Abstract
Intracavernous (IC) injection of stem cells (SCs) has been shown to improve erectile function in various erectile dysfunction (ED) animal models. However, the tissue distribution of the injected cells remains unknown. In this study we tracked IC injected adipose-derived stem cells (ADSCs) in various tissues. Rat paratesticular fat was processed for ADSC isolation and culture. The animals were then subject to cavernous nerve (CN) crush injury or sham operation, followed by IC injection of one million autologous or allogeneic ADSCs that were labeled with 5-ethynyl-2-deoxyuridine (EdU). Another group of rats received IC injection of EdU-labeled allogeneic penile smooth muscle cells (PSMCs). At 2 and 7 days post-injection, penises and femoral bone marrow were processed for histological analyses. Whole femoral bone marrows were also analyzed for EdU-positive cells by flow cytometry. The results show that ADSCs exited the penis within days of IC injection and migrated preferentially to bone marrow. Allogenicity did not affect ADSC's bone marrow appearance either at 2 or 7 days, while CN injury reduced the number of ADSCs in bone marrow significantly at 7 but not 2 days. The significance of these results in relation to SC therapy for ED is discussed.
Keywords: Adipose-derived stem cells, bone marrow, intracavernous injection, erectile dysfunction
Introduction
Stem cells (SCs) are well known for their regenerative potential. Their application to treat damaged or degenerative tissues is usually done by injection to the target tissue. However, increasingly more studies are reporting that intravenously (IV) injected SCs are capable of homing to injury sites and exerting similar therapeutic effects as locally injected SCs 1-3. Thus, because of its ease of application and sometimes being less invasive than local injection, IV injection may become a standard procedure for most SC treatments 3. However, the realization of this potential requires a clear understanding of these cells' tissue distribution following IV injection and whether they pose any danger to the host tissues due to their systemic distribution.
Since the first report of SC therapy for the treatment of neurogenic erectile dysfunction (ED) that employed intracavernous (IC) injection 4, all subsequent studies of SC treatment for various types of ED have used the same injection method 5-12. This method, which delivers the treatment cells into the corpus cavernosum, may seem overtly a local injection. However, the reality is somewhat more complicated. First, in the case of neurogenic ED, the injury site is not the corpus cavernosum but the cavernous nerves whose cell bodies reside some distance away in the major pelvic ganglia (MPG) 13. Thus, IC injected SCs cannot be expected to have local effects on the MPG. Second, the corpus cavernosum is composed of endothelium-lined sinusoids that are anatomically and physiologically similar to arteries and veins 14. Thus, IC injection is analogous to IV injection in that the injected cells can be transported by blood to distant locales, including the target tissue. Together, these considerations point to the possibility that the therapeutic effect of IC injected SCs on neurogenic ED is attributable to their home-in to the MPG through the blood stream. However, it remains poorly understood in regard to the overall tissue distribution of IC or IV injected SCs. In the present study we show that IC injected ADSCs preferentially travel to the bone marrow.
Materials and Methods
Animals
Male Sprague-Dawley rats 3 months of age were obtained from Charles River Laboratories (Wilmington, MA, USA). Their care and treatments were approved by the Institutional Animal Care and Use Committee at our institution.
ADSC Isolation
Under 2% isoflurane anesthesia, each rat received a midline abdominal incision and a specimen of its paratesticular fat was excised and placed in phosphate-buffered saline (PBS) on ice. The wound was then closed in two layers with absorbable suture. After rinsing with PBS, the harvested fat was minced into small pieces and then incubated in a solution containing 0.075% collagenase type IA (Sigma-Aldrich, St. Louis, MO, USA) for 1 h at 37°C with vigorous shaking for 15 sec in 20-min intervals. The top lipid layer was removed and the remaining liquid portion was centrifuged at 1000g for 10 min at room temperature. The pellet was treated with 160 mM NH4Cl for 10 min to lyse red blood cells. The remaining cells were suspended in 10 ml of Dulbecco's Modified Eagle Medium (DMEM) supplemented with Streptomycin, Fungizone, Penicillin and 10% fetal bovine serum (FBS). The suspension was filtered through a 70-μm cell strainer, plated at a density of 1 × 106 cells in a 10-cm dish, and cultured at 37°C in 5% CO2.
Preparation of Cells for Intracavernous Injection
For tracking purpose, all cells to be used for transplantation were labeled for 24 h with thymidine analog 5-ethynyl-2-deoxyuridine (EdU; Invitrogen, Carlsbad, CA, USA) as previously described 15. For autologous transplantation, the above-described ADSCs were cultured for 3 to 5 days before being labeled with EdU. For allogeneic transplantation, previously isolated rat ADSCs or penile smooth muscle cells (PSMCs) 16,17 at passage 2 were used. Approximately 1 × 106 EdU-labeled cells in 0.4 ml of PBS were used for each IC injection.
Induction of Cavernous Nerve Injury and Cell Injection
Under 2% isoflurane anesthesia, a lower abdomen midline incision was made and the prostate gland exposed. The CN and MPG were then identified posterolaterally on both sides of the prostate. In rats designated as Sham, no further manipulation was performed except for closing the wound. In rats designated as CN injury, the CN were isolated and crushed for 2 minutes per side, using a dedicated needle holder. Next, the penis was exposed and its base constricted with a PE-90 tube. Each of these rats then received injection of one million ADSCs or PSMCs in 0.4 ml PBS into the left corpus cavernosum. Following injection, the needle was left in place for 5 min to allow diffusion of the injected materials. The wound was then closed in one layer with absorbable suture.
Histology
Penile and bone marrow tissues were obtained from the above-described animal groups and from a previous study (Fandel et al., manuscript submitted). They were fixed for 4 h with cold 2% formaldehyde and 0.002% picric acid in 0.1 M phosphate buffer, followed by overnight immersion in buffer solution containing 30% sucrose. Tissues were frozen in optimum cutting temperature compound (Sakura Finetek, Torrance, CA, USA), and stored at −80°C until use. Sections were cut at 6 μm, adhered to charged slides, air dried for 5 min, and rehydrated with 0.05 M PBS. After rinsing, sections were washed in PBS followed by 30 minutes room-temperature incubation with 3% goat serum/PBS/0.3% triton X-100. For tracking of transplanted cells, slides were incubated with freshly made Click-iT reaction cocktail, which contained Alexa-594 fluor (Invitrogen), for 30 min at room temperature 15. Nuclear staining was performed with 4′,6-diamidino-2-phenylindole (DAPI; D-3571, Invitrogen).
Image Analysis
The stained tissues were examined with a Nikon Eclipse E600 fluorescence microscope (Nikon Instruments, Melville, NY, USA) and photographed with a Retiga 1300 QImaging camera (QImaging, Surrey, BC, Canada) using the ACT-1 software (Nikon Instruments). Computerized histomorphometric analysis was performed using Image-Plus 5.1 software (Media Cybernetics, Bethesda, MD, USA). To quantify EdU staining, penis and bone marrow were analyzed at 200× magnification and expressed as the number of EdU-positive nuclei per high power field (HPF).
Flow Cytometric Analysis of Transplanted Cells in Bone Marrow
Bone marrow was flushed out of the femur with PBS, incubated in 0.75% collagenase at 37°C for 20 min, washed 3 times with PBS, and treated in a fixation and permeabilization solution (BD Biosciences, San Jose, CA, USA) at room temperature for 10 min. Afterward, the individualized bone marrow cells were washed with 3% bovine serum albumin (BSA) and then incubated in Click-iT reaction cocktail (Invitrogen, Carlsbad, CA, USA) for 30 min at room temperature without light. Thereafter, the cells were further washed with PBS, resuspended in 2ml of PBS, and analyzed in a fluorescence-activated cell sorter (FACSVantage SE System, BD Biosciences). The resulting data were further analyzed with FlowJo software (Tree Star, Inc., Ashland, OR) to determine the percentage of EdU-positive cells among 1 × 105 bone marrow cells.
Statistical Analysis
Data was analyzed with Prism 4 (GraphPad Software, Inc., San Diego, CA, USA) and expressed as mean ± standard error of the mean for continuous variables. The continuous data was compared the groups using one-way analysis of variance. The Tukey-Kramer test was used for post-hoc comparisons. Statistical significance was set at p < 0.05.
Results
Tissue Distribution of Intracavernously Injected ADSCs
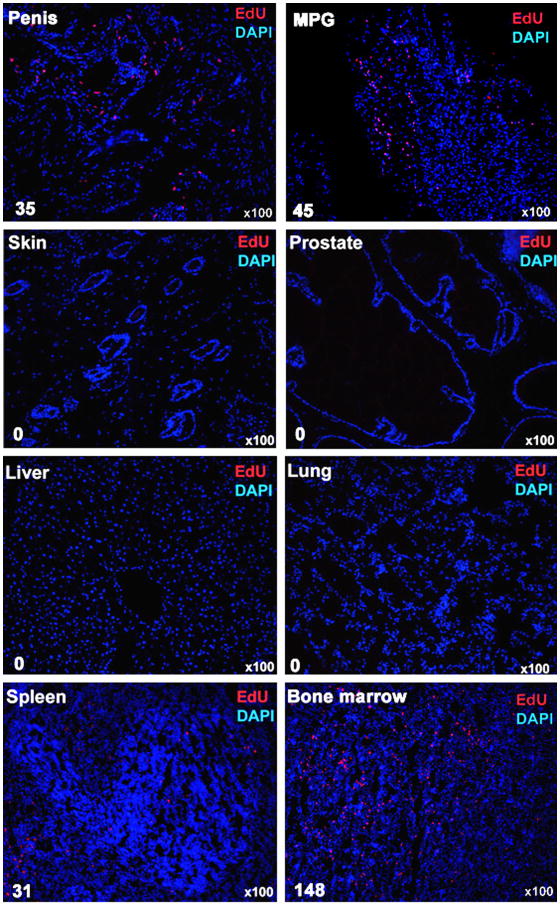
Various tissues were examined for the presence of ADSCs 7 days after their autologous injection into the corpus cavernosum of rats that received bilateral CN crush. The results show that ADSCs were detected in the penis, major pelvic ganglia (MPG), spleen, and bone marrow, but not in liver, lung, skin, or prostate (Fig. 1). Of note is that, despite being the injection site, the penis contained relatively few ADSCs. Instead, bone marrow appeared to be a preferred destination for IC transplanted ADSCs.
Fig. 1.
Tissue distribution of IC injected ADSCs. The indicated tissues were obtained from rats at 7 days after IC injection of one million ADSCs. All cell nuclei were stained blue by DAPI. ADSCs, which stained red by EdU, were counted manually and the results shown at the lower left corner of each tissue image.
Distribution of Intracavernously Injected ADSCs and PSMCs in the Penis and Bone Marrow
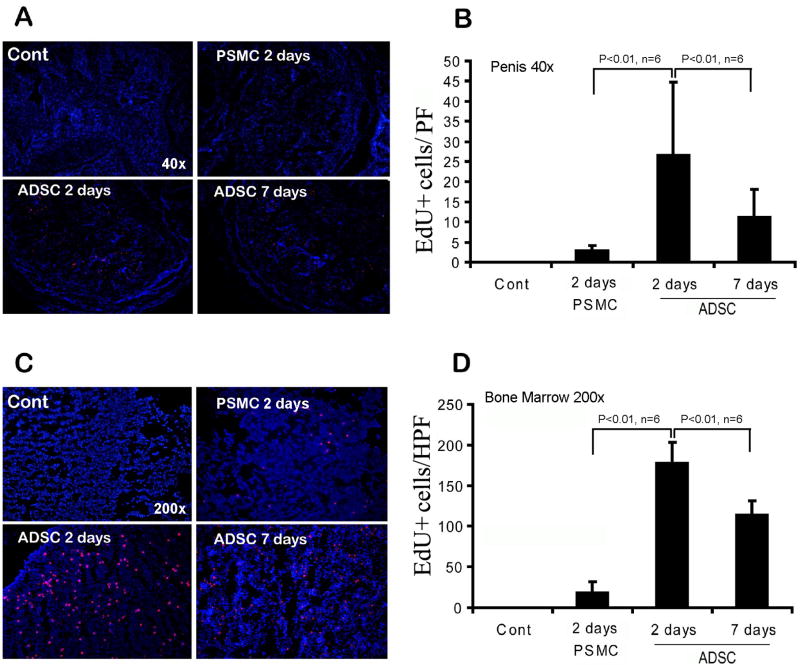
To investigate ADSC's bone marrow connection, we compared them to penile smooth muscle cells (PSMCs) in distribution to the penis and bone marrow. The results show that, at 2 days after IC injection, the number of PSMCs was significantly lower than that of ADSCs in both the penis and bone marrow (Fig. 2). We further examined the distribution of ADSCs at 7 days after IC injection, and the results show that the number of cells was lower in both the penis and bone marrow when compared to the respective 2-day samples (Fig. 2).
Fig. 2.
Quantification of IC injected cells in penis and bone marrow. Control rats (Cont) received no injection. The other rats received IC injection of either PSMCs (PSMC) or ADSCs (ADSC), and their penis and femoral bone marrow harvested at 2 or 7 days later. Representative images of tissue sections of penis (40× magnification) and bone marrow (200× magnification) are shown in panels A and C, respectively. All cell nuclei were stained blue by DAPI. PSMCs and ADSCs, which stained red by EdU, were counted manually and the results shown in panels B and D, respectively.
Cytometric Analysis of Intracavernously Injected ADSCs and PSMCs in the Bone Marrow
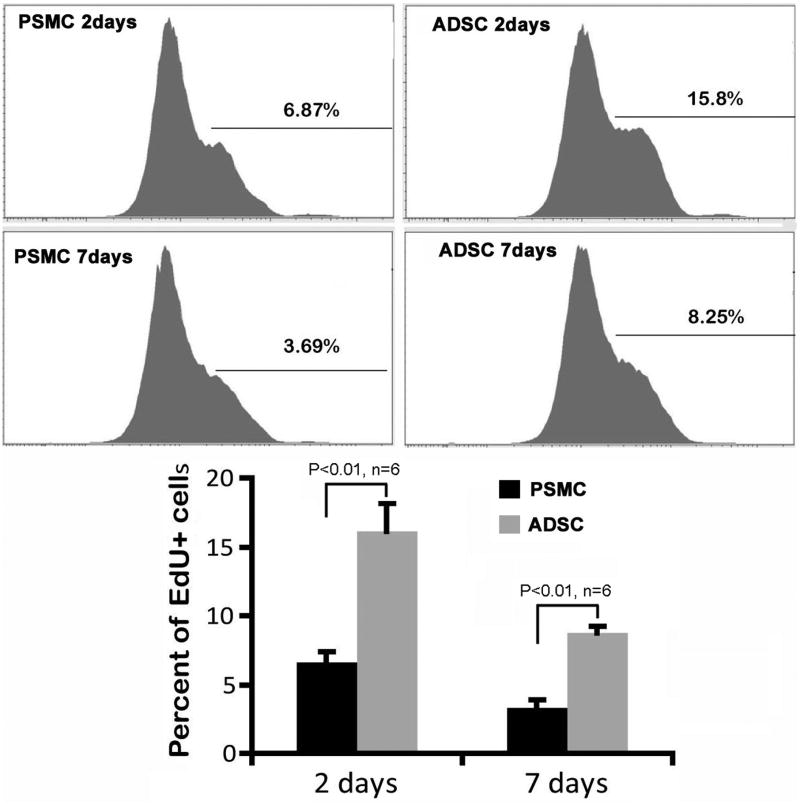
The comparisons between ADSCs and PSMCs and between different-day bone marrow samples were further examined by cytometric analysis. The results again show that the differences were significant in both comparisons (Fig. 3).
Fig. 3.
Quantification of IC injected cells in bone marrow. Rats received IC injection of either PSMCs (PSMC) or ADSCs (ADSC), and their femoral bone marrow harvested at 2 or 7 days later for FACS analysis. Percentages shown in the FACS charts are the ratio of EdU+ cells versus total bone marrow cells (represented by 1 × 105 cells). They are further compared in the bar chart below.
Distribution of Autologous Versus Allogenic ADSCs in the Bone Marrow
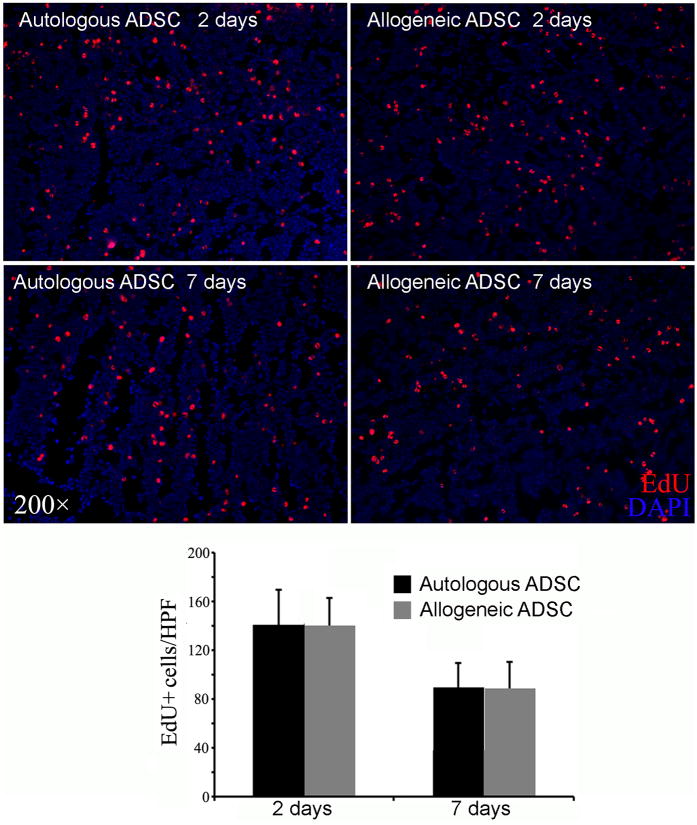
Bone marrows from rats that received IC injection of autologous or allogenic ADSCs were examined for the presence of ADSCs. The results show that there was no significant difference between autologous and allogenic ADSCs in either the 2 or 7-day samples (P>0.05, n=6, Fig. 4).
Fig. 4.
Comparison of autologous versus allogeneic ADSCs. Rats received IC injection of either autologous or allogeneic ADSCs, and their femoral bone marrow harvested at 2 or 7 days later for histology. Representative images are shown in the upper panel. All cell nuclei were stained blue by DAPI. ADSCs, which stained red by EdU, were counted manually and the results shown in the bar chart in the lower panel.
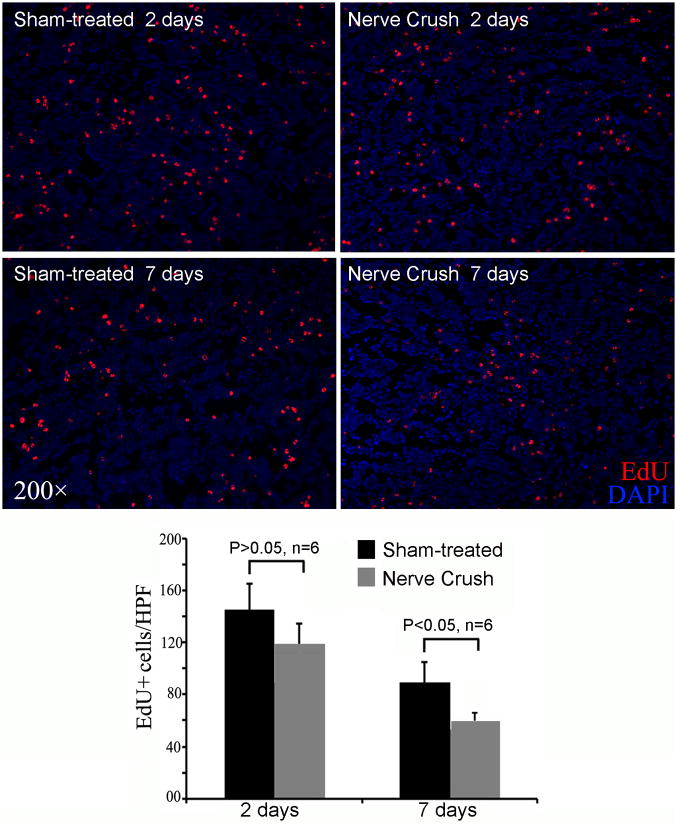
Distribution of Intracavernously Injected ADSCs in the Bone Marrow of Normal Versus CN Injury Rats
Rats were treated with sham operation or bilateral CN crush, followed by IC injection of ADSCs. Two and 7 days later their bone marrows were analyzed for the presence of ADSCs. The results show that, at 2 days, there was a trend of fewer cells in the bone marrow of CN injury rats than in those of sham-treated rats, but the difference was not statistically significant (Fig. 5). At 7 days, the trend continued and this time the difference was significant.
Fig. 5.
Comparison of sham-treated versus CN injury rats. Sham-treated and CN injury rats received IC injection of ADSCs, and their femoral bone marrow harvested at 2 or 7 days later for histology. Representative images are shown in the upper panel. All cell nuclei were stained blue by DAPI. ADSCs, which stained red by EdU, were counted manually and the results shown in the bar chart in the lower panel.
Discussion
To this date, IC injection has been used in every study that investigated SC therapy for ED. However, while generally demonstrating favorable treatment outcome, these studies, including our own, have so far not been able to explain why cells injected into the cavernosum can treat the underlying diseases, particularly in targets that lie outside the penis. Furthermore, in studies that have looked for the transplanted cells in the penis, the results invariably indicate their scant presence. Thus, it appears that most of the transplanted cells have exited the penis not long after their injection, and if so, which parts of the body do these cells travel to? To address this question we examined in the present study several tissues in rats that received IC injection of ADSCs. The results show that, among several tissues examined, bone marrow harbored the highest number of such cells.
In stem cell biology, the bone marrow play's a pivotal role in housing and controlling the bone marrow stem cells, which are the prototype of all mesenchymal stem cells, including ADSCs. Furthermore, a recent study has shown that a fraction of IV injected ADSCs homed to bone marrow 18. Thus, we considered the possibility that ADSCs might originate from bone marrow, and consequently home in to bone marrow is an intrinsic property of ADSCs. To test this hypothesis, we performed quantitative and time-course analyses using PSMCs as a cell type control. We first used the histology-based analysis that permits visualization of the fluorescently labeled cells in representative bone marrow tissue sections. We then used the FACS analysis that permits the quantification of the fluorescently labeled cells in the entire femoral bone marrow. Together, these two complimentary approaches consistently demonstrated that significantly more ADSCs traveled to bone marrow than PSMCs, whether at 2 or 7 days after IC injection.
IC injection of PSMCs can only be done allogeneically; thus, the above-described experimental results raised the question whether host immunity could influence the outcome. To address this question, we performed quantitative and time-course analyses comparing autologous and allogeneic ADSCs, and the results show no significant difference. Furthermore, since the practical purpose of IC injection is to treat ED, we also wondered whether a disease status such as CN injury could influence the migration of ADSCs to bone marrow. Thus, we performed quantitative and time-course analyses comparing sham-operated and CN injury rats, and the results show a trend of fewer ADSCs in the bone marrow of CN injury rats than in those of sham-treated rats, but the difference was significant only at 7 days.
In our recent study (Fandel et al., Submitted) we noted that a significantly larger number of ADSCs traveled to the MPG of CN injury rats as compared to sham-treated rats at 1, 3, and 7 days after IC injection. Also noteworthy is that the number of ADSCs in the MPG of CN injury rats increased incrementally from 1 to 7 days. Thus, while the number of ADSCs decreased from 2 to 7 days in bone marrow (see last paragraph), it increased in the MPG. Such an inverse relationship suggests that IC injected ADSCs exist the penis and travel preferentially to bone marrow; however, when an injury is present - such as CN injury, a portion of the IC injected ADSCs is redirected from penis to the injury site. Alternatively, it has been shown that contact with bone marrow altered the tissue distribution of transplanted SCs 19; thus, ADSCs that travel to bone marrow may establish a reservoir of repair cells that can be recruited to injury sites at later times and thereby enable a sustained therapy. While these hypothetical mechanisms await future investigation, the present study provides evidence that IC injection resulted in ADSC migration to bone marrow; and researchers who study other SC types for ED therapy may want to examine whether bone marrow migration also occurs in their research models.
Conclusions
Within days of IC injection, ADSCs exited the penis and traveled preferentially to bone marrow. Allogenicity did not affect ADSC's ability to migrate to bone marrow. Disease status, such as CN injury, negatively influenced ADSC's bone marrow appearance and appeared to redirect them to injury sites, such as the MPG. These results provide further explanation for the therapeutic efficacy of IC injected SCs toward ED.
Acknowledgments
This work was supported by grants from the Arthur Rock Foundation and the National Institutes of Health (DK045370).
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Huang YC, Shindel AW, Ning H, Lin G, Harraz AM, Wang G, et al. Adipose derived stem cells ameliorate hyperlipidemia associated detrusor overactivity in a rat model. J Urol. 2010;183(3):1232–1240. doi: 10.1016/j.juro.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin G, Wang G, Banie L, Ning H, Shindel AW, Fandel TM, et al. Treatment of stress urinary incontinence with adipose tissue-derived stem cells. Cytotherapy. 2010;12(1):88–95. doi: 10.3109/14653240903350265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev. 2011 doi: 10.1089/scd.2010.0466. [DOI] [PubMed] [Google Scholar]
- 4.Bochinski D, Lin GT, Nunes L, Carrion R, Rahman N, Lin CS, et al. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int. 2004;94(6):904–909. doi: 10.1111/j.1464-410X.2003.05057.x. [DOI] [PubMed] [Google Scholar]
- 5.Lin CS. Advances in stem cell therapy for the lower urinary tract. world J Stem Cells. 2010;2(1):1–4. doi: 10.4252/wjsc.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin CS, et al. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7(10):3331–3340. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia MM, Fandel TM, Lin G, Shindel AW, Banie L, Lin CS, et al. Treatment of erectile dysfunction in the obese type 2 diabetic ZDF rat with adipose tissue-derived stem cells. J Sex Med. 2010;7(1 Pt 1):89–98. doi: 10.1111/j.1743-6109.2009.01541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang YC, Ning H, Shindel AW, Fandel TM, Lin G, Harraz AM, et al. The effect of intracavernous injection of adipose tissue-derived stem cells on hyperlipidemia-associated erectile dysfunction in a rat model. J Sex Med. 2010;7(4 Pt 1):1391–1400. doi: 10.1111/j.1743-6109.2009.01697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel Aziz MT, El-Haggar S, Mostafa T, Atta H, Fouad H, Mahfouz S, et al. Effect of mesenchymal stem cell penile transplantation on erectile signaling of aged rats. Andrologia. 2010;42(3):187–192. doi: 10.1111/j.1439-0272.2009.00977.x. [DOI] [PubMed] [Google Scholar]
- 10.Bahk JY, Jung JH, Han H, Min SK, Lee YS. Treatment of diabetic impotence with umbilical cord blood stem cell intracavernosal transplant: preliminary report of 7 cases. Exp Clin Transplant. 2010;8(2):150–160. [PubMed] [Google Scholar]
- 11.Qiu X, Lin H, Wang Y, Yu W, Chen Y, Wang R, et al. Intracavernous transplantation of bone marrow-derived mesenchymal stem cells restores erectile function of streptozocin-induced diabetic rats. J Sex Med. 2011;8(2):427–436. doi: 10.1111/j.1743-6109.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- 12.Kendirci M, Trost L, Bakondi B, Whitney MJ, Hellstrom WJ, Spees JL. Transplantation of nonhematopoietic adult bone marrow stem/progenitor cells isolated by p75 nerve growth factor receptor into the penis rescues erectile function in a rat model of cavernous nerve injury. J Urol. 2010;184(4):1560–1566. doi: 10.1016/j.juro.2010.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin G, Bella AJ, Lue TF, Lin CS. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part 2. J Sex Med. 2006;3(5):821–827. doi: 10.1111/j.1743-6109.2006.00292.x. discussion 828-829. [DOI] [PubMed] [Google Scholar]
- 14.Christ GJ. The penis as a vascular organ. The importance of corporal smooth muscle tone in the control of erection. Urol Clin North Am. 1995;22(4):727–745. [PubMed] [Google Scholar]
- 15.Lin G, Huang YC, Shindel AW, Banie L, Wang G, Lue TF, et al. Labelling and tracking of mesenchymal stromal cells with EdU. Cytotherapy. 2009;11(7):864–873. doi: 10.3109/14653240903180084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Ning H, Banie L, Wang G, Lin G, Lue TF, et al. Adipose tissue-derived stem cells secrete CXCL5 cytokine with chemoattractant and angiogenic properties. Biochem Biophys Res Commun. 2010;402(3):560–564. doi: 10.1016/j.bbrc.2010.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Yang R, Wang Z, Lin G, Lue TF, Lin CS. Adipose Tissue-Derived Stem Cells Secrete CXCL5 Cytokine with Neurotrophic Effects on Cavernous Nerve Regeneration. J Sex Med. 2011;8(2):437–446. doi: 10.1111/j.1743-6109.2010.02128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han J, Koh YJ, Moon HR, Ryoo HG, Cho CH, Kim I, et al. Adipose tissue is an extramedullary reservoir for functional hematopoietic stem and progenitor cells. Blood. 2010;115(5):957–964. doi: 10.1182/blood-2009-05-219923. [DOI] [PubMed] [Google Scholar]
- 19.Massollo M, Podesta M, Marini C, Morbelli S, Cassanelli C, Pinto V, et al. Contact with the bone marrow microenvironment readdresses the fate of transplanted hematopoietic stem cells. Exp Hematol. 2010;38(10):968–977. doi: 10.1016/j.exphem.2010.06.003. [DOI] [PubMed] [Google Scholar]