Abstract
Objective
Slow and variable reaction times (RTs) on fast tasks are such a prominent feature of Attention Deficit Hyperactivity Disorder (ADHD) that any theory must account for them. However, this has proven difficult because the cognitive mechanisms responsible for this effect remain unexplained. Although speed and variability are typically correlated, it is unclear whether single or multiple mechanisms are responsible for group differences in each. RTs are a result of several semi-independent processes, including stimulus encoding, rate of information processing, speed-accuracy trade-offs, and motor response, which have not been previously well characterized.
Method
A diffusion model was applied to RTs from a forced-choice RT paradigm in two large, independent case-control samples (NCohort 1= 214 and N Cohort 2=172). The decomposition measured three validated parameters that account for the full RT distribution, and assessed reproducibility of ADHD effects.
Results
In both samples, group differences in traditional RT variables were explained by slow information processing speed, and unrelated to speed-accuracy trade-offs or non-decisional processes (e.g. encoding, motor response).
Conclusions
RT speed and variability in ADHD may be explained by a single information processing parameter, potentially simplifying explanations that assume different mechanisms are required to account for group differences in the mean and variability of RTs.
Keywords: ADHD, reaction time, reaction time variability, diffusion model
When tasks call for quick, accurate response decisions, children with ADHD are typically less accurate, slower, and have more variable reaction times (RTs) compared to their same aged peers (Castellanos et al., 2005; Douglas, 1999; Hervey et al., 2006; Kuntsi & Stevenson, 2001; Leth-Steensen, Elbaz, & Douglas, 2000). This phenomenon has been highlighted in all theories of ADHD, yet in itself has not been well understood. Quantitative genetic analyses indicate higher heritability estimates for simple RT variables as compared to other specific task or accuracy measures (Groot, Leo, Stins, & Boomsma, 2004; Kuntsi et al., 2006; Stins et al., 2005; Stins, Van Baal, Polderman, Verhulst, & Boomsma, 2004), as well as considerable overlap between the genes associated with ADHD symptoms and those associated with RT speed and variability (Wood, Asherson, van der Meere, & Kuntsi, 2010). The prevalence of slow and variable RTs in the ADHD cognitive landscape has led some to suggest that these performance characteristics reflect important cognitive mechanisms in the disorder that could be linked to underlying neural dysfunction (Castellanos & Tannock, 2002).
However, theories and gene finding are handicapped by the absence of an adequate cognitive (or process) interpretation for ADHD-related slow RT speed and high variability. Although slow and variable RTs are common in ADHD, there remains important between-study heterogeneity in the presence and size of effects for RT measures (Frazier, Demaree, & Youngstrom, 2004; Huang-Pollock, Karalunas, Tam, & Moore, in press). In addition, although mean and variability of RT are highly correlated, they are often interpreted as reflecting separate cognitive problems in ADHD. Thus, whether these measures should be considered separately in cognitive theories or gene-finding studies, or whether they can be considered to reflect a single process remains unclear. Further complicating interpretation of underlying mechanisms, in neural terms RT speed and variability do not appear to be very specific, tending to show broad, rather than well-delineated, associations with structural and functional imaging measures (Yarkoni, Barch, Gray, Conturo, & Braver, 2009).
Also contributing to the problem of identifying mechanisms, RT speed and variability reflect the influence of multiple interacting processes, including stimulus encoding, rate of information processing, motor preparation and output, speed-accuracy trade-off effects, and response bias (Ratcliff, 2002). Indeed, each of these component processes have been variously hypothesized to be involved in ADHD (Banaschewski et al., 2008; Halperin & Schulz, 2006; Hurks et al., 2005; Klimkeit, Mattingley, Sheppard, Lee, & Bradshaw, 2005; Lajoie et al., 2005; Sergeant, 2000; Sergeant & Scholten, 1985; Steger et al., 2001), and any one may be the source of group differences in RT speed or variability in ADHD or for that matter in subsets of children with ADHD who may have distinct deficits. Current approaches to quantifying speed and variability in ADHD rarely take these multiple influences into account. Thus, clarification of cognitive mechanisms involved in this phenomenon is needed in order for the field to move forward, and this in turn requires an information-processing model that takes into account the multiple processes influencing RTs.
Recently, efforts to address this problem have appealed to alternative analysis approaches, such as frequency-domain analyses (Castellanos, et al., 2005; Di Martino et al., 2008; Helps, Broyd, Bitsakou, & Sonuga-Barke, 2011; Johnson et al., 2008; Johnson et al., 2007a; Vaurio, Simmonds, & Mostofsky, 2009) and ex-Gaussian models (Buzy, Medoff, & Schweitzer, 2009; Epstein et al., 2011; Geurts et al., 2008; Leth-Steensen, et al., 2000) in an effort to provide more specific description of the RT distributions of children with ADHD. In the case of frequency-domain analyses, theory suggests that children with ADHD should be characterized by specific low-frequency patterns in the RT distribution (i.e. long RTs occurring once avery 20–40 seconds, Castellanos, Kelly, & Milham, 2009). However, confirmation of this claim has not been convincing. Although some studies are positive (Castellanos, et al., 2005; Di Martino, et al., 2008; Johnson, et al., 2007a; Vaurio, et al., 2009) others find equal effects at other frequencies (Helps, et al., 2011; Johnson, et al., 2008; Johnson et al., 2007b; Vaurio, et al., 2009) and pooled data confirm equal effects across frequency ranges (Karalunas, Huang-Pollock, & Nigg, in press). Thus, this approach seems not to have better characterized RT distributions in ADHD.
Ex-Gaussian analyses separately quantify the mean (μ) and standard deviation (σ) of the normal portion of the RT distribution, as well as the mean and standard deviation of the exponential tail of the RT distribution (τ). The most consistent significant ADHD-control group differences are reported in the exponential upper tail of the RT distribution (τ), suggesting that children with ADHD have a large number of excessively long RTs relative to non-ADHD controls (Buzy, et al., 2009; Epstein, et al., 2011; Leth-Steensen, et al., 2000). However, the same studies also note group differences in both μ and σ (Buzy, et al., 2009; Epstein, et al., 2011). Although the patterns of statistical significance are less consistent for μ and σ, the size of the effects for the three ex-Gaussian parameters have not been directly compared, and it is not clear if the effect for τ is reliably larger than for μ and σ.
Even if we accept that the largest group differences are seen in the exponential tail of the RT distribution, this is only partial progress because the cognitive interpretations of ex-Gaussian parameters remain uncertain. τ has sometimes been interpreted as reflecting higher-order processing while μ and σ relate more strongly to motor responding. This is consistent with interpretations of increased τ as reflecting ADHD-related deficits in attention(Hervey, et al., 2006; Leth-Steensen, et al., 2000). However, the opposite interpretations have also been made (for review see Matzke & Wagenmakers, 2009), and so it is equally plausible that group differences in motor responding could account for the patterns observed. Such conflicting interpretations mean that we still have not arrived at a satisfying explanation of ADHD RT effects using ex-Gaussian models.
Analyses based on a drift diffusion model of RTs (Ratcliff & Rouder, 1998) provide another, and perhaps more satisfying, way of disentangling the multiple factors influencing RTs. Widely used in the cognitive psychology literature (Balota & Yap, 2011; Kühn et al., 2011; Ratcliff, Thapar, Gomez, & McKoon, 2004; Schmiedek, Lövdén, & Lindenberger, 2009; Spaniol & Bayen, 2005; Thapar, Ratcliff, & McKoon, 2003), diffusion models have been developed to explain decision-making in forced-choice paradigms for which relatively rapid (~1 second) response decisions are required (Ratcliff & Rouder, 1998). Thus, they may be quite helpful for further understanding RTs on the cognitive paradigms often used with children with ADHD in both clinical and research settings.
As used herein, a diffusion model assumes that information about a stimulus is accumulated via a noisy information accumulation process until a decision criterion is met, at which point a response is initiated. That is, when faced with a forced-choice decision, participants are assumed to set criteria for how much information they require to commit to either of the response options. Individuals who require less information will respond more quickly but will also make more errors than those who require more information. Thus, how quickly a person responds is related both to the conservativeness of the criteria they have set for responding, and to the rate at which information is accumulated in favor of one of the response criterion. The process is described as “noisy” because random neural activity unrelated to the decision process influences the speed and efficiency with which a person is able to accumulate information relevant to the decision itself. Finally, processes that are not directly related to the response decision, such as stimulus encoding and motor preparation, also influence the final RT and so must be modeled.
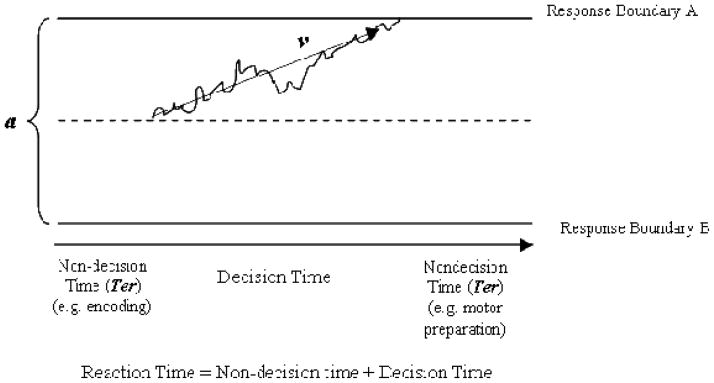
Practically, the diffusion model provides estimates of: 1) drift rate (v; an index of how quickly and efficiently an individual can accumulate information to inform their response decision, which is theoretically linked to neural signal:noise ratio); 2) boundary separation (a; how “sure” a person needs to be before committing to a response, or their speed-accuracy trade-off setting); and 3) non-decision time (Ter; the time it takes to complete all other information processes not involved in stimulus discrimination, e.g., encoding, memory access, motor preparation; Ratcliff, 2006). Figure 1 depicts these processes conceptually. Note that another advantage of these parameters is that they can be related conceptually to signal detection parameters discussed in ADHD theory (Sergeant et al., 1999). Both conceptually and mathematically, the drift rate parameter is similar to discriminability (d′) in traditional signal detection theory, whereas the boundary separation parameter is similar to response bias (β). Signal detection theory, however, accounts only for accuracy data, and its parameters are most reliable in the context of moderate accuracy rates, which produce ample numbers of hits, false alarms, misses, and correct rejections (Sarter, Givens, & Bruno, 2001). On many cognitive tasks used in ADHD this assumption is not met because all children achieve relatively high or low accuracy rates. In this case, RT measures become especially critical for understanding performance differences.
Figure 1.
(adapted from Ratcliff & McKoon, 2008). Diffusion model parameters are depicted for a hypothetical single trial. Drift rate (v) is the rate at which information accumulates towards a decision boundary, as reflected by the average slope of the line. It is determined by speed of information processing and “noise” unrelated to the decision processes (which is represented by the hypothetical jagged deviations from the average slope shown in the Figure). Larger values of v indicate faster processing. Boundary separation (a) indicates the conservativeness of the response criterion with wider separations indicating more conservative responding. Finally, non-decision time (Ter) includes all non-decision processes, such as stimulus encoding and motor preparation. Larger values of Ter indicate longer non-decisional processing times.
Diffusion models are well-validated and show strong convergent and divergent validity. Manipulations of stimulus quality uniquely influence drift rate and manipulations of motor response complexity uniquely influence non-decision time (Voss, Rothermund, & Voss, 2004). In contrast to ex-Gaussian and frequency-domain parameters, which vary widely in their interpretation, diffusion parameters have consensus cognitive interpretations (Ratcliff, 2006; Schmiedek, Oberauer, Wilhelm, Süß, & Wittmann, 2007). Further, recent studies in both humans and non-human primates have demonstrated correspondence between diffusion model parameters and real-time functioning of neural networks (Beck et al., 2008; Philiastides, Ratcliff, & Sajda, 2006; Philiastides & Sajda, 2006; Ratcliff, Cherian, & Segraves, 2003; Ratcliff, Philiastides, & Sajda, 2009). In all, the diffusion model approach has sufficient advantages that its application to the problem of RT variability in ADHD appears to be well advised.
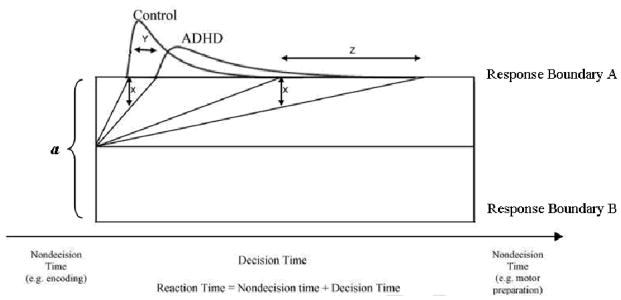
Within the diffusion model framework, slow drift rates in ADHD would be consistent with ex-Gaussian approaches that have identified the most consistent group differences in τ. This is because small changes in drift rates have a larger effect in the tail of the distribution than in the leading edge (see Figure 2 for additional explanation of why this is the case; (see Figure 2 for additional explanation of why this is the case, Ratcliff & McKoon, 2008; Wagenmakers, Grasman, & Molenaar, 2005). Consistent with this hypothesis, slow drift rates in ADHD were identified in a meta-analysis of continuous performance tasks (Huang-Pollock, Karalunas, Tam, & Moore, 2012). However, all of the CPTs included had rare targets (thus a small number of RTs) and used a single-choice RT paradigm (as opposed to the forced two-choice paradigm for which diffusion models were initially developed). Further, because it was a meta-analysis, parameters were estimated using only the mean, variance, and percent correct reported by group rather than using the full RT distributions. Two case-control studies have also applied diffusion modeling in ADHD (Karalunas & Huang-Pollock, revise and resubmit; Mulder et al., 2010). Although one found the expected group differences in drift rate, in the other drift rate was held constant across participants via manipulating the difficulty of stimulus discrimination, and so group differences in drift rate could not be determined (Mulder, et al., 2010). Thus, whether children with ADHD can be characterized as having slow drift rate remains unclear.
Figure 2.
(adapted from Ratcliff & McKoon, 2008). Hypothetical RT distributions for children with and without ADHD. If the drift rate for children with ADHD is “x” degrees slower than controls, the slowdown results in large changes in the tail of the distribution (Z), but only small changes at the leading edge (Y).
The evidence for ADHD group differences in speed-accuracy trade-off and non-decision time is less clear. Two studies have found no group differences in speed-accuracy trade-off (Huang-Pollock, et al., 2012; Karalunas & Huang-Pollock, revise and resubmit), while a third did find that children with ADHD traded speed for accuracy to a greater extent than non-ADHD controls, but only when specifically instructed to prioritize accuracy over speed (Mulder, et al., 2010). The sample for the latter study was small, however, and so the confidence interval around the effect is large. For non-decision time, two studies have found no group differences in non-decision times (Huang-Pollock, et al., 2012; Mulder, et al., 2010), while a third found that children with ADHD had faster non-decision times than non-ADHD controls (Karalunas & Huang-Pollock, revise and resubmit). Thus, the processes responsible for slow and variable RTs in ADHD remain in need of further clarification.
In the current study, we apply diffusion model analyses to two large, independent datasets that were not utilized in any of the prior studies, to determine which RT components: speed/efficiency of information processing (drift rate), speed-accuracy trade-offs (boundary separation), or non-decisional processes, explain slower and more variables RTs under speeded conditions in childhood ADHD, and the replicability of these findings. We expect that children in both samples will show unique, robust group differences in drift rate, and only smaller group differences in speed-accuracy trade-off and non-decision time.
Methods
Data are presented for two cohorts of children, obtained in two different cities, in different states, using identical recruitment and screening procedures (unless noted). The goal of this approach was to evaluate reproducibility (replicability) of results in two independent samples, i.e., built-in cross-replication of any findings.
Participants and Diagnostic Procedures
Cohort #1 included 164 children with ADHD and 50 non-ADHD controls between the ages of 7 and 18. Cohort #2 included an additional 81 children with ADHD and 91 non-ADHD controls between the ages of 7 and 12. Ethics approval was obtained from the Institutional Review Boards at Michigan State University and Oregon Health & Science University, respectively. In both cohorts, a parent/legal guardian provided written informed consent, and children provided written assent for the study. Children were recruited using a community-based recruitment strategy based on public advertising and outreach. After an initial screening phone call, diagnoses were established via a multi-gate process. A parent/guardian and teacher completed standardized rating scales, including Child Behavior Checklist/Teacher Report Form Attention Problems subscale (CBCL/TRF, Achenbach & Rescorla, 1991), Conners’ Rating Scales-Revised (CRS-R, Conners, 2003), and the ADHD Rating Scale (ADHD-RS, DuPaul, Power, Anastopoulos, & Reid, 1998). The parent/guardian also completed a semi-structured clinical interview administered by a Master’s-level clinician who had achieved research reliability on the interview (Kiddie Schedule for Affective Disorders and Schizophrenia, K-SADS, Kaufman et al., 1997). In the case of the Cohort #1, children ages 13 and older also completed a K-SADS interview to report on their own symptoms. Both parent and child reported on lifetime and current symptom levels. For all children, IQ was estimated based on a reliable and valid three-subtest short form of the WISC-IV (Vocabulary, Block Design, and Information, Wechsler, 2003).
Final diagnosis for all children was made by a clinical diagnostic team that included a board certified child psychiatrist and licensed clinical psychologist, who took into account data from the parent and teacher ratings, and parent and teenager clinical interviews, IQ and achievement testing, and behavioral observations. In both cohorts, agreement ratings between the members of the diagnostic team were acceptable for ADHD diagnosis (both kappas > .75) and for other disorders with >5% base rate in the sample (all kappas >.70).
Children were excluded if they: were prescribed long-acting psychotropic medications; had neurological impairment, seizure history, head injury with loss of consciousness, other major medical conditions, or substance abuse; had prior diagnosis of mental retardation, autism spectrum disorder, or psychosis; were currently experiencing a major depressive episode; or had estimated IQ <70. Children with ADHD taking stimulant medications (27% of Cohort #1, 37% of Cohort #2) were included in the study but were required to be off medication for 24 (for short-acting preparations) to 48 hours (for long-acting preparations) prior to testing. These procedures were identical in the two cohorts.
Cognitive Measure
For the evaluation of RT speed and variability using the diffusion model, we used data from a tracking version of the dual-task “Stopping Task” (described in detail in Logan, 1994; Logan, Schachar, & Tannock, 1997; Nigg, 1999). This is a dual-task experiment in which the child completes a series of fast decision trials, generally with high accuracy, so it is ideal for application of the diffusion model. This task embeds a choice reaction time task (go trials) and a stop task (stop trials). For each trial, a central fixation point appeared for 500 ms. An “X” or an “O” then appeared for 1000 ms. On 75% of trials (“go” trials), children were asked to indicate with a key press whether an “X” or an “O” appeared in the center of the screen. Children were given a total of 2000 ms to respond after which the next trial automatically commenced. Diffusion parameters were estimated from the go trials of the task using the full distribution of correct (pressed X when X was presented or pressed O when O was presented) and error (pressed X when O was presented or pressed O when X was presented) RTs. Diffusion modeling has been previously used successfully in the analysis of go-trials of a stop task in adults (Verbruggen & Logan, 2009). This was a familiar tracking version of the stop task, such that on 25% of trials (“stop” trials), an auditory tone presented after the stimulus (timing varied adaptively based on participant performance) indicated that the child should not respond. These trials were ignored in this analysis because (a) stopping is already heavily described in ADHD and (b) the diffusion model cannot be applied to the inferred stop time. After 32 practice trials, children completed 4 blocks of 64 experimental trials each. Pros and cons of using this task are considered again later.
Statistical Analyses
Mean and standard deviation of RT on correct go trials and percent accuracy on go trials were calculated for each child. In addition, diffusion parameters were estimated from the trial-by-trial data using the Fast-dm modeling technique and the downloadable program from the author’s website (Voss & Voss, 2007). Drift rate (v), boundary separation (a), and non-decision time (Ter) were computed for each participant based on their performance on go trials as an index of speeded responses in the context of a forced-choice reaction time task. Anticipation RTs (those <150ms) were removed from the distribution because these outlier RTs negatively impact estimation of the diffusion parameters (Vandekerckhove & Tuerlinckx, 2007). Note that individuals tend to have slower RTs on the go trials following failed inhibitions (Schachar et al., 2004; Verbruggen, Logan, Liefooghe, & Vandierendonck, 2008). For this reason, trials immediately following the stop trials (referred to as stop + 1 trials) were initially removed from the RT distribution. However, because diffusion parameters estimates did not differ when stop+1 trials were included/excluded (all interactions with stop+1 trial presence were non-significant at p >.07, η2 < .03), the results reported are with the stop+1 trials included.
The Fast-dm program uses an iterative distribution fitting approach with downhill-simplex method to compare the observed RT distribution to the distribution predicted to occur with specific values of v, a, and Ter. Both the correct and error RT distributions are used. Diffusion model parameter estimates are adjusted and distributions re-compared on successive trials until the maximum fit between the predicted and observed distributions is achieved (for additional information on fitting procedures see Voss & Voss, 2007). Participants for whom the diffusion model was not able to adequately fit the data, as indicated by significant values for the Kolmogorov–Smirnov statistic, would have been excluded from analyses; however, no participants needed to be excluded for this reason. Smaller absolute values of v indicate slower drift rates. Smaller values of a indicate greater speed-accuracy trade-offs, and smaller values of Ter indicate faster non-decision time (again, see Figure 1 for additional explanation of parameters.)
In each cohort, multivariate analysis of covariance (MANCOVA) tests were used to compare ADHD and control groups for descriptive purposes on mean RT, standard deviation of RT, and go-trial accuracy. The primary analysis used the same MANCOVA approach to examine the three diffusion parameters. Given well-known associations of age and IQ with speed and variability of RTs, results are reported with these covariates included. Of note, however, is that patterns of significance did not change regardless of whether or not IQ was included as a covariate. Gender composition, parent-reported symptoms of oppositional defiant disorder (ODD), and medication status were also initially included as covariates to ensure they did not account for the results. However, none significantly contributed to the model (all p> .428) and so results reported are without these covariates.
ADHD Subtypes
In Cohort #1 57% of children met criteria for the combined subtype and the remainder for the inattentive subtype. In Cohort #2 68% of children met criteria for the ADHD DSM-IV combined subtype and the remainder met criteria for the DSM-IV inattentive subtype. (Note: Children assigned to the ADHD-I subtype must never have previously met criteria for ADHD-C based on lifetime symptom reports on the clinical interview.) In both samples, subtypes did not significantly differ on any of the outcome variables (mean RT, standard deviation of RT, accuracy, or the three diffusion model parameters), and so subtypes were collapsed into a single ADHD group for all analyses reported below.
Results
Cohort #1
Sample Characteristics
Table 1 provides a summary of group demographic and clinical characteristics. Clinical scores were consistent with validity of the diagnostic group assignments. Groups did not differ in age (p= .489); however, children in the ADHD group had significantly lower estimated IQs than non-ADHD controls (p= .006, d = 0.38). The ADHD group also had a higher proportion of boys and a higher proportion of children meeting criteria for ODD than non-ADHD controls (all p < .005). As noted above, these variables did not affect results reported.
Table 1.
Cohort #1 Characteristic and Task Performance Measures
| Control | ADHD | F(1, 210) | Effect Size (95% CI) | |
|---|---|---|---|---|
| Basic Demographics | ||||
| N | 50 | 164 | ||
| (Boys:Girls) | 24:26 | 116:50 | χ2(1)= 8.07** | |
| Full Scale IQ | 109.22 (14.23) | 102.63 (14.80) | 7.72** | 0.38 (0.13 – 0.77) |
| Age (years) | 10.78 (1.81) | 11.02 (2.27) | 0.48 | 0.12 (−0.20 – 0.43) |
| Conners’ Hyperactivity | 50.40 (10.75) | 65.62 (13.91) | 50.53*** | 0.97 (0.88 – 1.56) |
| Conners’ Cognitive Problems | 49.98 (10.88) | 71.48 (10.08) | 167.72*** | 1.78 (1.67 – 2.41) |
| ODD (n) | 1 | 48 | χ2(1)= 15.87*** | |
| CD (n) | 0 | 1 | χ2(1)= 0.30 | |
| GAD (n) | 11 | 35 | χ2(1)= 0.10 | |
| Traditional RT Variables | ||||
| Accuracy | 0.88 (0.11) | 0.84 (0.12) | 4.12* | 0.28 (0.03 – 0.66) |
| Mean RT | 587.34 (92.67) | 588.96 (85.24) | 0.01 | 0.02 (−0.30 – 0.33) |
| SD RT | 141.82 (21.03) | 155.57 (27.35) | 11.89*** | 0.48 (0.24 – 0.88) |
| Diffusion Model Components | ||||
| Drift rate (v) | 3.65 (0.83) | 3.32 (0.82) | 5.97* | 0.34 (0.08 – 0.72) |
| Boundary Separation (a) | 1.35 (0.24) | 1.30 (0.24) | 1.58 | 0.17 (−0.11 – 0.52) |
| Non-Decision Time (Ter) | 0.40 (0.09) | 0.38 (0.09) | 2.02 | 0.19 (−0.10 – 0.54) |
Note: Results reported are with age and estimated IQ included as covariates.
ODD = oppositional defiant disorder, CD = conduct disorder, GAD = Generalized Anxiety Disorder. RT = Reaction time. SD = Standard Deviation.
Smaller absolute values of v indicate slower drift rates.
Smaller values of a indicate greater speed-accuracy trade off, and smaller values of Ter indicate faster non-decisional time.
Task Performance Measures
By way of data exploration, a MANCOVA that included mean RT, standard deviation of RT, and percent accuracy as between-groups measures, with age and estimated IQ as covariates, indicated significant between-groups differences on traditional task performance measures (F[3,208]= 3.55, p= .015). Children with ADHD were less accurate (F[1, 210]= 4.12, p= .044, d= 0.28) and had more variable RTs (F[1, 210]= 11.89, p= .001, d= 0.48) than non-ADHD controls. Groups did not differ in mean RTs (see Table 1). This result is similar to many other ADHD studies, as noted in the introduction.
We then moved to the diffusion model. A MANCOVA that included the three diffusion model parameters as between-groups measures, with age and estimated IQ as covariates, indicated significant between-group differences (F[5,206]= 2.55, p= .029). Children with ADHD had slower drift rates than non-ADHD controls (F[1,210]= 5.97, p= .015, d= 0.34), but did not differ in either their speed-accuracy trade-off setting or non-decision time (all p> .157; see Table 1).
Cohort #2
Sample Characteristics
The second cohort offered an independent sample in which replication of results could be assessed. Table 2 provides a summary of group demographic and clinical characteristics. Clinical scores were again consistent with validity of the diagnostic group assignments. Groups did not differ in their age or estimated IQs (all p> 0.10). The ADHD group had a higher proportion of boys and a higher proportion of children meeting criteria for ODD than non-ADHD controls (all p < .007).
Table 2.
Cohort #2 Characteristics and Task Performance Measures
| Control | ADHD | F(1, 168) | Effect Size (95% CI) | |
|---|---|---|---|---|
| Basic Demographics | ||||
| N | 91 | 81 | ||
| (Boys:Girls) | 47:44 | 58:23 | χ2(2)= 7.18** | |
| Full Scale IQ | 114.63 (13.09) | 111.24 (14.62) | 2.73 | 0.26 (−0.07 – 0.56) |
| Age (years) | 8.55 (1.10) | 8.64 (1.20) | 0.27 | 0.09 (−0.23 – 0.39) |
| Conners’ Hyperactivity | 45.93 (8.82) | 72.41 (14.04) | 250.68*** | 2.48 (1.87 – 2.63) |
| Conners’ Inattention | 46.59 (8.82) | 73.97 (11.10) | 399.28*** | 2.56 (2.31 – 3.12) |
| ODD (n) | 0 | 13 | χ2(2)= 15.80*** | |
| CD (n) | 0 | 1 | χ2(2)= 1.13 | |
| GAD (n) | 3 | 7 | χ2(2)= 2.24 | |
| Traditional RT Variables | ||||
| Accuracy | 0.93 (0.06) | 0.89 (0.06) | 17.93*** | 0.65 (0.34 – 0.98) |
| Mean RT | 718.34 (122.77) | 714.85 (122.76) | 0.04 | 0.03 (−0.29 – 0.34) |
| SD RT | 218.03 (66.30) | 269.94 (66.60) | 25.67*** | 0.78 (0.45 – 1.10) |
| Diffusion Model Components | ||||
| Drift rate (v) | 2.91 (0.57) | 2.52 (0.58) | 19.49*** | 0.68 (0.35 – 1.00) |
| Boundary Separation (a) | 1.49 (0.27) | 1.52 (0.27) | 0.59 | 0.13 (-.21 – 0.43) |
| Non-Decision Time (Ter) | 0.47 (0.10) | 0.45 (0.11) | 0.77 | 0.14 (0.13 – 0.50) |
Note: Results reported are with age and estimated IQ included as covariates.
ODD = oppositional defiant disorder, CD = conduct disorder, GAD = Generalized Anxiety Disorder. RT = Reaction time. SD = Standard Deviation.
Smaller absolute values of v indicate slower drift rates.
Smaller values of a indicate greater speed-accuracy trade-off, and smaller values of Ter indicate faster non-decisional time.
Task Performance Measures
A MANCOVA that included mean RT, standard deviation of RT, and percent accuracy as between-groups measures, with age and estimated IQ as covariates, indicated significant between-group differences on traditional task performance measures (F[3,166]= 11.40, p< .001). Children with ADHD were less accurate (F[1, 168]= 17.93, p< .001, d = 0.65) and had more variable RTs (F[1, 168]= 25.67, p< .001, d = 0.78) than non-ADHD controls. However, groups did not differ in their mean RTs (Table 2).
We then proceeded to the diffusion analysis. A MANCOVA that included all three diffusion model parameters as between-groups measures, with age and estimated IQ as covariates, indicated significant between-group differences (F[5,164]= 8.17, p< .001). As with the previous sample, children with ADHD had slower drift rates than non-ADHD controls (F[1, 168]= 19.49, p< .001, d = 0.68), but did not differ in either speed-accuracy trade-off setting or non-decision times (all p> 0.381).
Comparison of Cohorts
When compared on task performance measures, one-way ANOVAs indicated that children in Cohort #1 had significantly lower accuracy than those in Cohort #2 (F[1, 365]= 24.71, p< .001, d = 0.52), as well as less variable RTs (F[1, 365]= 277.10, p< .001, d = 1.74) and faster drift rates (F[1, 365]= 66.17, p< .001, d = 0.85).
As compared to Cohort 2, Cohort 1 was also older (F[1, 365]= 146.44, p< .001, d = 1.27), had lower estimated IQ (F[1, 365]= 33.11, p< .001, d = 0.60), and had a greater proportion of families making less than the federal poverty limit for a family of four (χ2[8]= 54.21, p< .001). However, group difference in cognitive outcome variables were not explained by the sample differences in age, IQ, or income (all p remained <.001). Further, a 2 (Group) ×2 (Cohort) MANOVA test demonstrated no Group × Cohort interaction for accuracy, standard deviation of RT, or drift rate, indicating that although the sample means differed, the sizes of the between-group effects were not significantly different between the samples (all p> .347). We thus concluded that the primary effect reproduced across two different samples, despite unavoidable sampling variation.
Discussion
In speeded-decision tasks, children with ADHD often perform slowly and more variably than non-ADHD controls, although effects have been somewhat more robust for variability than for mean RT. Currently, however, there is no agreement about the mechanisms underlying these group differences. Diffusion model analyses, which separately estimate the impact of information processing speed/efficiency, speed-accuracy trade-offs, and non-decisional processes (e.g. encoding, motor response), here indicate that children with ADHD have slower/less efficient information processing speed, but do not differ from non-ADHD controls in their speed-accuracy trade-offs or non-decision times. Slow drift rates, as documented in these two cohorts, are consistent with a recent meta-analysis of CPT performance (Huang-Pollock, et al., 2012), as well as with a case-control comparison study that also employed a stop task paradigm(Karalunas & Huang-Pollock, revise and resubmit). The only other study applying diffusion modeling in ADHD used a motion coherence paradigm and specifically manipulated stimulus discrimination difficulty to be equivalent across participants, thereby intentionally minimizing group differences in drift rate, preventing comparison with current results (Mulder, et al., 2010). Thus, the current study adds to initial evidence that children with ADHD have slower drift rates than non-ADHD controls.
Within a diffusion model framework, drift rate is hypothesized to reflect signal:noise ratio in the neural networks supporting decision-making, an interpretation corroborated by single-cell recordings in non-human primates (Beck, et al., 2008; Ratcliff, et al., 2003) and imaging studies in human adults (Heekeren, Marrett, & Ungerleider, 2008; Ratcliff, et al., 2009). Using this interpretation, slow drift rates in ADHD are indicative of a lower neural signal:noise ratio. This finding is consistent with recent suggestions that children with ADHD also have difficulty suppressing activation in neural networks associated with rest (Castellanos, et al., 2009). A failure to suppress this resting-state activation would be expected to result in lower signal:noise ratios and slower drift rates. Similarly, because myelin is critical for preserving signal strength in neural communication, slow drift rates and low signal:noise ratios are also consistent with growing evidence that children with ADHD show less mature patterns of myelination relative to non-ADHD controls (Nagel et al., 2011). Thus, theories that account for these neural findings in ADHD and seek a link to a behavioral endophenotypes, may be advised to focus not on global RT variability, but on the information gain parameter represented by the diffusion drift rate.
In both cohorts, slow drift rate was detected in the absence of differences in mean RT and the effect size was similar to that of standard deviation of RT (with substantially overlapping confidence intervals for drift rate and standard deviation of RT in both cohorts). On the face, this may seem contradictory in that mean RTs are most often interpreted as an index of information processing speed, while standard deviation is often interpreted as reflecting the processes unrelated to information processing (e.g. attention lapses). However, because slow drift rates exert the largest effects on the RTs at the upper end of the distribution (for additional explanation of why this is the case see Figure 2, Ratcliff & McKoon, 2008; Wagenmakers, van der Maas, & Grasman, 2007), group differences in information processing speed may, in fact, be best captured in increased spread of the distribution rather than by traditional measures of central tendency. With this in mind, slow drift rates in ADHD are consistent with findings from the ex-Gaussian analyses, which often identify the largest group differences in the upper tail of the RT distribution. However, because ex-Gaussian parameters lack clear cognitive interpretations, group differences in the upper tail are often interpreted as reflecting unique cognitive mechanisms from those affecting the mean of the distribution(Hervey, et al., 2006; Leth-Steensen, et al., 2000). Use of the diffusion model clarifies that it is possible for slow/inefficient information processing to result in large differences in the upper tail of the RT distribution.
Diffusion model results are also consistent with prior literature suggesting that ADHD was characterized by poor signal detection as represented by d′ (Losier, McGrath, & Klein, 1996; Sergeant, Oosterlaan, & van der Meere, 1999). That finding has often been interpreted as evidence of reduced alertness. However, as noted earlier, the d′ parameter is vulnerable to artifact when error rates depart markedly from 50%, which is often the case in ADHD studies using cognitive tasks. Thus, the present results provide a clarification and, perhaps, a more robust parameter for use in modeling ADHD cognitive processing in future work, although direct comparison of the size of effects for diffusion, ex-Gaussian, and signal detection parameters will be needed.
In the current study, both cohorts of children were asked to place equal emphases on the speed and accuracy of the go process, and in this context children with ADHD did not trade speed for accuracy to a greater degree than controls. This is consistent with two previous studies which also found no group difference in speed-accuracy trade-offs (Huang-Pollock, et al., 2012; Karalunas & Huang-Pollock, revise and resubmit). Mulder et al. (2010), in contrast, found that children with ADHD traded accuracy for speed to a greater degree than non-ADHD controls, but only when specifically instructed to prioritize accuracy over speed. Thus, some of the evidence suggests that children with ADHD do not have baseline differences in their tendency to prioritize speed over accuracy, but are less able than non-ADHD controls to adjust speed-accuracy settings in different contexts (e.g., depending on instructional set). Overall, we conclude that speed-accuracy trade-off is not a primary explanation for ADHD-associated effects related to RT variability because robust group differences in variability were seen in the current study even in the absence of these trade-offs. Additional studies systematically varying instructional set and response contingencies will be helpful in fully characterizing speed-accuracy trade-offs in ADHD.
In regards to non-decision time, the current study found no group differences in non-decision times in either sample. This is consistent with two other studies which also found no group differences in non-decision times (Huang-Pollock, et al., 2012; Mulder, et al., 2010). However, results contradict findings in another study suggesting that children with ADHD have faster non-decision times than non-ADHD controls (Karalunas & Huang-Pollock, revise and resubmit). One possibility is that the pattern of results for non-decision times and speed-accuracy trade-offs reflects cognitive heterogeneity in ADHD in general. In particular, the effect sizes for both non-decision time and speed-accuracy trade-offs are substantially smaller than those for drift rate, even when significant group differences have been found. Thus, it is possible that deficits in speed-accuracy trade-offs and non-decision processes characterize a small, but important, subgroups of children with ADHD. This would make the detection of group-level differences more sample specific. Future studies applying methods such as latent class or graph theory approaches to identify subgroups will be an important step in characterizing cognition in ADHD and may enrich attempts to characterize ADHD subtypes based on cognitive features (e.g. Fair, Bathula, Nikolas, & Nigg, 2012).
Better characterizing cognition in ADHD will also help to clarify neural mechanisms of observed behavioral differences. Whereas traditional RT variables show broad patterns of association with structural and functional neuroimaging measures, initial evidence suggests that more specific associations can be achieved using diffusion parameters. In particular, drift rate has been associated with activation in the dorsolateral prefrontal cortex (DLPFC, Kühn, et al., 2011; Philiastides, Auksztulewicz, Heekeren, & Blankenburg, 2011) while the speed-accuracy trade-off parameter has been associated with activation in the basal ganglia (Forstmann et al., 2008). Initial evidence from direct single-cell recordings in primates (Kim & Shadlen, 1999), as well as functional imaging studies (Heekeren, Marrett, Bandettini, & Ungerleider, 2004; Heekeren, Marrett, Ruff, Bandettini, & Ungerleider, 2006) and transcranial magnetic stimulation studies in human(Philiastides, et al., 2011), suggests that the DLPFC is involved in decision-making independent of stimulus modality, and may be responsible for comparing inputs from modality-specific sensory neurons.
DLPFC structural and functional differences are also associated with ADHD and with performance on tasks demanding cognitive control (for reviews see Bush, Valera, & Seidman, 2005; Paloyelis, Mehta, Kuntsi, & Asherson, 2007). Given what is currently known about neural determinants of drift rate, we can now speculate that ADHD-related deficits in DLPFC functioning may interfere with the ability to efficiently compare evidence for and decide between response options. This, in turn, results in slow drift rates, which would influence any task requiring a decision between competing response options. Impairment in this general mechanism may also explain why children with ADHD are slow and variable even on many non-executive decision tasks, such as simple RT tasks (Kalff et al., 2005; Konrad, Gunther, Hanisch, & Herpertz-Dahlmann, 2004; Rommelse et al., 2008). Drift rate is therefore a promising target for new neuroimaging studies in ADHD, and if subgroups of children with specific deficits in regulating speed-accuracy trade-offs or non-decision processes can also be identified, this may eventually lead to characterization of neurobiologically-based ADHD subgroups.
Limitations
The present results should be considered in the context of possible limitations. Significant differences between the two cohorts were identified in task performance measures, including for standard deviation of RT and drift rate. These between-cohort difference could not be explained by sample differences in demographic characteristics, such as age, estimated IQ, or income, but instead likely reflect unmeasured sampling variation or undetected procedural variations across sites. Such variations are quite typical in ADHD research, so it is reassuring that effects essentially reproduced across these samples. At the same time, this suggests that estimation of the population effect of drift rate in ADHD will require additional samples. Nonetheless, the size of the between-group effects did not significantly differ between cohorts, and within each study the effect size for drift rate was similar to that for standard deviation of RT, suggesting that in both cohorts the diffusion model provided adequate and consistent mechanistic description of the group differences.
Although the diffusion model has numerous advantages over other models, it is not without limitations. In particular, although based on our data it is possible that between-group differences in RT variability may be explained by slow drift rates, there may also be other factors contributing to increased standard deviation of RT in ADHD that the diffusion model does not address. Theories of RT variability have generally interpreted RTs in the extended upper tails of the distribution as reflecting attention lapses, in which the child is temporarily disengaged from the decision process. The diffusion model is intended to characterize RTs that result from continuous engagement in the decision process. Attention lapses, in a diffusion model context, would be considered contaminant RTs in the distribution. Diffusion modeling is generally robust to these contaminant RTs (Ratcliff & Tuerlinckx, 2002), giving us confidence in the current finding that children with ADHD have slower drift rates than non-ADHD controls; however, for future studies it will be important to specifically characterize the role of attention lapses in creating the RT distribution, possibly via direct electrophysiological recording that can be time-locked to stimulus presentation or response.
The diffusion model has been applied successfully to a wide range of tasks including the stop task, with consistently good fit to the data (Ratcliff & McKoon, 2008). However, previous work in adults using the diffusion model suggests that individuals make strategic adjustments to their speed-accuracy trade-off settings on go trials to maximize their ability to inhibit responses on stop trials (Verbruggen & Logan, 2009). Similarly, the demands of the stop task may change how motor preparatory processes are carried out, impacting non-decision times. Thus, while the diffusion model may accurately produce parameter estimates when applied in the context of a stop task, it cannot answer questions about how the task context affects individual’s strategies for decision-making when applied to a single task. In the current sample, results did not change when we removed all trials immediately following a stop trial, supporting our interpretation of parameters estimates from this task; however, it will be critical for future studies of ADHD to apply the diffusion model in other choice RT paradigms and directly compare results across tasks with different cognitive demands.
Similarly, the diffusion model has been developed to explain single-step decision-making on tasks requiring fast, accurate responses. Although these types of decisions are critical in everyday life, it is not clear how group differences in diffusion parameters may be related to the decision-making required for multi-step problem solving, which is also impaired in ADHD. In order to understand the impact of slow/inefficient information processing speed in ADHD as captured by the drift rate parameter, future studies focused examining the relationships between deficits in rapid response decisions and multi-step, deliberative decision-making will be critical.
Conclusion
Overall, we present a mechanistic description of cognitive processing deficits in ADHD that highlights the role of slow/inefficient information processing speed. That, in turn, is conceptually related to neural signal:noise ratio. Based on current results, it is possible that a single mechanism could account for the apparent consistency of group differences in RT variability in ADHD, as well as for less consistently identified group differences in mean RT measures. Thus, only one parameter would be needed to address slow and variable RT in ADHD, simplifying neural and process models. Future work can address whether this is the case or whether additional mechanisms are required to fully explain group differences in RT speed and variability. Results also suggest a new phenotypic target for imaging analyses. Prior neuroscience studies lead us to infer that these results are consistent with ADHD-related findings in white matter integrity, as well as with functional imaging studies suggesting that children with ADHD showing an inability to suppress resting-state activation. Future work should consider using the drift rate parameter as a target in imaging, genetic, and diagnostic studies of ADHD.
References
- Achenbach TM, Rescorla LA. Manual for the aseba school-age forms & profiles. Burlington, VT: Research Center for Children, Youth, & Families; 1991. [Google Scholar]
- Balota DA, Yap MJ. Moving beyond the mean in studies of mental chronometry: The power of response time distributional analyses. Current Directions in Psychological Science. 2011;20(3):160–166. [Google Scholar]
- Banaschewski T, Yordanova J, Kolev V, Heinrich H, Albrecht B, Rothenberger A. Stimulus context and motor preparation in attention-deficit/hyperactivity disorder. Biological Psychology. 2008;77(1):53–62. doi: 10.1016/j.biopsycho.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Beck JM, Ma WJ, Kiani R, Hanks T, Churchland AK, Roitman J, et al. Probabilistic population codes for bayesian decision making. Neuron. 2008;60(6):1142–1152. doi: 10.1016/j.neuron.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: A review and suggested future directions. Biological Psychiatry. 2005;57(11):1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Buzy WM, Medoff DR, Schweitzer JB. Intra-individual variability among children with adhd on a working memory task: An ex-gaussian approach. Child Neuropsychology. 2009;15(5):441–459. doi: 10.1080/09297040802646991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Kelly C, Milham MP. The restless brain: Attention-deficit hyperactivity disorder, resting-state functional connectivity, and intrasubject variability. Canadian Journal of Psychiatry-Revue Canadienne De Psychiatrie. 2009;54(10):665–672. doi: 10.1177/070674370905401003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJS, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biological Psychiatry. 2005;57(11):1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nature Reviews Neuroscience. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners’ rating scales: Revised technical manual. New York, NY: Multi-Health Systems; 2003. [Google Scholar]
- Di Martino A, Ghaffari M, Curchack J, Reiss P, Hyde C, Vannucci M, et al. Decomposing intra-subject variability in children with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;64(7):607–614. doi: 10.1016/j.biopsych.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas VI. Cognitive control processes in attention-deficit/hyperactivity disorder. In: Quay HC, Hogan AE, editors. Handbook of disruptive behavior disorders. xiii. Dordrecht, Netherlands: Kluwer Academic Publishers; 1999. pp. 105–138. [Google Scholar]
- DuPaul G, Power T, Anastopoulos A, Reid R. Adhd rating scale—iv: Checklists, norms, and clinical interpretation. NY, NY: Guilford Press; 1998. [Google Scholar]
- Epstein JN, Langberg JM, Rosen PJ, Graham A, Narad ME, Antonini TN, et al. Evidence for higher reaction time variability for children with adhd on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology. 2011;25(4):427–441. doi: 10.1037/a0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D, Bathula D, Nikolas M, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with adhd. Proceedings of the National Academy of Sciences of the United States of America. 2012 doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann B, Dutilh G, Brown S, Neumann J, von Cramon D, Ridderinkhof K, et al. Striatum and pre-sma facilitate decision-making under time pressure. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(45):17538. doi: 10.1073/pnas.0805903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18(3):543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Grasman RPPP, Verté S, Oosterlaan J, Roeyers H, van Kammen SM, et al. Intra-individual variability in adhd, autism spectrum disorders and tourette’s syndrome. Neuropsychologia. 2008;46(13):3030–3041. doi: 10.1016/j.neuropsychologia.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Groot A, Leo MJdS, Stins J, Boomsma D. Familial influences on sustained attention and inhibition in preschoolers. Journal of Child Psychology and Psychiatry. 2004;45(2):306–314. doi: 10.1111/j.1469-7610.2004.00222.x. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychological Bulletin. 2006;132(4):560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG. A general mechanism for perceptual decision-making in the human brain. Nature. 2004;431:859–862. doi: 10.1038/nature02966. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Ruff DA, Bandettini PA, Ungerleider LG. Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10023–10028. doi: 10.1073/pnas.0603949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Ungerleider LG. The neural systems that mediate human perceptual decision making. Nature Reviews Neuroscience. 2008;9(6):467–479. doi: 10.1038/nrn2374. [DOI] [PubMed] [Google Scholar]
- Helps SK, Broyd SJ, Bitsakou P, Sonuga-Barke EJS. Identifying a distinctive familial frequency band in reaction time fluctuations in adhd. Neuropsychology. 2011;25(6):711–719. doi: 10.1037/a0024479. [DOI] [PubMed] [Google Scholar]
- Hervey AS, Epstein JN, Curry JF, Tonev S, Arnold LE, Conners CK, et al. Reaction time distribution analysis of neuropsychological performance in an adhd sample. Child Neuropsychology. 2006;12(2):125–140. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Karalunas SL, Tam H, Moore AN. Evaluating vigilance deficits in adhd: A meta-analysis of cpt performance. Journal of Abnormal Psychology. 2012;121(2):360–371. doi: 10.1037/a0027205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang-Pollock CL, Karalunas SL, Tam H, Moore AN. Evaluating vigilance deficits in adhd: A meta-analysis of cpt performance. Journal of Abnormal Psychology. doi: 10.1037/a0027205. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurks PPM, Adam JJ, Hendriksen JGM, Vles JSH, Feron FJM, Kalff AC, et al. Controlled visuomotor preparation deficits in attention-deficit/hyperactivity disorder. Neuropsychology. 2005;19(1):66–76. doi: 10.1037/0894-4105.19.1.66. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Barry E, Bellgrove MA, Cox M, Kelly SP, Dáibhis A, et al. Dissociation in response to methylphenidate on response variability in a group of medication naïve children with adhd. Neuropsychologia. 2008;46(5):1532–1541. doi: 10.1016/j.neuropsychologia.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Kelly SP, Bellgrove MA, Barry E, Cox M, Gill M, et al. Response variability in attention deficit hyperactivity disorder: Evidence for neuropsychological heterogeneity. Neuropsychologia. 2007a;45(4):630–638. doi: 10.1016/j.neuropsychologia.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Robertson IH, Kelly SP, Silk TJ, Barry E, Dáibhis A, et al. Dissociation in performance of children with adhd and high-functioning autism on a task of sustained attention. Neuropsychologia. 2007b;45(10):2234–2245. doi: 10.1016/j.neuropsychologia.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalff AC, De Sonneville LMJ, Hurks PPM, Hendriksen JGM, Kroes M, Feron FJM, et al. Speed, speed variability, and accuracy of information processing in 5 to 6-year-old children at risk of adhd. Journal of the International Neuropsychological Society. 2005;11(2):173–173–183. doi: 10.1017/s1355617705050216. [DOI] [PubMed] [Google Scholar]
- Karalunas SL, Huang-Pollock CL. Integrating evidence of slow rts and impaired executive functions using a diffusion model framework. Journal of Abnormal Child Psychology. doi: 10.1007/s10802-013-9715-2. (revise and resubmit) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalunas SL, Huang-Pollock CL, Nigg JT. Is reaction time variability in adhd mainly at low frequencies? Journal of Clinical Child Psychology. doi: 10.1111/jcpp.12028. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children–present and lifetime version (k-sads-pl): Initial reliability and validity. Pittsburgh: Department of Psychiatry, University of Pittsburgh School of Medicine; 1997. [DOI] [PubMed] [Google Scholar]
- Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nature Neuroscience. 1999;2:176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- Klimkeit EI, Mattingley JB, Sheppard DM, Lee P, Bradshaw JL. Motor preparation, motor execution, attention, and executive functions in attention deficit/hyperactivity disorder (adhd) Child Neuropsychology. 2005;11(2):153–173. doi: 10.1080/092970490911298. [DOI] [PubMed] [Google Scholar]
- Konrad K, Gunther T, Hanisch C, Herpertz-Dahlmann B. Differential effects of methylphenidate on attentional functions in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43(2):191–198. doi: 10.1097/00004583-200402000-00015. [DOI] [PubMed] [Google Scholar]
- Kühn S, Schmiedek F, Schott B, Ratcliff R, Heinze H-J, Düzel E, et al. Brain areas consistently linked to individual differences in perceptual decision-making in younger as well as older adults before and after training. Journal of Cognitive Neuroscience. 2011;23(9):2147–2158. doi: 10.1162/jocn.2010.21564. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Rogers H, Swinard G, Borger N, van der Meere J, Rijsdijk F, et al. Reaction time inhibition, working memory and ‘delay aversion’ performance: Genetic influences and their interpretation. Psychological Medicine. 2006;36(11):1613–1624. doi: 10.1017/S0033291706008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Stevenson J. Psychological mechanisms in hyperactivity: Ii the role of genetic factors. Journal of Child Psychology and Psychiatry. 2001;42(2):211–219. [PubMed] [Google Scholar]
- Lajoie G, Anderson V, Anderson P, Tucker AR, Robertson IH, Manly T. Effects of methylphenidate on attention skills in children with attention deficit/hyperactivity disorder. Brain Impairment. 2005;6(1):21–32. [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability and skew in the responding of adhd children: A response time distributional approach. Acta Psychologica. 2000;104(2):167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Logan G. On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In: Dagenbach D, Carr T, editors. Inhibitory processes in attention, memory, and language. San Diego, CA: Academic Press; 1994. pp. 189–239. [Google Scholar]
- Logan G, Schachar R, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8(1):60–64. [Google Scholar]
- Losier B, McGrath P, Klein R. Error patterns on the continuous performance test in non-medicated and medicated samples of children with and without adhd: A meta-analytic review. Journal of Child Psychology and Psychiatry. 1996;37(8):971–987. doi: 10.1111/j.1469-7610.1996.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Matzke D, Wagenmakers E-J. Psychological interpretation of the ex-gaussian and shifted wald parameters: A diffusion model analysis. Psychonomic Bulletin & Review. 2009;16(5):798–817. doi: 10.3758/PBR.16.5.798. [DOI] [PubMed] [Google Scholar]
- Mulder MJ, Bos D, Weusten JMH, van Belle J, van ZDijk SC, Simen P, et al. Basic impairments in regulating the speed-accuracy tradeoff predict symptoms of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2010;68(12):1114–1119. doi: 10.1016/j.biopsych.2010.07.031. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Bathula D, Herting M, Schmitt C, Kroenke CD, Fair D, et al. Altered white matter microstructure in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(3):283–292. doi: 10.1016/j.jaac.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. The adhd response-inhibition deficit as measured by the stop task: Replication with dsm-iv combined type, extension, and qualification. Journal of Abnormal Child Psychology. 1999;27(5):393–402. doi: 10.1023/a:1021980002473. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Kuntsi J, Asherson P. Functional mri in adhd: A systematic literature review. Expert Review of Neurotherapeutics. 2007;7(10):1337–1356. doi: 10.1586/14737175.7.10.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philiastides M, Auksztulewicz R, Heekeren H, Blankenburg F. Causal role of dorsolateral prefrontal cortex in human perceptual decision making. Current Biology. 2011;21(11):980–983. doi: 10.1016/j.cub.2011.04.034. [DOI] [PubMed] [Google Scholar]
- Philiastides MG, Ratcliff R, Sajda P. Neural representation of task difficulty and decision making during perceptual categorization: A timing diagram. The Journal of Neuroscience. 2006;26(35):8965–8975. doi: 10.1523/JNEUROSCI.1655-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philiastides MG, Sajda P. Temporal characterization of the neural correlates of perceptual decision making in the human brain. Cerebral Cortex. 2006;16(4):509–518. doi: 10.1093/cercor/bhi130. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. A diffusion model account of response time and accuracy in a brightness discrimination task: Fitting real data and failing to fit fake but plausible data. Psychonomic Bulletin & Review. 2002;9(2):278–291. doi: 10.3758/bf03196283. [DOI] [PubMed] [Google Scholar]
- Ratcliff R. Modeling response signal and response time data. Cognitive Psychology. 2006;53(3):195–237. doi: 10.1016/j.cogpsych.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Cherian A, Segraves M. A comparison of macaque behavior and superior colliculus neuronal activity to predictions from models of two-choice decisions. Journal of Neurophysiology. 2003;90(3):1392–1407. doi: 10.1152/jn.01049.2002. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G. The diffusion decision model: Theory and data for two-choice decision tasks. Neural Computation. 2008;20(4):873–922. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Philiastides MG, Sajda P. Quality of evidence for perceptual decision making is indexed by trial-to-trial variability of the eeg. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2009;106(16):6539–6544. doi: 10.1073/pnas.0812589106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Rouder JN. Modeling response times for two-choice decisions. Psychological Science. 1998;9(5):347–356. [Google Scholar]
- Ratcliff R, Thapar A, Gomez P, McKoon G. A diffusion model analysis of the effects of aging in the lexical-decision task. Psychology and Aging. 2004;19(2):278–289. doi: 10.1037/0882-7974.19.2.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff R, Tuerlinckx F. Estimating parameters of the diffusion model: Approaching to dealing with contaminant reaction and parameter variability. Psychonomic Bulletin & Review. 2002;9(3):438–481. doi: 10.3758/bf03196302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelse NN, Altink ME, Oosterlaan J, Beem L, Buschgens CJM, Buitelaar JK, et al. Speed, variability, and timing of motor output in adhd: Which measures are useful for endophenotypic research. Behavior Genetics. 2008;38(2):121–132. doi: 10.1007/s10519-007-9186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: Where top-down meets bottom-up. Brain Research Reviews. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Schachar R, Chen S, Logan G, Ornstein TJ, Crosbie J, Ickowicz A, et al. Evidence for an error monitoring deficit in attention deficit hyperactivity disorder. Journal of Abnormal Child Psychology. 2004;32(3):285–293. doi: 10.1023/b:jacp.0000026142.11217.f2. [DOI] [PubMed] [Google Scholar]
- Schmiedek F, Lövdén M, Lindenberger U. On the relation of mean reaction time and intraindividual reaction time variability. Psychology and Aging. 2009;24(4):841–857. doi: 10.1037/a0017799. [DOI] [PubMed] [Google Scholar]
- Schmiedek F, Oberauer K, Wilhelm O, Süß H-M, Wittmann WW. Individual differences in components of reaction time distributions and their relations to working memory and intelligence. Journal of Experimental Psychology: General. 2007;136(3):414–429. doi: 10.1037/0096-3445.136.3.414. [DOI] [PubMed] [Google Scholar]
- Sergeant J. The cognitive-energetic model: An empirical approach to attention-deficit hyperactivity disorder. Neuroscience and Biobehavioral Reviews. 2000;24(1):7–12. doi: 10.1016/s0149-7634(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Sergeant J, Oosterlaan J, van der Meere J. Information processing and energetic factors in attention deficit/hyperactivity disorder. In: Quay H, Hogan A, editors. Handbook of disruptive behavior disorders. NY: Kluwer Academic; 1999. [Google Scholar]
- Sergeant JA, Scholten CA. On data limitations in hyperactivity. Journal of Child Psychology and Psychiatry. 1985;26(1):111–124. doi: 10.1111/j.1469-7610.1985.tb01632.x. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Bayen UJ. Aging and conditional probability judgments: A global matching approach. Psychology and Aging. 2005;20(1):165–181. doi: 10.1037/0882-7974.20.1.165. [DOI] [PubMed] [Google Scholar]
- Steger J, Imhof K, Coutts E, Gundelfinger R, Steinhausen HC, Brandeis D. Attentional and neuromotor deficits in adhd. Developmental Medicine & Child Neurology. 2001;43(3):172–179. [PubMed] [Google Scholar]
- Stins J, Leo MJdS, Groot A, Polderman T, Caroline GCMvB, Boomsma D. Heritability of selective attention and working memory in preschoolers. Behavior Genetics. 2005;35(4):407–416. doi: 10.1007/s10519-004-3875-3. [DOI] [PubMed] [Google Scholar]
- Stins J, Van Baal GCM, Polderman T, Verhulst F, Boomsma D. Heritability of stroop and flanker performance in 12-year old children. BMC Neuroscience. 2004;5 doi: 10.1186/1471-2202-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Ratcliff R, McKoon G. A diffusion model analysis of the effects of aging on letter discrimination. Psychology and Aging. 2003;18(3):415–429. doi: 10.1037/0882-7974.18.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J, Tuerlinckx F. Fitting the ratcliff diffusion model to experimental data. Psychonomic Bulletin & Review. 2007;14(6):1011–1026. doi: 10.3758/bf03193087. [DOI] [PubMed] [Google Scholar]
- Vaurio RG, Simmonds DJ, Mostofsky SH. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47(12):2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan G, Liefooghe B, Vandierendonck A. Short-term aftereffects of response inhibition: Repetition priming or between-trial control adjustments. Journal of Experimental Psychology of Human Performance. 2008;34(2):413–426. doi: 10.1037/0096-1523.34.2.413. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Proactive adjustments of response strategies in the stop-signal paradigm. Journal of Experimental Psychology: Human Perception and Performance. 2009;35(3):835–854. doi: 10.1037/a0012726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss A, Rothermund K, Voss J. Interpreting the parameters of the diffusion model: An empirical validation. Memory & Cognition. 2004;32(7):1206–1220. doi: 10.3758/bf03196893. [DOI] [PubMed] [Google Scholar]
- Voss A, Voss J. Fast-dm: A free program for efficient diffusion model analysis. Behavior Research Methods. 2007;39(4):767–775. doi: 10.3758/bf03192967. [DOI] [PubMed] [Google Scholar]
- Wagenmakers E-J, Grasman RPPP, Molenaar PCM. On the relation between the mean and the variance of a diffusion model response time distribution. Journal of Mathematical Psychology. 2005;49 [Google Scholar]
- Wagenmakers E-J, van der Maas HLJ, Grasman RPPP. An ez-diffusion model for response time and accuracy. Psychonomic Bulletin & Review. 2007;14(1):3–22. doi: 10.3758/bf03194023. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children, 4th ed (wisc-iv) technical and interpretive manual. San Antonio: Harcourt Brace; 2003. [Google Scholar]
- Wood AC, Asherson P, van der Meere JJ, Kuntsi J. Separation of genetic influences on attention deficit hyperactivity disorder symptoms and reaction time performance from those on iq. Psychological Medicine: A Journal of Research in Psychiatry and the Allied Sciences. 2010;40(6):1027–1037. doi: 10.1017/S003329170999119X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Barch DM, Gray JR, Conturo TE, Braver TS. Bold correlates of trial-by-trial reaction time variability in gray and white matter: A multi-study fmri analysis. PLoS ONE. 2009;4(1):e4257. doi: 10.1371/journal.pone.0004257. [DOI] [PMC free article] [PubMed] [Google Scholar]