Abstract
Within the last decade several genes have been identified as candidate risk genes for developmental dyslexia. Recent research using animal models and embryonic RNA interference (RNAi) has shown that a subset of the candidate dyslexia risk genes—DYX1C1, ROBO1, DCDC2, KIAA0319—regulate critical parameters of neocortical development, such as neuronal migration. For example, embryonic disruption of the rodent homolog of DYX1C1 disrupts neuronal migration and produces deficits in rapid auditory processing (RAP) and working memory—phenotypes that have been reported to be associated with developmental dyslexia. In the current study we used a modified prepulse inhibition paradigm to assess acoustic discrimination abilities of male Wistar rats following in utero RNA interference targeting Kiaa0319. We also assessed spatial learning and working memory using a Morris water maze (MWM) and a radial arm water maze. We found that embryonic interference with this gene resulted in disrupted migration of neocortical neurons leading to formation of heterotopia in white matter, and to formation of hippocampal dysplasia in a subset of animals. These animals displayed deficits in processing complex acoustic stimuli, and those with hippocampal malformations exhibited impaired spatial learning abilities. No significant impairment in working memory was detected in the Kiaa0319 RNAi treated animals. Taken together, these results suggest that Kiaa0319 plays a role in neuronal migration during embryonic development, and that early interference with this gene results in an array of behavioral deficits including impairments in rapid auditory processing and simple spatial learning.
Keywords: Dyslexia, KIAA0319, Rapid auditory processing, RNA interference, Neuronal migration
1. Introduction
Developmental dyslexia is a common disorder affecting 5–10% of school-aged children worldwide. It is defined as a significant impairment in reading despite adequate educational opportunity and normal non-verbal cognition (Fisher and Francks, 2006). The etiology of developmental dyslexia is still not well understood, but twin studies suggest that it is a heritable disorder (see Bishop, 2009; Willcutt et al., 2010 for review). Within the last two decades, researchers have used genetic linkage studies to identify several chromosomal regions that are significantly associated with dyslexia (see Kang and Drayna, 2011 for a complete review). Interestingly, the genomic region with the most consistently replicated linkage to dyslexia is at chromosome 6p23-21.3. In fact, linkage to this region has been detected in five separate dyslexic populations from the United Kingdom and United States (Cardon et al., 1994, 1995; Gayan and Olson, 2001; Grigorenko et al., 1997; Fisher et al., 1999; Fisher and DeFries, 2002). More recent studies have used candidate-gene association analyses to identify specific dyslexia-associated genes within this identified region on chromosome 6. Such studies have found correlations between variants of the gene KIAA0319 and impairments in dyslexic individuals in language-related cognitive abilities, including phoneme awareness, phonological decoding, orthographic coding, spelling, and single word reading (Cope et al., 2005; Deffenbacher et al., 2004; Elbert et al., 2011; Francks et al., 2004; Newbury et al., 2011; Rice et al., 2009). Little is known about the function of KIAA0319, but the presence of a polycystic kidney disease domain on the KIAA0319 protein has lead to speculation that it may play a role in cell adhesion (Paracchini et al., 2006). Additional research has suggested that it may also play a role in cellular signaling (Velayos-Baeza et al., 2007, 2008, 2010). Assays have shown that KIAA0319 is expressed extensively in the superior parietal cortex, primary visual cortex, and occipital cortex throughout the human lifespan, and similar patterns have been reported for rodent brains (Paracchini et al., 2006; Velayos-Baeza et al., 2007). Using animal models, the rodent homologs of several CDSGs (Kiaa0319, Dyx1c1, Dcdc2) have been shown to be involved in neuronal migration (Burbridge et al., 2008; Rosen et al., 2007; Wang et al., 2006; Peschansky et al., 2010). This is interesting in light of research byGalaburda et al. (1985), who reported neuronal migration disruptions in the cerebral cortex of post-mortem dyslexics.
Developmental dyslexia is characterized by a constellation of behavioral phenotypes, ranging from impairments in the phonological domain to short-term memory and attention deficits (Beneventi et al., 2010; Bishop, 2009; Gathercole et al., 2006; Menghini et al., 2010, 2011; Paracchini et al., 2007; Smith-Spark and Fisk, 2007). Several studies have also illustrated a relationship between rapid auditory processing (RAP) – defined as the ability to detect rapidly presented acoustic verbal and non-verbal cues – and language impairment and dyslexia, with indices of RAP ability associating with concurrent or longitudinal language scores. Several studies have demonstrated that behavioral and electrophysiological measures of RAP abilities in early childhood are significantly correlated with later measures of reading ability (or measures of reading subprocesses, such as phoneme awareness and phonological processing) in dyslexic individuals (Benasich et al., 2006; Boets et al., 2011; Boscariol et al., 2010; Cohen-Mimran and Sapir, 2007; Fitch and Tallal, 2003; Gaab et al., 2007; King et al., 2008; Tallal and Piercy, 1973; Vandermosten et al., 2011). These data provide compelling evidence that rapid auditory processing skills are related to and fundamental for reading acquisition. Different CDSGs have been related to specific “core” behavioral components of dyslexia, including KIAA0319, which has been associated with phonological/language-related deficits (Harold et al., 2006; Newbury et al., 2011; Rice et al., 2009), and DYX1C1, which has been specifically linked to short-term memory impairment in some dyslexic populations (Dahdouh et al., 2009; Marino et al., 2007). Parallels to some of these “core” behaviors can be assessed in rodents. Previous studies from our lab have used a modified prepulse inhibition paradigm to assess rodent RAP abilities following various neurodevelopmental disruptions, including Dyx1c1 knockdown (Fitch et al., 2008a; Threlkeld et al., 2007). Other studies have assessed working memory in rats following disrupted neuronal migration using a radial arm water maze. In fact, one recent study from our lab reported significant spatial working memory impairments in rats following early interference with the gene Dyx1c1 (Fitch et al., 2008b; Szalkowski et al., 2011). In the current study, in utero RNA interference (RNAi) was used to create a Kiaa0319 knockdown model in male rats. A modified pre-pulse inhibition paradigm was used to assess rapid auditory processing abilities. Simple spatial learning and spatial working memory abilities were also assessed in order to better characterize the impact of Kiaa0319 on behavioral processes relevant to dyslexic populations.
2. Methods
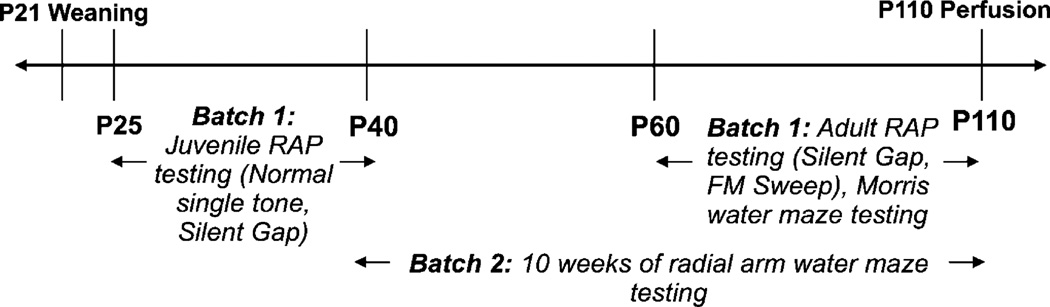
Two different experimental cohorts (Batch 1 and Batch 2) were used for behavioral testing. Batch 1 consisted of a total of 27 male Wistar rats (Charles River Laboratory, Wilmington, MA, USA) that participated in the rapid auditory processing and spatial learning studies (Batch 1; Kiaa0319 RNAi n = 18, Sham n = 9). Batch 2 consisted of a total of 32 male Sprague–Dawley rats (Charles River Laboratory, Wilmington, MA, USA) that participated in the working memory study (Batch 2; Kiaa0319 RNAi n = 20, Sham n = 12). Note that Wistar and Sprague–Dawley rats were specifically chosen for these two batteries of tests in the interest of comparing the current data to different literature bases that have predominantly used these strains in these specific tasks. Additionally, there is evidence that Sprague–Dawley rats are adept at performing complex spatial mazes, making them good candidates for the eight arm radial water maze task (Clements and Wainwright, 2006). All animals were pair-housed with same-sex littermates in Plexiglass tubs. They were maintained on a 12 h/12 h light/dark cycle, and food and water were available ad libitum. All procedures were performed in accordance with guidelines established by the National Institutes of Health, and were approved by the University of Connecticut’s Institutional Animal Care and Use Committee.
2.1. Transfection
In utero electroporation of Kiaa0319 shRNA was performed by C.F. at the University of Connecticut in accordance with the procedures outlined inBai et al. (2003). Two batches of surgeries were performed. All surgeries were performed on embryonic day 15 (E15). In all Kiaa0319 RNAi treatments, plasmids encoding short hairpin RNA pU6shRNA-Kiaa0319 (Kiaa0319 shRNA) were transfected into the fetal ventricular zone by in utero electroporation, following externalization of the uterine horns. Note that previous studies using the same exact Kiaa0319 shRNA vector and in utero electroporation procedure have demonstrated the effectiveness of this vector at specifically targeting and knocking down KIAA0319 protein translation in rats (see Paracchini et al., 2006; Peschansky et al., 2010 for reports on this shRNA vector from the labs of JJL and GDR, respectively). For Batch 1 and Batch 2, timemated Wistar dams and Sprague–Dawley dams were used, respectively. At E15, dams were anesthetized (Ketamine/Xylazine, 100/10 mixture, 0.1 mg/g intraperitoneally) and abdominal incisions were made through skin and muscle. The uterine horns were exposed and injections were made as follows: Batch 1, Kiaa0319 shRNA plasmids (1.5 µg/µL) + eGFP (enhanced green fluorescent protein, 0.5 µg/µL) + fast green dye, while shams received injections of mRFP (monomeric red fluorescent protein) plasmids (pCAGGS-RFP, 0.5 µg/µL) + fast green dye; Batch 2, Kiaa0319 shRNA plasmids (1.5 µg/µL) + mRFP plasmids (0.5 µg/µL) + fast green dye, while shams received injections of eGFP plasmids (0.5 µg/µL) + fast green dye. All solutions were microinjected into fetal ventricles through the uterine wall by mechanical pressure (General Valve Picospritzer, Pine Brook, NJ, USA). One randomly chosen lateral ventricle of each embryo was injected using a pulled glass capillary (Drummond Scientific, Broomall, PA, USA). Electroporation was achieved by discharge of a 500 mV capacitor charged to 70 mV. Specifically, a pair of copper alloy plates (1 ×.05 cm) was positioned around the head of the embryo and they served as the conduit for the voltage pulse. The voltage current was discharged through both sides of the brain. Since only males were used for testing and embryos were unable to be sexed at this age, equal numbers of Sham and Kiaa0319 RNAi injections were made, at roughly double the numbers needed. Males were used exclusively for testing because of previous research that has demonstrated that male rodents exhibit more robust behavioral deficits than females following early disruption of cortical development (Fitch et al., 1997; Peiffer et al., 2002, 2004). Additionally, developmental dyslexia occurs more frequently in males than females (Flannery et al., 2000; Katusic et al., 2001; Rutter et al., 2004).
A total of 59 male subjects were weaned on postnatal day 21 (P21) and received right or left ear marking. Subjects were housed in same-sex littermate pairs as treatment could not be identified at weaning. (Treatment was later identified postmortem via fluorescence of GFP in Batch 1 and fluorescence of RFP in Batch 2.)
2.2. Auditory testing: startle reduction paradigm (Batch 1)
Juvenile auditory testing began on P25 (for a complete timeline of behavioral testing, see Fig. 1). Rapid auditory processing testing utilized a startle response paradigm that has been discussed extensively elsewhere (Fitch et al., 2008a; Peiffer et al., 2002, 2004). Briefly, a modified prepulse inhibition paradigm was used in which an auditory cue is presented prior to a startle-eliciting stimulus (SES). The SES elicits an acoustic startle response (ASR) – a gross motor startle reflex. If the subject is able to detect the pre-SES cue, the amplitude of the ASR is reduced. In this way, the subject’s inhibition of the startle response acts as a measure of whether or not the subject can discriminate the cue (Faraday and Grunberg, 2000). During testing, subjects were placed on a load cell platform (Med Associates, Georgia, VT) to record movement. Auditory stimuli were generated using a Pentium 4 Dell PC with custom programmed sound files and a Tucker Davis Technologies (RP2) real time processor. Sound files were played through a Marantz integrated amplified connected to nine Cambridge Sound Works speakers, whose sound levels were calibrated prior to testing with a decibel meter. Each pair of platforms had one speaker centered 30 cm above it. The voltage of each platform was passed through a load cell amplifier (PHM- 250-60) into a Biopac MP100WS acquisition system (Biopac Systems, Santa Barbara, CA). The Biopac system was connected to two Macintosh computers which recorded the amplitude of the subject’s ASR in millivolts following each presentation of the SES using the program Acqknowledge v. 3.2.9. The maximum peak value of each subject’s ASR was extracted for the 150 ms time period following the onset of the SES. Attenuated response scores (ATT score) for each subject were calculated using the formula ([mean cued ASR amplitude/mean uncued ASR amplitude] × 100). This gave a ratio of the amplitude of each animal’s displacement of the load cell during a cued trial compared to that on an uncued trial. These ATT scores were expressed as percentages, and these represented the main dependent variable of interest for auditory testing.
Fig. 1.
A timeline of the behavioral testing schedule for animals in Batch 1 and Batch 2. Note that “P21” refers to postnatal day 21.
2.3. Auditory testing: normal single tone (Batch 1)
Starting on P25, all subjects were tested for one day each on two normal single tone procedures. The normal single tone procedures served as measures of the subjects’ baseline auditory acuity, gross motor startle reflex and prepulse inhibition abilities. Two versions of the normal single tone task were utilized. The normal single tone variable frequency test was presented first. This test consisted of 104 trials (cued and uncued) that were presented in a pseudo-random order. Uncued trials consisted of a silent background interrupted by a 105 dB, 50 ms white noise burst (SES). During cued trials, a 75 dB, 7 ms tone pip of varying frequency (2300 Hz, 5000 Hz, 8000 Hz, or 12,000 Hz) preceded the SES by 50 ms. This task verified the subjects’ ability to discriminate a range of frequencies.
The second day of normal single tone testing involved the normal single tone variable cue-burst interval test and it consisted of 104 trials (cued or uncued) presented in a pseudo-random order. Uncued trials were the same as those previously described. During cued trials, a 75 dB, 7 ms, 2300 Hz tone pip preceded the SES by an interval of varying lengths: 25 ms, 50 ms, 75 ms, 100 ms. The variable cue-burst interval test validated the subjects’ ability to attenuate their startle responses when the benign acoustic cue was presented 50 ms prior to the SES. The 50 ms cue-burst interval was used throughout the rest of RAP testing.
2.4. Auditory testing: long/short silent gap (Batch 1)
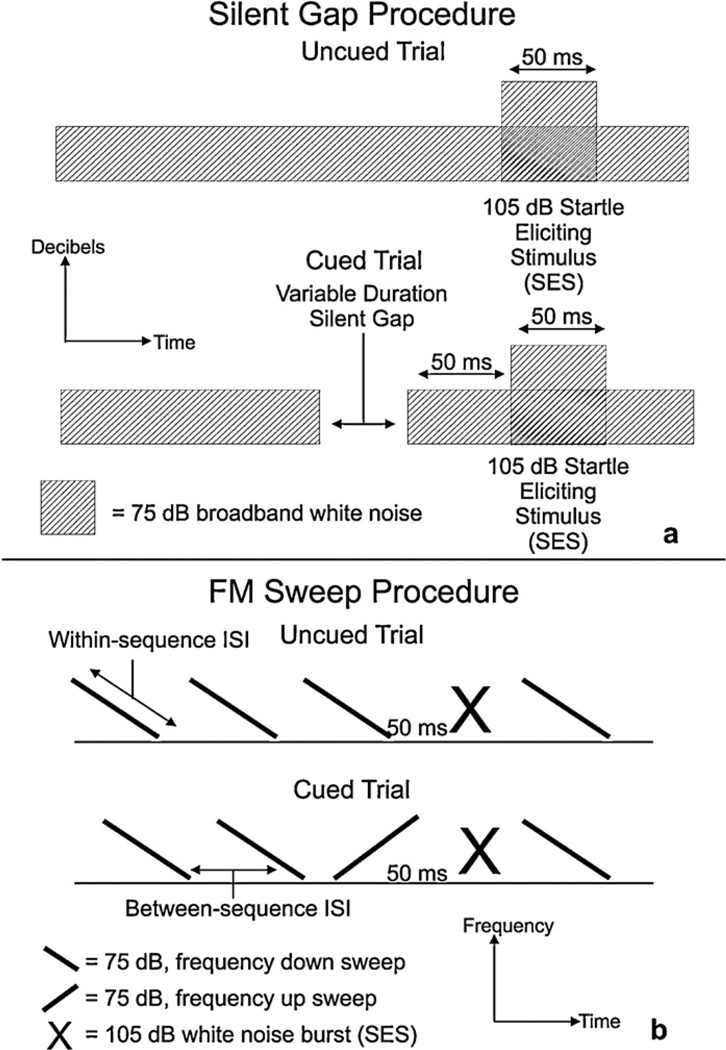
A silent gap detection task was used in the juvenile period of P29–40 (with repeat testing in the adult period, P60+; see Figs. 1 and 2a). A long silent gap task was presented first over a period of four days to assess long gap detection thresholds. One daily long silent gap session consisted of 300 trials. Uncued trials consisted of a 75 dB broadband white noise background interrupted by a 105 dB, 50 ms burst of white noise. On cued trials, a silent gap of varying duration interrupted the background white noise 50 ms before the SES. On the long silent gap task, the gap lengths were 2, 5, 10, 20, 50, 75, and 100 ms and these were presented in a pseudo-random order. The inter trial interval (ITI)—the interval between the presentation of the SES and presentation of the next cue—was between 16 and 24 ms long, and the length of the ITI varied in a pseudorandom order.
Fig. 2.
Schematic representations of two of the sound files used for rapid auditory processing testing. (a) A schematic of the silent gap task. Note that this is representative of both the long and short versions of the silent gap task, with the only difference between the two being the durations of the silent gaps. (b) A schematic of the FM sweep task. In the FM sweep task, a single reversal of the repeating background high-to-low frequency sweep serves as the pre-SES cue. In all versions of the task the high frequency is 2300 Hz and the low frequency is 1100 Hz. The within-sequence ISI is gradually reduced (over days) to make the change in frequency, and thus the cue, more difficult to discriminate.
A short silent gap task followed the four days of long silent gap testing to assess gap detection abilities at shorter intervals. Short silent gap was administered for five days. The basic structure of the task was the same as the long silent gap task, except that in the short silent gap test, the gap lengths were 2, 3, 4, 5, 6, 7, 8, 9, and 10 ms in length (0 ms = uncued trials).
2.5. Auditory testing: FM sweep (Batch 1)
The FM sweep task was administered over four days in the adult period (P60+). The FM sweep procedure included 104 trials (cued and uncued) presented in a pseudo-random order (see Fig. 2b). This procedure consisted of the presentation of a standard 75 dB, high/low frequency sweep stimulus (2300–1100 Hz). The frequency sweep was extended over a within-stimulus inter-stimulus interval (ISI) of 275, 225, 175, 125 ms. One ISI was used per session, beginning on the first day of FM sweep testing with the longest ISI of 275 ms and gradually stepping down to the shorter ISI’s over three days of testing. This allowed us to test the subject’s ability to detect the cue as a function of how rapidly the frequency sweep was presented. Each FM sweep task used a fixed between-stimulus ISI, which was always 200 ms greater than the within-stimulus ISI. Inter trial intervals ranged from 16 ms to 24 ms in duration and were presented in a pseudo-random order. Uncued trials consisted of 50 ms of silence after the last background frequency sweep, followed by a 105 dB, 50 ms SES. Cued trials involved the reversal of the standard high/low frequency sweep to a low/high sweep, followed by 50 ms of silence and the SES.
2.6. Water escape and Morris water maze (Batch 1)
Spatial learning testing began at the completion of auditory testing in the adult period. All subjects from Batch 1 were tested on a water (swim) escape task before beginning the Morris water maze. The water escape task was employed to rule out motivational, visual, and motor impairments as a cause for performance differences on the Morris water maze (MWM). The water escape task utilized a visible platform (4 in. diameter) placed at one end of an oval-shaped metal tub (40.5 in. ×21.5 in.) filled with room temperature water (8 in.). Subjects were released at the opposite end of the tub, and the latency to swim to and climb onto the platform was recorded. Morris water maze testing began the following day, and was administered over a period of five days. Testing was conducted in a round 48 in. diameter black Plexiglass tub. An 8 in. diameter submerged (invisible) platform was placed in the same location in the southeast quadrant of the tub every day of testing. Fixed extra-maze cues were present in the room (the doorway, the light source, the experimenter, and large black shapes painted onto the surrounding walls). The tub and the platform were both painted flat black to eliminate intramaze cues. On each testing day, subjects underwent four trials each. On day 1 trial 1, each subject was placed on the platform for 10 s before the start of testing, then removed from the platform and released from the designated starting location. On each trial, the subject was placed into the maze at a randomly selected compass point (N, E, S, W), from which they had to navigate to the same goal location. The SMART video-tracking system (Harvard Apparatus USA, Holliston, Massachusetts) and a ceiling mounted digital camera were used to record distance, latency, and swim speed data throughout Morris water maze testing
2.7. Radial arm water maze (Batch 2)
The testing procedures used for the radial arm delayed match to sample water maze have been described extensively elsewhere (Chrobak et al., 2008). Briefly, a black metal radial arm water maze was housed in a black Plexiglass tub filled with cool water. A removable black plastic platform was used as the escape platform and was submerged beneath the water. Fixed extra-maze cues were present throughout testing. Testing began on P40 for the animals in Batch 2. All rats were assessed on an initial acclimation trial and were found to be capable of navigating the maze and mounting the platform. Testing of animals consisted of four daily sessions per week. Each daily session consisted of one forced-choice sample trial and one test trial. On the forced-choice sample trial, all of the arms to the radial arm maze were blocked except for the arm the animal was placed in (the start arm) and the arm that housed the escape platform (the goal arm). This forced the animal to navigate directly to the platform. After locating the platform the animal was immediately removed from the tub and returned to its home cage for a 10 min period before the test trial. During the test trial, all of the arms of the maze were open and the animal was placed in a new start arm. The goal location remained fixed between the sample and test trials. Unlike the Morris water maze, the goal location in the radial arm water maze changed everyday, and thus the animals were required to learn and retain a new spatial location during each daily testing session. Experimenters recorded the latency to reach the platform, as well as the number of incorrect arm entries made prior to locating the goal arm. Testing was carried out for 10 weeks. Sequences of start and goal arms varied systematically among 48 patterns.
2.8. Histological analysis
At the end of testing, all subjects were transcardially perfused for assessment of brain tissue and identification of the relevant fluorescent proteins to identify treatment groups (Sham vs. Kiaa0319 shRNA). It is important to note that all experimenters were blind to treatment condition throughout testing. Before transcardial perfusion, all subjects were weighed and deeply anesthetized with Ketamine/Xylazine (100 and 15 mg/kg, respectively). Subjects were perfused with 0.1 M phosphate buffered saline followed by chilled 4% paraformaldehyde. Heads were removed and brains were extracted and shipped to GDR and AMG at Beth Israel Deaconess medical center for histological preparation. Brains were placed in a 30% sucrose buffer solution before being cut in the coronal plane at 40 µm thickness. A 1-in-10 series of slices was mounted and stained for Nissl substance with Thionin. An adjacent series of free-floating sections were mounted and screened using fluorescence microscopy to detect the presence of GFP or RFP. An additional series of sections were immunohistochemically processed for visualization of RFP or GFP (Chemicon, 1:200) using ABC protocols. Light microscopy was used to visualize the position of transfected cells and identify dysplasia in shRNA and Sham treated subjects.
2.9. Data analyses
Multivariate analyses of variance (ANOVA) were used to analyze the ATT scores for the various acoustic discrimination tasks. Analyses of variance were also used to analyze the latency measures from the Morris water maze and the error data from the radial arm water maze. All reported P-values are two-tailed. All statistical analyses were carried out using IBM SPSS Statistics Standard Edition.
3. Results
3.1. Histology
Qualitative observation of GFP and RFP labeling in animals from Batch 1 and Batch 2 revealed no differences in the relative size of the sham and Kiaa0319 shRNA transfections between the two batches. In each batch, and in both sham and Kiaa0319 shRNA transfected animals, the majority of labeled cells were observed in regions of parietal cortex. [Note that in Kiaa0319 shRNA transfected animals, the majority of the labeled cells were observed in heterotopic malformations present in the white matter, and in some case the hippocampus, subjacent to parietal cortex, and not in the cortex itself.] Transfected cells in sham animals (i.e., cells that migrated normally) were primarily found in Layer 2/3 of the neocortex. The specific transfection of Layer 2/3 neocortical pyramidal cells destined for parietal cortex occurred as a result of the timing of the transfection and the placement of the electrodes during electroporation (see LoTurco et al., 2009 for more details).
It is also worth noting that there was not a clear correlation between the apparent size of the transfection and the severity of malformations. Roughly half (n = 19) of all Kiaa0319 shRNA transfected animals exhibited qualitatively small transfections with large rostral to caudal malformations. On a similar note, it is worth mentioning that many of the observed heterotopic malformations in Kiaa0319 shRNA transfected animals contained non-transfected neurons. Non-cell autonomous effects of Kiaa0319 shRNA transfection have previously been reported, and suggest that there are indeed off-target, downstream effects of the transfection (Peschansky et al., 2010). The exact mechanism of the off-target effects has not yet been characterized.
Processing brain tissue for the presence of GFP or RFP in Batch 1 revealed 9 sham subjects and 18 Kiaa0319 shRNA treated subjects. Three categories of gross neocortical disruption were observed as follows (see Fig. 3): (1) Injection site ectopia (shRNA treated n = 8, sham n = 6), resulting from the injection puncture wound. These anomalies were observed as collections of ectopic cells in Layer 1 of the cortex. (2) Unmigrated neurons in the white matter (shRNA treated n = 16, sham n = 0). These anomalies were observed as collections of neocortical neurons that failed to migrate to their target layers and instead formed heterotopic pockets in the white matter. And (3) hippocampal dysplasia (shRNA treated n = 9, sham n = 0), characterized as unmigrated neocortical neurons that disrupted the structure of the hippocampus proper, (and specifically the dentate gyrus). Note that 2 of the Kiaa0319 shRNA treated subjects did not display any gross cortical malformation and that 12 of the Kiaa0319 shRNA treated subjects displayed more than one form of disruption. It is also worth noting that with the exception of Injection site ectopia, the sham animals did not display any type of cortical disruption. Statistical analyses revealed that the shams with injection site ectopia did not perform significantly differently from those without ectopia, and so shams were analyzed as a pooled group for the remainder of statistical testing.
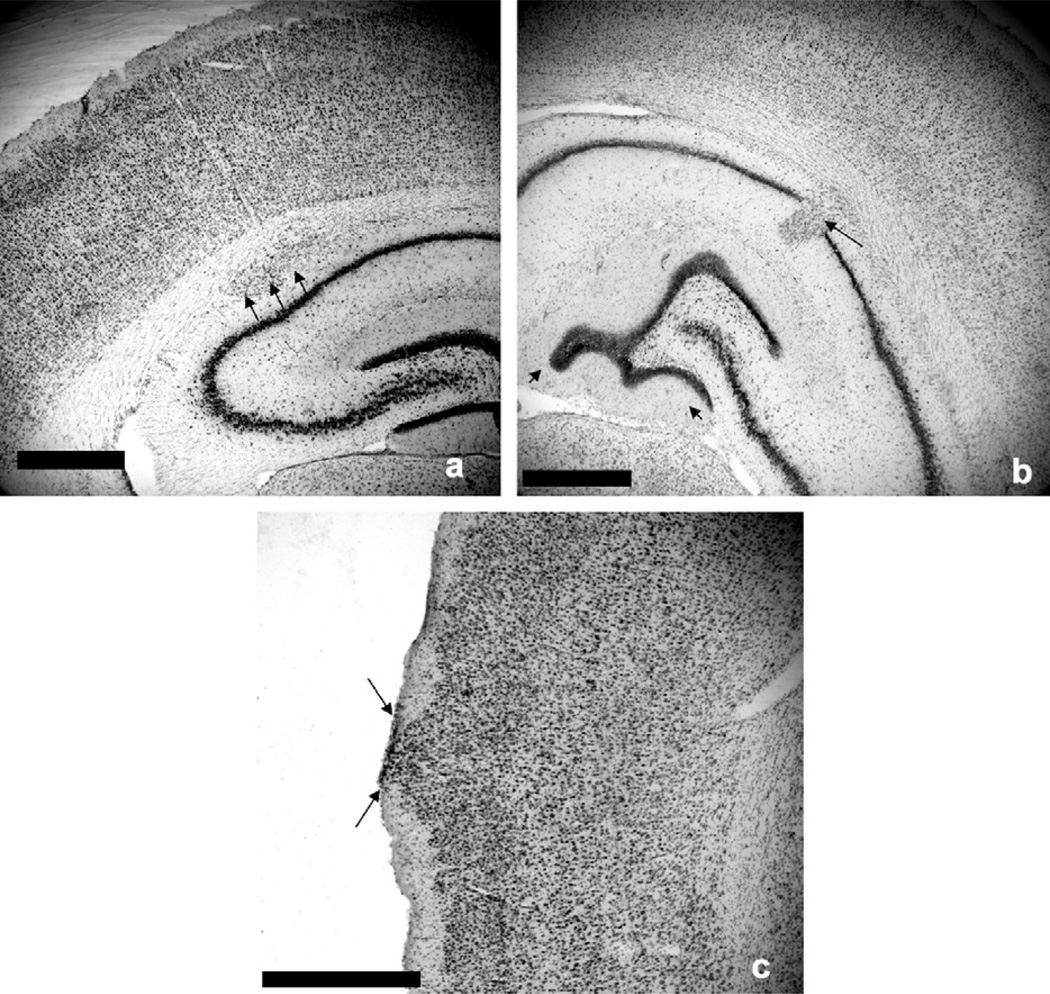
Fig. 3.
Coronal Nissl stained sections from Kiaa0319 RNAsh subjects. Histology revealed three categories of cortical disruption. (a) Unmigrated neurons in the white matter (Batch 1, shRNA treated n = 16, Sham n = 0; Batch 2, shRNA treated n = 20, Sham n = 0). These anomalies were observed as collections of neocortical neurons that failed to migrate to their target layers and instead formed heterotopic pockets in the white matter (black arrows). Scale bar = 1 mm. [Note that the pictured heterotopia is located in the subcortical white matter adjacent to, but not penetrating, the hippocampal formation.] (b) Hippocampal dysplasia (Batch 1, shRNA treated n = 9, Sham n = 0; Batch 2, shRNA treated n = 13, Sham n = 0), characterized as unmigrated neocortical neurons that disrupted the structure of the hippocampus proper (black arrow), along with atypical folding of the dentate gyrus (black arrowheads). Scale bar = 1 mm. (c) Injection site ectopia (Batch 1, shRNA treated n = 8, Sham n = 6; Batch 2, shRNA treated n = 9, Sham n = 8), resulting from the injection puncture wound. These anomalies were observed as collections of ectopic cells in Layer 1 of the cortex (black arrows). Scale bar = 1 mm.
In Batch 2, post mortem tissue analysis revealed 12 sham subjects and 20 Kiaa0319 RNAi subjects. Three categories of cortical disruption similar to those described in Batch 1 were observed: (1) Injection site ectopia (shRNA treated n = 9, sham n = 8). (2) Unmigrated neurons in white matter (shRNA treated n = 20, sham n = 0). And (3) Hippocampal dysplasia (shRNA treated n = 13, sham n = 0). As with Batch 1, statistical analyses revealed no significant differences between sham animals with and without Injection site ectopia, and so sham subjects remained pooled for all subsequent analyses.
Note that in addition to being analyzed as a function of Treatment (Kiaa0319 shRNA vs. Sham) all data were analyzed with the Kiaa0319 shRNA animals divided into these histological subgroups. However, with the exception of the data from Morris water maze testing (see Section 3.5), all Kiaa0319 shRNA animals performed all behavioral tasks equivalently to each other (and worse than shams)—regardless of histological subgroup. Thus, histological subgroups did not account for any significant main effects and so those data are not reported here.
3.2. Auditory processing: normal single tone, P25–26
Paired samples t-tests were used to compare the mean cued scores and the mean uncued scores on the NST tasks. Analyses revealed significant attenuation of the startle response on cued trials in both shams and Kiaa0319 shRNA treated animals on each normal single tone task (p < .05), and a one way ANOVA with Treatment (2 levels) showed no main effect on ATT scores (the ratio of cued response to uncued response) for either task [F(2, 24) < 1, NS; F(1, 25) < 1, NS]. These results indicate that there was no significant difference between the Treatment groups on a basic auditory discrimination task, indicating that Kiaa0319 shRNA treated animals did not have significant impairments in baseline hearing, frequency discrimination or prepulse inhibition.
3.3. Auditory processing: long/short silent gap, P29–40
Analysis of mean cued and uncued scores on the long and short silent gap tasks showed that both Kiaa0319 shRNA and sham treated subjects were able to significantly detect silent gaps down to 5 ms. A repeated measures ANOVA on ATT scores for Long silent gap (Treatment (2 levels) × Day (4 levels) × Gap (9 levels)) revealed no main effect of Treatment [F(1, 22) < 1, NS], indicating similar long gap detection abilities between the two groups. Significant Day [F(4, 88) > 100, p < .001] and Gap [F(8, 176) > 100, p < .001] effects indicated that average performance of all animals significantly improved across the four days of testing and as the silent gaps increased in duration.
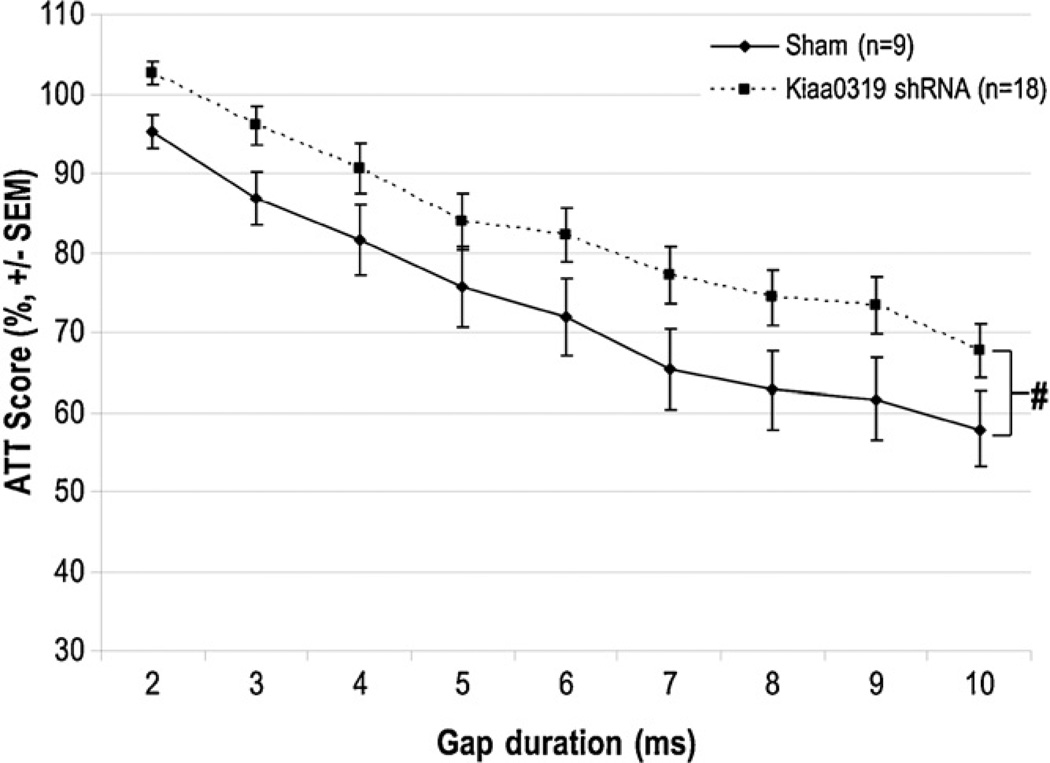
A repeated measures ANOVA was also computed for the ATT scores from the short silent gap task. Similar to the long silent gap task, significant day [F(4, 100) > 10, p < .001] and Gap [F(8, 200) > 100, p < .001] effects indicated that on average all animals improved across the five days of testing and as the gap length increased. A marginal effect of Treatment was detected, indicating a trend for the Kiaa0319 shRNA animals to perform slightly worse than shams [F(1, 25) = 3.7, p = .07], as indicated by their higher ATT scores (see Fig. 4). Interestingly, this trend was absent upon retesting in adulthood [F(1, 25) < 1, NS], which is consistent with evidence that more difficult RAP tasks are required to elicit deficits in adults (Friedman et al., 2004).
Fig. 4.
Juvenile short silent gap attenuation scores. This graph illustrates a near significant effect of treatment on detection of short silent gaps in background white noise. The Kiaa0319 shRNA treated animals performed marginally worse than Shams on all gaps, as indicated by their higher attenuation scores. This effect was ameliorated in adulthood (data not shown) (# indicates p = .07).
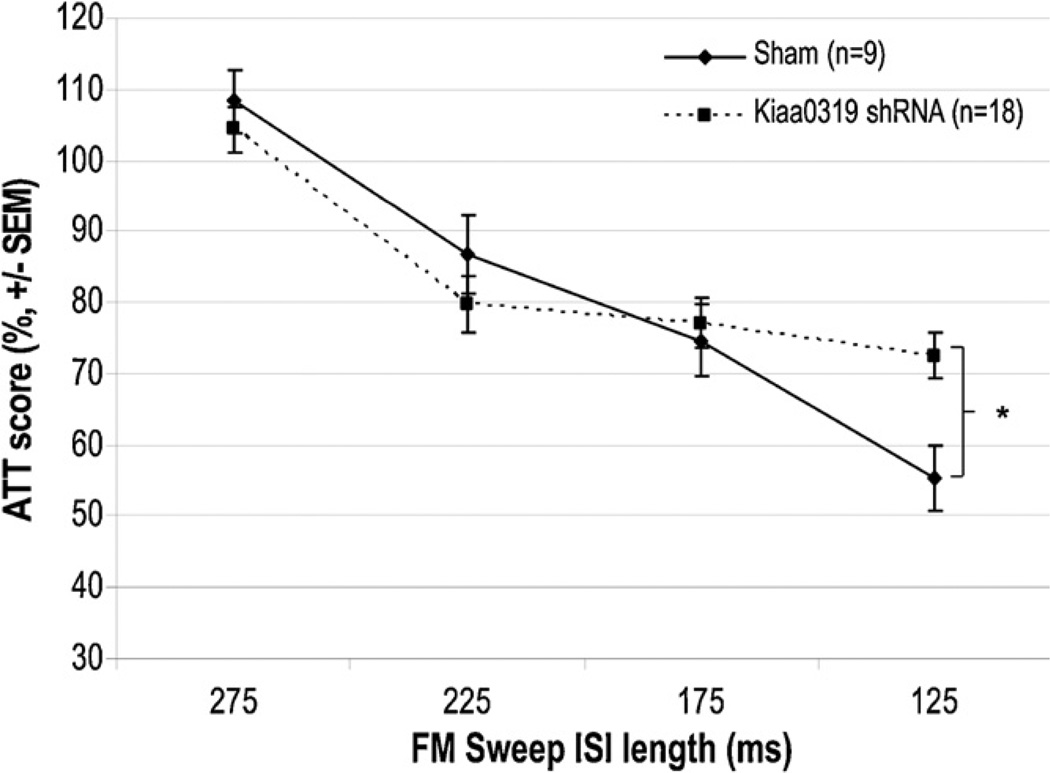
3.4. Auditory processing: FM sweep, P60+
An overall repeated measures ANOVA on FM sweep ATT scores (Treatment (2 levels) × ISI (4 levels)) revealed no significant main effect of Treatment [F(1, 25) < 1, NS]. However, a significant Treatment × ISI interaction was found, reflecting the fact that the performance of the two treatment groups differed as a function of the inter-stimulus interval presented [F(3, 75) = 3.7, p < .05] (Fig. 5). Specifically, simple effects analysis revealed a significant Treatment effect at the shortest interstimulus interval of 125 ms, with the Kiaa0319 shRNA animals showing significantly less startle response attenuation than shams [One way ANOVA: F(2, 26) = 4.6, p < .01] (Fig. 5). Shams and Kiaa0319 shRNA animals performed similarly at the three longer FM sweep ISIs.
Fig. 5.
Adult FM sweep attenuation scores. While there was not an overall effect of treatment on FM sweep performance, there was a significant Treatment × ISI interaction (p = .02). Post-hoc analysis revealed that Kiaa0319 RNAi animals had significantly more trouble detecting the stimulus than shams only when the duration of the sweep was shortened to 125 ms (* indicates p < .01).
3.5. Water escape and Morris water maze, P60+
For the analysis of the water escape and Morris water maze, the subjects were separated a priori into three groups. Based on the well-documented evidence of the role of the hippocampus in spatial navigation tasks (see Poucet et al., 2010), the subjects were divided into Shams (n = 9), Kiaa0319 shRNA without hippocampal malformations (n = 9), and Kiaa0319 shRNA with hippocampal malformations (n = 9). A one-way ANOVA revealed no significant differences in water escape latency among the three groups, indicating similar baseline performance of the motor and motivational aspects of a swim escape task [F(2, 26) < 1, NS]. For the Morris water maze data, a repeated measures ANOVA (Treatment (3 levels) × Day (5 levels)) was carried out to compare average swim speed across all four trials throughout five days of testing. The analysis revealed no main effects of Day [F(4, 96) = 1.559, NS], Treatment [F(2, 24) < 1, NS], nor Day × Treatment interaction [F(8, 96) < 1, NS], indicating that all animals swam at roughly the same speed throughout testing and thus exhibited no significant gross motor differences.
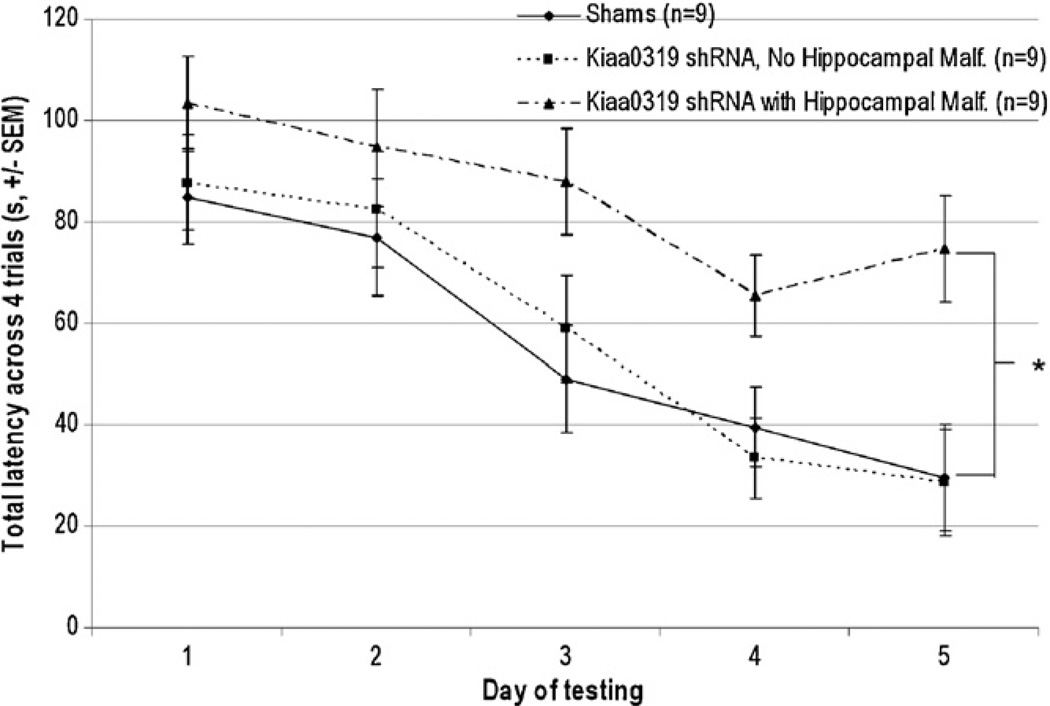
To assess differences in spatial learning, a repeated measures ANOVA (Treatment (3 levels) × Day (5 levels)) was carried out to compare total latency to find the platform among the three treatment groups across all four trials throughout five days of testing. The analysis revealed main effects of Day [F(4, 96) = 27.601, p < .001] and Treatment [F(2, 24) = 4.7, p < .05], indicating that, while all groups increased their latency over days of testing, significant differences in total latency to reach the platform were present (Fig. 6). Separate Treatment (2 levels) × Day (5 levels) analyses revealed that Kiaa0319 shRNA treated animals with hippocampal malformations took significantly longer to locate the platform than shams [F(1, 16) = 6.1, p < .05], and also took marginally longer than Kiaa0319 shRNA without hippocampal malformations [F(1, 16) = 4.5, p = .051]. There was no significant difference in MWM performance between shams and Kiaa0319 shRNA animals without hippocampal malformations [F(1, 16) < 1, NS]. A repeated measures ANOVA (Treatment (3 levels) × Day (5 levels)) comparing average path length to reach the goal revealed similar results, with a significant effect of Day [F(4, 96) = 32.853, p < .001] and a marginal effect of Treatment [F(2, 24) = 2.722, p=.086], with the Kiaa0319 shRNA animals with hippocampal malformations tending to swim longer distances to find the platform across all five days than both Kiaa0319 shRNA animals without hippocampal malformations and shams (who swam equivalent distances throughout testing).
Fig. 6.
Morris water maze, latency to reach platform. A Treatment (3 levels) × Day (5 levels) ANOVA revealed that Kiaa0319 shRNA treated animals with hippocampal malformations took significantly longer to locate the platform than both shams and Kiaa0319 shRNA treated animals without hippocampal malformations. This effect was present despite equivalent swim speed (* indicates p < .05).
3.6. Radial arm water maze: errors to find the platform, P40–110
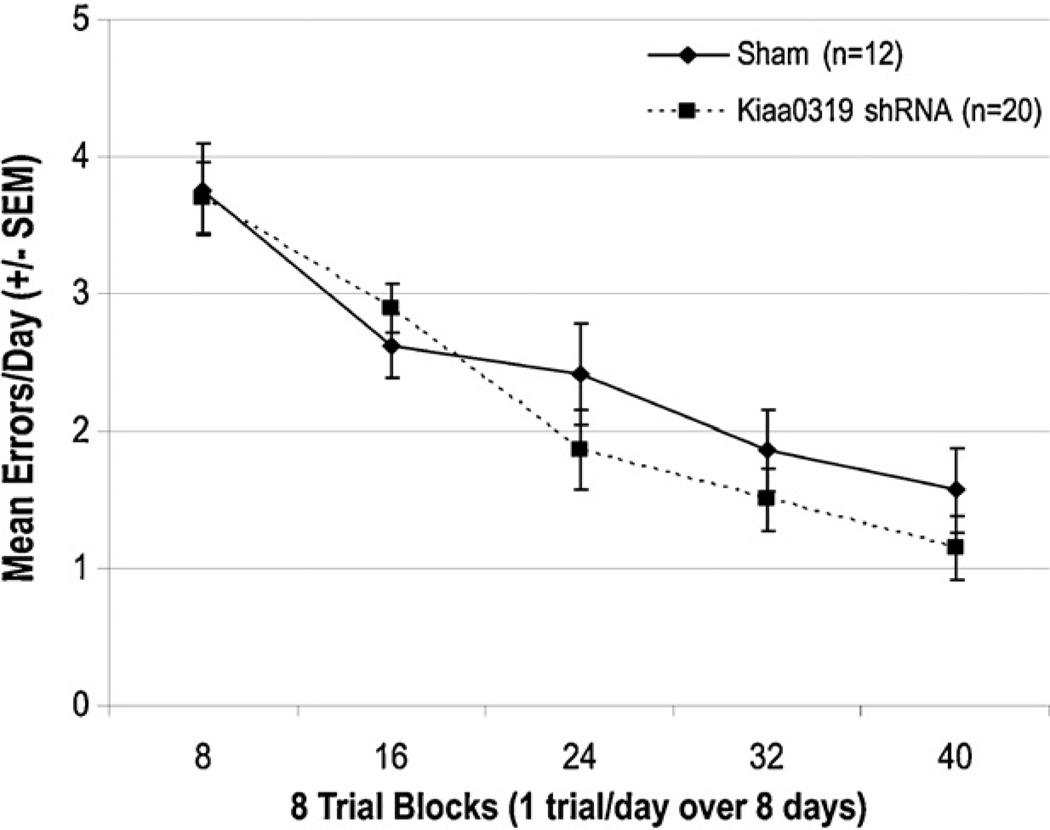
Analysis of the mean number of incorrect arm entries (errors) made during the test trial across all 10 weeks of testing revealed no significant effect of Treatment [F(1, 30) < 1, NS] (Fig. 7). This indicates that the Kiaa0319 shRNA treated animals learned and performed the task in a similar manner to shams. The results were also analyzed with the animals of Batch 2 divided into three treatment groups: Shams n = 12, Kiaa0319 shRNA animals without hippocampal malformations n = 7, Kiaa0319 shRNA animals with hippocampal malformations n = 13. Again, no significant effect of Treatment was detected, indicating that the presence or absence of hippocampal malformations did not significantly affect performance on this task [F(2, 29) < 1, NS]. There was a significant Block effect (referring to two week blocks of testing), which indicates that significant learning of the task occurred across the 10 weeks of testing [F(4, 120) = 33.301, p < .001]. There was no Block ×Treatment interaction, indicating that the treatment groups all learned the task at a similar pace. Importantly, these scores match closely with baseline error scores observed for comparably treated intact male rats on this same task run over the same time period in a previous study (Szalkowski et al., 2011).
Fig. 7.
Mean errors made by Kiaa0319 shRNA treated animals and Shams across five 8-trial blocks (10 weeks/40 days) of testing. There was no effect of treatment on the average number of incorrect arm entries (errors) made by Kiaa0319 shRNA treated subjects and shams, nor any Treatment × Block interaction. This indicates that the two treatment groups were able to perform the task in a similar manner across all weeks of testing.
4. Discussion
We found that in utero disruption of Kiaa0319 lead to disruptions in neuronal migration, and was associated with rapid auditory processing impairments, with no effect on simple spatial learning (Morris water maze) unless specific disruptions of hippocampus were present. Early interference with Kiaa0319 did not lead to deficits in spatial working memory in this study. This study provides support for the role of Kiaa0319 in neuronal migration and behavioral features that parallel those associated with dyslexia in humans.
4.1. Kiaa0319 RNAi disrupts neuronal migration
The observation that transfection with Kiaa0319 shRNA leads to disrupted neuronal migration is a replication of previous results (Paracchini et al., 2006; Peschansky et al., 2010). As previously discussed, the majority of transfected cells (that migrated into the cortex) in both Kiaa0319 shRNA and sham animals were found in regions of parietal cortex. Kiaa0319 shRNA transfected neurons that failed to migrate into the cortical plate were found in heterotopic collections in white matter, or subcortical structures such as the hippocampus, subjacent to parietal cortex. This was a result of the position of the electrodes during electroporation (see LoTurco et al., 2009). It is worth noting that previous reports of other animal models of early brain injury—specifically focal freezing lesions to the cortex—demonstrated that the location of the lesion (frontal, parietal, or occipital cortex) had no effect on auditory processing abilities (Herman et al., 1997). Moreover, lesions in all three areas of cortex were associated with similar changes in cell size within the medial geniculate nucleus of the thalamus. Thus, it seems possible that early disruptions to the developing cortex (such as focal freezing lesions, or RNA interference induced malformations) trigger a top-down cascade of reorganizational events distal to the malformations themselves, and that timing of the injury may be more salient to the resultant behavioral outcome than the exact location of the injury. This may also account for the observation that, in shRNA-transfected animals, disparate heterotopic malformations ultimately give rise to similar deficits in rapid auditory processing and simple spatial learning. Further analysis is needed to confirm this hypothesis, however.
4.2. Kiaa0319 RNAi behavioral results: rapid auditory processing
Rapid auditory processing is defined as an inability to detect rapidly presented acoustic cues (verbal and non-verbal). RAP has been associated with dyslexia and other language impairments (Benasich et al., 2006; Boets et al., 2011; Boscariol et al., 2010; Cohen-Mimran and Sapir, 2007; Gaab et al., 2007; King et al., 2008; Vandermosten et al., 2011). Here we report that Kiaa0319 shRNA treated animals have impaired rapid auditory processing as compared to shams. Specifically, in the juvenile period, Kiaa0319 shRNA animals showed no deficits on long gap detection tasks, although a marginal effect of Treatment was seen for shorter gaps (see Fig. 4). Upon re-testing in adulthood this effect was absent, with Kiaa0319 shRNA and sham subjects performing equivalently. However, in adulthood the Kiaa0319 shRNA treated animals performed significantly worse than shams when presented with the more complex FM sweep task (Fig. 5). This suggests that task difficulty is an important factor in eliciting a deficit, which has been reported in previous studies using this modified prepulse inhibition paradigm in rodents (Fitch et al., 2008a; Friedman et al., 2004). Additionally, the juvenile short silent gap and adult FM sweep data suggest that RAP deficits in Kiaa0319 shRNA treated animals are subtle but significant. Importantly, the lack of significant impairment on the easier tasks (normal single tone; long silent gap) presented during the juvenile and adult periods indicate that the Kiaa0319 shRNA animals are not globally impaired in auditory processing as compared to shams. That is, when the task was easy enough, the two groups performed similarly. However, a complex rapid auditory processing task (FM sweep) was able to elicit a significant deficit in the Kiaa0319 shRNA animals, with deficits exclusively at the shortest stimulus duration. Taken together, our results indicate that task complexity and temporal threshold are both important factors in eliciting a RAP deficit in Kiaa0319 shRNA treated animals.
These results parallel evidence of RAP deficits in dyslexic and language impaired individuals. In these populations, auditory processing impairments are also observed to be subtle and specific, rather than broad and global. Additionally, in human studies rapid auditory processing deficits can also be elicited as a function of task complexity (i.e., simple gap detection tasks versus more complex frequency discrimination tasks) or as a function of temporal demands of the task (i.e., rapid versus slow presentation of stimuli). These specific deficits in RAP have been correlated with measures of reading ability in dyslexic and language impaired populations (Benasich et al., 2006; Boets et al., 2011; Boscariol et al., 2010; Cohen-Mimran and Sapir, 2007; Gaab et al., 2007; Fitch and Tallal, 2003; King et al., 2008; Vandermosten et al., 2011).
4.3. Kiaa0319 RNAi behavioral results: spatial learning
Results from the Morris water maze are similar to those reported byThrelkeld et al. (2007) for Dyx1c1. That is, shRNA treated animals with malformations in the hippocampus took significantly longer to locate the platform in the Morris water maze than shams and shRNA treated animals without hippocampal malformations, in spite of comparable swim speeds (Fig. 6). This finding is not surprising, given the well-established role of the hippocampus in spatial navigation (Poucet et al., 2010 for review). The lack of an overall significant Treatment effect on this task suggests that the observed auditory processing deficits are domain-specific, and are not due to a generalized learning impairment.
4.4. Kiaa0319 RNAi behavioral results: working memory
Working memory is another cognitive domain that is affected in developmental dyslexia (Gathercole et al., 2006; Jeffries and Everatt, 2004; Marino et al., 2007; Smith-Spark and Fisk, 2007). Recent work from our lab has shown that in utero RNAi against Dyx1c1 leads to impairment in spatial working memory in Sprague–Dawley rats as measured by performance on this same radial arm water maze delayed match to sample task (Szalkowski et al., 2011). Interestingly, the current results were different from the patterns observed in Dyx1c1 shRNA rats, with the Kiaa0319 shRNA treated animals performing the task as well as shams throughout testing (see Fig. 5). Moreover, the presence of hippocampal malformations did not make a significant difference in performance of this task (as it did in the Morris water maze with the Kiaa0319 shRNA treated animals from Batch 1). Interestingly, to date, there is no clinical evidence for or against linking Kiaa0319 variants to memory impairment. This stands in contrast to DYX1C1, which has been significantly associated with short-term memory deficits in some dyslexic populations (Dahdouh et al., 2009; Marino et al., 2007).
4.5. Defining dyslexia: intermediate phenotypes and genetics
Recently the focus of genetic and epidemiological dyslexia research has shifted from categorical diagnosis to identifying subtypes of the disorder, characterized by specific behavioral impairments (see Grigorenko, 2009 for review). Recent studies have defined “intermediate phenotypes”—such as impairments in rapid auditory processing, phonological processing, or short-term memory—that associate with developmental dyslexia. This shift in focus has had an important impact on the interpretation of the results of genetic association studies, since the classic expectation of genetic disease studies has been to find a specific gene that associates with a specific disorder. Conversely, as we have previously discussed, several genes have been identified as dyslexia-risk genes (Francks et al., 2004; Hannula-Jouppi et al., 2005; Konig et al., 2011; Meng et al., 2005; Poelmans et al., 2009; Scerri et al., 2010; Taipale et al., 2003). Moreover, associations between these genes and dyslexia are variable, with many studies reporting failures to replicate previously-reported associations in different populations (Bellini et al., 2005; Marino et al., 2005; Scerri et al., 2004).
In response to this inconsistency, some researchers have begun to search for linkage between genomic regions and core processes that are deficient in disorders like developmental dyslexia. The first identified dyslexia risk gene, DYX1C1, was derived from a single family (Taipale et al., 2003). Efforts to replicate the association in broader dyslexic populations were inconsistent. One negative finding came from Marino et al., 2007, who failed to find an association between DYX1C1 and the categorical diagnosis of developmental dyslexia in an Italian population (2005). In 2007, Marino et al., 2007 looked again at DYX1C1 in their dyslexic population, but specifically in relation to deficits in intermediate phenotypes of the disorder: text reading, single word reading, single nonword reading, single word spelling, single nonword spelling, orthographic coding, and auditory short-term memory. Interestingly, DYX1C1 was significantly associated with impaired short-term memory in the dyslexic population, in spite of the lack of association to the categorical diagnosis of dyslexia. A similar association was detected between DYX1C1 variants and the memory impairment phenotype in German dyslexics (Dahdouh et al., 2009). Other studies have linked Kiaa0319 variants to similarly specific language-related processes, such as orthographic choice, phonological decoding, spelling, and phoneme awareness (Francks et al., 2004; Harold et al., 2006; Newbury et al., 2011; Rice et al., 2009). Perhaps even more compelling are the recent findings that suggest that these genephenotype relationships exist in unaffected populations as well (Luciano et al., 2007; Paracchini et al., 2008).
4.6. Defining dyslexia: comparing risk genes
The focus on dyslexia as a variable constellation of intermediate phenotypes, and the growing body of evidence suggesting relationships between genes and specific phenotypes are compelling epidemiological and molecular biology research to define CDSGs in terms of their cellular function and relevance to specific cognitive abilities. In this context, the observed similarities and differences between the effects of Kiaa0319 knockdown and Dyx1c1 knockdown raise interesting points for consideration. All studies to date have reported similar types of neural malformation following interference with Kiaa0319 and Dyx1c1 in rodents. For example, interference with either gene results in disruptions of neuronal migration in the cerebral cortex and hippocampus (Peschansky et al., 2010; Rosen et al., 2007). Yet evidence of different behavioral effects suggests that these patterns may be oversimplified. For example, transfection with Dyx1c1 shRNA, but not Kiaa0319 shRNA, has been shown to create spatial working memory impairments in Sprague–Dawley rats. Conversely, transfection with Dyx1c1 shRNA and transfection with Kiaa0319 shRNA have both been linked to auditory processing impairments—though different in characterization—in Wistar rats. Specifically, animals transfected with Dyx1c1 shRNA exhibited impairments at both long and short stimulus durations on a complex RAP task, while the current data shows that Kiaa0319 shRNA transfection leads to deficits that are specific to rapid stimulus presentation (Threlkeld et al., 2007). Moreover, there are differences in the ways that disruption of each gene affects neuronal migration. For example, cortical neurons transfected with Kiaa0319 shRNA displayed a largely orthogonal orientation with respect to radial glial fibers (Paracchini et al., 2006). This has not been observed after Dyx1c1 RNAi. Also, while neither protein’s function is fully understood, important structural clues suggest that Dyx1c1 and Kiaa0319 play different cellular roles in neuronal migration. For example, it has been proposed that the DYX1C1 protein is important for cell dynamics and protein-protein interactions, whereas KIAA0319 may play a role in cell-cell adhesion (potentially including the adhesion that mediates neuron-glia interactions during migration; Velayos-Baeza et al., 2007, 2008, 2010; Wang et al., 2006).
5. Conclusion
The current results provide evidence for a role for Kiaa0319 in rapid auditory processing, and provide further evidence for its role in neuronal migration. Our results provide an interesting comparison between the behavioral and neuroanatomical impact of early interference with Kiaa0319 and other CDSGs. Future studies of Kiaa0319 interference will attempt to hone in on a more defined temporal threshold necessary to elicit a behavioral deficit in rapid auditory processing, and may be able to further delineate genetic–neural–behavioral relationships through the comparison of different timing of injections (transfecting different cortical layers) and/or conditional knockdowns and knockouts that restrict the genetic loss to specific regions or structures in the brain. Further stereological characterization of the neuroanatomical changes that result from early interference with this gene will also help us understand the mechanism by which it influences sensory and cognitive abilities pertinent to language and reading.
Acknowledgments
The authors wish to thank Dr. James Chrobak at the University of Connecticut for his contributions to this manuscript. This work was supported by NIH grant P01HD57853.
Abbreviations
- CDSG
candidate dyslexia susceptibility gene
- RAP
rapid auditory processing
- RNAi
RNA interference
- shRNA
short hairpin RNA
- RFP
red fluorescent protein
- GFP
green fluorescent protein
- SES
startle eliciting stimulus
- ATT
attenuation
- ISI
inter-stimulus interval
- ITI
inter-trial interval.
References
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat. Neurosci. 2003;6:1277–1283. doi: 10.1038/nn1153. [DOI] [PubMed] [Google Scholar]
- Bellini G, Bravaccio C, Calamoneri F, Cocuzza MD, Fiorillo P, Gagliano A, Mazzone D, Miraglia del Guidice E, Scuccimarra G, Militerni R, Pascotto A. No evidence for association between dyslexia andDYX1C1functional variants in a group of children and adolescents from southern Italy. J. Mol. Neurosci. 2005;27:311–314. doi: 10.1385/jmn:27:3:311. [DOI] [PubMed] [Google Scholar]
- Benasich AA, Choudhury N, Friedman JT, Realpe-Bonilla T, Chojnowski C, Gou Z. The infant as a prelinguistic model for language learning impairments: predicting from event-related potentials to behavior. Neuropsychologia. 2006;44:396–411. doi: 10.1016/j.neuropsychologia.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneventi H, Tonnessen FE, Ersland L, Hugdahl K. Working memory deficit in dyslexia: behavioral and fMRI evidence. Int. J. Neurosci. 2010;120:51–59. doi: 10.3109/00207450903275129. [DOI] [PubMed] [Google Scholar]
- Bishop DVM. Genes, cognition, and communication: insights from neurodevelopmental disorders. Ann N.Y. Acad. Sci. 2009;1156:1–18. doi: 10.1111/j.1749-6632.2009.04419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boets B, Vandermosten M, Poelmans H, Luts H, Wouters J, Ghesquiere P. Preschool impairments in auditory processing and speech perception uniquely predict future reading problems. Res. Dev. Disabil. 2011;32:560–570. doi: 10.1016/j.ridd.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Boscariol M, Guimaraes CA, Hage SR, Cendes F, Guerreiro MM. Temporal auditory processing: correlation with developmental dyslexia and cortical malformation. Pro. Fono. 2010;22:537–542. doi: 10.1590/s0104-56872010000400030. [DOI] [PubMed] [Google Scholar]
- Burbridge TJ, Wang Y, Volz AJ, Peschansky VJ, Lisann L, Galaburda AM, LoTurco JJ, Rosen GD. Postnatal analysis of the effect of embryonic knockdown and overexpression of candidate dyslexia susceptibility gene homologDcdc2in the rat. Neuroscience. 2008;152:723–733. doi: 10.1016/j.neuroscience.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon LR, Smith SD, Fulker DW, Kimberling WJ, Pennington BF, DeFries JC. Quantitative trait locus for reading disability on chromosome 6. Science. 1994;266:276–279. doi: 10.1126/science.7939663. [DOI] [PubMed] [Google Scholar]
- Cardon LR, Smith SD, Fulker DW, Kimberlin WJ, Pennington BF, DeFries JC. Quantitative trait locus for reading disability: correction. Science. 1995;268:1553. doi: 10.1126/science.7777847. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Hinman JR, Sabolek HR. Revealing past memories: proactive interference and ketamine-induced memory deficits. J. Neurosci. 2008;28:4512–4520. doi: 10.1523/JNEUROSCI.0742-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements KM, Wainwright PE. Spontaneously hypertensive Wistar-Kyoto and Sprague-Dawley rats differ in performance on a win-shift task in the water radial arm maze. Behav. Brain Res. 2006;167:295–304. doi: 10.1016/j.bbr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Cohen-Mimran R, Sapir S. Auditory temporal processing deficits in children with reading disabilities. Dyslexia. 2007;13:175–192. doi: 10.1002/dys.323. [DOI] [PubMed] [Google Scholar]
- Cope N, Harold D, Hill G, Moskvina V, Stevenson J, Holmans P, Owen MJ, O’Donovan MC, Williams J. Strong evidence thatKIAA0319on chromosome 6p is a susceptibility gene for developmental dyslexia. Am. J. Hum. Genet. 2005;76:581–591. doi: 10.1086/429131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahdouh F, Anthoni H, Tapia-Paez I, Peyrard-Janvid M, Schulte-Korne G, Warnke A, Remschmidt H, Ziegler A, Kere J, Muller-Myhsok B, Nothen MM, Schumacher J, Zucchelli M. Further evidence forDYX1C1as a susceptibility factor for dyslexia. Psychiat. Genet. 2009;19:59–63. doi: 10.1097/YPG.0b013e32832080e1. [DOI] [PubMed] [Google Scholar]
- Deffenbacher KE, Kenyon JB, Hoover DM, Olson RK, Pennington BF, DeFries JC, Smith SD. Refinement of the 6p21.3 quantitative trait locus influencing dyslexia: linkage and association analyses. Hum. Genet. 2004;115:128–138. doi: 10.1007/s00439-004-1126-6. [DOI] [PubMed] [Google Scholar]
- Elbert A, Lovett MW, Cate-Carter T, Pitch A, Kerr EN, Barr CL. Genetic variation in theKIAA03195’ region as a possible contributor to dyslexia. Behav. Genet. 2011;41:77–89. doi: 10.1007/s10519-010-9434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraday MM, Grunberg NE. The importance of acclimation in acoustic startle amplitude and pre-pulse inhibition testing of male and female rats. Pharmacol. Biochem. Behav. 2000;66:375–381. doi: 10.1016/s0091-3057(00)00212-4. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Marlow AJ, Lamb J, Maestrini E, Williams DF, Richardson AJ, Weeks DE, Stein JF, Monaco AP. A quantitative-trait locus on chromosome 6p influences different aspects of developmental dyslexia. Am. J. Hum. Genet. 1999;64:146–156. doi: 10.1086/302190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SE, DeFries JC. Developmental dyslexia: genetic dissection of a complex cognitive trait. Nat. Rev. Neurosci. 2002;3:767–780. doi: 10.1038/nrn936. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Francks C. Genes, cognition and dyslexia: learning to read the genome. Trends Cogn. Sci. 2006;10:250–257. doi: 10.1016/j.tics.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Brown CP, Rosen GD, Tallal P. Effects of sex and MK-801 on auditory-processing deficits associated with developmental microgyric lesions in rats. Behav. Neurosci. 1997;111:404–412. doi: 10.1037//0735-7044.111.2.404. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Tallal P. Neural mechanisms of language-based learning impairments: insights from human populations and animal models. Behav. Cogn. Neurosci. Rev. 2003;2:155–178. doi: 10.1177/1534582303258736. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Threlkeld SW, McClure MM, Peiffer AM. Use of a modified prepulse inhibition paradigm to assess complex auditory discrimination in rodents. Brain Res. Bull. 2008a;76:1–7. doi: 10.1016/j.brainresbull.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch RH, Breslawski H, Rosen GD, Chrobak JJ. Persistent spatial working memory deficits in rats with bilateral cortical microgyria. Behav. Brain Funct. 2008b;4:45. doi: 10.1186/1744-9081-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery KA, Liederman J, Daly L, Schultz J. Male prevalence for reading disability is found in a large sample of black and white children free from ascertainment bias. J. Int. Neuropsychol. Soc. 2000;6:433–442. doi: 10.1017/s1355617700644016. [DOI] [PubMed] [Google Scholar]
- Francks C, Paracchini S, Smith SD, Richardson AJ, Scerri TS, Cardon LR, Marlow AJ, MacPhie L, Walter J, Pennington BF, Fisher SE, Olson RK, DeFries JC, Stein JF, Monaco AP. A 77-kilobase region of chromosome 6p22.2 is associated with dyslexia in families from the United Kingdom and from the United States. Am. J. Hum. Genet. 2004;75:1046–1058. doi: 10.1086/426404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JT, Peiffer AM, Clark MG, Benasich AA, Fitch RH. Age and experience-related improvements in gap detection in the rat. Dev. Brain Res. 2004;152:83–91. doi: 10.1016/j.devbrainres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Gaab N, Gabrieli JD, Deutsch GK, Tallal P, Temple E. Neural correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: an fMRI study. Restor. Neurol. Neurosci. 2007;25:295–310. [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Ann. Neurol. 1985;18:222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Alloway TP, Willis C, Adams A. Working memory in children with reading disabilities. J. Exp. Child Psychol. 2006;93:265–281. doi: 10.1016/j.jecp.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Gayan J, Olson RK. Genetic and environmental influences on orthographic and phonological skills in children with reading disabilities. Dev. Neuropsychol. 2001;20:483–507. doi: 10.1207/S15326942DN2002_3. [DOI] [PubMed] [Google Scholar]
- Grigorenko EL, Wood FB, Meyer MS, Hart LA, Speed WC, Shuster A, Pauls DL. Susceptibility loci for distinct components of developmental dyslexia on chromosomes 6 and 15. Am. J. Hum. Genet. 1997;60:27–39. [PMC free article] [PubMed] [Google Scholar]
- Grigorenko EL. At the height of fashion: what genetics can teach us about neurodevelopmental disabilities. Curr. Opin. Neurol. 2009;22:126–130. doi: 10.1097/WCO.0b013e3283292414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula-Jouppi K, Kaminen-Ahola N, Taipale M, Eklund R, Nopola-Hemmi J, Kaariainen H, Kere J. The axon guidance receptor geneROBO1is a candidate gene for developmental dyslexia. PLoS Genet. 2005;1:e50. doi: 10.1371/journal.pgen.0010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold D, Paracchini S, Scerri T, Dennis M, Cope N, Hill G, Moskvina V, Walter J, Richardson AJ, Owen MJ, Stein JF, Green ED, O’Donovan MC, Williams J, Monaco AP. Further evidence that theKIAA0319gene confers susceptibility to developmental dyslexia. Mol. Psychiatry. 2006;11:1085–1091. doi: 10.1038/sj.mp.4001904. [DOI] [PubMed] [Google Scholar]
- Herman AE, Galaburda AM, Fitch RH, Carter AR, Rosen GD. Cerebral microgyria, thalamic cell size, and auditory temporal processing in male and female rats. Cereb. Cortex. 1997;7:453–464. doi: 10.1093/cercor/7.5.453. [DOI] [PubMed] [Google Scholar]
- Jeffries S, Everatt J. Working memory: its role in dyslexia and other specific learning difficulties. Dyslexia. 2004;10:196–214. doi: 10.1002/dys.278. [DOI] [PubMed] [Google Scholar]
- Kang C, Drayna D. Genetics of speech and language disorders. Annu. Rev. Genomics Hum. Genet. 2011;12:5.1–5.20. doi: 10.1146/annurev-genom-090810-183119. [DOI] [PubMed] [Google Scholar]
- Katusic SK, Colligan RC, Barbaresi WJ, Schaid DJ, Jacobsen SJ. Incidence of reading disability in a population-based birth cohort: 1976–1982, Rochester, Minn. Mayo Clin. Proc. 2001;76:1081–1092. doi: 10.4065/76.11.1081. [DOI] [PubMed] [Google Scholar]
- King B, Wood C, Faulkner D. Sensitivity to visual and auditory stimuli in children with developmental dyslexia. Dyslexia. 2008;14:116–141. doi: 10.1002/dys.349. [DOI] [PubMed] [Google Scholar]
- Konig IR, Schumacher J, Hoffmann P, Kleensang A, Ludwig KU, Grimm T, Neuhoff N, Preis M, Roeske D, Warnke A, Propping P, Remschmidt H, Nothen MM, Ziegler A, Muller-Myhsok B, Schulte-Korne G. Mapping for dyslexia and related cognitive trait loci provides strong evidence for further risk genes on chromosome 6p21. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156:36–43. doi: 10.1002/ajmg.b.31135. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Manent JB, Sidiqi F. New and improved tools forin uteroelectroporation studies of developing cerebral cortex. Cereb. Cortex. 2009;19:i120–i125. doi: 10.1093/cercor/bhp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Lind PA, Duffy DL, Castles A, Wright MJ, Montgomery GW, Martin NG, Bates TC. A haplotype spanningKIAA0319andTTRAPis associated with normal variation in reading and spelling ability. Biol. Psychiatry. 2007;62:811–817. doi: 10.1016/j.biopsych.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Marino C, Giorda R, Lorusso ML, Vanzin L, Salandi N, Nobile M, Citterio A, Beri S, Crespi V, Battaglia M, Molteni M. A family-based association study does not supportDYX1C1on 15q21.3 as a candidate gene in developmental dyslexia. Eur. J. Hum. Genet. 2005;13:491–499. doi: 10.1038/sj.ejhg.5201356. [DOI] [PubMed] [Google Scholar]
- Marino C, Citterio A, Giorda R, Facoetti A, Menozzi G, Vanzin L, Lorusso ML, Nobile M, Molteni M. Association of short-term memory with a variant withinDYX1C1in developmental dyslexia. Genes Brain Behav. 2007;6:640–646. doi: 10.1111/j.1601-183X.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- Meng H, Smith SD, Hager K, Held M, Liu J, Olson RK, Pennington BF, DeFries JC, Gelernter J, O’Reilly-Pol T, Somlo S, Skudlarski P, Shaywitz SE, Shaywitz BA, Marchione K, Wang Y, Paramasivam M, LoTurco JJ, Page GP, Gruen JR. Proc. Natl. Acad. SciU.SA. 2005;102:17053–17058. doi: 10.1073/pnas.0508591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menghini D, Finzi A, Benassi M, Facoetti A, Giovagnoli S, Ruffino M, Vicari S. Different underlying neurocognitive deficits in developmental dyslexia: a comparative study. Neuropsychologia. 2010;48:863–872. doi: 10.1016/j.neuropsychologia.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Menghini D, Finzi A, Carlesimo GA, Vicari S. Working memory impairment in children with developmental dyslexia: is it just a phonological deficit? Dev. Neuropsychol. 2011;36:199–213. doi: 10.1080/87565641.2010.549868. [DOI] [PubMed] [Google Scholar]
- Newbury DF, Paracchini S, Scerri TS, Winchester L, Addis L, Richardson AJ, Walter J, Stein JF, Talcott JB, Monaco AP. Investigation of dyslexia and SLI risk variants in reading- and language-impaired subjects. Behav. Genet. 2011;41:90–104. doi: 10.1007/s10519-010-9424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paracchini S, Thomas A, Castro S, Lai C, Paramasivam M, Wang Y, Keating BJ, Taylor JM, Hacking DF, Scerri T, Francks C, Richardson AJ, Wade-Martins R, Stein JF, Knight JC, Copp AJ, LoTurco JJ, Monaco AP. The chromosome 6p22 haplotype associated with dyslexia reduces the expression ofKIAA0319a novel gene involved in neuronal migration. Hum. Mol. Genet. 2006;15:1659–1666. doi: 10.1093/hmg/ddl089. [DOI] [PubMed] [Google Scholar]
- Paracchini S, Scerri T, Monaco AP. The genetic lexicon of dyslexia. Annu. Rev. Genomics Hum. Genet. 2007;8:57–79. doi: 10.1146/annurev.genom.8.080706.092312. [DOI] [PubMed] [Google Scholar]
- Paracchini S, Steer CD, Buckingham L, Morris AP, Ring S, Scerri T, Stein J, Pembrey ME, Ragoussis J, Golding J, Monaco AP. Association of theKIAA0319dyslexia susceptibility gene with reading skills in the general population. Am. J. Psychiatry. 2008;165:1576–1584. doi: 10.1176/appi.ajp.2008.07121872. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Rosen GD, Fitch RH. Sex differences in rapid auditory processing deficits in ectopic BXSB/MpJ mice. Neuroreport. 2002;13:2277–2280. doi: 10.1097/00001756-200212030-00021. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Rosen GD, Fitch RH. Sex differences in rapid auditory processing deficits in microgyric rats. Dev. Brain Res. 2004;148:53–57. doi: 10.1016/j.devbrainres.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Peschansky VJ, Burbridge TJ, Volz AJ, Fiondella CG, Wissner-Gross Z, Galaburda AM, LoTurco JJ, Rosen GD. Cereb. Cortex. 2010;20:884–897. doi: 10.1093/cercor/bhp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelmans G, Engelen JJM, Van Lent-Albrechts J, Smeets HJ, Schoenmakers E, Franke B, Buitelaar JK, Wuisman-Frerker M, Erens W, Steyaert J, Schrander-Stumpel C. Identification of novel dyslexia candidate genes through the analysis of a chromosomal deletion. Am. J. Med. Genet. Part B. 2009;150B:140–147. doi: 10.1002/ajmg.b.30787. [DOI] [PubMed] [Google Scholar]
- Poucet B, Alvernhe A, Hok V, Renaudienau S, Sargolini F, Save E. The hippocampus and the neural code of spatial memory. Biol. Aujourdhui. 2010;204:103–112. doi: 10.1051/jbio/2010009. [DOI] [PubMed] [Google Scholar]
- Rice ML, Smith SD, Gayan J. Convergent genetic linkage and associations to language, speech and reading measures in families or probands with specific language impairment. J. Neurodevelop. Disord. 2009;1:264–282. doi: 10.1007/s11689-009-9031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen GD, Bai J, Wang Y, Fiondella CG, Threlkeld SW, LoTurco JJ, Galaburda AM. Disruption of neuronal migration by RNAi ofDyx1c1results in neocortical and hippocampal malformations. Cereb. Cortex. 2007;17:2562–2572. doi: 10.1093/cercor/bhl162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Caspi A, Fergusson D, Horwood LJ, Goodman R, Maughan B, Moffitt TE, Meltzer H, Carroll J. Sex differences in developmental reading disability: new findings from 4 epidemiological studies. JAMA. 2004;291:2007–2012 . doi: 10.1001/jama.291.16.2007. [DOI] [PubMed] [Google Scholar]
- Scerri TS, Fisher SE, Francks C, MacPhie IL, Paracchini S, Richardson AJ, Stein JF, Monaco AP. Putative functional alleles ofDYX1C1are not associated with dyslexia susceptibility in a large sample of sibling paris from the UK. J. Med. Genet. 2004;41:853–857. doi: 10.1136/jmg.2004.018341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerri TS, Paracchini S, Morris A, MacPhie IL, Talcott J, Stein J, Smith SD, Pennington BF, Olson RK, DeFries JC, Monaco AP. Identification of candidate genes for dyslexia susceptibility on chromosome 18. PLoS ONE. 2010;5:e13712. doi: 10.1371/journal.pone.0013712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Spark JH, Fisk JE. Working memory functioning in developmental dyslexia. Memory. 2007;15:34–56. doi: 10.1080/09658210601043384. [DOI] [PubMed] [Google Scholar]
- Szalkowski CE, Hinman JR, Threlkeld SW, Wang Y, LePack A, Rosen GD, Chrobak JJ, LoTurco JJ, Fitch RH. Persistent spatial working memory deficits in rats followingin uteroRNAi ofDyx1c1. Genes Brain Behav. 2011;10:244–252. doi: 10.1111/j.1601-183X.2010.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale M, Kaminen N, Nopola-Hemmi J, Haltia T, Myllyluoma B, Lyytinen H, Muller K, Kaaranen M, Lindsberg PJ, Hannula-Jouppi K, Kere J. A candidate gene for developmental dyslexia encodes a nuclear tetratricopeptide repeat domain protein dynamically regulated in brain. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11553–11558. doi: 10.1073/pnas.1833911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallal P, Piercy M. Defects of non-verbal auditory perception in children with developmental aphasia. Nature. 1973;241:468–469. doi: 10.1038/241468a0. [DOI] [PubMed] [Google Scholar]
- Threlkeld SW, McClure MM, Bai J, Wang Y, LoTurco JJ, Rosen GD, Fitch RH. Developmental disruptions and behavioral impairments in rats followingin uteroRNAi ofDyx1c1. Brain Res. Bull. 2007;71:508–514. doi: 10.1016/j.brainresbull.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M, Boets B, Luts H, Poelmans H, Wouters J, Ghesquiere P. Impairments in speech and nonspeech sound categorization in children with dyslexia are driven by temporal processing difficulties. Res. Dev. Disabil. 2011;32:593–603. doi: 10.1016/j.ridd.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Velayos-Baeza A, Toma C, da Roza S, Paracchini S, Monaco AP. Alternative splicing in the dyslexia-associated geneKIAA0319Mamm. Genome. 2007;9:627–634. doi: 10.1007/s00335-007-9051-3. [DOI] [PubMed] [Google Scholar]
- Velayos-Baeza A, Toma C, Paracchini S, Monaco AP. The dyslexiaassociated geneKIAA0319encodes highly N- and O-glycosylated plasma membrane and secreted isoforms. Hum. Mol. Genet. 2008;17:859–871. doi: 10.1093/hmg/ddm358. [DOI] [PubMed] [Google Scholar]
- Velayos-Baeza A, Levecque C, Kobayashi K, Holloway ZG, Monaco AP. The dyslexia-associated KIAA0319 protein undergoes proteolytic processing with {gamma}-secretaste-independent intramembrane cleavage. J. Biol. Chem. 2010;285:40148–40162. doi: 10.1074/jbc.M110.145961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Paramasivam M, Thomas A, Bai J, Kaminen-Ahola N, Kere J, Voskuil J, Rosen GD, Galaburda AM, LoTurco JJ. DYX1C1 functions in neuronal migration in developing neocortex. Neuroscience. 2006;143:515–522. doi: 10.1016/j.neuroscience.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Duncan L, Smith SD, Keenan JM, Wadsworth S, DeFries JC, Olson RK. Understanding the complex etiologies of developmental disorders: behavioral and molecular genetic approaches. J. Dev. Behav. Pediatr. 2010;31:533–544. doi: 10.1097/DBP.0b013e3181ef42a1. [DOI] [PMC free article] [PubMed] [Google Scholar]