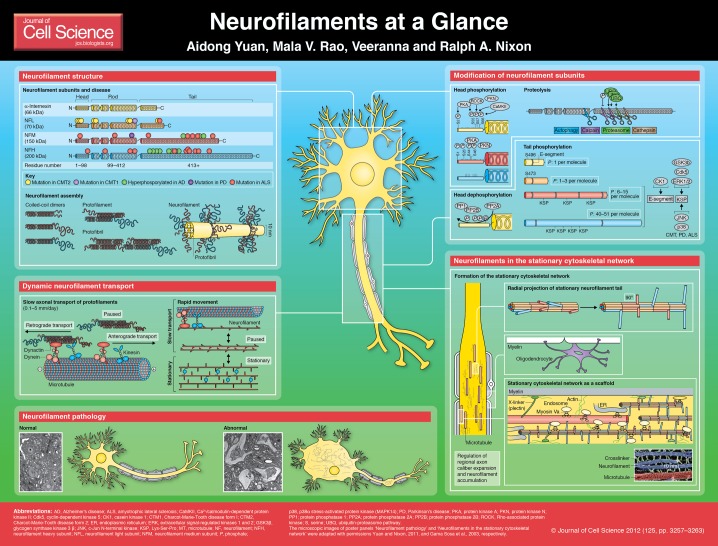
Neurofilaments (NFs) are intermediate filaments with a diameter of 10 nm, similar to that of neurons. Although they are present in perikarya and dendrites, neurofilaments are particularly abundant in axons, where they are essential for the radial growth of axons during development, the maintenance of axon caliber and the transmission of electrical impulses along axons, i.e. velocity of nerve conduction (Eyer and Peterson, 1994; Friede and Samorajski, 1970; Ohara et al., 1993; Yum et al., 2009; Zhu et al., 1997). In some pathological conditions, neurofilaments can accumulate in large numbers within cell bodies and proximal axons of affected neurons (Liu et al., 2009; Munoz et al., 1988). In patients with amyotrophic lateral sclerosis (ALS), these accumulations are a hallmark pathological lesion, but they are also prominent in sufferers of other neurological diseases, such as Charcot-Marie-Tooth (CMT) disease, neurofilament inclusion disease (NFID), giant axonal neuropathy (GAN), diabetic neuropathy, spinal muscular atrophy (SMA) and spastic paraplegia, and are present in those people that suffer from Alzheimer’s disease (AD) and Parkinson’s disease (PD) (Abe et al., 2009; Perrot and Eyer, 2009; Szaro and Strong, 2010). Transgenic mouse models support the idea that these aberrant NF accumulations contribute to the death of the affected neurons, rather than simply being by-products of the pathogenic process (Côté et al., 1993; Couillard-Després et al., 1998; Williamson et al., 1998). In this Cell Science at a Glance article, we review the current understanding of neurofilament functions in health and disease.
Neurofilament structure and function
Neurofilaments from the central nervous system (CNS) are heteropolymers that are composed of four subunits, namely neurofilament heavy, medium and light polypeptides (NFH, NFM and NFL, respectively; also known as NEFH, NEFM and NEFL), as well as α-internexin (Int), whereas in the peripheral nervous system, neurofilaments are made up of NFH, NFM, NFL and peripherin (Beaulieu et al., 1999; Yan et al., 2007; Yuan et al., 2006b). Neurons may also express other intermediate filament proteins, including nestin, synemin, syncoilin and vimentin (Perrin et al., 2005). Mouse NFH, NFM and NFL subunits are unable to self-assemble into homopolymer filaments, although – at least in vitro – human NFL can do so (Carter et al., 1998). Among the notable properties of NFs are their exceptionally long half-lives (Millecamps et al., 2007; Nixon and Logvinenko, 1986; Yuan et al., 2009) and their elastic fibrous properties that enable them to maintain the markedly asymmetrical shape of neurons (Wagner et al., 2007). Neurofilaments are required for axon radial growth (Eyer and Peterson, 1994; Hoffman et al., 1987) and the NFL and NFM subunits are especially important (Elder et al., 1998; Ohara et al., 1993; Zhu et al., 1997). Although deletion of the phosphorylated tail domain of NFM inhibits radial growth of axons and reduces their conduction velocities (Garcia et al., 2003; Rao et al., 2003), mice expressing NFM subunits that lack phosphorylation at KSP (Lys-Ser-Pro) sites along the tail domain have normal axon calibers and conduction velocities (Garcia et al., 2009), indicating that the NFM tail domain but not tail phosphorylation is crucial for axon radial growth. Decreased axon caliber that is accompanied by reduced conduction velocity has been observed in mutant Japanese quail that lack NFs because of a nonsense mutation in the NFL gene (Sakaguchi et al., 1993), in transgenic mice expressing a NFH–β-galactosidase fusion protein that interferes with NF transport into axons (Perrot et al., 2007), and in knockout mice that lack Nefl or Nefm (Križ et al., 2000). However, normal axonal caliber but decreased conduction velocity is observed in Nefh-null mice, indicating that NFs have roles beyond being determinants of the physical dimensions of axons (Križ et al., 2000).
A number of specific roles have been identified for the protein domains in each NF subunit, which confer to the heteropolymer (see poster panel, NF assembly) the general properties of a scaffold for the docking and organization of different axoplasmic constituents (Balastik et al., 2008; Kim et al., 2011; Rao et al., 2011). NF subunits contain a globular head, an α-helical rod domain, and variable tail domains that differ in length and amino acid composition. Each neurofilament subunit contains a highly conserved 310 amino acid rod domain that is important for the co-assembly with other NF subunits to form filaments. The head domains of all NFs have a microtubule (MT) polymerization inhibitory domain that regulates the number of MTs in the axon (Bocquet et al., 2009). The rod domains have important roles in the polymerization of NF subunits into NFs, and serve as a binding site for the myosin Va motor protein, which modulates levels and local topography of specific vesicular organelles (ER, endosomes, synaptic vesicles) within the axoplasm (Rao et al., 2011). Loss of NFL or myosin Va partially depletes these organelles from axons (Rao et al., 2011). The C-terminal domains of both NFH and NFM form fine lateral extensions that increase the spacing between NFs, thereby maximizing their ability to occupy space during axon caliber expansion. The C-terminal domain of NFM is more important for radial growth of axons than that of NFH (Rao et al., 2003; Rao et al., 2002). Crossbridging between neurofilaments through the tail domains of NFH and NFM is believed to be influenced by the phosphorylation level of these tails (Eyer and Leterrier, 1988; Gou et al., 1998) and mediated by divalent cations (Kushkuley et al., 2009; Kushkuley et al., 2010).
Interestingly, a splice variant of the N-methyl-D-aspartate (NMDA) receptor subunit NR1 associates with NFL (Ehlers et al., 1998), and the dopamine D1 receptor selectively associates with the C-terminus of NFM (Kim et al., 2002), although the functional significance of these intriguing interactions is not yet clear. In humans, mutations in NFL are associated with Charcot-Marie-Tooth disease. These mutations affect the assembly of NFs in the neurons (Sasaki et al., 2006) and, upon that, can inhibit NF transport (Yates et al., 2009). Mutations of the NFM rod domain have occasionally been identified in early-onset PD (Lavedan et al., 2002). Mutations in the NFH gene have been identified in a small number of sporadic ALS patients (Al-Chalabi and Miller, 2003).
Neurofilament transport
Most neurofilament proteins are synthesized within the cell body and must travel long distances along axons to reach their sites of function. The mechanisms that underly axonal transport of neurofilament proteins are elusive, although recent genetic and live-cell imaging approaches have yielded general principles regarding the dynamic behaviors of neurofilaments (Roy et al., 2000; Wang et al., 2000; Yabe et al., 1999; Yuan et al., 2003; Yuan et al., 2006b; Yuan et al., 2009). For example, by transfecting green fluorescent protein (GFP)-labeled NF subunits into developing sympathetic neurons in culture, Brown and colleagues were able to directly visualize movements of individual short filaments (1.0–15.8 µm in length) along the relatively NF-poor growing axons (Trivedi et al., 2007; Wang et al., 2000). Studies in mice that lack one or more NF subunit genes have shown, however, that transport of NF subunits does not require the formation of complete neurofilaments (Yuan et al., 2003; Yuan et al., 2006b). For instance, we have demonstrated that the number of neurofilaments in optic axons of NFH-NFL double knockout mice is less than 10% of the usual number of neurofilaments, yet these mice have 50% of the usual level of NFM subunits, which move along axons at typical transport rates (Yuan et al., 2003).
Further studies with different combinations of NF subunit deletions have shown that the minimal requirement for axonal transport is the formation of heterodimers that involve specific NF subunits. NFM and α-internexin have been identified as the subunits that are crucial for the transport of dimers or NFs, as deleting both prevents transport of NFL and/or NFH, whereas deleting either NFH, NFL or both, only minimally alters NFM or α-internexin transport in optic axons (Yuan et al., 2006a; Yuan et al., 2003; Yuan et al., 2006b). The early presence of α-internexin in developing rat optic axons explains why NFM and NFL subunits are detectable in these axons before morphologically definable neurofilaments appear, further indicating its crucial role for neurofilament partnership and transport (Pachter and Liem, 1984). The state of assembly of neurofilament proteins during axonal transport became an active area of investigation and debate during the past three decades, with considerably indirect evidence being amassed to support movement of polymers or subunit or oligomer assemblies (Baas and Brown, 1997; Hirokawa et al., 1997). Movement of fluorescent puncta that represent non-filamentous assemblies of GFP-labeled NF subunits have been reported (Prahlad et al., 2000; Yabe et al., 1999); however, short neurofilaments have also been observed in other types of cultured cells and in the squid giant axon, depending on the methods used (Ackerley et al., 2003; Galbraith et al., 1999; Roy et al., 2000; Yan and Brown, 2005). Transport of both non-filamentous NF oligomers and filaments has also been reported in neuroblastoma cells (Yabe et al., 2001), as well as movement of vimentin non-filamentous particles and filaments in spreading baby hamster kidney cells (Prahlad et al., 1998). Our recent photobleaching analyses of GFP-tagged NFL protein in cultured cortical neurons further reconcile these, apparently conflicting, observations by demonstrating that both non-filamentous NF subunit assemblies and short NF polymers can be transported in the same axon (Yuan et al., 2009). At proximal axonal levels in these neurons, the transport of subunit assemblies predominates, whereas the transport of short NFs predominates at distal levels of the same axon. Collectively, these observations establish that NF proteins might exist in multiple assembly forms during axonal transport and suggest that the transported NF subunits assemble into filaments during axonal transport (Yuan et al., 2009).
The molecular motors that regulate NF transport are believed to be the fast microtubule-based motors kinesin and dynein (Prahlad et al., 2000; Sunil et al., 2012), and microfilament-based motor myosin Va (Alami et al., 2009). Kinesin-I has been proposed to be an anterograde motor for NF because it interacts with the NFH or NFM subunits (Jung et al., 2005; Yabe et al., 2000) and because antibody against kinesin-I blocks NF transport (Yabe et al., 1999). However, later studies have found that NFH is dispensable for NF transport (Rao et al., 2002; Yuan et al., 2006a; Yuan et al., 2006b), implying that, if kinesin-I were the NF transport motor, it would have additional NF subunit partners. Studies from kinesin-IA (an isoform of kinesin-I) knockout mice (Xia et al., 2003) and analyses of the effect of kinesin-I mutation on NF transport in cultured neurons (Wang and Brown, 2010) suggest that it acts as an anterograde motor for NF transport. The dynein-dynactin complex is believed to be the retrograde motor for axonal transport of NF because the dynein–dynactin complex co-purifies with NF (Shah et al., 2000) and dynein interacts with the rod domain of the NFM subunit in yeast two hybrid assays (Wagner et al., 2004). Knockdown of expression of the dynein heavy chain using small interference RNA (siRNA) has been shown to significantly decrease retrograde NF transport (He et al., 2005; Uchida et al., 2009). Despite these advances, the interaction between NFs and motors has not yet been directly visualized by live-cell imaging in cultured neurons or in live animals.
The earliest in vivo pulse-labeling studies of neurofilament protein transport by Lasek and colleagues were initially interpreted to support the idea that the labeled neurofilaments that undergo transport constitute the entire NF cytoskeleton within axons, which, according to this model, is continuously moving (Lasek et al., 1984; Lasek et al., 1992). A different model, however, was proposed by the authors on the basis of studies of short and exceptionally long-term (6 months) pulse-labeling by using the mouse optic system (Nixon and Logvinenko, 1986). These studies supported the idea that neurofilaments (or oligomeric assemblies) that undergo slow transport in myelinated axons are a small precursor pool that maintains a large pre-existing fixed NF lattice. This lattice, in turn, exists within a complex stationary cytoskeletal network that is composed of the various cytoskeletal elements (NF, microtubules, actin filaments) that are visible in ultrastructural images of the cytoskeleton. Later studies added support to this general model (Millecamps et al., 2007; Yuan et al., 2009), including an in vivo study of NF turnover by Julien and colleagues, in which NFL expression was acutely shut off in a conditional NFL knockout mouse, followed by measuring the fate of the pre-existing neurofilaments (Millecamps et al., 2007). This study demonstrates that pre-existing neurofilaments in the axon remain stationary and display an exceptionally slow turnover (>2.5 months) – much longer than could be explained by loss through axonal transport. On the basis of this current evidence (Millecamps et al., 2007; Yuan et al., 2009), the neurofilament network is now viewed as a large stationary and metabolically stable structure that is assembled from transported elements (Yuan et al., 2009). These elements can either be short polymers or oligomers of NF subunits. The proportions of these different assembly forms might vary depending on cell type and developmental state (Nixon and Shea, 1992; Yabe et al., 2001). Regardless of the assembly form that neurofilament proteins take during transport (i.e. dimer, oligomer or short filament), they subsequently undergo additional steps of integration into a stationary neurofilament network, which involves regulatory events that still remain to be fully explored (Yuan et al., 2003; Yuan et al., 2009).
Neurofilament phosphorylation
Neurofilaments undergo various post-translational modifications, such as phosphorylation, glycosylation, nitration, oxidation and ubiquitylation (Perrot et al., 2008). The head domains of NFL, NFM, NFH and α-internexin are phosphorylated and glycosylated (Dong et al., 1996; Manser et al., 2008; Tanaka et al., 1993; Vosseller et al., 2006). Phosphorylation of the NF head domain is mediated by the second messenger dependent kinases protein kinase A (PKA) and C (PKC) (Sihag et al., 1988; Sihag and Nixon, 1989; Sihag and Nixon, 1990) and possibly also by Cam kinase II (Hashimoto et al., 2000). The occurrence of NF head phosphorylation in the cell body soon after subunit synthesis reflects its suggested role in maintaining the disassembled state of NFs, as head phosphorylation of NFL inhibits NF assembly (Hashimoto et al., 2000; Hisanaga et al., 1990; Sihag et al., 1999; Sihag and Nixon, 1991). Phosphorylation of the NF head domain is also known to modulate their interaction with fodrin, an important protein of the sub-axolemmal cytoskeleton (Frappier et al., 1987). However, as neurofilaments are transported along axons, many of the initially incorporated phosphate groups are removed (Nixon and Lewis, 1986). The assembly of neurofilaments prior to their entry into the axon and the rapid turnover of phosphates during their transit along the axon requires dephosphorylation of the head domain, which is effected by protein phosphatase 2A (PP2A) (Nixon and Lewis, 1986; Saito et al., 1995).
The C-terminal domains of NFM and NFH have multiple lysine-serine-proline (KSP) sites, the phosphorylation status of which is regulated by multiple protein kinases and phosphatases (Veeranna et al., 2011). Soon after the NFs enter and move along the axon they are extensively phosphorylated on the tail domains of NFM and NFH (Julien and Mushynski, 1983; Nixon and Lewis, 1986; Nixon et al., 1989; Nixon et al., 1987). Phosphorylation of the tail domain is specific for particular regions within the cell, with non-phosphorylated tails predominately found in the cell bodies, which become highly phosphorylated in mature axons (see poster panel Modification of neurofilaments: NF head phosphorylation, Tail phosphorylation). This developmentally regulated phosphorylation appears to depend on myelination and synaptogenesis (Carden et al., 1987; Sánchez et al., 2000). Although the dendrites also harbor NFs, these filaments are, for unknown reasons, not highly phosphorylated (Lee et al., 1987; Sternberger and Sternberger, 1983). Most phosphorylation of the tail domain occurs on KSP repeats (Geisler et al., 1987; Jaffe et al., 1998; Lee et al., 1988; Xu et al., 1992), although non-SP sites are also phosphorylated. KSPxK motifs are phosphorylated by proline-directed cyclin-dependent kinase 5 (Cdk5) (Shetty et al., 1993; Sun et al., 1996; Veeranna et al., 1995). Both KSPxK and KSPxxxK sites are phosphorylated by mitogen-activated protein kinases (MAPKs), such as extracellular-signal-regulated kinases (Erks) (Veeranna et al., 1998), c-Jun N-terminal kinases (JNKs) (Giasson and Mushynski, 1997) and p38 (MAPK14) (Ackerley et al., 2004), which constitute signaling cascades that respond to growth factors (Li et al., 1999b; Pearson et al., 2001), Ca2+ influx (Li et al., 1999a), integrins (Li et al., 2001) and myelination (Nixon et al., 1994). Myelination has an effect on NF phosphorylation. This is evidenced by the observations that NF phosphorylation is decreased in dysmyelinating mutant Trembler mice (de Waegh et al., 1992) and at the initial segment of optic nerves and nodes of Ranvier (the gaps formed between the myelin sheaths generated by different cells) (Hsieh et al., 1994; Mata et al., 1992; Reles and Friede, 1991), as well as by the possible role of myelin associated glycoprotein (MAG) as a mediator of myelin signals that alter NF phosphorylation (Dashiell et al., 2002; Yin et al., 1998). Phosphorylation of the tail domain can regulate both the interactions between the NF domains themselves and with microtubules (Hisanaga and Hirokawa, 1989; Hisanaga et al., 1991). Tail phosphorylation is also the crucial modification that confers their exceptional proteolysis resistance to NFs (Pant, 1988). Although the activity of NF kinases decreases during maturation and aging (Veeranna et al., 2011), neurofilament tail domains become increasingly phosphorylated mainly owing to decreased activities of PP2A and protein phosphatase (PP1). However, PP1 has only a minor role in the regulation of NF phosphorylation compared with that of PP2A (Strack et al., 1997). Aberrant phosphorylation of NFs leads to their accumulation in cell bodies and has been observed in the brains of AD patients and those suffering from other neurodegenerative disorders (Rudrabhatla et al., 2011). The abnormal phosphorylation of NFs in AD patients has been attributed to a decrease in the levels of PP2A and PP1 (Gong et al., 2005; Gong et al., 1995; Gong et al., 1993), and to elevated levels of NF kinases, including Cdk5, ERK1 and ERK2 (Veeranna et al., 2004), and JNKs (Zhu et al., 2001). These observations are further supported by mass spectrometric analyses of NFM and NFH in brains of AD patients, in which phosphorylation of KSP repeats was increased approximately four- to eightfold compared with the phosphorylation of these sites in brains of control patients (previously documented) (see Rudrabhatla et al., 2011). Also in the brains of AD patients, Deng and colleagues observed a reciprocal relationship between O-GlcNAcylation and phosphorylation of NFM, in that a decreased O-GlcNAcylation of NF due to lower glucose uptake in AD patients is accompanied with increased KSP phosphorylation of NFM (Deng et al., 2008). The mechanisms underlying the topographic regulation of phosphorylation of NFs (phosphorylation in axons and cell bodies is modulated by different phosphatases and kinases associated with compartment-specific multimeric complexes), the identification of NF-interacting proteins and the role of phosphorylation in guiding those interactions remains a challenge for future investigations.
Perspectives
Although the studies reviewed here have increased our understanding of neurofilament biology and pathophysiology, many unanswered questions remain. NFM is important for axonal transport of NFs in vivo and for regulating their transport rates; however, it is not known which domain(s) of NFM regulate the transport kinetics of NFs. Another remaining question is how formation and stabilization of the stationary NF network are affected by the phosphorylation of NFM heads and tails. How do kinesin and dynein–dynactin interact with NFM for slow NF transport? The functional significance of the interactions between specific NF subunits and neurotransmitter receptors in vivo is also not entirely clear. Finally, how do NF mutations that have been linked to neurodegenerative diseases affect NF function? For example – as yet – little is known about the mechanisms that regulate protein turnover of NF subunits, and the relevance of NF turnover for aging and neurodegenerative diseases. With this in mind, a deeper understanding of neurofilament function and dysfunction in health and disease is clearly desired.
Supplementary Material
Acknowledgments
Owing to space limitations, we were unable to cite many worthy additional contributions. We thank Corrine Peterhoff for assistance with the poster. The microscopic images of poster panels ‘Neurofilament pathology’ and ‘Neurofilaments in the stationary cytoskeletal network’ are adapted with permissions from Yuan and Nixon, and Gama Sosa et al., respectively (Yuan and Nixon, 2011) and (Gama Sosa et al., 2003).
Footnotes
Funding
This work was supported by a National Institute on Aging Grant [grant number AG05604 to R.A.N.]. Deposited in PMC for release after 12 months.
A high-resolution version of the poster is available for downloading in the online version of this article at jcs.biologists.org. Individual poster panels are available as JPEG files at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.104729/-/DC1.
References
- Abe A., Numakura C., Saito K., Koide H., Oka N., Honma A., Kishikawa Y., Hayasaka K. (2009). Neurofilament light chain polypeptide gene mutations in Charcot-Marie-Tooth disease: nonsense mutation probably causes a recessive phenotype. J. Hum. Genet. 54, 94–97 10.1038/jhg.2008.13 [DOI] [PubMed] [Google Scholar]
- Ackerley S., Thornhill P., Grierson A. J., Brownlees J., Anderton B. H., Leigh P. N., Shaw C. E., Miller C. C. (2003). Neurofilament heavy chain side arm phosphorylation regulates axonal transport of neurofilaments. J. Cell Biol. 161, 489–495 10.1083/jcb.200303138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerley S., Grierson A. J., Banner S., Perkinton M. S., Brownlees J., Byers H. L., Ward M., Thornhill P., Hussain K., Waby J. S., et al. (2004). p38alpha stress-activated protein kinase phosphorylates neurofilaments and is associated with neurofilament pathology in amyotrophic lateral sclerosis. Mol. Cell. Neurosci. 26, 354–364 10.1016/j.mcn.2004.02.009 [DOI] [PubMed] [Google Scholar]
- Al–Chalabi A., Miller C. C. (2003). Neurofilaments and neurological disease. Bioessays 25, 346–355 10.1002/bies.10251 [DOI] [PubMed] [Google Scholar]
- Alami N. H., Jung P., Brown A. (2009). Myosin Va increases the efficiency of neurofilament transport by decreasing the duration of long-term pauses. J. Neurosci. 29, 6625–6634 10.1523/JNEUROSCI.3829-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. W., Brown A. (1997). Slow axonal transport: the polymer transport model. Trends Cell Biol. 7, 380–384 10.1016/S0962-8924(97)01148-3 [DOI] [PubMed] [Google Scholar]
- Balastik M., Ferraguti F., Pires–da Silva A., Lee T. H., Alvarez–Bolado G., Lu K. P., Gruss P. (2008). Deficiency in ubiquitin ligase TRIM2 causes accumulation of neurofilament light chain and neurodegeneration. Proc. Natl. Acad. Sci. USA 105, 12016–12021 10.1073/pnas.0802261105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J. M., Robertson J., Julien J. P. (1999). Interactions between peripherin and neurofilaments in cultured cells: disruption of peripherin assembly by the NF-M and NF-H subunits. Biochem. Cell Biol. 77, 41–45 10.1139/o99-003 [DOI] [PubMed] [Google Scholar]
- Bocquet A., Berges R., Frank R., Robert P., Peterson A. C., Eyer J. (2009). Neurofilaments bind tubulin and modulate its polymerization. J. Neurosci. 29, 11043–11054 10.1523/JNEUROSCI.1924-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden M. J., Trojanowski J. Q., Schlaepfer W. W., Lee V. M. (1987). Two-stage expression of neurofilament polypeptides during rat neurogenesis with early establishment of adult phosphorylation patterns. J. Neurosci. 7, 3489–3504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J., Gragerov A., Konvicka K., Elder G., Weinstein H., Lazzarini R. A. (1998). Neurofilament (NF) assembly; divergent characteristics of human and rodent NF-L subunits. J. Biol. Chem. 273, 5101–5108 10.1074/jbc.273.9.5101 [DOI] [PubMed] [Google Scholar]
- Côté F., Collard J. F., Julien J. P. (1993). Progressive neuronopathy in transgenic mice expressing the human neurofilament heavy gene: a mouse model of amyotrophic lateral sclerosis. Cell 73, 35–46 10.1016/0092-8674(93)90158-M [DOI] [PubMed] [Google Scholar]
- Couillard–Després S., Zhu Q., Wong P. C., Price D. L., Cleveland D. W., Julien J. P. (1998). Protective effect of neurofilament heavy gene overexpression in motor neuron disease induced by mutant superoxide dismutase. Proc. Natl. Acad. Sci. USA 95, 9626–9630 10.1073/pnas.95.16.9626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashiell S. M., Tanner S. L., Pant H. C., Quarles R. H. (2002). Myelin-associated glycoprotein modulates expression and phosphorylation of neuronal cytoskeletal elements and their associated kinases. J. Neurochem. 81, 1263–1272 10.1046/j.1471-4159.2002.00927.x [DOI] [PubMed] [Google Scholar]
- de Waegh S. M., Lee V. M., Brady S. T. (1992). Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell 68, 451–463 10.1016/0092-8674(92)90183-D [DOI] [PubMed] [Google Scholar]
- Deng Y., Li B., Liu F., Iqbal K., Grundke–Iqbal I., Brandt R., Gong C. X. (2008). Regulation between O-GlcNAcylation and phosphorylation of neurofilament-M and their dysregulation in Alzheimer disease. FASEB J. 22, 138–145 10.1096/fj.07-8309com [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D. L-Y., Xu Z-S., Hart G. W., Cleveland D. W. (1996). Cytoplasmic O-GlcNAc modification of the head domain and the KSP repeat motif of the neurofilament protein neurofilament-H. J. Biol. Chem. 271, 20845–20852 10.1074/jbc.271.34.20845 [DOI] [PubMed] [Google Scholar]
- Ehlers M. D., Fung E. T., O’Brien R. J., Huganir R. L. (1998). Splice variant-specific interaction of the NMDA receptor subunit NR1 with neuronal intermediate filaments. J. Neurosci. 18, 720–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder G. A., Friedrich V. L., Jr, Bosco P., Kang C., Gourov A., Tu P. H., Lee V. M., Lazzarini R. A. (1998). Absence of the mid-sized neurofilament subunit decreases axonal calibers, levels of light neurofilament (NF-L), and neurofilament content. J. Cell Biol. 141, 727–739 10.1083/jcb.141.3.727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyer J., Leterrier J. F. (1988). Influence of the phosphorylation state of neurofilament proteins on the interactions between purified filaments in vitro. Biochem. J. 252, 655–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyer J., Peterson A. (1994). Neurofilament-deficient axons and perikaryal aggregates in viable transgenic mice expressing a neurofilament-beta-galactosidase fusion protein. Neuron 12, 389–405 10.1016/0896-6273(94)90280-1 [DOI] [PubMed] [Google Scholar]
- Frappier T., Regnouf F., Pradel L. A. (1987). Binding of brain spectrin to the 70-kDa neurofilament subunit protein. Eur. J. Biochem. 169, 651–657 10.1111/j.1432-1033.1987.tb13657.x [DOI] [PubMed] [Google Scholar]
- Friede R. L., Samorajski T. (1970). Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat. Rec. 167, 379–387 10.1002/ar.1091670402 [DOI] [PubMed] [Google Scholar]
- Galbraith J. A., Reese T. S., Schlief M. L., Gallant P. E. (1999). Slow transport of unpolymerized tubulin and polymerized neurofilament in the squid giant axon. Proc. Natl. Acad. Sci. USA 96, 11589–11594 10.1073/pnas.96.20.11589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama Sosa M. A., Friedrich V. L., Jr, DeGasperi R., Kelley K., Wen P. H., Senturk E., Lazzarini R. A., Elder G. A. (2003). Human midsized neurofilament subunit induces motor neuron disease in transgenic mice. Exp. Neurol. 184, 408–419 10.1016/S0014-4886(03)00206-1 [DOI] [PubMed] [Google Scholar]
- Garcia M. L., Lobsiger C. S., Shah S. B., Deerinck T. J., Crum J., Young D., Ward C. M., Crawford T. O., Gotow T., Uchiyama Y., et al. (2003). NF-M is an essential target for the myelin-directed “outside-in” signaling cascade that mediates radial axonal growth. J. Cell Biol. 163, 1011–1020 10.1083/jcb.200308159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M. L., Rao M. V., Fujimoto J., Garcia V. B., Shah S. B., Crum J., Gotow T., Uchiyama Y., Ellisman M., Calcutt N. A., et al. (2009). Phosphorylation of highly conserved neurofilament medium KSP repeats is not required for myelin-dependent radial axonal growth. J. Neurosci. 29, 1277–1284 10.1523/JNEUROSCI.3765-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler N., Vandekerckhove J., Weber K. (1987). Location and sequence characterization of the major phosphorylation sites of the high molecular mass neurofilament proteins M and H. FEBS Lett. 221, 403–407 10.1016/0014-5793(87)80964-X [DOI] [PubMed] [Google Scholar]
- Giasson B. I., Mushynski W. E. (1997). Study of proline-directed protein kinases involved in phosphorylation of the heavy neurofilament subunit. J. Neurosci. 17, 9466–9472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C. X., Singh T. J., Grundke–Iqbal I., Iqbal K. (1993). Phosphoprotein phosphatase activities in Alzheimer disease brain. J. Neurochem. 61, 921–927 10.1111/j.1471-4159.1993.tb03603.x [DOI] [PubMed] [Google Scholar]
- Gong C. X., Shaikh S., Wang J. Z., Zaidi T., Grundke–Iqbal I., Iqbal K. (1995). Phosphatase activity toward abnormally phosphorylated tau: decrease in Alzheimer disease brain. J. Neurochem. 65, 732–738 10.1046/j.1471-4159.1995.65020732.x [DOI] [PubMed] [Google Scholar]
- Gong C. X., Liu F., Grundke–Iqbal I., Iqbal K. (2005). Post-translational modifications of tau protein in Alzheimer’s disease. J. Neural Transm. 112, 813–838 10.1007/s00702-004-0221-0 [DOI] [PubMed] [Google Scholar]
- Gou J. P., Gotow T., Janmey P. A., Leterrier J. F. (1998). Regulation of neurofilament interactions in vitro by natural and synthetic polypeptides sharing Lys-Ser-Pro sequences with the heavy neurofilament subunit NF-H: neurofilament crossbridging by antiparallel sidearm overlapping. Med. Biol. Eng. Comput. 36, 371–387 10.1007/BF02522486 [DOI] [PubMed] [Google Scholar]
- Hashimoto R., Nakamura Y., Komai S., Kashiwagi Y., Tamura K., Goto T., Aimoto S., Kaibuchi K., Shiosaka S., Takeda M. (2000). Site-specific phosphorylation of neurofilament-L is mediated by calcium/calmodulin-dependent protein kinase II in the apical dendrites during long-term potentiation. J. Neurochem. 75, 373–382 10.1046/j.1471-4159.2000.0750373.x [DOI] [PubMed] [Google Scholar]
- He Y., Francis F., Myers K. A., Yu W., Black M. M., Baas P. W. (2005). Role of cytoplasmic dynein in the axonal transport of microtubules and neurofilaments. J. Cell Biol. 168, 697–703 10.1083/jcb.200407191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Funakoshi S. T., Tekeda S. (1997). Slow axonal transport: the subunit transport model. Trends Cell Biol. 7, 384–388 10.1016/S0962-8924(97)01133-1 [DOI] [PubMed] [Google Scholar]
- Hisanaga S., Hirokawa N. (1989). The effects of dephosphorylation on the structure of the projections of neurofilament. J. Neurosci. 9, 959–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisanaga S., Gonda Y., Inagaki M., Ikai A., Hirokawa N. (1990). Effects of phosphorylation of the neurofilament L protein on filamentous structures. Cell Regul. 1, 237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisanaga S., Kusubata M., Okumura E., Kishimoto T. (1991). Phosphorylation of neurofilament H subunit at the tail domain by CDC2 kinase dissociates the association to microtubules. J. Biol. Chem. 266, 21798–21803 [PubMed] [Google Scholar]
- Hoffman P. N., Cleveland D. W., Griffin J. W., Landes P. W., Cowan N. J., Price D. L. (1987). Neurofilament gene expression: a major determinant of axonal caliber. Proc. Natl. Acad. Sci. USA 84, 3472–3476 10.1073/pnas.84.10.3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S. T., Kidd G. J., Crawford T. O., Xu Z., Lin W. M., Trapp B. D., Cleveland D. W., Griffin J. W. (1994). Regional modulation of neurofilament organization by myelination in normal axons. J. Neurosci. 14, 6392–6401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe H., Veeranna, Shetty K. T., Pant H. C. (1998). Characterization of the phosphorylation sites of human high molecular weight neurofilament protein by electrospray ionization tandem mass spectrometry and database searching. Biochemistry 37, 3931–3940 10.1021/bi972518u [DOI] [PubMed] [Google Scholar]
- Julien J. P., Mushynski W. E. (1983). The distribution of phosphorylation sites among identified proteolytic fragments of mammalian neurofilaments. J. Biol. Chem. 258, 4019–4025 [PubMed] [Google Scholar]
- Jung C., Lee S., Ortiz D., Zhu Q., Julien J. P., Shea T. B. (2005). The high and middle molecular weight neurofilament subunits regulate the association of neurofilaments with kinesin: inhibition by phosphorylation of the high molecular weight subunit. Brain Res. Mol. Brain Res. 141, 151–155 10.1016/j.molbrainres.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Kim O. J., Ariano M. A., Lazzarini R. A., Levine M. S., Sibley D. R. (2002). Neurofilament-M interacts with the D1 dopamine receptor to regulate cell surface expression and desensitization. J. Neurosci. 22, 5920–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. K., Kim H., Yang Y. R., Suh P. G., Chang J. S. (2011). Phosphatidylinositol phosphates directly bind to neurofilament light chain (NF-L) for the regulation of NF-L self assembly. Exp. Mol. Med. 43, 153–160 10.3858/emm.2011.43.3.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Križ J., Zhu Q., Julien J. P., Padjen A. L. (2000). Electrophysiological properties of axons in mice lacking neurofilament subunit genes: disparity between conduction velocity and axon diameter in absence of NF-H. Brain Res. 885, 32–44 10.1016/S0006-8993(00)02899-7 [DOI] [PubMed] [Google Scholar]
- Kushkuley J., Chan W. K., Lee S., Eyer J., Leterrier J. F., Letournel F., Shea T. B. (2009). Neurofilament cross-bridging competes with kinesin-dependent association of neurofilaments with microtubules. J. Cell Sci. 122, 3579–3586 10.1242/jcs.051318 [DOI] [PubMed] [Google Scholar]
- Kushkuley J., Metkar S., Chan W. K., Lee S., Shea T. B. (2010). Aluminum induces neurofilament aggregation by stabilizing cross-bridging of phosphorylated c-terminal sidearms. Brain Res. 1322, 118–123 10.1016/j.brainres.2010.01.075 [DOI] [PubMed] [Google Scholar]
- Lasek R. J., Garner J. A., Brady S. T. (1984). Axonal transport of the cytoplasmic matrix. J. Cell Biol. 99, 212s–221s 10.1083/jcb.99.1.212s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasek R. J., Paggi P., Katz M. J. (1992). Slow axonal transport mechanisms move neurofilaments relentlessly in mouse optic axons. J. Cell Biol. 117, 607–616 10.1083/jcb.117.3.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavedan C., Buchholtz S., Nussbaum R. L., Albin R. L., Polymeropoulos M. H. (2002). A mutation in the human neurofilament M gene in Parkinson’s disease that suggests a role for the cytoskeleton in neuronal degeneration. Neurosci. Lett. 322, 57–61 10.1016/S0304-3940(01)02513-7 [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W., Trojanowski J. Q. (1987). Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J. Neurosci. 7, 3474–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V. M., Otvos L., Jr, Carden M. J., Hollosi M., Dietzschold B., Lazzarini R. A. (1988). Identification of the major multiphosphorylation site in mammalian neurofilaments. Proc. Natl. Acad. Sci. USA 85, 1998–2002 10.1073/pnas.85.6.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. S., Veeranna, Grant P., Pant H. C. (1999a). Calcium influx and membrane depolarization induce phosphorylation of neurofilament (NF-M) KSP repeats in PC12 cells. Brain Res. Mol. Brain Res. 70, 84–91 10.1016/S0169-328X(99)00142-4 [DOI] [PubMed] [Google Scholar]
- Li B. S., Veeranna, Jianguo G., Grant P., Pant H. C. (1999b). Activation of mitogen-activated protein kinases (Erk1 and Erk2) cascade results in phosphorylation of NF-M tail domains in transfected NIH 3T3 cells. Eur. J. Biochem. 262, 211–217 10.1046/j.1432-1327.1999.00372.x [DOI] [PubMed] [Google Scholar]
- Li B. S., Daniels M. P., Pant H. C. (2001). Integrins stimulate phosphorylation of neurofilament NF-M subunit KSP repeats through activation of extracellular regulated-kinases (Erk1/Erk2) in cultured motoneurons and transfected NIH 3T3 cells. J. Neurochem. 76, 703–710 10.1046/j.1471-4159.2001.00064.x [DOI] [PubMed] [Google Scholar]
- Liu Y. L., Guo Y. S., Xu L., Wu S. Y., Wu D. X., Yang C., Zhang Y., Li C. Y. (2009). Alternation of neurofilaments in immune-mediated injury of spinal cord motor neurons. Spinal Cord 47, 166–170 10.1038/sc.2008.90 [DOI] [PubMed] [Google Scholar]
- Manser C., Stevenson A., Banner S., Davies J., Tudor E. L., Ono Y., Leigh P. N., McLoughlin D. M., Shaw C. E., Miller C. C. J. (2008). Deregulation of PKN1 activity disrupts neurofilament organisation and axonal transport. FEBS Lett. 582, 2303–2308 10.1016/j.febslet.2008.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata M., Kupina N., Fink D. J. (1992). Phosphorylation-dependent neurofilament epitopes are reduced at the node of Ranvier. J. Neurocytol. 21, 199–210 10.1007/BF01194978 [DOI] [PubMed] [Google Scholar]
- Millecamps S., Gowing G., Corti O., Mallet J., Julien J. P. (2007). Conditional NF-L transgene expression in mice for in vivo analysis of turnover and transport rate of neurofilaments. J. Neurosci. 27, 4947–4956 10.1523/JNEUROSCI.5299-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz D. G., Greene C., Perl D. P., Selkoe D. J. (1988). Accumulation of phosphorylated neurofilaments in anterior horn motoneurons of amyotrophic lateral sclerosis patients. J. Neuropathol. Exp. Neurol. 47, 9–18 10.1097/00005072-198801000-00002 [DOI] [PubMed] [Google Scholar]
- Nixon R. A., Lewis S. E. (1986). Differential turnover of phosphate groups on neurofilament subunits in mammalian neurons in vivo. J. Biol. Chem. 261, 16298–16301 [PubMed] [Google Scholar]
- Nixon R. A., Logvinenko K. B. (1986). Multiple fates of newly synthesized neurofilament proteins: evidence for a stationary neurofilament network distributed nonuniformly along axons of retinal ganglion cell neurons. J. Cell Biol. 102, 647–659 10.1083/jcb.102.2.647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon R. A., Shea T. B. (1992). Dynamics of neuronal intermediate filaments: a developmental perspective. Cell Motil. Cytoskeleton 22, 81–91 10.1002/cm.970220202 [DOI] [PubMed] [Google Scholar]
- Nixon R. A., Lewis S. E., Marotta C. A. (1987). Posttranslational modification of neurofilament proteins by phosphate during axoplasmic transport in retinal ganglion cell neurons. J. Neurosci. 7, 1145–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon R. A., Lewis S. E., Dahl D., Marotta C. A., Drager U. C. (1989). Early posttranslational modifications of the three neurofilament subunits in mouse retinal ganglion cells: neuronal sites and time course in relation to subunit polymerization and axonal transport. Brain Res. Mol. Brain Res. 5, 93–108 10.1016/0169-328X(89)90001-6 [DOI] [PubMed] [Google Scholar]
- Nixon R. A., Paskevich P. A., Sihag R. K., Thayer C. Y. (1994). Phosphorylation on carboxyl terminus domains of neurofilament proteins in retinal ganglion cell neurons in vivo: influences on regional neurofilament accumulation, interneurofilament spacing, and axon caliber. J. Cell Biol. 126, 1031–1046 10.1083/jcb.126.4.1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara O., Gahara Y., Miyake T., Teraoka H., Kitamura T. (1993). Neurofilament deficiency in quail caused by nonsense mutation in neurofilament-L gene. J. Cell Biol. 121, 387–395 10.1083/jcb.121.2.387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachter J. S., Liem R. K. (1984). The differential appearance of neurofilament triplet polypeptides in the developing rat optic nerve. Dev. Biol. 103, 200–210 10.1016/0012-1606(84)90021-6 [DOI] [PubMed] [Google Scholar]
- Pant H. C. (1988). Dephosphorylation of neurofilament proteins enhances their susceptibility to degradation by calpain. Biochem. J. 256, 665–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G., Robinson F., Beers Gibson T., Xu B. E., Karandikar M., Berman K., Cobb M. H. (2001). Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22, 153–183 10.1210/er.22.2.153 [DOI] [PubMed] [Google Scholar]
- Perrin F. E., Boisset G., Docquier M., Schaad O., Descombes P., Kato A. C. (2005). No widespread induction of cell death genes occurs in pure motoneurons in an amyotrophic lateral sclerosis mouse model. Hum. Mol. Genet. 14, 3309–3320 10.1093/hmg/ddi357 [DOI] [PubMed] [Google Scholar]
- Perrot R., Eyer J. (2009). Neuronal intermediate filaments and neurodegenerative disorders. Brain Res. Bull. 80, 282–295 10.1016/j.brainresbull.2009.06.004 [DOI] [PubMed] [Google Scholar]
- Perrot R., Lonchampt P., Peterson A. C., Eyer J. (2007). Axonal neurofilaments control multiple fiber properties but do not influence structure or spacing of nodes of Ranvier. J. Neurosci. 27, 9573–9584 10.1523/JNEUROSCI.1224-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot R., Berges R., Bocquet A., Eyer J. (2008). Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol. Neurobiol. 38, 27–65 10.1007/s12035-008-8033-0 [DOI] [PubMed] [Google Scholar]
- Prahlad V., Yoon M., Moir R. D., Vale R. D., Goldman R. D. (1998). Rapid movements of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J. Cell Biol. 143, 159–170 10.1083/jcb.143.1.159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahlad V., Helfand B. T., Langford G. M., Vale R. D., Goldman R. D. (2000). Fast transport of neurofilament protein along microtubules in squid axoplasm. J. Cell Sci. 113, 3939–3946 [DOI] [PubMed] [Google Scholar]
- Rao M. V., Garcia M. L., Miyazaki Y., Gotow T., Yuan A., Mattina S., Ward C. M., Calcutt N. A., Uchiyama Y., Nixon R. A., et al. (2002). Gene replacement in mice reveals that the heavily phosphorylated tail of neurofilament heavy subunit does not affect axonal caliber or the transit of cargoes in slow axonal transport. J. Cell Biol. 158, 681–693 10.1083/jcb.200202037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. V., Campbell J., Yuan A., Kumar A., Gotow T., Uchiyama Y., Nixon R. A. (2003). The neurofilament middle molecular mass subunit carboxyl-terminal tail domains is essential for the radial growth and cytoskeletal architecture of axons but not for regulating neurofilament transport rate. J. Cell Biol. 163, 1021–1031 10.1083/jcb.200308076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. V., Mohan P. S., Kumar A., Yuan A., Montagna L., Campbell J., Veeranna, Espreafico E. M., Julien J. P., Nixon R. A. (2011). The myosin Va head domain binds to the neurofilament-L rod and modulates endoplasmic reticulum (ER) content and distribution within axons. PLoS ONE 6, e17087 10.1371/journal.pone.0017087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reles A., Friede R. L. (1991). Axonal cytoskeleton at the nodes of Ranvier. J. Neurocytol. 20, 450–458 10.1007/BF01252273 [DOI] [PubMed] [Google Scholar]
- Roy S., Coffee P., Smith G., Liem R. K., Brady S. T., Black M. M. (2000). Neurofilaments are transported rapidly but intermittently in axons: implications for slow axonal transport. J. Neurosci. 20, 6849–6861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudrabhatla P., Jaffe H., Pant H. C. (2011). Direct evidence of phosphorylated neuronal intermediate filament proteins in neurofibrillary tangles (NFTs): phosphoproteomics of Alzheimer’s NFTs. FASEB J. 25, 3896–3905 10.1096/fj.11-181297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Shima H., Osawa Y., Nagao M., Hemmings B. A., Kishimoto T., Hisanaga S. (1995). Neurofilament-associated protein phosphatase 2A: its possible role in preserving neurofilaments in filamentous states. Biochemistry 34, 7376–7384 10.1021/bi00022a010 [DOI] [PubMed] [Google Scholar]
- Sakaguchi T., Okada M., Kitamura T., Kawasaki K. (1993). Reduced diameter and conduction velocity of myelinated fibers in the sciatic nerve of a neurofilament-deficient mutant quail. Neurosci. Lett. 153, 65–68 10.1016/0304-3940(93)90078-Y [DOI] [PubMed] [Google Scholar]
- Sánchez I., Hassinger L., Sihag R. K., Cleveland D. W., Mohan P., Nixon R. A. (2000). Local control of neurofilament accumulation during radial growth of myelinating axons in vivo. Selective role of site-specific phosphorylation. J. Cell Biol. 151, 1013–1024 10.1083/jcb.151.5.1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T., Gotow T., Shiozaki M., Sakaue F., Saito T., Julien J. P., Uchiyama Y., Hisanaga S. (2006). Aggregate formation and phosphorylation of neurofilament-L Pro22 Charcot-Marie-Tooth disease mutants. Hum. Mol. Genet. 15, 943–952 10.1093/hmg/ddl011 [DOI] [PubMed] [Google Scholar]
- Shah J. V., Flanagan L. A., Janmey P. A., Leterrier J. F. (2000). Bidirectional translocation of neurofilaments along microtubules mediated in part by dynein/dynactin. Mol. Biol. Cell 11, 3495–3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty K. T., Link W. T., Pant H. C. (1993). cdc2-like kinase from rat spinal cord specifically phosphorylates KSPXK motifs in neurofilament proteins: isolation and characterization. Proc. Natl. Acad. Sci. USA 90, 6844–6848 10.1073/pnas.90.14.6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihag R. K., Nixon R. A. (1989). In vivo phosphorylation of distinct domains of the 70-kilodalton neurofilament subunit involves different protein kinases. J. Biol. Chem. 264, 457–464 [PubMed] [Google Scholar]
- Sihag R. K., Nixon R. A. (1990). Phosphorylation of the amino-terminal head domain of the middle molecular mass 145-kDa subunit of neurofilaments. Evidence for regulation by second messenger-dependent protein kinases. J. Biol. Chem. 265, 4166–4171 [PubMed] [Google Scholar]
- Sihag R. K., Nixon R. A. (1991). Identification of Ser-55 as a major protein kinase A phosphorylation site on the 70-kDa subunit of neurofilaments. Early turnover during axonal transport. J. Biol. Chem. 266, 18861–18867 [PubMed] [Google Scholar]
- Sihag R. K., Jeng A. Y., Nixon R. A. (1988). Phosphorylation of neurofilament proteins by protein kinase C. FEBS Lett. 233, 181–185 10.1016/0014-5793(88)81380-2 [DOI] [PubMed] [Google Scholar]
- Sihag R. K., Jaffe H., Nixon R. A., Rong X. (1999). Serine-23 is a major protein kinase A phosphorylation site on the amino-terminal head domain of the middle molecular mass subunit of neurofilament proteins. J. Neurochem. 72, 491–499 10.1046/j.1471-4159.1999.0720491.x [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. (1983). Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc. Natl. Acad. Sci. USA 80, 6126–6130 10.1073/pnas.80.19.6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strack S., Westphal R. S., Colbran R. J., Ebner F. F., Wadzinski B. E. (1997). Protein serine/threonine phosphatase 1 and 2A associate with and dephosphorylate neurofilaments. Brain Res. Mol. Brain Res. 49, 15–28 10.1016/S0169-328X(97)00117-4 [DOI] [PubMed] [Google Scholar]
- Sun D., Leung C. L., Liem R. K. H. (1996). Phosphorylation of the high molecular weight neurofilament protein (NF-H) by Cdk5 and p35. J. Biol. Chem. 271, 14245–14251 10.1074/jbc.271.24.14245 [DOI] [PubMed] [Google Scholar]
- Sunil N., Lee S., Shea T. B.2012). Interference with kinesin-based anterograde neurofilament axonal transport increases neurofilament-neurofilament bundling. Cytoskeleton 69, 371–379 [DOI] [PubMed] [Google Scholar]
- Szaro B. G., Strong M. J. (2010). Post-transcriptional control of neurofilaments: New roles in development, regeneration and neurodegenerative disease. Trends Neurosci. 33, 27–37 10.1016/j.tins.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Tanaka J., Ogawara M., Ando S., Shibata M., Yatani R., Kusagawa M., Inagaki M. (1993). Phosphorylation of a 62 kd porcine alpha-internexin, a newly identified intermediate filament protein. Biochem. Biophys. Res. Commun. 196, 115–123 10.1006/bbrc.1993.2223 [DOI] [PubMed] [Google Scholar]
- Trivedi N., Jung P., Brown A. (2007). Neurofilaments switch between distinct mobile and stationary states during their transport along axons. J. Neurosci. 27, 507–516 10.1523/JNEUROSCI.4227-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida A., Alami N. H., Brown A. (2009). Tight functional coupling of kinesin-1A and dynein motors in the bidirectional transport of neurofilaments. Mol. Biol. Cell 20, 4997–5006 10.1091/mbc.E09-04-0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranna, Shetty K. T., Link W. T., Jaffe H., Wang J., Pant H. C. (1995). Neuronal cyclin-dependent kinase-5 phosphorylation sites in neurofilament protein (NF-H) are dephosphorylated by protein phosphatase 2A. J. Neurochem. 64, 2681–2690 10.1046/j.1471-4159.1995.64062681.x [DOI] [PubMed] [Google Scholar]
- Veeranna, Amin N. D., Ahn N. G., Jaffe H., Winters C. A., Grant P., Pant H. C. (1998). Mitogen-activated protein kinases (Erk1,2) phosphorylate Lys-Ser-Pro (KSP) repeats in neurofilament proteins NF-H and NF-M. J. Neurosci. 18, 4008–4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranna, Kaji T., Boland B., Odrljin T., Mohan P., Basavarajappa B. S., Peterhoff C., Cataldo A., Rudnicki A., Amin N., et al. (2004). Calpain mediates calcium-induced activation of the erk1,2 MAPK pathway and cytoskeletal phosphorylation in neurons: relevance to Alzheimer’s disease. Am. J. Pathol. 165, 795–805 10.1016/S0002-9440(10)63342-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeranna, Yang D. S., Lee J. H., Vinod K. Y., Stavrides P., Amin N. D., Pant H. C., Nixon R. A. (2011). Declining phosphatases underlie aging-related hyperphosphorylation of neurofilaments. Neurobiol. Aging 32, 2016–2029 10.1016/j.neurobiolaging.2009.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosseller K., Trinidad J. C., Chalkley R. J., Specht C. G., Thalhammer A., Lynn A. J., Snedecor J. O., Guan S., Medzihradszky K. F., Maltby D. A., et al. (2006). O-linked N-acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry. Mol. Cell. Proteomics 5, 923–934 10.1074/mcp.T500040-MCP200 [DOI] [PubMed] [Google Scholar]
- Wagner O. I., Ascaño J., Tokito M., Leterrier J. F., Janmey P. A., Holzbaur E. L. (2004). The interaction of neurofilaments with the microtubule motor cytoplasmic dynein. Mol. Biol. Cell 15, 5092–5100 10.1091/mbc.E04-05-0401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner O. I., Rammensee S., Korde N., Wen Q., Leterrier J. F., Janmey P. A. (2007). Softness, strength and self-repair in intermediate filament networks. Exp. Cell Res. 313, 2228–2235 10.1016/j.yexcr.2007.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Brown A. (2010). A hereditary spastic paraplegia mutation in kinesin-1A/KIF5A disrupts neurofilament transport. Mol. Neurodegener. 5, 52 10.1186/1750-1326-5-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Ho C. L., Sun D., Liem R. K., Brown A. (2000). Rapid movement of axonal neurofilaments interrupted by prolonged pauses. Nat. Cell Biol. 2, 137–141 10.1038/35004008 [DOI] [PubMed] [Google Scholar]
- Williamson T. L., Bruijn L. I., Zhu Q., Anderson K. L., Anderson S. D., Julien J. P., Cleveland D. W. (1998). Absence of neurofilaments reduces the selective vulnerability of motor neurons and slows disease caused by a familial amyotrophic lateral sclerosis-linked superoxide dismutase 1 mutant. Proc. Natl. Acad. Sci. USA 95, 9631–9636 10.1073/pnas.95.16.9631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C. H., Roberts E. A., Her L. S., Liu X., Williams D. S., Cleveland D. W., Goldstein L. S. (2003). Abnormal neurofilament transport caused by targeted disruption of neuronal kinesin heavy chain KIF5A. J. Cell Biol. 161, 55–66 10.1083/jcb.200301026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. S., Liu W. S., Willard M. B. (1992). Identification of six phosphorylation sites in the COOH-terminal tail region of the rat neurofilament protein M. J. Biol. Chem. 267, 4467–4471 [PubMed] [Google Scholar]
- Yabe J. T., Pimenta A., Shea T. B. (1999). Kinesin-mediated transport of neurofilament protein oligomers in growing axons. J. Cell Sci. 112, 3799–3814 [DOI] [PubMed] [Google Scholar]
- Yabe J. T., Jung C., Chan W. K., Shea T. B. (2000). Phospho-dependent association of neurofilament proteins with kinesin in situ. Cell Motil. Cytoskeleton 45, 249–262 [DOI] [PubMed] [Google Scholar]
- Yabe J. T., Chan W. K., Chylinski T. M., Lee S., Pimenta A. F., Shea T. B. (2001). The predominant form in which neurofilament subunits undergo axonal transport varies during axonal initiation, elongation, and maturation. Cell Motil. Cytoskeleton 48, 61–83 [DOI] [PubMed] [Google Scholar]
- Yan Y., Brown A. (2005). Neurofilament polymer transport in axons. J. Neurosci. 25, 7014–7021 10.1523/JNEUROSCI.2001-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Jensen K., Brown A. (2007). The polypeptide composition of moving and stationary neurofilaments in cultured sympathetic neurons. Cell Motil. Cytoskeleton 64, 299–309 10.1002/cm.20184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. M., Manser C., De Vos K. J., Shaw C. E., McLoughlin D. M., Miller C. C. (2009). Neurofilament subunit (NFL) head domain phosphorylation regulates axonal transport of neurofilaments. Eur. J. Cell Biol. 88, 193–202 10.1016/j.ejcb.2008.11.004 [DOI] [PubMed] [Google Scholar]
- Yin X., Crawford T. O., Griffin J. W., Tu P., Lee V. M., Li C., Roder J., Trapp B. D. (1998). Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J. Neurosci. 18, 1953–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A., Nixon R. A. (2011). Axonal transport mechanisms in cytoskeleton formation and regulation. Cytoskeleton of the Nervous System, Vol. 3 (ed. Yuan A, Nixon R A.), pp. 503–527 New York, NY: Springer [Google Scholar]
- Yuan A., Rao M. V., Kumar A., Julien J. P., Nixon R. A. (2003). Neurofilament transport in vivo minimally requires hetero-oligomer formation. J. Neurosci. 23, 9452–9458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A., Nixon R. A., Rao M. V. (2006a). Deleting the phosphorylated tail domain of the neurofilament heavy subunit does not alter neurofilament transport rate in vivo. Neurosci. Lett. 393, 264–268 10.1016/j.neulet.2005.10.029 [DOI] [PubMed] [Google Scholar]
- Yuan A., Rao M. V., Sasaki T., Chen Y., Kumar A., Veeranna, Liem R. K., Eyer J., Peterson A. C., Julien J. P., et al. (2006b). Alpha-internexin is structurally and functionally associated with the neurofilament triplet proteins in the mature CNS. J. Neurosci. 26, 10006–10019 10.1523/JNEUROSCI.2580-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan A., Sasaki T., Rao M. V., Kumar A., Kanumuri V., Dunlop D. S., Liem R. K., Nixon R. A. (2009). Neurofilaments form a highly stable stationary cytoskeleton after reaching a critical level in axons. J. Neurosci. 29, 11316–11329 10.1523/JNEUROSCI.1942-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yum S. W., Zhang J., Mo K., Li J., Scherer S. S. (2009). A novel recessive Nefl mutation causes a severe, early-onset axonal neuropathy. Ann. Neurol. 66, 759–770 10.1002/ana.21728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q., Couillard–Després S., Julien J. P. (1997). Delayed maturation of regenerating myelinated axons in mice lacking neurofilaments. Exp. Neurol. 148, 299–316 10.1006/exnr.1997.6654 [DOI] [PubMed] [Google Scholar]
- Zhu X., Raina A. K., Rottkamp C. A., Aliev G., Perry G., Boux H., Smith M. A. (2001). Activation and redistribution of c-jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer’s disease. J. Neurochem. 76, 435–441 10.1046/j.1471-4159.2001.00046.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.