ml for UAMS-1782). This is therapeutically relevant as our group has previously documented the benefit of a reduced capacity to form a biofilm (using sarA mutants) on increased antibiotic efficacy in vivo (Weiss et al., 2009b).
Figure 2.
Microtiter plate biofilm assays were undertaken with UAMS-1 (top) or UAMS-1782 (bottom) after the addition of 002-1-D at the indicated concentrations or excipient (DMSO) to the growth medium. Confocal images were obtained after 20 hours of incubation. An orthogonal view (0.9 × 0.9 mm section) is included to illustrate overall biofilm architecture at a magnification of 10×. Isogenic sarA mutants grown in BM with DMSO were included as negative controls. A dose-dependent effect is evident, and approximates that of the isogenic sarA controls.
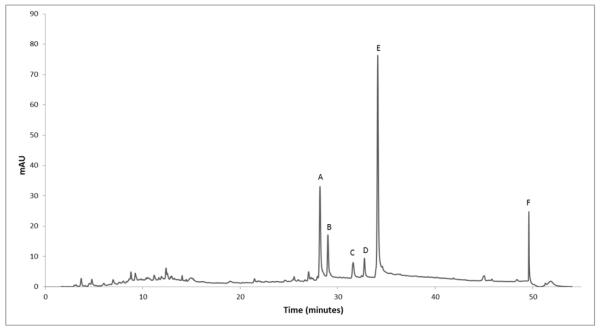
HPLC analysis of 002-1-D revealed the presence of 15 minor and 6 major constituents (representing >3% of the % area) labeled as A-F and present at retention times of 28.17, 28.99, 31.57, 32.73, 34.1, and 49.59 minutes, respectively (Figure 3). Further work using semi-preparative HPLC to isolate, test, and structurally elucidate compounds A-F is planned.
Figure 3.
Chromatogram of 002-1-D, the fraction with the greatest anti-biofilm activity without growth-inhibitory effects. Future work will focus on the isolation, structural elucidation and testing of compounds A-F in biofilm models.
4. Conclusion
We have demonstrated that the butanol fraction of the crude stem/fruit Q. cerris extract is responsible for the biofilm-inhibitory activity of this plant. The properties of this plant in interfering with staphylococcal pathogenesis contribute to the validation of its use in traditional therapies for infections and wounds. Further bioassay-guided fractionation efforts are planned in order to isolate and elucidate the active constituent(s) in 002-1-D responsible for this activity. Likewise, microarray analysis with this extract is planned in effort to investigate the mechanism of action. Such studies, which contribute to the discovery and development of novel drugs to treat intrinsically resistant biofilm-associated staphylococcal infection, are crucial to the future of our anti-infective therapeutic repertoire.
Acknowledgements
This project was supported by an F32 Fellowship to CLQ (AT005040) from the NIH National Center for Complementary and Alternative Medicine (NCCAM) and by the US Department of Defense grant USAMRAA OR090571 to MSS. The content is solely the responsibility of the authors and does not necessarily reflect the official views of DOD, NCCAM or NIH. The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- BM
Biofilm medium
- MRSA
Methicillin resistant Staphylococcus aureus
- MSSA
Methicillin sensitive Staphylococcus aureus
- PBS
Phosphate buffered saline
- TSB
Tryptic soy broth
- USDA
United States Department of Agriculture
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonone R, Simone FD, Morrica P, Ramundo E. Traditional phytotherapy in the Roccamonfina volcanic group, Campania, Southern Italy. J. Ethnopharmacol. 1988;22:295–306. doi: 10.1016/0378-8741(88)90240-1. [DOI] [PubMed] [Google Scholar]
- Beenken KE, Blevins JS, Smeltzer MS. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infection and Immunity. 2003;71:4206–4211. doi: 10.1128/IAI.71.7.4206-4211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenken KE, Mrak LN, Griffin LM, Zielinska A, Shaw LN, Rice KC, Horswill AR, Bayles KW, Smeltzer MS. Epistatic relationships between sarA and agr in Staphylococcus aureus biofilm formation. PloS ONE. 2010;5:e10790. doi: 10.1371/journal.pone.0010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomaria B, Della ML. Novita nell’uso delle piante officinali per la zona di Matelica (Macerata) anche in confronto con altre zone delle Marche. Archivio Botanico e Biogeografico Italiano. 1985;61:51–81. [Google Scholar]
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the Natural environment to infectious diseases. Nat Rev Micro. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Isenberg HD. Clinical Microbiology Procedures Handbook. ASM Press; Washington, DC: 2004. [Google Scholar]
- Pieroni A. Medicinal plants and food medicines in the folk traditions of the upper Lucca Province, Italy. Journal of Ethnopharmacology. 2000;70:235–273. doi: 10.1016/s0378-8741(99)00207-x. [DOI] [PubMed] [Google Scholar]
- Pignatti S. Flora d’Italia. Edizioni Edagricole; Bologna, Italy: 2002. [Google Scholar]
- Quave CL, Estévez-Carmona M, Compadre CM, Hobby G, Hendrickson H, Beenken KE, Smeltzer MS. Ellagic Acid Derivatives from Rubus ulmifolius Inhibit Staphylococcus aureus Biofilm Formation and Improve Response to Antibiotics. PLoS One. 2012;7:e28737. doi: 10.1371/journal.pone.0028737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quave CL, Plano LWR, Pantuso T, Bennett BC. Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus. Journal of Ethnopharmacology. 2008;118:418–428. doi: 10.1016/j.jep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EC, Spencer HJ, Daily SJ, Weiss BD, Smeltzer MS. Impact of sarA on antibiotic susceptibility of Staphylococcus aureus in a catheter-associated in vitro model of biofilm formation. Antimicrobial Agents and Chemotherapy. 2009a;53:2475–2482. doi: 10.1128/AAC.01432-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EC, Zielinska A, Beenken KE, Spencer HJ, Daily SJ, Smeltzer MS. Impact of sarA on daptomycin susceptibility of Staphylococcus aureus biofilms in vivo. Antimicrobial Agents and Chemotherapy. 2009b;53:4096–4102. doi: 10.1128/AAC.00484-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO guidelines on good agricultural and collection practices (GACP) for medicinal plants. Geneva: 2003. [Google Scholar]