Abstract
Alzheimer's disease (AD) is a progressive, neurodegenerative disorder that impairs memory and semantic processing. AD patients and MCI patients at risk for AD show altered N400 ERP responses to incongruent visual and verbal stimuli. AD patients exhibit neuropathology in olfactory brain areas before cognitive symptoms, suggesting the potential for olfactory processing to reflect early pathology. Despite this, odor congruency has not been examined. We investigated odor-image congruency in older adults at genetic risk for AD. ApoE ε4 carriers and non-carriers were screened for anosmia, severe hyposmia, and dementia. Olfactory ERPs were measured 600-1300ms following odor-image pairs. Odors were each presented once congruently and once incongruently via olfactometer. Right dorsal and ventral sites were vulnerable to the ε4 allele, consistent with a compensation hypothesis. Pz amplitude differences on congruous and incongruous trials were larger in non carriers. Regression indicated that congruency showed very high sensitivity and specificity for correctly classifying ε4 carriers from non-carriers.
Keywords: Alzheimer's disease, ApoE ε4, olfactory ERP, semantic congruency, olfaction, smell impairment
1. Introduction
1.1 Alzheimer's Disease
Alzheimer's disease (AD) progressively debilitates emotion and memory systems in the brain. The disease first impairs entorhinal, transentorhinal and hippocampal regions, extending later throughout the neocortex. A critical progressive disconnection between the entorhinal cortex and the hippocampal formation occurs in AD that insulates subcortical processing from cortical association areas (Braak & Braak, 1995). The diagnosis of AD remains a post-mortem classification based upon the presence and distribution of histopathological features at autopsy, though clinical research has linked this disconnection to an assortment of deficits in encoding and retrieving information (Davis, Price, Kaplan, & Libon, 2002; Morgan, Nordin, & Murphy, 1995; Olichney et al., 2002). Very early AD pathology is also observed in the olfactory bulb and anterior olfactory nucleus, regions that project to the entorhinal cortex (Christen-Zaech, et al., 2003; Esiri & Wilcock, 1984; Ohm & Braak, 1987; Haberly & Price, 1978; Price et al., 1991; Reyes et al., 1985; Struble & Clark, 1992), suggesting the particular vulnerability of the olfactory system and the processing of olfactory information in AD.
The principal histopathological features of AD include neurofibrillary tangles and Aβ plaques. Tangles result from destabilization of the microtubule associated protein (MAP) tau, which, under normal conditions tau maintains the integrity of axonal transport pathways. Tau function is impeded in AD due to excessive interaction at phosphorylation sites, facilitating conformational change into a paired helical filament (PHF) with reduced binding affinity to the axon. The PHF elevates risk for detachment and toxic aggregates (Trojanowski & Lee, 1995; Bancher et al., 1989). Plaques, on the other hand, are formed through elevated levels of polymorphic beta-amyloid, an unstable peptide variant with greater susceptibility for plaque formation (Voet, Voet, & Pratt, 2008; Hardy & Selkoe, 2002).
1.2 The Apolipoprotein ε4 Allele
Notable interactions have been suggested between the apolipoprotein and AD histopathology. While involved in lipid transport throughout the body, the protein facilitates neural growth and repair in the CNS. The three isoforms found in humans—coded on chromosome 19 (ApoE ε2, ApoE ε3, and ApoE ε4)—have slightly different amino acid sequences. Both ApoE ε2 and ApoE ε3 share a common Cys112 residue that is substituted for Arg 112 in ApoE ε4. The ε4 and ε3 variants do, however, share an Arg158 residue not observed in E2 (Rocchi et al., 2003; Selkoe, 2001). These substitutions, though subtle, have widespread implications for the CNS function (Kitamura et al., 2004; Levi, Jongen-Relo, Feldon, & Michaelson, 2005).
The ε4 allele is a primary risk factor for late onset or sporadic AD. Homozygote carriers (ε4/ε4) have a higher risk and a lower mean age of onset than heterozygote carriers (ε4/ε3 or ε4/ε2), indicating the allele strongly influences AD pathogenesis (Rocchi et al., 2003). Researchers link ε4 expression to altered calcium dynamics, reduced resiliency to oxidative stress, and enhanced beta-amyloid aggregation in vitro (Buttini et al., 1999; Vienbergs, Everson, Sagara, & Masliah 2002; Hatters, Zhong, Rutenberg, Weisgraber, 2006; Mahley, Weisgraber, & Huang, 2006). Struble and colleagues have hypothesized that regeneration and repair of degenerating neurons in the CNS are compromised in ε4 carriers (Nathan et al., in press; Nathan et al., 2002), contributing to pathological burden.
1.3 Olfactory Measures
Crossmodal odor identification tasks show particular vulnerability in AD (Serby, 1986; Morgan, Nordin & Murphy, 1995). Studies confirm this sensitivity extends to ApoE ε4 carriers (Murphy, Bacon, Bondi & Salmon, 1998) and individuals with mild cognitive impairment (Devanand, Tabert, Cuasay, Manly, Schupf, Brickman, Andrews, Brown, DeCarli, & Mayeux, 2010). Individuals with poor odor identification are more likely to go on to develop cognitive impairment (Schubert, Carmichael, Murphy, Klein, Klein & Cruickshanks, 2008; Wilson, Schneider, Arnold, Tang, Boyle & Bennett, 2007), especially in the presence of an ε4 allele (Graves, Bowen, Rajaram, McCormick, McCurry, Schellenberg, & Larson, E.B., 1999). Morgan, Nordin, and Murphy (1995) showed olfactory detection contributes to but does not fully explain the impairment in odor identification; that is, the crossmodal integration and identification remain important.
One measure of olfactory function that has exquisite sensitivity to the timing of the brain's response to odor is the olfactory event-related potential (OERP). The methodology for recording olfactory ERP includes rapid build-up of odorant concentration (rise time of under 20 msec), exact timing of stimulus onset, and avoidance of simultaneous stimulation of other sensory modalities; e.g., presenting odors in a constant air stream to avoid trigeminal responses (Lorig, 2000). Air-dilution olfactometers provide stimulus precision while warming and humidifying the stimulus to prevent somatosensory cues. Reaction times to odors lie in the 800-900 ms range but vary based on stimulus and subject characteristics (Lorig, 2009). Thus the P300 often occurs about 300-400 msec later in olfactory than in auditory ERPs. The neuronal recovery time for the olfactory system is significantly longer than that of both the auditory and visual systems (Ekman et al., 1967; Morgan et al., 1997; Wilson & Linster, 2008). Auditory and visual stimuli can typically be given within 2-3 sec in an ERP experiment without significant sensory adaptation (Polich, 1990a, 1990b, 1993). Slow recovery after stimulation in the olfactory system is partly due to olfactory receptor cells that rapidly adapt and slowly recover (Moore, 1994) and partly due to habituation (Wilson & Linster, 2008). It is difficult to discriminate the processes of adaptation and habituation at the level of scalp recordings, but it is likely that both influence the processing of olfactory information reflected in OERPs. As a result of neuronal recovery time in the olfactory system, a longer ISI (30-45 msec) is utilized; and, to offset potential fatigue and loss of vigilance, fewer trials are presented. A narrower filter is typically used to compensate for the smaller number of trials.
OERPs have shown promise for sensitivity to AD in the latency and amplitude of the P300 (Morgan & Murphy, 2002), though these characteristics are generated by a variety of neural structures, and the optimum ERP task for early sensitivity for reliable AD prediction has yet to be determined (Olichney & Hillert, 2004; Johnson, 1993).
1.4 Semantic Congruency
Kutas and Hillyard (1980) found semantic priming tasks resulted in enhanced negative amplitude at approximately 400ms following words that they had presented incongruously with others. Studies have since shown N400 signal generators include parahippocampus and fusiform gyrus, areas important in olfactory processing and visual processing respectively (McCarthy et al, 1995; Nobre & McCarthy, 1995). Other potential generators such as superior temporal sulcus, posterior parietal and ventral prefrontal cortex (Halgren et al., 2002) are also typically activated in fMRI experiments with olfactory tasks.
The traditional verbal-based semantic congruency paradigm adds additional insight into behavioral measures in AD patients (Ford et al., 2001), and recent studies suggest the potential to enhance the sensitivity and specificity for transition from MCI into AD (Olichney et al., 2008; Chapman, 2009). The heightened vulnerability of the olfactory system in AD leads to the hypothesis that a semantic congruity task with OERP measurement may be particularly sensitive and specific in early AD.
Lorig, Mayer, Moore, and Warrenburg (1993) first experimented with integrating olfactory and verbal stimuli while observing VERP amplitude changes. Later efforts focused specifically on possible VERP changes within N400 windows emerging from verbal-based semantic congruency paradigms.
The adaptation of Lorig et al. (1993) by Grigor (1995) evidenced a congruency effect through VERPs with paired food images and odors. On each trial there was a 75% probability the pair would be congruent and a 25% probability it would be incongruent. Grigor et al. (1999) confirmed the priming effect was not restricted to food-related stimuli, extending the same model to non-food odors and images as well. Castle et al. (2000) applied this model to pairs of pleasant odors and pictures of freshly laundered clothing but failed to find significant VERP differences between congruent and incongruent trials. Interestingly, a change from pleasant to unpleasant odors was sufficient to replicate previous findings (Grigor, 1995; Grigor et al., 1999).
Congruency effects persist not only with olfaction but also multiple non-verbal stimulus combinations (Hamm, Johnson, & Kirk, 2002; Balconi & Pozzoli, 2005). It is the extent of generalization that suggests the underlying neural response is dependent upon the congruency paradigm rather than stimulus characteristics per se (Nigam, Hoffman, & Simmons, 1992). To date, semantic congruency has not been widely applied with OERP measurement and has not been investigated in AD.
To examine whether it was feasible that such an OERP congruency paradigm could have diagnostic potential in AD, we investigated its potential to discriminate between individuals with differential risk for AD, i.e., older (aged 65+) ApoE ε4 carriers and non-carriers. The ERP study presented here investigated the neural processes that operated while subjects appraised paired visual and olfactory stimuli, observed through late onset changes in OERP mean amplitude for congruent and incongruent stimulus pairs. Reaction time to odors average between 800-900 msec with wide variability (Lorig, 2000) so that the P3 component often occurs about 300-400 msec later in olfactory ERPs. It was hypothesized that incongruous pairs would yield greater mean negative amplitude in the selected post-odor interval (600-1300ms), and ApoE ε4 carriers and non-carriers would produce differential responses.
2. Results
2.1 Demographic and Behavioral Data
MANOVA showed subjects did not significantly differ overall in terms of age (p=.764), education level (p=.214), DRS scores (p=.671), odor identification (p=.250) or AST (p=.091). Although subjects were prescreened and met a criterion of thresholds of 3 or better and odor identification scores of 3 or better, it was of interest to compare the groups further, as olfactory threshold scores were overall significantly different (p=.001). Follow up univariate tests indicated ε4 positives carriers had poorer thresholds than non-carriers (F(1,16)=31.56, p<.001) (Table 1).
Table 1. Olfactory and dementia screening by ε4 status.
| ApoE ε4 | ||
|---|---|---|
|
|
||
| Measure | Positive | Negative |
| DRS | 140.6 (1.4) | 140.8 (2.1) |
| Odor ID | 4.8 (1.5) | 6.0 (1.6) |
| AST | 16.3 (6.8) | 23.0 (4.4) |
| Threshold | 4.8 (1.0) | 7.1 (1.1) |
| Age | 69.6 (5.0) | 69.0 (3.5) |
Note: SD in parentheses
The repeated measures ANOVA for congruency judgments showed subjects provided, on average, 16 more correct than incorrect congruency judgments (F (1,16)= 142.22, p<.001), with successful classification occurring about 80% of the time. This result did not significantly interact with ApoE status (F (1,16)=.80, p>.05), gender (F (1,16)=.089, p>.05), or the condition (F (1,16)=.218, p>.05), whether congruent or incongruent. Moreover, there were no significant 3 or 4-way interactions amongst the variables.
2.2 Event Related Potential Data
Olfactory threshold was tested as a potential covariate but it did not provide a significant contribution to the ERP analysis of variance and was removed F (1,15) = .174, p =.683).
Repeated measures ANOVA identified a significant main effect for congruency (F (1,16) = 27.08, p < .001). Contrasts specified an average amplitude difference of 1.77 between the congruous and incongruous odor-image pairs inside 600-1300 post odor; the mean amplitude falling within the post-odor interval was negative for incongruous odor-image pairs. Two way interactions between congruency and the topographic dimensions were not significant; however, the 3-way interaction with AP, DV, and congruency approached significance (F (1,16) = 4.09, p = .06), which suggested the congruency effect was strongest at posterior ventral sites (Figure 1).
Figure 1.
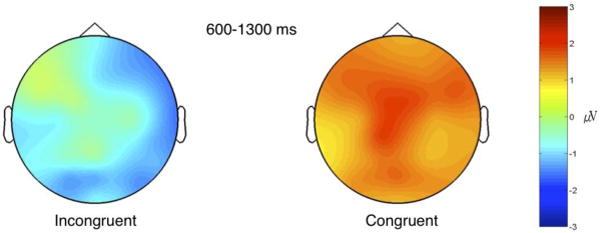
Overall mean amplitude 600-1300 ms following incongruent and congruent odor-image pairs.
ApoE ε4 status influenced congruency and its topographic distribution. There was a significant 4-way interaction between LR, DV, congruency and ApoE4 (F (1,16) = 9.41, p = .007). Paired samples t confirmed ε4 carriers had a larger difference in amplitude between congruous and incongruous odor-image pairs at right ventral electrode sites than non-carriers, t (9) = 3.97, p =.003. The difference is illustrated in Figures 2 and 3. Statistics for the lower level, 2 and 3-way interactions of the 4-way interaction have been included in Table 2.
Figure 2.
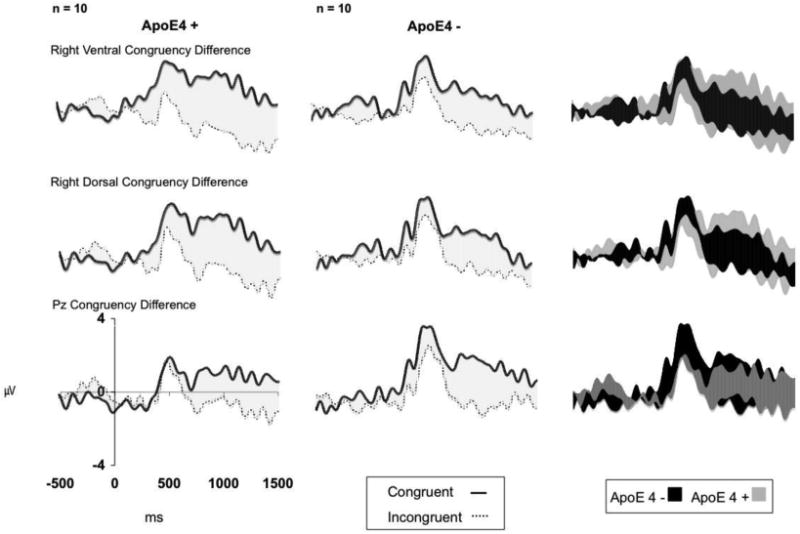
Grand average ERP congruency differences recorded from the Pz site, (Lower), right dorsal regions (Middle), and the right ventral regions (Upper), displayed by ApoE ε4 status. The shading illustrates differences in response between congruent and incongruent stimuli. Solid lines represent congruent ERPs while incongruent ERPs are represented by dotted lines.
Figure 3.
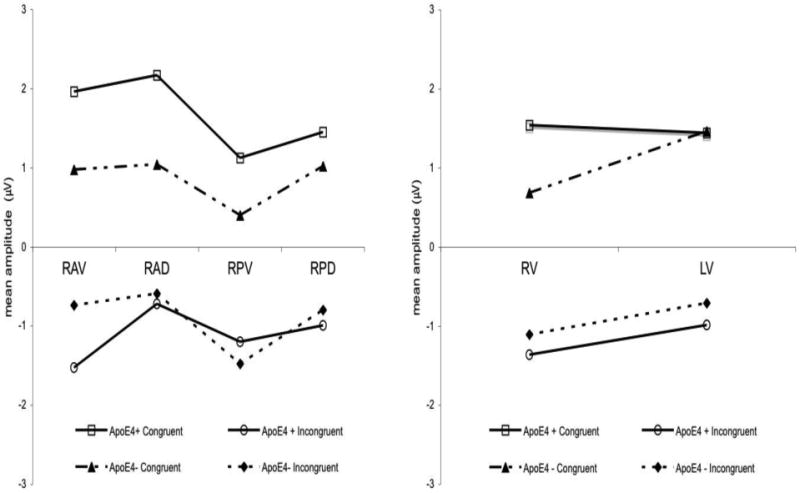
(Left) Congruous and incongruous mean amplitudes (600-1300 ms post-odor) are displayed for the right hemispheric regions by ApoE4 status (RAD = Right Anterior Dorsal; RPD = Right Posterior Dorsal; RAV = Right Anterior Ventral; RPV = Right Posterior Ventral). (Right). The same comparison is made between ApoE4 carriers and non-carriers at right ventral (RV) and left ventral (LV) regions.
Table 2. ANOVA Summary.
| Source | F (1,16) | p | Partial Eta Squared |
|---|---|---|---|
| Congruency | 27.08** | .000 | .63 |
| LR × DV | .09 | .771 | .01 |
| AP × DV | 4.09 | .060 | .20 |
| LR × AP × DV | .19 | .672 | .01 |
| Congruency × ApoE4 | .02 | .893 | .00 |
| LR | .31 | .586 | .02 |
| DV | .92 | .353 | .05 |
| LR × DV | 9.41** | .007 | .37 |
| AP × DV | 1.51 | .237 | .09 |
| LR × AP × DV | 1.34 | .263 | .08 |
| Congruency × ApoE4 × Gender | 4.24 | .056 | .21 |
| LR × DV | .05 | .829 | .00 |
| AP × DV | 4.48* | .050 | .22 |
| LR × AP × DV | 5.21* | .036 | .25 |
Note: L= Left Hemisphere; R= Right Hemisphere; A= Anterior; P= Posterior; D= Dorsal; V= Ventral.
p < 0.01.
p < 0.05.
Further, the 3-way gender, congruency and ApoE4 interaction was marginally significant (F (1,16) = 4.24, p = .056. Significant 5 and 6-way interactions involving ApoE ε4, congruency, and gender were found. For brevity, these have been included in Table 2.
The congruency effect is typically largest at Pz. Repeated measures ANOVA was also run on Pz for 19 of the 20 subjects who had provided normal Pz recordings as a reference for effects that might have occurred along the midline electrodes that were excluded from the regional averages. Gender was not included. Here, the congruency difference remained strongly significant (F (1,17) = 18.25, p < .001), characterized by greater negative ERP amplitude following incongruous odor-image pairs. ApoE status influenced the difference between congruous and incongruous pairs. ApoE ε4 carriers had, on average, a significantly smaller ERP amplitude difference than non-carriers (F (1,17) = 5.12, p = .037).
Intra Class Correlation was run (600-1300ms post-odor) within Neuroscan on grand averaged ERPs to better visualize the ANOVA results. A comparison between ApoE ε4 status for the congruent ERP average showed low correlations at ventral electrode sites. When the incongruent ERP average was compared between subjects with different ApoE ε4 status, high correlations were found at central and parietal electrodes. To explore gender, congruency was compared between male and female ε4 carriers. High ERP correlations were found at right frontal electrode sites for the incongruent ERP average, contrasted with low correlations from left frontal and left into right centro-parietal sites for these trials (Figure 4 for correlations).
Figure 4.
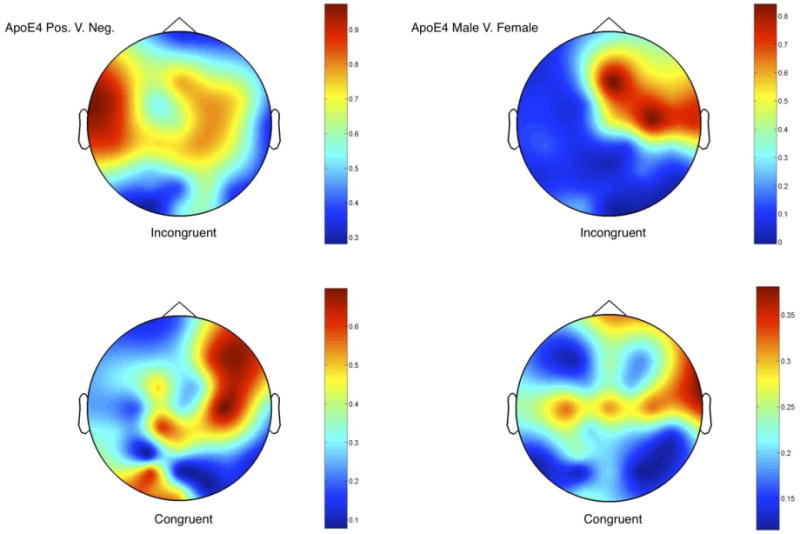
The Intra Class Correlations comparing waveshape and amplitude similarities for averaged congruent (left) and incongruent (right) ERPs (600-1300ms) between ApoE ε4 positive and negative carriers (top) and positive male and female carriers are displayed topographically. Scales show the correlations and are relative for each comparison. Lowest correlations are in blue while highest correlations are in red.
The congruency differences at right dorsal and ventral sites were assessed as predictors of ε4 in binary logistic regression. Given existing support for the sensitivity of olfactory threshold to AD and ε4, these two ERP variables and olfactory threshold were forced onto ε4 status in two separate blocks. A classification rate of 85% (Sensitivity = 90% Specificity = 80%), χ2 (1) = 15.97, p < .001 was achieved with olfactory threshold. Following entry of the ERP variables (χ2 (2) = 11.76, p = .008), the classification rate was 100% (Sensitivity = 100%, Specificity = 100%), χ2 (3) = 27.73, p < .001. For comparison, congruency differences at left ventral and dorsal regions failed to improve upon the 85% classification rate of threshold alone, χ2 (2) = 4.27, p = .118. The right hemispheric predictors, despite their improvement upon the overall classification rate, did not however account for a significant amount of variance within ApoE status individually.
The Pz congruency difference accounted for more variance in genetic status than either the right ventral or dorsal differences, as assessed with 19 of the 20 subjects who had provided normal Pz recordings. This model, which contained Pz, dorsal and ventral congruency differences, classified the 19 subjects at a rate of 84% (Sensitivity = 90% Specificity = 78%), χ2 (3) = 10.79, p < .01; a rate that improved to 100 % following entry of olfactory threshold, χ2 (1) = 15.50, p < .001.
3. Discussion
The ERPs suggested greater negative amplitude followed odors that were incongruous with visual primes as was originally hypothesized. The ε4 carriers did have a larger congruency difference at right ventral electrodes than non-carriers, which was coupled with a smaller difference at Pz than non-carriers. While the weakened response was hypothesized, the larger congruency differences at right hemispheric electrode regions were not. These two effects are very likely linked however and indicate a combination of structural and functional abnormalities in the ε4 carriers, consonant with previous neuroimaging studies that support a strong association between the progression of Alzheimer's disease and the ε4 allele.
MRI scans conducted on non-demented ε4 brains evidence accelerated cortical thinning of regions previously demonstrated to decline in thickness with age, as well as thinning in areas that develop Alzheimer's pathology (Espeseth, Westlye, Fjell, Walhovd, Rootwelt, & Reinvang, 2008), particularly entorhinal cortex and subiculum (Burggren, Zeineh, Ekstrom, Braskie, Thompson, Small & Bookheimer, 2008) as well as a reduction in fractional anisotropy within the corpus callosum and parahippocampus (Persson et al., 2006; Nierenberg et al., 2005). A PET study has shown hypometabolism at posteriomedial and frontal regions in ε4 carriers (Mosconi et al., 2004). Functional MRI experiments have demonstrated limited task-dependent deactivation with ε4. Importantly, Han et al. (2007) showed ε4 carriers showed greater fMRI activation in right hemisphere regions associated with verbal episodic memory encoding and consolidation. Similarly, Seidenberg et al. (2009) found unfamiliar faces could produce elevated activation in ε4 carriers compared to non-carriers and Haase et al. (2011) observed differences in task-related functional connectivity between ε4 carriers and non-carriers that were compatible with dissociations among brain regions associated with prodromal changes in the ε4 carriers.
These more robust metabolic responses, coupled with regions that are impaired, may evidence compensation in lieu of ongoing degenerative processes. Some have proposed the right hemisphere may carry a substantive burden of the compensatory response (Han et al., 2007). ERPs in verbal congruency tasks have been shown to favor the right hemisphere in probable AD patients but not cognitively intact controls (Ford et al., 2001). Similarly, we observed diminished ERP effects at Pz alongside enhanced effects at right hemispheric regions, which would offer additional support for this view.
Intra-class Correlation, a measurement of waveshape and amplitude similarities amongst grand-averaged ERPs, largely confirmed our statistical findings. The high correlation between the average incongruent trial ERPs for ε4 males and females at right anterior regions further emphasized the importance of the right hemisphere in these data. But there were many other areas of the right hemisphere where ERPs were not well correlated. We can only speculate about the source of variability at this time, although a brief discussion may help in directing additional studies.
The SOA, or duration between target and prime, has served as an interpretive tool in similar semantic congruency models. Deacon, Hewit, Yang, and Negata (2000) demonstrated that longer durations diminish the influence a prime has on the target whereas shorter durations tend to strengthen it. We might speculate the small SOA used in the present study enhanced the influence of the visual primes on the olfactory targets. Influences from such characteristics as edibility, caloric content and perspective should be considered in future research, given the interactions between apolipoprotein E polymorphism, gender, olfaction, cardiovascular disease, diabetes, and AD pathogenesis (Vegeto, Benedusi, & Maggi, 2008; Frisardi et al., in press; Bourdel-Marchasson, Lapre, Laksir, & Puget, 2010; Beydoun et al., in press; Sundermann, Gilbert, & Murphy, 2008)
Sundermann, Gilbert, and Murphy (2007), for instance, found a gender-specific pattern in performance when comparing male and female ApoE ε4 carries and non-carriers' recognition memory for faces, symbols, and odors. The comparison was made both within cognitively intact adults and probable AD patients. In contrast to their non-carrier counterparts, healthy ε4 males were impaired on odor recognition whereas healthy ε4 females were not. It was only in the AD group that odor recognition memory was more impaired for female ε4 carriers than non-carriers, where males did not perform differently. They found no significant differences for faces and symbols. Clearly, in addition to information about gender differences in aging and disease progression, complex biochemical interactions (e.g., AD pathology, hormones, cholesterol ratio, and blood glucose) may inform these differing trends. Addiction researchers have found success conceptualizing similarly complex biochemical phenomena in decision making-processes (Fishbein et al., 2005). The same might be true for the appraisal of paired olfactory and visual stimuli, as was studied here.
Conclusions
This study utilized an olfactory-visual semantic congruency task to investigate cross-modal odor identification disturbances in persons at genetic risk for AD. The results support significant differences in olfactory performance as assessed by OERPs between ε4 carriers, who are genetically predisposed to enhanced AD risk, and non-carriers.
The ApoE ε4 carriers had a scalp topography that was consistent with morphological and hypometabolic abnormalities found in PET, fMRI and MRI studies. OERPs reflected hemispheric asymmetries in ε4 carriers that were line with a compensatory mechanism. Unique influence from the congruency judgments of the odor-image pairs is supported by past experiments (Morgan, et al., 1995) and by the differing ICCs from congruent and incongruent trials, especially when comparing ε4 males and females.
ERP recordings combined with olfactory thresholds had very good sensitivity (100%) and specificity (100%) for ε4 status.
4. Experimental Procedures
4.1 Participants
Twenty-seven participants were recruited. Four were eliminated because of extreme odor threshold values and 3 for technical problems (see below), thus a total of 20 older adult subjects aged 65 and above (M=69.3, SD=4.2) participated in the study. The sample was divided into ApoE ε4 positive (n=10) and negative carriers (n=10), including equal numbers of positive males and females as well as negative males and females. The ε4+ and ε4− groups were matched for years of education, and prescreened with questionnaires for nasal sinus disease, allergic rhinitis, upper respiratory infection (Harris et al., 2006). They were tested for odor identification and detection. To meet minimum inclusion criteria, subjects were required to have butanol odor thresholds at or above 3 (Murphy, Gilmore, Seery, Salmon, & Lasker, 1990) and odor identification scores at or above 3 (Murphy et al, 2002). The subjects were prior participants at the Lifespan Human Senses Laboratory at San Diego State University and ApoE status had been determined through polymerase chain reaction. Participants in this study gave informed consent and their rights were protected in accordance with the IRB policies of San Diego State University and the University of California, San Diego, which approved the research.
4.2 Olfactory Screening
4.2.1 Odor Threshold
The odor threshold is a forced choice ascending staircase that assesses a subject's ability to discriminate distilled water from butanol (Murphy, et al., 1990; Cain Gent, Catalanotto, Godspeed, 1983). The butanol and distilled water (or blank) solutions are prepared in plastic bottles that the subject positions below the right or left nostril and squeezes to release the stimulus. There are 10 serial dilutions of butanol concentrations from 0 through 9. If the subject correctly chooses the butanol solution over the blank, the process is repeated with the same concentration. If not, a greater concentration is given. Each new presentation alternates nostrils. The threshold in a given nostril is determined after 5 correct choices at a single concentration have been given.
4.2.2 Odor Identification
The Odor Identification test is a measurement of the ability to name 8 common household odors (Murphy, Anderson, & Markison, 1994; Murphy et al., 2002). The stimuli are prepared in 8 opaque jars and then randomly presented to a subject. A subject closes the eyes and is given approximately 5 seconds to sample the odor. At the conclusion, a subject opens the eyes and identifies the presented odor on a picture board containing 20 possibilities. A 45 second interval is maintained between successive odor presentations.
4.3 ERP Methods
4.3.1 ERP Paradigm and Stimulus Presentation
During EEG recording, fourteen odors followed images that were either semantically congruous or incongruous. Each odor was presented once congruently and once incongruently, so that rejection criteria could be applied more conservatively. This produced 28 total trials while maintaining a 50/50 distribution for stimulus congruency. The visual stimuli were comprised of 28 high-resolution (1028 × 786) images of familiar household items, food (e.g., chocolate cake) and non-food items (e.g., roses) from iStockPhoto (See Table 3). Several distinctly non-odorous images were used to serve as controls and limit strategies or emergent expectations concerning congruency. Each image was matched with the others according to color scheme, luminosity, and resolution to avoid novelty responses. Color, contrast, and brightness of the images was enhanced by projecting them onto a 22-inch high-definition flat screen monitor, situated approximately 125cm from the subject's view.
Table 3. List of pictures used.
| Pictures |
|---|
| Blank baby powder bottle |
| Bananas |
| Peppermint candies |
| Cherries |
| Shredded cheese |
| Coffee |
| Chocolate cake |
| Plain cinnamon buns |
| Laundered towels |
| Colored pencil shavings |
| Pine trees |
| Wood burning in fireplace |
| Oranges and orange juice |
| Lemons |
| Blank yellow mustard bottle |
| Ocean scene |
| Colored plastic plates with plastic utensils |
| Pizza |
| Colored paper clips |
| Peanut butter on bread |
| Strawberries |
| Rocks |
| Brown and black leather shoes |
| Rose bush |
| Tulip bouquet |
| Toothpaste and mouthwash |
| Vanilla ice cream in cones |
| Tire heap |
The 14 odors (See Table 4) included stimuli from both food and non-food classifications and were delivered through a continuous flow olfactometer (Morgan & Murphy, 2002). Humidification was added via a bypass between the air supply and the subject's nose. Each odor was selected on the basis of it being readily identifiable by a sample of 40 young, middle aged, and older adults. They were asked to identify 17 total odors from written multiple-choice options. A sub-set of these subjects then reported whether each odor was supra-threshold and iso-intensive when delivered via the olfactometer. The odors were initially paired with food or non-food images based upon response frequencies from the odor identification survey to produce congruent and incongruent stimuli. For instance, responses that were lowest in frequency for each odor were translated into images and paired. These pairs were then confirmed according to subjects' congruency judgments during a pilot study. A program designed by Neuroscan, STIM, was used to synchronize the olfactometer with the images. To facilitate offline analysis, a unique electronic signature was assigned to each of the fourteen odors. A template sequence of the odor-image pairs was exported into a third party application for Microsoft Excel to create randomized sequence files.
Table 4. List of odors used.
| Odors |
|---|
| Pine Tree |
| Cinnamon |
| Orange |
| Lemon |
| Banana |
| Wintergreen |
| Rose |
| Vanilla |
| Coffee |
| Chocolate |
| Peanut butter |
| Leather |
| Baby powder |
| Chocolate |
At the beginning of the experiment subjects were presented with a series of instructions on the monitor. They were told that several images would be presented; each followed by an odor. “You might find these odors if you were to actually smell the objects or experience the events you see in the pictures. Just before each image, a red fixation cross will appear. Please focus on the cross and limit eye movement to any black screen that follows an odor.”
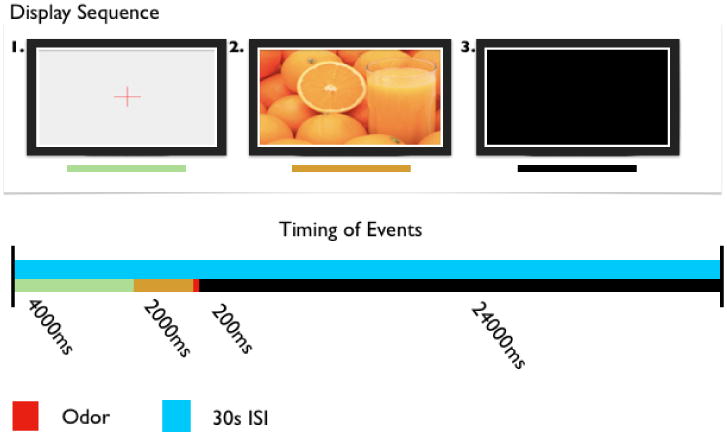
All trials began with a 4000ms duration fixation cross, then transitioned to an image that was displayed for 2000ms. An odor (congruent or incongruent) was delivered for 200ms immediately following the image. There was approximately a 30 ISI between successive odor presentations to avoid habituation and adaptation, which are greater considerations in the olfactory modality than other modalities. During the ISI, only a black screen was displayed. A new trial began whenever the red fixation-cross replaced the black screen (Figure 1).
Congruency judgments were collected after the experiment. The trial sequence was restarted with a “Did they Match?” prompt added 4000ms after each pair. Subjects used a Logitech joystick that included buttons labeled “yes” and “no” for congruency judgments.
4.3.2 ERP Recording
Continuous measurement of subjects' brain responses to the stimuli was obtained from a 64-electrode Neuroscan cap. The signal was sampled at 20kHz, amplified and digitized for computer recording by SynAmps™. The signal impedance was kept below 10kΩ through QuikCell™ electrolyte solution, manufactured by Neuroscan. Reference electrodes were applied to both ears (A1 and A2) and, to measure eye movement, placed below the left eye as well as directly adjacent to both the left and right eyes, situated beneath the temple.
4.3.3. ERP Processing
An initial .1 HZ to 9 HZ zero phase shift bandpass filter was used to process the recorded EEG. The EEG recording was divided into 28 epochs reflecting 500ms pre and 1500ms post odor presentation. Congruent and incongruent epochs were handled in two different phases because the coding scheme assigned to each odor was unique but duplicated. Every epoch was re-referenced to A1 and A2 and baseline corrected. The data were similarly swept along an array (3×4) of centrally distributed electrodes for outlying positive and negative amplitudes falling outside −50 μV and 50 μV. Additional corrections were made to the data for eye blinks across the trials based on 10% amplitude threshold and 400ms duration criteria. If the number of bad electrodes exceeded 20 % in any trial, it was not included in the average. Similarly, unacceptable averages were defined as those wherein approximately 30% of the trials were bad per condition (i.e., 4 trials were eliminated in any one condition). The application of this rule resulted in the elimination of 3 subjects during ERP processing. For each acceptable average, mean amplitude was reported from all electrode sites within 600-1300ms. These values were exported into a spreadsheet.
4.3.4. Statistical Analysis
MANOVA (2 × 2), including subjects' E4 status (ApoE ε4+, ApoE ε4−) and gender (male/female), was used to assess differences in education level and age as well as cognitive and olfactory performance that would require inclusion in the ERP analysis.
Subjects' congruency judgments were analyzed in a mixed model design, and run within repeated measures ANOVA. Trial type (incongruent and congruent) and correct and incorrect varied within subjects whereas gender (male and female) as well as ApoE4 status (ApoE ε4+, ApoE ε4−) varied between subjects.
Similarly, a mixed model design was utilized for topographical ERP data. Repeated measures ANOVA assessed the mean amplitude 600-1300ms post odor at 52 electrodes, grouped along the anterior-posterior (AP), dorsal-ventral (DV) and left-right (LR) dimensions (Handy, 2004). Amplitudes that were recorded within these different regions—in both congruent and incongruent conditions—were transformed into z scores and examined for outliers. Each region was then averaged, resulting in 16 variables (Left Anterior Dorsal Congruent: F1, FC3, C5, FC1, C3, C1; Left Anterior Ventral Congruent: AF3, F3, FC5, F7, FT7, F5, T7; Left Anterior Dorsal Incongruent; Left Anterior Ventral Incongruent; Left Posterior Dorsal Congruent: CP5, P3, PO3, CP3, P1, CP1; Left Posterior Ventral Congruent: TP7, P5, PO5, O1, P7, PO7, CB1; Left Posterior Dorsal Incongruent; Left Posterior Ventral Incongruent; Right Anterior Dorsal Congruent: F2, FC4, C6, FC2, C4, C2; Right Anterior Ventral Congruent: AF4, F4, FC6, T8, F6, FT8, F8; Right Anterior Dorsal Incongruent; Right Anterior Ventral Incongruent; Right Posterior Dorsal Congruent: CP6, P4, PO4, CP4, P2, CP2; Right Posterior Ventral Congruent: TP8, P6, PO6, O2, P8, PO8, CB2; Right Posterior Dorsal Incongruent; Right Posterior Ventral Incongruent). Subjects' gender (male/female) and ApoE4 status (ApoE ε4+, ApoE ε4−) varied between (2 × 2) while congruency (congruent/incongruent) and the three topographic dimensions (LR, AP, and DV) varied within (2 × 2 × 2 × 2).
Because the congruency effect is typically largest at Pz, two factor ANOVA on trial type (incongruent and congruent) and ApoE ε4 status (ApoE ε4+, ApoE ε4−) was used to assess differences in mean amplitude 600-1300ms post odor at Pz. One subject was eliminated from the analysis because the Pz value fell more than 2 SD from the mean.
No repeated measure exceeded 2 levels, thus significance was assessed at p < .05 without Greenhouse Geisser correction. Paired samples t was used when appropriate as a follow-up for significant main effects and interactions. Reported t values are significant following bonferroni correction. Significant interactions involving congruency and ApoE were broken down and examined as predictors of E4 status in logistic regression using forced entry.
In order to compare the ERP grand averages in terms of waveshape and absolute voltage values between ApoE ε4+ and ApoE ε4− persons and between ApoE ε4+ males and females on congruent and incongruent trials, the Intra-Class Correlation (ICC), a measure of overlap and related variability between two waveforms, was computed in Neuroscan. The ICCs are displayed in topographical map format.
Note, that four subjects (1 ApoE ε4 negative male, 1 negative female, 1 positive male and 1 positive female) were eliminated for having olfactory threshold scores that fell approximately 2 z scores above or below the mean. Behavioral, demographic and topographical ERP statistics reported here are from the 20 subjects that remained.
Figure 5. Sample trial, displaying duration and sequence of events.
Highlights.
Olfactory ERPs in an odor/visual congruency task differentiated ApoE ε4+ and ApoE ε4−.
Pz amplitude significantly decreased on incongruent trials in non-carriers.
Amplitude in right dorsal and ventral sites suggested a compensation hypothesis.
High sensitivity and specificity for classifying ε4 carriers from non -carriers.
Acknowledgments
Supported by NIH grants R01 DC02064-14 from the National Institute of Deafness and other Communicative Disorders and R01 AG04085-24 from the National Institute on Aging to Claire Murphy. We are grateful to the participants who contributed their time and to the UCSD Alzheimer's Disease Research Center for genotyping (P50 AG005131-28). We especially thank Jessica Bartholow, Charlie D. Morgan, and Jeremy Chartier for research assistance and expertise. The authors have no actual or potential conflicts of interest, financial or otherwise. JK and CM designed the experiment, JK and others collected the data, JK conducted data analysis with advice from CM, JK and CM prepared the article, and both authors approved the final article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychological Association. Publication manual of the American Psychological Association. 5th. Washington, DC: American Psychological Association; 2001. [Google Scholar]
- Balconi M, Pozzoli U. Morphed facial expressions elicited a N400 ERP effect: A domain-specific semantic module? Scandinavian Journal of Psychology. 2005;46(6):467–474. doi: 10.1111/j.1467-9450.2005.00478.x. [DOI] [PubMed] [Google Scholar]
- Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM. Accumulation of abnormally phosphorylated τ precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Research. 1989;477(1-2):90–99. doi: 10.1016/0006-8993(89)91396-6. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Boueiz A, Abougergi MS, Kitner-Triolo MH, Beydoun HA, Resnick SM, O'Brien R, Zonderman AB. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiology of Aging. doi: 10.1016/j.neurobiolaging.2010.05.017. In Press, Corrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdel-Marchasson I, Lapre E, Laksir H, Puget E. Insulin resistance, diabetes and cognitive function: Consequences for preventative strategies. Diabetes & Metabolism. 2010;36(3):173–181. doi: 10.1016/j.diabet.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer's Disease-Related Neurofibrilary Changes. Neurobiology of Aging. 1995;16(3):271–284. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Burggren AC, Zeineh MM, Ekstrom AD, Braskie WN, Thompson PM, Small GW, Bookheimer SY. Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein Ee4 carriers. NeuroImage. 2008;4:1177–1183. doi: 10.1016/j.neuroimage.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttini M, Orth M, Bellosta S, Akeefe H, Pitas RE, Wyss-Coray T, Mahley RW. Expression of human apolipoprotein E3 or E4 in the brains of apoe -/- mice: isoforms specific effects on neurodegeneration. Journal of Neuroscience. 1999;19(12):4867–4880. doi: 10.1523/JNEUROSCI.19-12-04867.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain WS, Gent JS, Catalanotto FA, Goodspeed RB. Clinical evaluation of olfaction. Am J Otolaryngol. 1983;4:252–256. doi: 10.1016/s0196-0709(83)80068-4. [DOI] [PubMed] [Google Scholar]
- Calhoun-Haney R, Murphy C. Apolipoprotein E4 is associated with more rapid decline in odor identification than in odor threshold or Dementia Rating Scale scores. Brain and Cognition. 2005;58:178–182. doi: 10.1016/j.bandc.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Castle P, Van Toller S, Milligan G. The effect of odour priming on cortical EEG and visual ERP responses. International Journal of Psychophysiology. 2000;36(2):123–131. doi: 10.1016/s0167-8760(99)00106-3. [DOI] [PubMed] [Google Scholar]
- Chapman RM, McCrary JW, Gardner MN, Sandoval TC, Guillily MD, Reilly LA, DeGrush E. Brain ERP components predict which individuals progress to Alzheimer's disease and which do not. Neurobiology of Aging. 2009 doi: 10.1016/jneurobiolaging.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christen-Zaech S, Kraftsik R, Pillevuit O, Kiraly M, Martins R, Khalili K, et al. Early olfactory involvement in Alzheimer's disease. The Canadian Journal of Neurological Sciences/Le Journal Canadien Des Sciences Neurologiques. 2003;30(1):20–25. doi: 10.1017/s0317167100002389. [DOI] [PubMed] [Google Scholar]
- Davis K, Price C, Kaplan E, Libon D. Error analysis of the nine-word California Verbal Learning Test (CVLT-9) among older adults with and without dementia. Clinical Neuropsychologist. 2002;16(1):81–89. doi: 10.1076/clin.16.1.81.8330. [DOI] [PubMed] [Google Scholar]
- Deacon D, Hewitt S, Yang C, Nagata M. Event-related potential indices of semantic priming using masked and unmasked words: Evidence that the N400 does not reflect a post-lexical process. Cognitive Brain Research. 2000;9(2):137–146. doi: 10.1016/s0926-6410(99)00050-6. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Tabert MH, Cuasay K, Manly JJ, Schupf N, Brickman AM, Andrews H, Brown TR, DeCarli C, Mayeux R. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiology of Aging. 2010;31:1593–1600. doi: 10.1016/j.neurobiolaging.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman GB, Berglund U, Berglund B, Lindwall T. Perceived intensity of odor: a function of time of adaptation. Scan J Psychol. 1967;8:177–186. doi: 10.1111/j.1467-9450.1967.tb01392.x. [DOI] [PubMed] [Google Scholar]
- Esiri MM, Wilcox GK. The olfactory bulbs in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1984;47:56–60. doi: 10.1136/jnnp.47.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeseth T, Westlye L, Fjell A, Walhovd K, Rootwelt H, Reinvang I. Accelerated age-related cortical thinning in healthy carriers of apolipoprotein E epsilon 4. Neurobiology Of Aging. 2008;29(3):329–340. doi: 10.1016/j.neurobiolaging.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Fishbein DH, Eldreth DL, Hyde C, Matochik JA, London ED, Contoreggi C, Kurian V, Kimes AS, Breeden A, Grant S. Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Cognitive Brain Research. 2005;23(1):119–136. doi: 10.1016/j.cogbrainres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Frisardi V, Solfrizzi V, Seripa D, Capurso C, Santamato A, Sancarlo D, Vendemiale G, Pilotto A, Panza F. Metabolic-cognitive syndrome: A cross-talk between metabolic syndrome and Alzheimer's disease. Ageing Research Reviews. doi: 10.1016/j.arr.2010.04.007. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- Ford JM, Askari N, Gabrieli JDE, Mathalon DH, Tinklenberg JR, Menon V, Yesavage J. Event-related brain potential evidence of spared knowledge in Alzheimer's disease. Psychology and Aging. 2001;16(1):161–176. doi: 10.1037/0882-7974.16.1.161. [DOI] [PubMed] [Google Scholar]
- Graves AB, Bowen JD, Rajaram L, Mc Cormick WC, McCurry SM, Schellenberg GD, Larson EB. Impaired olfaction as a marker for cognitive decline: Interaction with apolipoprotein E e4 status. Neurology. 1999;53:1480–1487. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- Grigor J. Do the eyes see what the nose knows? An investigation of the effects of olfactory priming on visual event related potentials. Chemical Senses. 1995;20:163. [Google Scholar]
- Grigor J, Van Toller S, Behan J, Richardson A. The effect of odour priming on long latency visual evoked potentials of matching and mismatching objects. Chemical Senses. 1999;24(2):137–144. doi: 10.1093/chemse/24.2.137. [DOI] [PubMed] [Google Scholar]
- Haase L, Wang M, Green E, Murphy C. Functional connectivity during recognition memory in individuals genetically at risk for Alzheimer's disease. Human Brain Mapping. 2011 doi: 10.1002/hbm.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. I. systems originating in the piriform cortex and adjacent areas. The Journal of Comparative Neurology. 1978;178(4):711–740. doi: 10.1002/cne.901780408. [DOI] [PubMed] [Google Scholar]
- Halgren E, Dhond RP, Christensen N, Van Petten C, Marinkovic K, Lewine JD, Dale AM. N400-like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. NeuroImage. 2002;17(3):1101–1116. doi: 10.1006/nimg.2002.1268. [DOI] [PubMed] [Google Scholar]
- Hamm J, Johnson B, Kirk I. Comparison of the N300 and N400 ERPs to picture stimuli in congruent and incongruent contexts. Clinical Neurophysiology. 2002;113(8):1339–1350. doi: 10.1016/s1388-2457(02)00161-x. [DOI] [PubMed] [Google Scholar]
- Han S, Houston W, Jak A, Eyler L, Nagel B, Fleisher A, Bondi MW. Verbal paired-associate learning by APOE genotype in non-demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiology of Aging. 2007;28(2):238–247. doi: 10.1016/j.neurobiolaging.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handy TC. Event related potentials: A methods handbook. 1st. Cambridge, MA: MIT Press; 2004. [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Harris R, Davidson TM, Murphy C, Gilbert PE, Chen M. Clinical evaluation and symptoms of chemosensory impairment: One thousand consecutive cases from the nasal dysfunction clinic in San Diego. American Journal of Rhinology. 2006;20(1):101–108. [PubMed] [Google Scholar]
- Hatters DM, Zhong N, Rutenberg E, Weisgraber KH. Amino-terminal domain stability mediates apolipoprotein E aggregation into neurotoxic fibrils. Journal of Molecular Biology. 2006;361:932–934. doi: 10.1016/j.jmb.2006.06.080. [DOI] [PubMed] [Google Scholar]
- Huang Y. Apolipoprotein E and Alzheimer disease. Neurology. 2006;66(2 Suppl 1):S79–S85. doi: 10.1212/01.wnl.0000192102.41141.9e. [DOI] [PubMed] [Google Scholar]
- Johnson R. On the neural generators of the P300 component of the event-related potential. Psychophysiology. 1993;30(1):90–97. doi: 10.1111/j.1469-8986.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- Kitamura HW, Hamanaka H, Watanabe M, Wada K, Yamazaki C, Fujita SC, Nukina N. Age-dependent enhancement of hippocampal long-term potentiation in knock-in mice expressing human apolipoprotein E4 instead of mouse apolipoprotein E. Neuroscience Letters. 2004;369(3):173–178. doi: 10.1016/j.neulet.2004.07.084. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: Brain potentials reflect semantic incongruity. Science. 1980;207(4427):203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Levi O, Jongen-Relo AL, Feldon J, Michaelson DM. Brain area- and isoform-specific inhibition of synaptic plasticity by apoE4. Journal of the Neurological Sciences. 2005:229–230. 241–248. doi: 10.1016/j.jns.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Lorig TS. The application of electroencephalographic techniques to the study of human olfaction: a review and tutorial. International Journal of Psychophysiology. 2000:91–104. doi: 10.1016/s0167-8760(99)00104-x. [DOI] [PubMed] [Google Scholar]
- Lorig TS. What was the question? fMRI and inference in psychophysiology. International Journal of Psychophysiology. 2009;73(1):17–21. doi: 10.1016/j.ijpsycho.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Lorig TS, Mayer T, Moore F, Warrenburg S. Visual event-related potentials during odor labeling. Chemical Senses. 1993;18:379–387. [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. PNAS. 2006;103(15):5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Nobre A, Bentin S, Spencer D. Language-related field potentials in the anterior-medial temporal lobe: I. intracranial distribution and neural generators. Journal of Neuroscience. 1995;15(2):1080–1089. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PA. A model of the role of adaptation and disadaptation in olfactory receptor neurons: implications for the coding of temporal and intensity patterns in odor signals. Chemical Senses. 1994;19(1):17–86. doi: 10.1093/chemse/19.1.71. [DOI] [PubMed] [Google Scholar]
- Morgan CD, Nordin S, Murphy C. Odor identification as an early marker for Alzheimer's disease: Impact of lexical functioning and detection sensitivity. Journal of Clinical and Experimental Neuropsychology. 1995;17(5):793–803. doi: 10.1080/01688639508405168. [DOI] [PubMed] [Google Scholar]
- Morgan CD, Covington JW, Geisler MW, Polich J, Murphy C. Olfactory event-related potentials: older males demonstrate the greatest deficits. Electroencephalography And Clinical Neurophysiology. 1997;104(4):351–358. doi: 10.1016/s0168-5597(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Morgan CD, Murphy C. Olfactory event-related potentials in Alzheimer's disease. Journal of the International Neuropsychological Society: JINS. 2002;8(6):753–763. doi: 10.1017/s1355617702860039. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Sorbi S, Nacmias B, De Cristofaro M, Fayyaz M, Bracco L, Pupi A. Age and ApoE genotype interaction in Alzheimer's disease: An FDG-PET study. Psychiatry Research: Neuroimaging. 2004;130(2):141–151. doi: 10.1016/j.pscychresns.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BR. Olfactory thresholds are associated with degree of dementia in Alzheimer's disease. Neurobiol Aging. 1990;11:465–469. doi: 10.1016/0197-4580(90)90014-q. [DOI] [PubMed] [Google Scholar]
- Murphy C, Anderson JA, Markison S. Psychophysical assessment of chemosensory disorders in clinical populations. In: Kurihara K, Suzuki N, Ogawa H, editors. Olfaction and Taste XI. Tokyo: Springer-Verlag; 1994. pp. 609–613. [Google Scholar]
- Murphy C, Bacon C, Bondi MW, Salmon DP. Apolipoproein E status is associated with odor identification deficits in nondemented older persons. Annals of the New York Academy of Sciences. 1998;855:744–750. doi: 10.1111/j.1749-6632.1998.tb10654.x. [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert C, Cruickshanks K, Klein B, Klein R, Nondahl D. Prevalence of olfactory impairment in older adults. JAMA: Journal of the American Medical Association. 2002;288(18):2307. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- Murphy C, Solomon E, Haase L, Wang M, Morgan C. Olfaction in aging and Alzheimer's disease: event-related potentials to a cross-modal odor-recognition memorytask discriminate ApoE epsilon4+ and ApoE epsilon 4- individuals. Annals of the New York Academy of Sciences. 2009;1170:647–657. doi: 10.1111/j.1749-6632.2009.04486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan BP, Gairhe S, Nwosu I, Clark S, Struble RG. Reconstitution of the olfactory epithelium following injury in ApoE-deficient mice. Experimental Neurology. doi: 10.1016/j.expneurol.2010.08.001. In Press, Uncorrected Proof. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan BP, Jiang Y, Wong GK, Shen F, Brewer GJ, Struble RG. Apolipoprotein E4 inhibits, and apolipoprotein E3 promotes neurite outgrowth in cultured adult mouse cortical neurons through the low-density lipoprotein receptor-related protein. Brain Research. 2002;928(1-2):96–105. doi: 10.1016/s0006-8993(01)03367-4. [DOI] [PubMed] [Google Scholar]
- Nierenberg J, Pomara N, Hoptman M, Sidtis J, Ardekani B, Lim K. Abnormal white matter integrity in healthy apolipoprotein E epsilon4 carriers. Neuroreport. 2005;16(12):1369–1372. doi: 10.1097/01.wnr.0000174058.49521.16. [DOI] [PubMed] [Google Scholar]
- Nigam A, Hoffman JE, Simmons RF. N400 to semantically anomalous pictures and words. Journal of Cognitive Neuroscience. 1992;4(1):15–22. doi: 10.1162/jocn.1992.4.1.15. [DOI] [PubMed] [Google Scholar]
- Nobre A, McCarthy G. Language-related field potentials in the anterior-medial temporal lobe: effects of word type and semantic priming. Journal of Neuroscience. 1995;15(2):1090–1098. doi: 10.1523/JNEUROSCI.15-02-01090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm TG, Braak H. Olfactory bulb changes in alzheimer's disease. Acta Neuropathologica. 1987;73(4):365–369. doi: 10.1007/BF00688261. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Morris S, Ochoa C, Salmon D, Thal L, Kutas M, Iragui VJ. Abnormal verbal event related potentials in mild cognitive impairment and incipient Alzheimer's disease. Journal of Neurology, Neurosurgery & Psychiatry. 2002;73(4):377–384. doi: 10.1136/jnnp.73.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Hillert DG. Clinical applications of cognitive event related potentials in Alzheimer's disease. Physical Medicine and Rehabilitation Clinics of North America. 2004;15:205–233. doi: 10.1016/s1047-9651(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Taylor J, Gatherwright J, Salmon D, Bressler A, Kutas M, Iragui-Madoz VJ. Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology. 2008;70(19 Part 2):1763–1770. doi: 10.1212/01.wnl.0000281689.28759.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Sleegers K, Van Broeckhoven C, Nyberg L. Altered deactivation in individuals with genetic risk for Alzheimer's disease. Neuropsychologia. 2008;46(6):1679–1687. doi: 10.1016/j.neuropsychologia.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Polich J. P300, probability, and inter-stimulus interval. Psychophysiology. 1990a;27:396–403. doi: 10.1111/j.1469-8986.1990.tb02333.x. [DOI] [PubMed] [Google Scholar]
- Polich J. Probability and inter-stimulus interval effects on the P300 from auditory stimuli. International Journal of Psychophysiology. 1990b;10:163–170. doi: 10.1016/0167-8760(90)90030-h. [DOI] [PubMed] [Google Scholar]
- Polich J. P300 in clinical applications: meaning, method, and measurement. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. 3rd. Wiliams and Wilkins; Baltimore, MD: 1993. pp. 1005–1018. [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The Distribution of Tangles, Plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiology of Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Reyes PF, Golden GT, Fariello RG, Fagel L, Zalewska M. Olfactory pathways in Alzheimer's disease (AD): Neuropathological studies. Society for Neurosciences [Abstract] 1985;168 [Google Scholar]
- Rocchi A, Pellegrini S, Siciliano G, Murri L. Causative and susceptibility genes for Alzheimer's disease: a review. Brain Research Bulletin. 2003;61(1):1–24. doi: 10.1016/s0361-9230(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Sample Images [Online Image] (n.d.). Retrieved 15, 2007, from istockphoto.com. http://www.istockphoto.com/index.php.
- Schubert CR, Carmichael LL, Murphy C, et al. Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. JAGS. 2008;56:1517–1521. doi: 10.1111/j.1532-5415.2008.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TJ, Kutas M, Butters N, Paulsen JS, Salmon DP. Electrophysiological insights into the nature of the semantic deficit in Alzheimer's disease. Neuropsychologia. 1996;34(8):827–841. doi: 10.1016/0028-3932(95)00164-6. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Guidotti L, Nielson K, Woodard J, Durgerian S, Antuono P, Zhang Q, Rao SM. Semantic memory activation in individuals at risk for developing Alzheimer disease. Neurology. 2009;73(8):612–620. doi: 10.1212/WNL.0b013e3181b389ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Serby M. Olfaction and Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:579–586. doi: 10.1016/0278-5846(86)90027-8. [DOI] [PubMed] [Google Scholar]
- Struble RG, Clark HB. Olfactory bulb lesions in Alzheimer's disease. Neurobiology of Aging. 1992;13(4):469–473. doi: 10.1016/0197-4580(92)90074-8. [DOI] [PubMed] [Google Scholar]
- Sundermann E, Gilbert P, Murphy C. The effect of hormone therapy on olfactory sensitivity is dependent on apolipoprotein E genotype. Hormones & Behavior. 2008;54(4):528–533. doi: 10.1016/j.yhbeh.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski JQ, Lee VM. Phosphorylation of paired helical filament tau in Alzheimer's disease neurofibrillary lesions: focusing on phosphatases. The FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 1995;9(15):1570–1576. doi: 10.1096/fasebj.9.15.8529836. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Benedusi V, Maggi A. Estrogen anti-inflammatory activity in brain: A therapeutic opportunity for menopause and neurodegenerative diseases. Frontiers in Neuroendocrinology. 2008;29(4):507–519. doi: 10.1016/j.yfrne.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinbergs I, Everson A, Sagara Y, Masliah E. Neurotoxic effects of apolipoprotein E4 are mediated via dysregulation of calcium homeostasis. Journal of Neuroscience Research. 2002;67(3):379–387. doi: 10.1002/jnr.10138. [DOI] [PubMed] [Google Scholar]
- Voet D, Voet JG, Pratt CW. Fundamentals of biochemistry: Life at the molecular level. 3rd. Hoboken, NJ: Wiley; 2008. [Google Scholar]
- Wetter S, Murphy C. Apolipoprotein E ε4 positive individuals demonstrate delayed olfactory event-related potentials. Neurobiology of Aging. 2001;22(3):439–447. doi: 10.1016/s0197-4580(01)00215-9. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Linster C. Neurobiology of a simple memory. J Neurophysiol. 2008;100:2–7. doi: 10.1152/jn.90479.2008. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identificatin and incidence of mild cognitive impairment. Arch Gen Psychiatry. 2007;64(7):802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]


