Abstract
Although males and females differ in eating behavior and prevalence rates for eating disorders and obesity, little is known about gender differences in cortical activation to pleasant and unpleasant pure tastes during the physiological states of hunger and satiety. Twenty-one healthy young adults (12 females and 9 males) underwent two functional magnetic resonance imaging scans. Using four pure tastants of differing qualities (i.e., salty, sour, bitter, sweet), the present study examined gender differences in fMRI activation during two motivational states (hunger and satiety). There was greater change in fMRI activation from hunger to satiety in males than females in response to all tastes within the middle frontal gyrus (BA 10), insula, and cerebellum. Males also had greater change in activation from hunger to satiety, relative to females, in limbic regions including dorsal striatum, amygdala, parahippocampal gyrus, and posterior and anterior cingulate; however, activation was stimulus dependent, despite equivalent ratings in perceived pleasantness and intensity. Interestingly, males and females showed significant change from hunger to satiety in response to citric acid, suggesting that in addition to gender and physiological condition, stimulus quality is an important factor in taste fMRI activation. These gender differences may have implications for the pathophysiology of eating disorders and obesity.
Keywords: Gender, Taste, Hunger, Satiety, Reward, fMRI
Introduction
Gender differences in eating behavior have been widely documented (Conner, Johnson, & Grogan, 2004; Fagerli & Wandel, 1999; Rolls, Fedoroff, & Guthrie, 1991; Wardle et al., 2004; Zylan, 1996). Clinical manifestations of eating disorders such as bulimia and anorexia nervosa have greater incidence rates among females relative to males (Jacobi, Hayward, de Zeaan, Kraemer, & Agars, 2004; Kjelsas, Bjornstrom, & Gotestam, 2004; Woodside et al., 2001) and prevalence of obesity is greater in females (Flegal, Carroll, Ogden, & Curtin, 2010). Males and females differ in caloric intake (Basiotis, Thomas, Kelsay, & Mertz., 1989), which may in part, be a result of differential eating styles (Green, 1987; Hill & McCutcheon, 1984; Mori & Pliner, 1987). In addition, differential cultural pressures to achieve ideal body shapes may influence the way males and females respond to food to a different degree (Rolls et al., 1991; Rozin, Trachtenberg, & Cohen, 2001; Ostovich & Rozin, 2004).
Food consumption and termination are regulated by a complex system of peripheral and central processes that interact with genetics and environmental factors (Lenard & Berthoud, 2008). The gustatory system is one of the first sensory systems involved in food intake. Interestingly, gustatory psychophysical experiments examining gender differences have been inconsistent (Enns, Van Itallie, & Grinker 1979; Robin, Rousmans, Dittmar, & Vernet-Maury, 2003). Enns et al (1979) found that while males and females reported identical perceptions of sucrose intensity (using subjective ratings), hedonic evaluations were significantly different. In particular, males perceived higher concentrations of sucrose as more pleasant when compared to females. Conversely, other studies have reported no significant gender differences in the perceived pleasantness of taste stimuli (sweet, sour, salty, bitter; Robin et al., 2003) or in the perceived pleasantness and intensity of calcium (Leshem, Katz-Levin, & Schulkin, 2003).
Gender differences in the psychophysical evaluation of taste stimuli are more consistently observed when hunger and satiety are controlled (Laeng, Berridge, & Butter, 1993). Females perceive the sweetness of sucrose as more intense relative to males across physiological condition, and also perceive sucrose as less pleasant than males after a meal (Laeng et al., 1993). Additionally, females rate food images as more pleasant than males after a 12 hour fast, and less pleasant than males in a non-fasted condition, (Stoeckel, Cox, Cook, & Weller, 2007). Although inconclusive, these findings suggest that physiological state may alter the subjective pleasantness of food-related stimuli to a greater extent in females than males.
Although neuroimaging studies have reported significant gender differences in brain activation during the physiological conditions of hunger and satiety in response to flavor (Del Parigi et al., 2002; Smeets et al., 2006) and food pictures (Cornier, Salzberg, Endly, Bessesen, & Tregllas, 2010; Frank et al., 2010; Uher, Treasure, Heining, Brammer, & Campbell, 2006), to date, no study has examined gender differences during hunger and satiety in response to pure tastes.
The perceived valence of a stimulus (e.g. pleasant or unpleasant) modulates patterns of cortical activation. In regard to chemosensory stimuli, valence specific brain activation has been localized within emotion processing regions such as the orbital frontal cortex (OFC) and amygdala (Francis et al., 1999; Kringelbach, O'Doherty, Rolls, & Andrews, 2003; O'Doherty, Rolls, Francis, Bowtell, & McGlone, 2001; Small, Zatorre, Dagher, Evans, & Jones-Gotman, 2001; Zald, Hagen, & Pardo, 2002). Findings from research involving other sensory modalities demonstrate that valence-specific activation is further influenced by gender. Specifically, gender differences have been reported in response to stimuli with various affective characteristics including olfaction (Berglunds, Lindstrom & Savic, 2006; Levy et al., 1997; Royet, Plailly, Delon-Martin, Kareken, & Segebarth, 2003; Savic, Berglund & Lindstrom, 2005; Yousem et al., 1999), visual cues (Killgore & Yurgelun-Todd, 2010; Klein et al., 2003; Schienle, Schafer, Stark, Walter, & Vaitl, 2005; Wrase et al., 2003), happiness and sadness (Azim, Mobbs, Jo, Menon, & Reiss, 2005; Schneider, Habel, Kessler, Salloum, & Posse, 2000), and unpleasant words associated with body image (Shirao, Okamoto, Mantani, Okamoto, & Yamawaki, 2005). While the direction of gender effects across experiments vary, consistent differences in activation are observed within the inferior frontal gyrus (IFG), anterior cingulate cortex (ACC), and amygdala.
We have previously shown that the physiological conditions of hunger and satiety influence brain activation in young (Haase, Cerf-Ducastel, & Murphy, 2009) and older adults (Jacobson, Green & Murphy, 2010) in response to pure taste stimuli. However, gender differences in brain activation during hunger and satiety in response to pure taste stimuli have yet to be examined. Additionally, it is unknown what role gender has in cortical activation in response to pleasant and unpleasant taste stimuli. Therefore, one aim of the present event-related fMRI study was to investigate gender differences in cortical fMRI activation in response to pure taste stimuli when subjects were hungry or satiated. In addition, using this experimental design we have previously reported decreases in fMRI activation in regions implicated in emotion and modulation of eating behavior (e.g., amygdala, hypothalamus, inferior insula, orbitofrontal cortex; Haase et al., 2009), so we also examined gender differences in the change in brain activation from hunger to satiety.
Methods
A more detailed description of the materials and methods used in this study can be found in Haase, Cerf-Ducastel, Buracas, & Murphy, 2007, in the Journal of Neuroscience Methods.
Participants
Twenty-one healthy young adults, 12 females and 9 males, ranging in age from 19 to 26 years (Males: M = 20.44; Females: M 21.58), participated in the study after providing written informed consent. They received monetary compensation for their participation. The Institutional Review Boards at both San Diego State University and the University of California, San Diego approved the research. Data from these subjects have been previously published (Haase et al., 2007; Haase et al., 2009; Jacobson et al., 2010).
Screening Session
In the first session, participants completed the chemosensory assessment to screen for ageusia and anosmia with taste and odor threshold measurements (Cain, Gent, Catalanotto, & Goodspeed, 1983; as modified in Murphy, Gilmore, Seery, Salmon, & Lasker, 1990). Exclusionary criteria consisted of ageusia, anosmia, and upper respiratory infection or allergies within the prior two weeks (Harris, Davidson, Murphy, Gilbert, & Chen, 2006). Participants also completed the Three-Factor Eating Questionnaire (Stunkard & Messick, 1985), to screen for restrained eating, and the preliminary fMRI safety screening. With regards to restrained eating, participants were within normal limits (≤ 12), with the exception of one female participant who scored 13 on the cognitive restraint factor.
Experimental Procedure
In the second and third sessions, the participants fasted for 12 hours prior to arrival and were randomly presented with either a pre-load consisting of 474ml (700kcal) of Vanilla flavored Ensure Plus (sated condition) or were not administered a pre-load (hungry condition) and then completed an fMRI session conducted on a 3T GE whole body scanner. Before, after, and during the scans, participants rated the pleasantness of the taste stimuli, using a modified version of the general Labeled Magnitude Scale (gLMS; Bartoshuk et al., 2004; Green, Shaffer, & Gilmore, 1993; Green et al., 1996). Participants also rated their perceived hunger before and after the scans using a modified gLMS.
Stimuli
Participants were administered 6 stimuli while in the scanner, 4 of which represented the basic tastes of bitter, sour, sweet and salty and were the focus of the present manuscript. The taste stimuli were presented dissolved in distilled water: caffeine, 0.04M; citric acid, 0.01M; sucrose, 0.64M; and sodium chloride (NaCl), 0.16M.
Stimulus Presentation
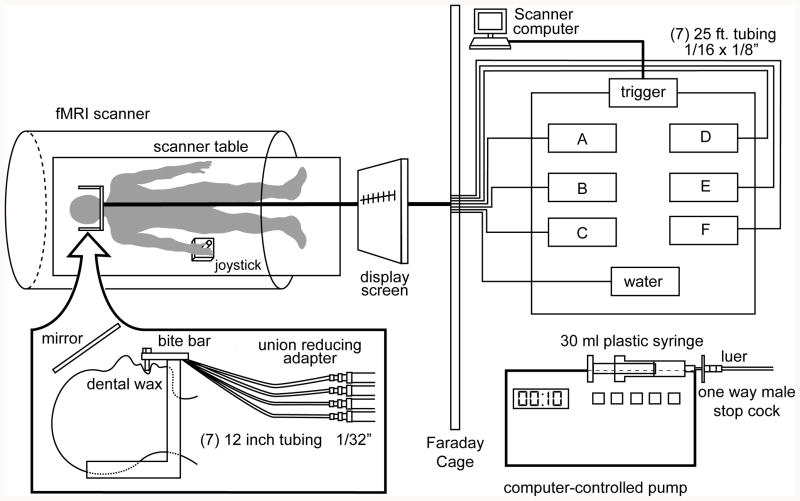
Inside the scanner, the participant lay supine and was fitted with a bite bar, which was positioned comfortably between the lips so that the tubes delivered stimuli to the tip of the tongue (See Figure 1). The taste stimuli and water were delivered at room temperature each through a unique 25 ft long plastic tube, which was connected to a different computer-programmable syringe pump. The pumps were programmed to present 0.3ml of solution in 1 s (Haase et al., 2007).
Figure 1.
The participant lays supine in the scanner and is delivered tastes to the mouth via a tubing system that is connected to computer-controlled pumps. After receiving each tastant, the participant uses a joystick to rate the perceived pleasantness on the gLMS, which is projected into the field of vision using a display screen and mirror. Figure reproduced from Haase, Cerf-Ducastel, Buracas, Murphy (2009), with permission.
During the functional run, the stimuli were counter-balanced and separated by a 10s inter-stimulus interval (ISI). Stimulus presentation was followed by two presentations of water; the first presentation of water was used as a rinse and the second presentation of water was used as a baseline comparison for each tastant. Instructions were displayed on a screen through a computer interface.
FMRI Scanning Paradigm
Experimental Design
Scans consisted of a pleasantness run 24 min (1440 s) in duration, where each stimulus was presented eight times. Two presentations of the same stimulus were separated by a minimum of 60 seconds due to the fact that there were two water presentations between each stimulus, and that no two stimuli were presented sequentially, thus minimizing adaptation (Bartoshuk, McBurney, & Pfaffmann, 1964; McBurney & Pfaffmann, 1963). Each of the computer-controlled pumps was programmed with a distinct series of stimulation periods so that the above conditions were met.
Image Acquisition
Imaging was conducted on a 3T General Electric (GE) Excite “shortbore” scanner. Structural images for anatomical localization of the functional images were collected first using a high-resolution T1-weighted whole-brain FSPGR sequence [Field of view (FOV) = 25 cm, slice thickness = 1 mm, resolution 1×1×1 mm3, echo time (TE) = 30 ms, Locs per slab = 136, flip angle = 15°]. A standard gradient echo EPI pulse sequence was used to acquire T2*-weighted functional images [24 axial slices, FOV = 19 cm, matrix size = 64×64, spatial resolution = 2.97×2.97×3 mm3, flip angle = 90°, echo time (TE) = 30 ms, repetition time (TR) = 2 s].
Image Analysis
Functional data were processed and analyzed using Analysis of Functional NeuroImage (AFNI) software (Cox, 1996). Preprocessing consisted of: motion correction, temporal and spatial smoothing, concatenation, and automasking. Deconvolution was applied to the concatenated runs at the individual level. In particular, in the 3dDconvolution analysis, for each stimulus, the main effects of pleasantness during the hunger condition and pleasantness during the satiety condition were investigated separately, for males and females, against a baseline of water. To conduct group analysis, for each contrast, a one-sample t-test was calculated on the fit coefficient at each voxel, separately for males and females. FMRI activation of males and females was also directly compared using independent samples t-tests for each stimulus during the hunger and sated conditions.
In order to investigate the effect of gender and physiological condition, voxel-by-voxel difference scores representing the change in activation from hunger to satiety for males and females, separately, were created. Independent samples t-test were then run to detect significant gender differences in the change from hunger to satiety for each stimulus. In other words, the gender by physiological state interaction was examined. Positive activation was associated with greater change in activation from hunger to satiety in females and negative activation was associated with greater change in activation from hunger to satiety in males.
Voxel by voxel analysis
The program 3dDeconvolution was used to analyze each individual dataset. 3dDeconvolution is a multiple regression program that estimates the impulse response function by examining the relationship between each individual time series dataset and the timing of stimulus onset and duration. The resulting output is a beta coefficient with corresponding t statistics, for each voxel, which corresponds to the size, direction, and fit of the impulse response function.
A whole brain statistical threshold correction for multiple statistical tests was performed. The statistical threshold was chosen based on the result of 10,000 Monte Carlo simulations run using the program AlphaSim (Ward, 1997). AlphaSim estimates the probability of obtaining false positives over an entire statistical map; in other words, the probability of generating a random cluster of noise by chance. This was done after voxels were individually thresholded at p ≤ 0.005 in an effort to control for multiple statistical tests. Therefore, only voxels activated according to this criterion (individual threshold p ≤ 0.005) and belonging to a cluster of at least 12 contiguous voxels (the whole brain corrected cluster threshold corresponding to a multiple comparison alpha of .05) were reported in the manuscript. The AlphaSim with an a priori Type I error rate set at .05 indicated that with the parameters for the present study (spatial blurring = 4 mm, voxel volume 3 mm, connectivity radius = separated by no more than one voxel width), less than 4% of clusters of a size corresponding to 12 voxels would be activated by chance in the complete explored brain volume.
Results
Demographics
Independent samples t-tests were conducted to examine potential differences in demographic characteristics [dependent variables: age, weight (kg), height (CM), body mass index (BMI), cognitive restraint as measured by the Three Factor Eating Questionnaire (TFEQ), odor and taste thresholds, hunger before and after each scan] between males and females (Table 1). As expected, males were significantly taller and weighed significantly more than females. There were no significant differences between males and females for BMI, cognitive restrain, disinhibition, or hunger factors of the TFEQ, odor threshold, and hunger before and after each scan. While males had slightly better taste thresholds than females, both groups fell within the normal range.
Table 1. Demographics and Taste Psychophysics.
| Demographics | ||||
|---|---|---|---|---|
| Mean (SD) | ||||
| Demographics | Males | Females | t | Significance |
| Age (years) | 22.25 (2.7) | 21.94 (1.9) | 1.64 | p > .05 |
| Weight (kg)* | 73.09 (10.7) | 61.43 (5.9) | -2.58 | p < .05 |
| Height (cm)* | 177.51 (6.9) | 166.16 (6.2) | -3.86 | p < .05 |
| BMI | 23.15 (3.5) | 22.76 (2.6) | -0.39 | p > .05 |
| TFEQ - Restraint | 4.25 (4.1) | 6.25 (4.5) | 0.89 | p > .05 |
| Odor threshold L | 6.56 (1.4) | 6.44 (1.4) | 0.44 | p > .05 |
| Odor threshold R | 7.00 (1.4) | 7.25 (1.4) | 0.75 | p > .05 |
| Taste threshold* | 0.002 (0.003) | 0.011 (0.01) | 2.28 | p < .05 |
| Hunger before ensurea | 48.31 (26.2) | 56.00 (34.2) | 0.78 | p > .05 |
| Hunger after ensurea | 16.88 (17.3) | 13.88 (16.9) | -0.16 | p > .05 |
| Hunger after ensure scana | 24.27 (19.9) | 13.13 (12.7) | -1.55 | p > .05 |
| Hunger beforeb | 58.00 (26.4) | 47.93 (32.2) | -1.13 | p > .05 |
| Hunger afterb | 63.38 (28.7) | 57.80 (26.0) | -1.74 | p > .05 |
| Taste Psychophyics** | ||||
| Mean (SD) | ||||
| Pleasantness | Males | Females | ||
| Sucrose H | 60.97 (9.9) | 58.02 (14.3) | ||
| Sucrose S | 59.61 (7.7) | 56.18 (15.5) | ||
| Caffeine H | 33.88 (8.9) | 25.33 (12.1) | ||
| Caffeine S | 31.53 (7.6) | 28.06 (13.0) | ||
| NaCl H | 35.46 (14.4) | 43.59 (10.9) | ||
| NaCl S | 36.68 (12.3) | 38.98 (10.4) | ||
| Citric Acid H | 43.46 (12.5) | 41.05 (9.3) | ||
| Citric Acid S | 44.85 (13.4) | 39.68 (12.8) | ||
| Intensity | ||||
| Sucrose H | 33.89 (15.2) | 28.77 (23.3) | ||
| Sucrose S | 31.35 (20.6) | 28.91 (25.6) | ||
| Caffeine H | 41.95 (20.3) | 38.41 (20.6) | ||
| Caffeine S | 42.28 (17.6) | 46.00 (25.1) | ||
| NaCl H | 38.32 (22.2) | 26.18 (14.6) | ||
| NaCl S | 36.97 (21.4) | 31.57 (17.9) | ||
| Citric Acid H | 36.63 (16.5) | 28.84 (19.4) | ||
| Citric Acid S | 34.85 (20.3) | 30.71 (23.5) | ||
Significant differences between males and females
Hunger ratings during the preload condition (satiety)
Hunger ratings during the no preload condition (hunger)
Magnitude estimates of pleasantness and intensity, collected during the fMRI hunger (H) and satiety (S) scans, using the gLMS.
Psychophysics
Two repeated measures ANOVAs were conducted to examine gender differences in the perceived pleasantness and intensity of the taste stimuli during hunger and satiety conditions (Table 1). There was a significant difference in the perceived pleasantness among the stimuli [F(3,16) = 30.234, p < .001, η = .85]. This finding was expected since stimuli were chosen based on their differences in pleasantness from one another. A follow up Newman-Keuls post hoc test indicated that sucrose was significantly more pleasant than the other stimuli and that citric acid and NaCl were significantly more pleasant than caffeine. No other significant interactions or differences were found for pleasantness. There were also no effects of gender, stimulus quality, physiological condition, or interactions between these factors for intensity ratings.
A repeated measures ANOVA was conducted to examine potential gender differences in the perceived hunger in the preload (satiety; hunger ratings before the preload and after the preload at the end of the fMRI scan, time points 1 and 2, respectively) and no preload (hunger; hunger ratings before the scan and after the fMRI scan, time points 3 and 4, respectively) conditions. There were no significant main effects of gender on hunger ratings. However, there was a significant interaction between hunger and physiology [F(1,19) = 47.95, p < .001, η = .72]. Newman-Keuls post hoc test indicated that time point 2 (M = 16.86, SD = 16.1) was significantly lower than time points 1 (M = 57.81, SD = 32.75), 3 (M = 54.43, SD = 30.93), and 4 (M = 59.81, SD = 26.53). That is, as expected, participants were more satiated after the preload than prior to the preload. Additionally, after ingestion of the preload, participants were less hungry compared to post-scan in the no preload condition. There were no other significant interactions or differences.
Voxel by voxel group analysis
See Tables 2-5 for coordinates and a comprehensive listings of significantly activated areas for each condition.
Table 2.
Voxel-by-voxel group analysis of activation during hunger for males and females uniquely considered.
| MALES | |||||
|---|---|---|---|---|---|
| Voxels | Max Int | X | Y | Z | Focus Points |
| NaCl relative to water | |||||
| No Clusters Found | |||||
| CAFFEINE relative to water | |||||
| No Clusters Found | |||||
| SUCROSE relative to water | |||||
| 268 | 9.60 | 41 | -7 | 2 | Right: Insula (BA 13), IFG (BA 47), Parahipp G (BA 34) |
| 86 | 6.97 | -37 | 23 | 8 | Left: IFG (BA 45, 47), Insula (BA 13) |
| 40 | 7.27 | 14 | -16 | 2 | Right: Thalamus |
| 27 | 7.23 | 56 | -4 | 20 | Right: Precentral G (BA 4) |
| 21 | 6.74 | -49 | -16 | 17 | Left: Precentral G (BA 43) |
| 18 | 7.46 | 13 | -64 | -9 | Right: Culmen, Declive |
| 18 | 5.44 | -2 | 46 | 6 | Left: Anterior Cingulate (BA 32) Right: MFG (BA 9) |
| 18 | 5.95 | 38 | 47 | 11 | Right MFG (BA 10) |
| 16 | 6.94 | -5 | 8 | 3 | Left/Right: Caudate |
| 16 | 4.90 | -2 | -29 | 27 | Left: Posterior Cingulate (BA 23) |
| 14 | 6.00 | -36 | 40 | 19 | Left: MFG (BA 10) |
| 12 | 4.77 | -5 | -14 | 6 | Left: Thalamus |
| 12 | 5.44 | 8 | -80 | 6 | Right: Cuneus |
| 12 | 6.64 | -35 | -14 | 12 | Left: Insula, Lentiform N |
| CITRIC ACID relative to water | |||||
| No Clusters Found | |||||
| FEMALES | |||||
| Voxels | Max Int | X | Y | Z | Focus Points |
| NaCl relative to water | |||||
| 42 | 6.71 | -10 | -19 | 5 | Left: Thalamus, Mamillary B |
| 35 | 5.11 | 38 | -10 | 14 | Right: Insula (BA 13) |
| 28 | 7.12 | 53 | -5 | 24 | Left: IFG (BA 9) |
| 23 | 4.79 | 35 | 11 | 8 | Right: Insula (BA 13) |
| 21 | 5.10 | -11 | 71 | 9 | Right: Cuneus (BA 17) |
| 13 | 5.25 | 11 | -17 | 9 | Right: Thalamus |
| 12 | 4.53 | -5 | -17 | -4 | Left/Right: Red N |
| CAFFEINE relative to water | |||||
| 29 | 7.64 | -52 | -37 | 5 | Left: Temporal G (BA 21, 22) |
| 21 | 8.61 | 59 | 50 | 12 | Left: Temporal G (BA 22) |
| SUCROSE relative to water | |||||
| 609 | 8.34 | -34 | -1 | 11 | Left: Insula (BA 13), Precentral G (BA 4), IFG (BA 44, 47), Lentiform N, Amygdala, Parahipp G (BA 28, 34) |
| 536 | 9.39 | 11 | -13 | -1 | Left/Right: Thalamus, Parahipp G, Substantia nigra, Cingulate G (BA 30), Mammillary B |
| 304 | 6.66 | 41 | 2 | -4 | Right: Insula (BA 13), IFG (BA 44, 47), Parahipp G (BA 28, 34), Amygdala |
| 233 | 10.18 | -4 | -70 | 11 | Left/Right: Cuneus (BA 17), Cingulate C (BA 30), Posterior Cingulate (BA 23, 30) Lingual G (BA 18) |
| 151 | 9.33 | -20 | 62 | 13 | Right: Declive, Culmen |
| 76 | 7.41 | 17 | 5 | 8 | Right: Lentiform N, Caudate |
| 53 | 4.49 | -25 | -46 | -16 | Left: Declive, Culmen, Dentate |
| 20 | 4.62 | 11 | -5 | 9 | Left: Caudate, Lentiform N |
| 12 | 5.16 | 58 | -2 | 12 | Right: Precentral G (BA 43) |
| CITRIC ACID relative to water | |||||
| 409 | 8.25 | 17 | -19 | -1 | Left/Right: Thalamus, Lentiform N, Mammillary B, Substantia Nigra, Left: Parahipp G (BA 28), Insula |
| 84 | 9.18 | -16 | -34 | 5 | Left: Thalamus, Parahipp G (BA 27), Posterior Cingulate (BA 31) |
| 73 | 8.39 | 17 | -61 | 17 | Right: Posterior Cingulate (BA 31), Cuneus |
| 68 | 5.69 | 20 | -61 | -13 | R Culmen, Declive, Lingual G, Fusiform G |
| 62 | 5.23 | -11 | -65 | -13 | Left/Right: Declive, Culmen, Lingual G |
| 56 | 6.64 | -49 | 2 | 20 | Left: IFG (BA 44) Precentral G (BA 4, 43) |
| 53 | 5.96 | 41 | 8 | 5 | Right: Insula (BA 13), Precentral G (BA 43) |
| 45 | 5.96 | -37 | 32 | 5 | Left: IFG (BA10, 46) |
| 33 | 6.16 | 26 | -46 | -4 | Right: Parahipp G |
| 31 | 5.40 | 8 | -55 | -4 | Right: Culmen |
| 31 | 5.20 | 8 | 8 | 5 | Right: Caudate, Lentiform N |
| 29 | 4.58 | 56 | -13 | 23 | Right: Precentral G (BA 43) |
| 28 | 4.80 | -49 | -19 | 14 | Left: Postcentral G (BA 43), Insula (BA 13) |
| 25 | 5.23 | 35 | 23 | 8 | Right: IFG (BA 45, 47), Insula (BA 13) |
| 23 | 5.60 | 38 | -4 | -1 | Right: Insula (BA 13) |
| 23 | 6.06 | -52 | 11 | 8 | Left: IFG (BA 44), Insula (BA 13) |
| 13 | 6.25 | 20 | -35 | 4 | Right: Parahipp G, Thalamus |
| 13 | 6.25 | -17 | -62 | 16 | Left: Posterior Cingulate (BA 31) |
Voxels: number of activated voxels in a cluster. Clusters were generated by the voxel-by-voxel group analysis and extracted with the 3dclust algorithm from AFNI (Cox, 1996). X, Y, and Z coordinates are based according to the Talairach & Tournoux Atlas within AFNI and represent the maximum intensity of a cluster. Abbreviations: L = left, R = right, G = gyrus, BA = brodmann area, N = nucleus, Parahipp = parahippocampus, IFG = inferior frontal gyrus, MFG = middle frontal gyrus, tlrc: talairach coordinates, Max Int: maximum intensity.
Table 5.
Voxel-by-voxel group analysis showing areas of significant change in activation from hunger to satiety in males and females.
| Voxels | Max Int | X | Y | Z | Focus Points |
|---|---|---|---|---|---|
| CAFFEINE | |||||
| 428 | -4.19 | 38 | -68 | 21 | Right: Middle Temporal G, Cuneus and Lingual G (BA 19), Cingulate (BA 30), Fusiform G (BA 37), Angular G (BA 39) |
| 291 | -4.46 | 33 | 13 | -1 | Right: Insula (BA 13), IFG (BA 47), MFG, Caudate, Lentiform N |
| 253 | -3.84 | 2 | 17 | 24 | Right/Left: Anterior Cingulate (BA 24) Left: Caudate |
| 127 | -3.61 | -38 | -75 | 4 | Left: Cerebellum, Fusiform G (BA 37) |
| 81 | -4.15 | -11 | 71 | 9 | Left: Cuneus (BA 19) |
| 74 | -3.26 | 33 | 62 | -3 | Right: Superior Frontal G (BA 10) |
| 59 | -3.21 | -38 | -47 | 18 | Left: Superior Temporal G, Angular G (BA 39) |
| 41 | 2.67 | 32 | 20 | 3 | Right: Cingulate G (BA 30), Anterior Cingulate (BA 24) |
| 39 | -3.13 | -2 | 62 | 3 | Left: MFG (BA 10) |
| 37 | -4.34 | 65 | -20 | -1 | Left: Middle Temporal G (BA 21) |
| 36 | 3.52 | 23 | -74 | -33 | Right: Cerebellum |
| 30 | -3.39 | 62 | -20 | 18 | Left: IFG (BA 44, 45) |
| 26 | -3.10 | 20 | -16 | 14 | Right: Thalamus |
| 20 | -3.73 | -65 | -28 | -7 | Left: Middle Temporal G (BA 21) |
| 20 | -3.31 | -38 | -23 | 18 | Left: Insula (BA 13) |
| 19 | -2.93 | -4 | -70 | 11 | Left: Posterior Cingulate (BA 30), Lingual G (BA 18) |
| 18 | -2.97 | 23 | -77 | 33 | Right: Cuneus (BA 19) |
| 16 | -3.04 | 11 | 62 | 9 | Right: Superior Frontal G (BA 10) |
| 16 | -3.25 | 44 | -68 | 39 | Right: Angular G (BA 39) |
| 13 | -2.57 | 35 | 2 | 3 | Right: Insula (BA 13) |
| 12 | -2.90 | 32 | -23 | -19 | Right: Parahipp G |
| 12 | -2.70 | 38 | 8 | -10 | Right: Insula (BA 13) |
| 12 | -3.16 | -44 | 41 | 3 | Left: IFG (BA 10) |
| CITRIC ACID | |||||
| 64 | 4.26 | -47 | -35 | 15 | Left: Superior Temporal G (BA 41), Insula (BA 13), Postcentral G (BA 40) |
| 40 | 3.44 | 44 | -71 | 33 | Right: Angular G (BA 39) |
| 37 | 2.79 | 14 | -16 | 2 | Left: IFG (BA 9), Insula (BA 13) |
| 34 | -3.44 | -35 | -59 | -34 | Left: Cerebellum |
| 32 | 3.33 | -36 | -44 | -6 | Left: Parahipp |
| 28 | 3.14 | -44 | 38 | 18 | Left: Middle Frontal G (BA 10), IFG (BA 46) |
| 25 | -3.44 | 32 | 26 | -7 | Right: IFG (BA 47) |
| 23 | -4.34 | 38 | -10 | -4 | Right: Insula (BA 13) |
| 23 | -3.65 | -4 | 61 | -1 | Left: Superior Frontal G (BA 10) |
| 19 | 3.13 | -23 | -59 | 30 | Left: Precuneus |
| 18 | 2.82 | 40 | -45 | 33 | Right: Supramarginal G (BA 40) |
| 18 | 2.96 | -56 | -35 | 39 | Left: Inferior Parietal Lobule |
| 12 | -3.42 | 3 | 47 | 14 | Right: Medial Frontal G (BA 10) |
| NaCl | |||||
| 84 | -3.54 | -21 | -10 | -25 | Left: Parahipp G (BA 35) |
| 55 | -3.68 | -8 | -35 | -13 | Left: Cerebellum |
| 37 | 3.50 | -55 | -65 | 6 | Left: Middle Temporal G, Fusiform G (BA 37) |
| 36 | -2.82 | -5 | -53 | -22 | Left/Right: Cerebellum |
| 32 | 4.88 | -45 | 6 | 24 | Left: IFG (BA 9), Insula (BA 13) |
| 20 | -3.07 | 25 | 68 | 4 | Right: Superior Frontal G (BA 10) |
| 15 | -2.96 | 21 | -11 | -26 | Right: Parahipp G (BA 35) |
| 15 | -3.55 | 26 | 2 | -22 | Right: Uncus, Amgydala, Parahipp G (BA 28) |
| 15 | -3.69 | -50 | -8 | 6 | Left: Precentral G (BA 6), Insula (BA 13) |
| 12 | -2.80 | 20 | 29 | 21 | Right: Anterior Cingulate |
| SUCROSE | |||||
| 442 | -4.33 | -2 | -59 | 3 | Left/Right: Cerebellum, Lingual G, Cuneus (BA 19) Right: Posterior Cingulate (BA 23, 31) |
| 266 | -4.30 | 23 | -5 | -22 | Right: Uncus, Amygdala, Parahipp G (BA 28, 34, 35), IFG |
| 110 | -3.78 | 59 | 2 | 9 | Right: Precentral G, Superior Teamporal G (BA 22), Insula (BA 13) |
| 102 | -3.50 | 47 | -55 | 20 | Right: Superior Temporal G (BA 22) |
| 74 | -3.99 | 53 | -41 | -1 | Right: Middle Temporal G (BA 21) |
| 65 | -2.95 | -32 | -58 | -13 | Left: Cerebellum, Fusiform G (BA 37), Cuneus (BA 19) |
| 59 | -3.69 | 47 | -13 | 24 | Right: Postcentral G, Insula (BA 13) |
| 59 | -3.14 | 32 | -32 | -7 | Right: Lingual G (BA 19) |
| 44 | -3.99 | 45 | -32 | 12 | Right: Superior Temporal G (BA 22, 41) |
| 43 | -3.10 | 29 | -20 | -13 | Right: Parahipp G (BA 28, 35), Hippocampus |
| 43 | -3.61 | 41 | -83 | 18 | Right: Middle Temporal G (BA 19) |
| 40 | -3.36 | -13 | -69 | 23 | Left: Posterior Cingulate (BA 31) |
| 35 | -3.58 | 53 | -53 | -13 | Right: Inferior Temporal G (BA 20) |
| 31 | -3.17 | 2 | 50 | 21 | Right: MFG (BA 9) |
| 27 | -2.92 | 44 | 29 | -10 | Right: IFG (BA 47) |
| 23 | -3.29 | -23 | -23 | -22 | Right: Parahipp G (BA 35) |
| 22 | -3.06 | 17 | 35 | 24 | Right: Anterior Cingulate (BA 32) |
| 21 | -3.22 | -64 | -27 | -1 | Left: Middle Temporal G (BA 21) |
| 20 | -3.92 | 6 | 2 | 6 | Right: Caudate, Lentiform N |
| 20 | -3.39 | -16 | 14 | 17 | Left: Caudate |
| 20 | -6.02 | 35 | -31 | -2 | Right: Caudate |
| 20 | -2.85 | 38 | 35 | 24 | Right: MFG (BA 10) |
| 16 | -3.35 | -20 | 8 | -16 | Left: IFG (BA 47) |
| 15 | -2.85 | 11 | -84 | 27 | Right: Cuneus |
See Table 2 caption.
Females in the hunger condition
Positive brain activation for females was consistently found for sucrose, citric acid, and NaCl within the insula (BA 13), thalamus, cuneus, and mamillary bodies (Table 2). Positive activation was also found for sucrose and citric acid within the inferior frontal gyrus (IFG; Brodmann areas [BA] 44 and 47), caudate, lentiform nucleus, parahippocampal gyrus (BA 28, 34), posterior cingulate (BA 23, 31), cingulate (BA 30), substantia nigra, pre- and postcentral gyri (BA 4, 43), and cerebellum. There was additional activation for sucrose within the amygdala and parahippocampal gyrus (BA 27) and for citric acid within the fusiform gyrus. Activation for caffeine was seen within the superior and middle temporal gyri (BA 21 and 22).
Males in the hunger condition
For sucrose, significant activation was localized within the insula (BA 13), IFG (BA 45, 47), middle frontal gyrus (MFG; BA 9, 10), caudate, lentiform nucleus, parahippocampal gyrus (BA 34), anterior cingulate (BA 32), posterior cingulate (BA 23), thalamus, pre- and postcentral gyri (BA 4, 43), cuneus (BA 17), and cerebellum (Table 2). There was no significant activation in response to citric acid, NaCl, or caffeine.
Females in the satiety condition
Significant activation in response to sucrose and caffeine was localized within the IFG (BA 9, 46; Table 3). For citric acid and caffeine, activation was found in the precentral gyrus (BA 43). Activation was also found in response to citric acid within the caudate; for sucrose within the IFG (BA 8); for caffeine within the insula (BA 13), IFG (BA 47), anterior cingulate (BA 32), MFG (BA 10); and for NaCl within the posterior cingulate (BA 31).
Table 3.
Voxel-by-voxel group analysis of activation during satiety for males and females uniquely considered.
| MALES | |||||
|---|---|---|---|---|---|
| Voxels | Max Int | X | Y | Z | Focus Points |
| NaCl relative to water | |||||
| No Clusters found | |||||
| CAFFEINE relative to water | |||||
| 42 | -7.38 | 47 | -53 | -10 | Right: Fusiform G (BA 37) |
| 41 | -5.51 | 28 | 28 | -5 | Right: IFG (BA 47), Lentiform N |
| 36 | -6.88 | 13 | -51 | 22 | Right: Posterior Cingulate (BA 31) |
| 30 | -8.40 | 47 | -55 | 20 | Right: Superior Temporal G (BA 22) |
| 21 | -5.65 | 46 | -52 | 4 | Right: Middle Temporal G (BA 39) |
| 21 | -6.95 | 8 | -68 | 24 | Right: Precuneus |
| 20 | -16.00 | 50 | 14 | -10 | Right: Superior Temporal G (BA 38), IFG (BA 47) |
| 14 | -6.00 | 32 | -32 | -7 | Right: Parahipp G |
| SUCROSE relative to water | |||||
| 295 | -10.66 | 5 | -40 | -13 | Left/Right Culmen Left: Parahipp G (BA 36) |
| 46 | -12.66 | 29 | -6 | -17 | Right: Parahipp G (BA 28, 34), Amygdala |
| 43 | -10.36 | 23 | -61 | 11 | Right: Posterior Cingulate (BA 31) |
| 37 | -7.48 | -22 | -7 | -16 | Left: Parahipp (BA 28, 34), Amygdala, Hippocampus |
| 35 | -6.72 | 29 | -28 | -13 | Right: Parahipp G (BA 28, 35) |
| 27 | -3.74 | -2 | 50 | 12 | Right: Posterior Cingulate (23) |
| CITRIC ACID relative to water | |||||
| 136 | -9.66 | -17 | -27 | 20 | Left: Caudate |
| 127 | -8.04 | 18 | -25 | 19 | Right: Caudate, Thalamus |
| 55 | -7.60 | -16 | 41 | -1 | Left: Anterior Cingulate (BA 32) |
| 40 | -6.02 | 35 | -31 | -2 | Right: Caudate |
| 32 | -6.47 | 5 | -49 | 20 | Left/Right: Cingulate (BA 30), Posterior Cingulate (BA 23), Precuneus |
| 28 | -5.91 | 43 | -8 | -23 | Right: Fusiform G (BA 37) |
| FEMALES | |||||
| Voxels | Max Int | X | Y | Z | Focus Points |
| NaCl relative to water | |||||
| 22 | -0.62 | 26 | 50 | 9 | Left: Posterior Cingulate (BA 31) |
| CAFFEINE relative to water | |||||
| 52 | 5.47 | 46 | 10 | 22 | Right: IFG (BA 9, 46) |
| 44 | 5.99 | 38 | 14 | 8 | Right: Insula (BA 13), IFG (BA 47) |
| 21 | 5.30 | 53 | -7 | 20 | Right: Precentral G (BA 43) |
| 16 | -6.60 | -2 | 2 | -4 | Left/Right: Anterior Cingulate (BA 32) |
| 16 | 5.60 | -41 | 29 | 15 | Left: MFG, IFG (BA 46) |
| 12 | 4.55 | 38 | 41 | 18 | Right: MFG (BA 10) |
| SUCROSE relative to water | |||||
| 18 | 4.70 | 41 | -2 | 24 | Left: IFG (BA 9) |
| 14 | 4.16 | -47 | 2 | 30 | Left: IFG (BA 8) |
| CITRIC ACID relative to water | |||||
| 33 | -4.68 | 21 | -31 | 18 | Right: Caudate |
| 21 | -5.80 | 35 | -31 | -1 | Right: Caudate |
| 16 | 4.55 | 52 | -9 | 24 | Right: Precentral G (BA 43) |
See Table 2 caption.
Males in the satiety condition
In response to sucrose, caffeine, and citric acid there was significant activation within the posterior cingulate (BA 31), caudate (Table 3). Several regions were activated by two stimuli: fusiform gyrus (BA 37; caffeine and citric acid); parahippocampal gyrus (BA 28, 34, 36; sucrose and caffeine). Additionally, in males sucrose elicited activation within the amygdala, hippocampus and cerebellum; citric acid elicited activation within the caudate, anterior cingulate (BA 32) and thalamus; and caffeine elicited actvation within the IFG (BA 47), lentiform nucleus, superior and middle temporal gyri (BA 22, 38, 39), and precuneus.
Males versus females
In order to examine significant gender differences in brain regions involved in processing taste stimuli during pleasantness evaluation, cortical activation in males was subtracted from activation in females, separately for the conditions of hunger and satiety (Table 4).
Table 4.
Voxel-by-voxel group analysis of activation in males minus activation in females during the hunger and satiety conditions.
| HUNGER | |||||
|---|---|---|---|---|---|
| Voxels | Max Int | X | Y | Z | Focus Points |
| NaCl relative to water | |||||
| 27 | 4.46 | -47 | 2 | 24 | Left: IFG (BA 9) |
| 16 | 3.77 | 26 | -75 | 27 | Right: Precuneus |
| CAFFEINE relative to water | |||||
| No clusters found | |||||
| SUCROSE relative to water | |||||
| No clusters found | |||||
| CITRIC ACID relative to water | |||||
| 236 | 5.63 | -48 | -20 | 16 | Left: Insula (BA 13), Superior Temporal Gyrus |
| 95 | 5.29 | 14 | -59 | 27 | Right: Precuneus, Posterior Cingulate (BA 31) |
| 64 | 4.68 | -26 | 2 | 6 | Left: Lentiform N, Insula |
| 56 | 4.62 | -23 | -74 | 19 | Left: Precuneus, Posterior Cingulate (BA 31) |
| 34 | 6.18 | 32 | -68 | 19 | Right: Middle Temporal G |
| 31 | 4.28 | 20 | -61 | -10 | Right Declive |
| 27 | 5.98 | -15 | -35 | 5 | Left: Parahipp (BA 27), Thalamus |
| 16 | 3.95 | 47 | -29 | 12 | Right: Temporal G (BA 41), Insula |
| 13 | 4.11 | 50 | -21 | 24 | Right: Postcentral G |
| 12 | 4.24 | 8 | -65 | -13 | Right: Declive |
| 12 | 4.55 | 25 | -60 | 32 | Right: Precuneus |
| SATIETY | |||||
| NaCl relative to water | |||||
| No clusters found | |||||
| CAFFEINE relative to water | |||||
| 165 | 5.49 | 26 | 11 | -1 | Right: Lentiform N, Insula (BA 13), IFG (BA 47) |
| 74 | 5.74 | 8 | -71 | 21 | Right: Cuneus, Precuneus |
| 17 | 4.49 | -23 | 8 | 6 | Left: Lentiform N |
| 17 | 3.97 | 17 | -58 | 6 | Right: Posterior Cingulate (BA 30), Lingual G |
| 17 | 4.08 | 38 | -62 | 9 | Right: Middle Temporal G |
| SUCROSE relative to water | |||||
| 103 | 4.65 | 20 | -65 | 12 | Right: Posterior Cingulate (BA 30), Cuneus, Lingual G |
| 39 | 4.30 | 5 | -47 | 2 | Right: Culmen |
| 17 | 3.99 | -2 | -59 | 3 | Left: Culmen |
| CITRIC ACID relative to water | |||||
| 100 | 5.60 | -2 | -50 | 18 | Left: Posterior Cingulate (BA 30) |
| 37 | 4.68 | -2 | -16 | 10 | Left/Right: Thalamus |
| 13 | 4.79 | 8 | -66 | -5 | Right: Culmen |
| 13 | 4.45 | -17 | -47 | -13 | Left: Culmen |
See Table 2 caption.
Hunger
During the hunger condition, females had significantly more activation than males in response to citric acid within the insula (BA 13), thalamus, parahippocampal gyrus (BA 27), lentiform nucleus, posterior cingulate (BA 31), precuneus, super and middle temporal gyri, postcentral gyrus (BA 43), and cerebellum. Females also had significantly more activation than males in response to NaCl within the IFG (BA 9) and precuneus. Differences in brain activation between males and females did not reach statistical significance during the hunger condition for caffeine or sucrose.
Satiety
Activation within the posterior cingulate (BA 31) was consistently greater for females relative to males in response to sucrose, caffeine, and citric acid. Females also demonstrated greater activation localized within the cerebellum in response to sucrose and citric acid; within the cuneus, and lingual gyrus in response to sucrose and caffeine; within the thalamus in response to citric acid; and within the insula (BA 13), IFG (BA 47), lentiform nucleus, and middle temporal gyrus in response to caffeine. Differences in brain activation between males and females did not reach statistical significance during the satiety condition for NaCl.
The effect of gender and physiological condition
In order to examine gender differences in the change in brain activation from hunger to satiety, an independent samples t test was run on difference scores related to the change in activity from hunger to satiety. For this analysis, positive activation represented a greater change in activation from hunger to satiety in females and negative activation represented a greater change in activation from hunger to satiety in males (Figure 2). Globally, males demonstrated greater changes in activation from hunger to satiety relative to females (Table 5).
Figure 2.
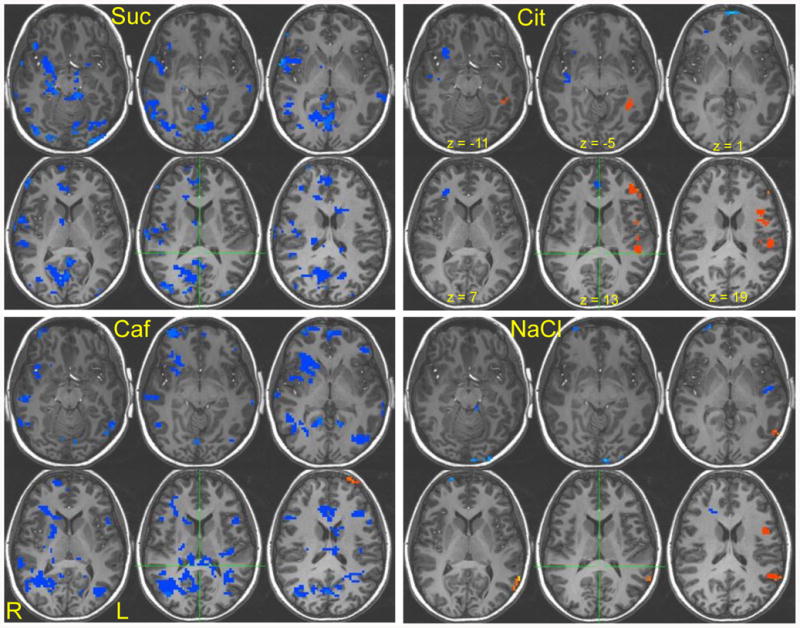
The right side of the brain appears on the left side of the image according to the radiological convention. Z coordinates correspond to -11, 5, 1, 7, 13, 19, respectively. Suc = sucrose, Cit = citric acid, Caf = caffeine, NaCl = sodium chloride, L = left, R = Right. An individual structural brain image is used as the anatomical underlay. Activation is corrected for multiple comparisons (p < .004, cluster threshold = 12). Red indicates greater change in activation from hunger to satiety in females and blue represents greater change in activation from hunger to satiety in males.
The change in activation from hunger to satiety was consistently greater in males relative to females for caffeine, NaCl, citric acid, and sucrose within the insula, IFG (BA 10), and cerebellum; in response to caffeine, NaCl, and sucrose within the parahippocampal gyrus and anterior cingulate; in response to caffeine, citric acid, and sucrose within the IFG (BA 47); in response to caffeine and sucrose within the caudate, lentiform nucleus, posterior cingulate, fusiform gyrus, middle and superior temporal gyri, lingual gryus and cuneus; and in response to NaCl and sucrose within the amygdala, uncus, and precentral gyrus.
Change in activation was greater in females relative to males in response to caffeine within the anterior cingulate and cerebellum; in response to NaCl within the middle temporal gyrus, fusiform gyrus, IFG (BA 9) and insula; and in response to citric acid within the superior temporal gyrus, insula, parahippocampal gyrus, angular gyrus, MFG (BA 10), and IFG (BA 46).
Discussion
The first aim of the present study was to examine potential gender differences in brain activation to taste stimuli during the physiological conditions of hunger and satiety. As expected, there was considerable overlap in the cortical regions activated in males and females, including gustatory, memory, and reward processing regions. However, significant differences in brain activation were also observed, which varied as a function of gender, physiological condition, and stimulus quality.
Hunger: Males and females uniquely considered
During the hunger condition, females had significant cortical activation in response to sucrose, citric acid, and NaCl within the regions previously shown to be involved in taste processing within non-human primates including the thalamus (Pritchard Hamilton, & Norgen, 1989), insula (Pritchard, Hamilton, Morse & Norgen, 1986) and frontal operculum (BA 44; Scott, Yaxley, Sienkiewicz, & Rolls, 1986; See Table 2 for a comprehensive list). Activation was also localized in response to sucrose within the amygdala; in response to sucrose and citric acid within the precentral gyrus (BA 43) and parahippocampal gyrus (BA 28); and in response to NaCl and citric acid within dorsolateral prefrontal cortex (BA 9, 46). These regions have been shown to respond to taste stimulation and have connections with the insula (Augustine, 1996; Baylis, Rolls & Baylis 1995; Rolls, Yaxley, & Sienkiewicz, 1990; Scott, et al., 1993). The present findings are also consistent with previously published reports that examined brain activation in humans, but without regard to gender, in response to taste stimuli (Cerf, Le Bihan, Van de Moortele, Mac Leod, & Faurion, 1998; Cerf-Ducastel, Van De Moortele, MacLeod, Le Bihan, & Faurion, 2001; de Araujo, Kringelbach, Rolls, & McGlone, 2003; Faurion et al., 1999; Francis et al., 1999; Frank et al., 2008; Kringelbach et al., 2003; Kringelbach, de Araujo & Rolls, 2004; O'Doherty et al., 2001; Zald et al., 2002) and fMRI experiments examining the effect of hunger on brain activation in response to taste and flavor stimuli (Del Parigi et al., 2002; Haase et al., 2007; 2009; Kringelbach et al., 2003; Tataranni et al., 1999; Uher et al., 2006).
Females also demonstrated cortical activation, during the hunger condition, within the limbic system. In particular, activation was localized within reward and memory processing regions including the amygdala, dorsal striatum (caudate, putamen), parahippocampal gyrus, entorhinal cortex (BA 28, 34), and cingulate gyrus, for sucrose and citric acid (Elliott, Dolan, & Frith, 2000; Elliott, Friston, & Dolan 2000; O'Doherty, 2004; Volkow, Fowler, & Wang 2002; for a review see Balleine, Mauricio, & Hikosaka, 2007). Activation within these regions may facilitate associative learning and long-term memory formation/retrieval (Cahill et al., 1996; Dolcos, LaBar, & Cabeza, 2004; Cerf-Ducastel & Murphy, 2006, 2009; Hamann, Eli, Grafton, & Kilts, 1999; Richardson, Strange, & Dolan, 2004; Shigemune et al., 2010). Interestingly, there were few brain regions where activation met statistical significance for caffeine. For males, there were no brain regions with activation that survived the threshold in response to NaCl, caffeine, and citric acid. However, males had brain activation in response to sucrose that was similar to females, after a 12-hour fast (See Table 2 for a comprehensive list). In response to sucrose and caffeine, brain activation in males and females appeared to be consistent after a 12 hour fast. On the other hand, brain activation in response to citric acid and NaCl only reached significance for females, despite equivalent pleasantness and intensity ratings. This discrepancy in activation, suggests that females process taste stimuli differently when hungry relative to males.
Satiety: Males and females uniquely considered
Relative to the hunger condition where brain activation was consistent across stimuli and all significantly activated clusters were greater for taste stimuli than water (positive activation), in the sated condition, cortical activation for females was less consistent across stimuli with regard to localization of activation and intensity. Females had positive (greater activation in comparison to water) and negative (less activation in comparison to water) activation; however, males had consistent negative activation, across all stimuli. These findings suggest that males have greater change in brain activation from hunger to satiety than females.
Females had positive activation within the dorsolateral prefrontal cortex (BA 9) in response to sucrose and caffeine, and within the frontal cortex (BA 43) in response to citric acid and caffeine (See Table 3 for a comprehensive list). Activation within the dorsolateral prefrontal cortex has previously been reported in response to taste stimuli (Kringelbach, de Araujo, & Rolls 2004) and may be associated with increased attentional or executive functioning demands (Frith & Dolan, 1996). Of note, negative activation was observed in females within limbic regions including the posterior cingulate (to NaCl), caudate nucleus (to citric acid), and the anterior cingulate (to caffeine).
Negative brain activation in males appeared to be more robust in the satiety condition within limbic regions for caffeine [i.e., IFG (BA 47), dorsal striatum (putamen), parahippocampal gyrus], sucrose (i.e., amygdala, parahippocampal gyrus, hippocampus), and citric acid [i.e., dorsal striatum (caudate)].
Hunger: Comparison of male and females
The greatest gender discrepancy in brain activation during the hunger condition occurred in response to the sour stimulus citric acid (See Table 4 for a comprehensive list). In response to citric acid, females had greater activation within the insula (BA 13), thalamus, posterior cingulate, parahippocampus (BA 27), dorsal striatum (i.e., putamen), postcentral gyrus, and superior and middle temporal gyri. Activation was also greater for females within the dorsolateral prefrontal cortex BA (9) in response to NaCl. These findings for pure taste stimuli are consistent with results of studies examining gender differences in brain response to flavor stimuli (Uher et al., 2006) and food images (Cornier et al., 2010; Frank et al., 2010). In particular, in response to flavor stimuli in a hunger condition, greater activation was observed in females in the anterior insula, frontal operculum and medial prefrontal cortex relative to males (Uher et al., 2006). Females in that cohort reported being more hungry than males, but there were no gender differences in response to the perceived pleasantness of the stimuli (Uher et al., 2006). More recently, Cornier and colleagues (2010) reported greater cortical activation during a fasted state, in response to food images, within the lateral and dorsolateral prefrontal cortices and parietal cortices for women relative to men.
Other studies have reported greater activation in males relative to females. In response to a liquid formula meal, Ensure-Plus, Del Parigi et al. (2002) reported greater activation for males during the physiological state of hunger within dorsolateral prefrontal cortex, middle temporal gyrus, posterior cingulate, and parahippocampus, relative to females. Of note, brain activation to citric acid and NaCl was greater for females than males in the current study, despite equivalent ratings of the perceived pleasantness and intensity, and equivalent hunger ratings. This suggests that significant differences in brain activation between males and females may be a function of the reward value of the stimulus below the level of consciousness.
When considered uniquely, no significant differences between males and females were found in response to caffeine and sucrose in the hunger condition. Bitter taste can serve to warn against the ingestion of toxic substances (Scott, Yan, & Rolls, 1995). The lack of significant differences in cortical activation for these stimuli may reflect the ecological importance of prototypically pleasant, nutritional (sucrose), and unpleasant, non-nutritional (caffeine) taste stimuli, particularly during the physiological state of hunger. Specifically, the lack of significant positive and negative activation for caffeine within primary taste regions for males and females and the failure to find differences in the direct comparison between males and females may reflect brain responses that are involved in avoiding the consumption of food that could be particularly dangerous if consumed. Conversely, the robust activation to sucrose and the lack of significant differences in brain activation in the direct comparison between males and females may stimulate eating when hungry. While these interpretations are purely speculative, they may stimulate further work that contributes to a greater understanding of the neural correlates of eating behavior.
Satiety: Comparison of males to females
Cortical activation in males was subtracted from cortical activation in females in order to examine significant gender differences in brain regions involved in processing taste stimuli during the satiety condition (See Table 4 for a comprehensive list). During satiety, females had significantly more brain activation than males for all pure taste stimuli. No significant differences were found for NaCl. However, females had greater activation than males in the posterior cingulate for sucrose, citric acid, and caffeine, and greater activation in the occipital cortex (i.e., lingual gyrus) for sucrose and caffeine. In addition, females had greater activation than males in response to caffeine within the insula, IFG (BA 47), dorsal striatum (putamen), and middle temporal gyrus. Greater activation with the amygdala and orbitofrontal cortex in females than in males has been reported in response to negative emotions (Koch et al., 2007). Studies that have examined gender differences in response to flavor stimuli during satiety have reported greater activation in females, relative to males in the DLPFC, precuneus, angular gyrus, occipital lobe, and posterior temporal lobe (Del Parigi et al., 2002), and hypothalamus and ventral striatum (Smeet et al., 2006). While the present study did not find any regions that were more activated to pure tastes in males than in females in the satiety condition, flavor studies have found greater prefrontal activation in males relative to females during satiety (Del Parigi et al., 2002; Smeet et al., 2006).
The effect of gender and physiology
The second aim was to examine gender differences in the change in brain activation to taste stimuli from hunger to satiety. In order to investigate this effect, the change in activation from hunger to satiety was compared between males and females (Table 5 and Figure 2). Negative activation reflected greater change from hunger to satiety in males relative to females. In other words, negative activation suggests that females had less of a decrease in activation from hunger to satiety than males. Changes in activation from hunger to satiety were consistently greater for males relative to females for all stimuli, in several areas including the middle frontal gyrus (BA 10), insula (BA 13), and cerebellum. Neuroimaging studies have consistently documented the role of the middle frontal gyrus (BA 10) in executive functioning. In particular, BA 10 is engaged during dual-task performance (Collette, Olivier, Van Der Liden, Laurys, Delfiore, Luxen & Salmon, 2005; Wager & Smith, 2003) and decision-making (Rolls & Grabenhorst, 2008). While little is known regarding potential gender differences in activation within BA 10, a recent study suggests that increased activation in females within this region may reflect greater “top-down” control in response to salient stimuli. Previous research has shown that during mental rotation tasks, males utilize “bottom-up” strategy, whereas females utilize a more effortful top-down strategy (Butler et al., 2006). Although purely speculative, the present findings may suggest that women engage more cognitive resources when processing taste hedonics, irrespective of physiological condition, which may be related to environmental factors such as cost/benefit analysis of calorie consumption.
In addition to processing taste and flavor information, the insula has been shown to be involved in the regulation of interoceptive states such as pain, thirst, and hunger (Cerf-Ducastel & Murphy, 2001; Critchley, Wiens, Rotshtein, Ohman, & Dolan 2004; Haase et al., 2009; Peyron, Laurent, & Garcia-Larrea, 2000; Pukall et al., 2005; Shibasaki, 2004; Small et al., 2001; Veldhuijzen, Greenspan, Kim, & Lenz, 2010; McKinley, Denton, Oldfield, De Oliveria, & Mathai, 2006) and in response to external states such as disgust (Wicker et al., 2003). Previous studies have reported significantly more brain activation for females relative to males within the insula during the observation of contemptuous faces (Aleman & Swart, 2008), during retrieval of negative memories (Piefke, Weiss, Markowitsch, & Fink, 2005) and in response to visual images of energy dense foods (Killgore et al., 2010). The present results support previous findings of gender differences in brain activation within the insula, and suggests that these differences may be modulated by physiological condition.
Relative to females, males demonstrated greater change in activation from hunger to satiety in response to sucrose and NaCl within the anterior cingulate. Activity in the anterior cingulate has been found to correlate with pleasantness ratings of water (de Araujo et al., 2003) and food-related stimuli (de Araujo, Rolls, Velazco, Margot, & Cayeux, 2005; Grabenhorst, Rolls, Parris, & d'Souza, 2009; Rolls, Kringelbach, & de Araujo, 2003). The pleasantness ratings did not significantly differ between males and females for any of the taste stimuli. The fact that brain activation showed less of a change from hunger to satiety in females than in males may reflect differences in neuronal response in females that were not captured in subjective pleasantness ratings. Interestingly, males and females demonstrated significant changes in activation from hunger to satiety within the anterior cingulate in response to caffeine.
Males also had greater change in brain activation from hunger to satiety in a number of limbic regions. In particular, males showed greater changes in response to sucrose, caffeine, and citric acid within the inferior frontal gyrus (BA 47); in response to sucrose and NaCl within the parahippocampal gyrus, entorhinal cortex (BA 38), perirhinal cortex, and amygdala; and in response to sucrose and within the dorsal striatum (caudate, putamen) and posterior cingulate. In a recent study, males demonstrated greater decreases in brain metabolism in response to food stimulation during cognitive inhibition within the orbitofrontal cortex, parahippocampus, amygdala, and putamen (Wang et al., 2009). The authors reported correlations between deactivation of the OFC and decreases in hunger ratings in males but not females, suggesting possible decreased neural signaling of the need for meal termination in females. This, in turn may lead to overeating and may contribute to higher obesity rates found in females. Greater activation within reward regions and memory processing regions during satiety may facilitate emotional learning. Therefore, it could be speculated that when females are presented with a pleasant taste stimulus, brain activation within these regions could trigger eating despite being satiated.
The IFG and posterior cingulate have been found to be involved in emotional processing for verbal (Kuchinke et al., 2005) and visual (Phillips et al., 1998) stimuli and may facilitate memory formation and retrieval. In addition, the posterior cingulate is associated with the evaluation of emotionally laden memories, particularly when the evaluative task is self-referential (Touryan et al., 2007). The dorsal striatum is hypothesized to play a modulatory role in food motivation and reward. Specifically, dopamine (DA) release in the dorsal striatum facilitates feeding (Szczypka et al., 2001), has been linked to the pleasantness derived from eating (Small, Jones-Gotman, & Dagher, 2003), and recent neuroimaging studies on flavor and food reward suggest that decreased activation of the dorsal striatum and levels of DA release in this region may be related to obesity (Green, Jacobson, Haase & Murphy, 2011; Stice, Spoor, Bohon, & Small, 2008; Wang et al., 2001). In the present study, females demonstrated less change in activation from hunger to satiety than males in regions involved in emotional processing and reward contingencies and continued to demonstrate significant activation in response to pleasant and unpleasant tastes in reward regions when sated.
The present results suggest that females exhibit less of a change in brain activation from hunger to satiety than males. This discrepancy may reflect differences in processing eating-related signals within the brain. We might speculate that greater activation in females when sated, compared to after a fast, may provide inefficient signaling for meal termination and potentially facilitate overconsumption. In addition, males demonstrated greater change in brain activation from hunger to satiety than females in response to sucrose, caffeine, and NaCl; however, in response to citric acid, the change in activation from hunger to satiety was consistently significant for males and females, suggesting the importance of both gender and stimulus quality. Moreover, given the lack of significant differences in perceived pleasantness and intensity of taste stimuli and perceived hunger, the present findings highlight the potential importance of using neuroimaging to measure physiological indices in an effort to elucidate gender differences in taste and reward processing when participants are hungry and sated. Differences in activation of cortical networks involved in processing stimulus valence may provide additional insight to subjective ratings of hunger/satiety, intensity, and pleasantness.
The phenomenon of sensory-specific satiety is hypothesized to be a mechanism encouraging a varied diet; after consuming a food to satiety, its specific qualities, including taste, smell, and texture are no longer as pleasant (B.J. Rolls, E.T. Rolls, Rowe, Sweeney, 1980). Although the present experimental paradigm was not designed to investigate sensory-specific satiety, it should be noted that the preload Ensure Plus and sucrose share the common taste quality, sweetness. Therefore, it is possible that sensory-specific satiety may have contributed to the differences in brain activation in response to sucrose. However, pleasantness ratings did not significantly differ before and after satiation for any of the stimuli, including sucrose. This is consistent with what we have found previously using this paradigm and identical satiety-inducing interventions (Haase et al., 2009; Green, Jacobson, Haase, Murphy, 2011).
Studies examining structural differences in brain volume have reported greater volumes in females relative to males in orbitofrontal (Gur, Gunning-Dixon, Bilker, & Gur, 2002) and frontal (Schlaepfer et al., 1995; Sowell et al., 2007) cortices. In addition, greater cortical thickness has also been reported in females relative to males within the inferior parietal and posterior temporal regions (Schlaepger et al., 1995; Sowell et al., 2007). These findings suggest a possible anatomic substrate for gender differences in brain function.
In conclusion, we have reported that, following a 12 hour fast, females appear to have more robust positive brain activation relative to males in response to different taste qualities, with the exception of the sweet taste, sucrose. When sated, females have both positive and negative activation that is more widespread across the brain, whereas males have only negative activation that is localized primarily within limbic regions. When examining the direct comparison between males and females, females have greater activation across stimuli and physiological conditions. However the most intriguing findings come from examining gender differences in the change in activation from hunger to satiety, which demonstrate that females show less change in activation from hunger to satiety relative to males. In a time when both obesity rates and the abundance of highly palatable foods are increasing, it becomes particularly important to elucidate mechanisms that might underlie unhealthy eating (e.g., eating in the presence of satiety) in various populations. The current findings suggest that examining these relationships in males and females independently will be beneficial to fully characterizing these brain/behavior relationships. Additional research on gender differences in processing food-related stimuli during the physiological states of hunger and satiety may provide insight into the mechanisms involved in disordered eating.
Highlights.
Females demonstrated greater brain activation when hungry and sated, relative to males.
Females showed less change in brain activation from hunger to satiety than males.
These differences may provide insight into the mechanisms of disordered eating.
Acknowledgments
We acknowledge and thank Drs. Barbara Cerf-Ducastel and Nobuko Kemmotsu for their fMRI expertise. We thank Aaron Jacobson, Delaney Downer and Lindsay Ramos for their assistance in data analysis. Supported by NIH grant AG04085-24 from the National Institute on Aging to CM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aleman A, Swart M. Sex Differences in neural activation to facial expressions denoting contempt and disgust. PLoS One. 2008;3(11):e3622. doi: 10.1371/journal.pone.0003622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates, including humans. Brain Res Brain Res Rev. 1996;22(3):229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Azim E, Mobbs D, Jo B, Menon V, Reiss AL. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102(45):16496–16501. doi: 10.1073/pnas.0408456102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Mauricio DR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;37:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis LL, Rolls ET, Baylis GC. Afferent connections of the orbitofrontal cortex taste area of the primate. Neuroscience. 1995;64:801–81. doi: 10.1016/0306-4522(94)00449-f. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina L, Weiffenbach JM, et al. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 2004;82(1):109–114. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, McBurney DH, Pfaffmann C. Taste of Sodium Chloride solutions after adaptation to Sodium Chloride: Implications for the “Water Taste.”. Science. 1964;143:967–968. doi: 10.1126/science.143.3609.967. [DOI] [PubMed] [Google Scholar]
- Basiotis PP, Thomas RG, Kelsay JL, Mertz W. Sources of variation in energy intake by men and women as determined from one year's daily dietary records. Am J Clin Nutr. 1989;50:448–453. doi: 10.1093/ajcn/50.3.448. [DOI] [PubMed] [Google Scholar]
- Berglunds H, Lindstrom P, Savic I. Brain response to putative pheromones in lesbian women. PNAS. 2006;103(21):8269–8274. doi: 10.1073/pnas.0600331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T, Imperato-McGinley J, Pan H, Voyer D, Codero J, Zhu YS, Stern E, Silbersweig D. Sex differences in mental rotation: Top-down versus bottom-up processing. Neuroimage. 2006;32:445–456. doi: 10.1016/j.neuroimage.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term free recall of emotional information. Proc Natl Acad Sci. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain WS, Gent J, Catalanotto FA, Goodspeed RB. Clinical evaluation of olfaction. Am J Otolaryngol. 1983;4(4):252–256. doi: 10.1016/s0196-0709(83)80068-4. [DOI] [PubMed] [Google Scholar]
- Cerf B, Le Bihan D, Van de Moortele PF, Mac Leod P, Faurion A. Functional lateralization of human gustatory cortex related to handedness disclosed by fMRI study. Ann N Y Acad Sci. 1998;855:575–578. doi: 10.1111/j.1749-6632.1998.tb10627.x. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Van De Moortele PF, MacLeod P, Le Bihan D, Faurion A. Interaction of gustatory and lingual somatosensory perceptions at the cortical level in the human: a functional magnetic resonance imaging study. Chem Senses. 2001;26(4):371–383. doi: 10.1093/chemse/26.4.371. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Murphy C. FMRI activation in response to odorants orally delivered in aqueous solutions. Chem Senses. 2001;26:625–637. doi: 10.1093/chemse/26.6.625. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Murphy C. Neural substrates of cross-modal olfactory recognition memory: An fMRI study. Neuroimage. 2006;31:386–396. doi: 10.1016/j.neuroimage.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Cerf-Ducastel B, Murphy C. Age-related differences in the neural substrates of cross-modal olfactory recognition memory: an fMRI investigation. Brain Res. 2009;1285:88–89. doi: 10.1016/j.brainres.2009.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette F, Olvier L, Van der Linden M, Laureys S, Delfiore G, Luxen A, Salmon E. Involvement of both prefrontal and inferior parietal cortex in dual-task performance. Brain Res Cogn Brain Res. 2005;24(2):237–251. doi: 10.1016/j.cogbrainres.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Conner M, Johnson C, Grogan S. Gender, sexuality, body image and eating behaviors. J Health Psychol. 2004;9(4):505–515. doi: 10.1177/1359105304044034. [DOI] [PubMed] [Google Scholar]
- Cornier MA, Salzberf AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99:538–543. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan R. Neural systems supporting interoceptive awareness. Nat Neuroscie. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Kringelbach ML, Rolls ET, McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol. 2003;90(3):1865–1876. doi: 10.1152/jn.00297.2003. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET, Velazco MI, Margot C, Cayeux I. Cognitive modulation of olfactory processing. Neuron. 2005;46:671–679. doi: 10.1016/j.neuron.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Del Parigi A, Chen K, Gautier JF, Salbe AD, Pratley RE, Ravussin E, Tataranni PA, et al. Sex differences in the human brain's response to hunger and satiation. Am J Clin Nutr. 2002;75(6):1017–1022. doi: 10.1093/ajcn/75.6.1017. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10:308–17. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Elliott R, Friston KJ, Dolan RJ. Dissociable Neural Responses in Human Reward Systems. J Neurosci. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enns MP, Van Itallie TB, Grinker JA. Contributions of age, sex and degree of fatness on preferences and magnitude estimations for sucrose in humans. Physiol Behav. 1979;22(5):999–1003. doi: 10.1016/0031-9384(79)90346-9. [DOI] [PubMed] [Google Scholar]
- Fagerli RA, Wandel M. Gender differences in opinions and practices with regard to a “healthy diet”. Appetite. 1999;32(2):171–190. doi: 10.1006/appe.1998.0188. [DOI] [PubMed] [Google Scholar]
- Faurion A, Cerf B, Van De Moortele PF, Lobel E, Mac Leod P, Le Bihan D. Human taste cortical areas studied with functional magnetic resonance imaging: evidence of functional lateralization related to handedness. Neurosci Lett. 1999;277(3):189–192. doi: 10.1016/s0304-3940(99)00881-2. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Odgen CL. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Francis S, Rolls ET, Bowtell R, McGlone F, O'Doherty J, Browning A, Smith E. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. NeuroReport. 1999;10(3):453–459. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- Frank GKW, Oberndorfer TA, Simmons AN, Paulus MP, Fudge JL, Yang TT, Kaye WH. Sucrose activates human taste pathways differently from artificial sweetener. NeuroImage. 2008;39:1559–1569. doi: 10.1016/j.neuroimage.2007.10.061. [DOI] [PubMed] [Google Scholar]
- Frank S, Laharnar N, Kullmann S, Veit R, Canova C, Li Hegner Y, Preissl H, et al. Processing of food pictures: influences of hunger, gender and calorie content. Brain Res. 2010;1350:159–166. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Frith C, Dolan R. The role of the prefrontal cortex in higher cognitive functions. Brain Res Cogn Brain Res. 1996;5:175–181. doi: 10.1016/s0926-6410(96)00054-7. [DOI] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET, Parris BA, d'Souza AA. How the brain represents the reward value of fat in the mouth. Cereb Cortex. 2009;20:1082–91. doi: 10.1093/cercor/bhp169. [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 1996;21(3):323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- Green BG, Shaffer SS, Gilmore MM. Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 1993;18(6):683–702. [Google Scholar]
- Green E, Jacobson A, Haase L, Murphy C. Reduced nucleus accumbens and caudate activation to a pleasant taste is associated with obesity in older adults. Brain Res. 2011;1386:109–117. doi: 10.1016/j.brainres.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J. Patterns of eating in normal men and women. J Hum Behav Soc Environ. 1987;24:1–14. [Google Scholar]
- Gur RC, Gunning-Dixon FM, Turetsky BI, Bilker WB, Gur RE. Brain region and sex differences in age association with brain volume: A quantitative MRI study of healthy young adults. Am J Geriatric Psychiatry. 2002;10:72–80. [PubMed] [Google Scholar]
- Haase L, Cerf-Ducastel B, Buracas G, Murphy C. On-line psychophysical data acquisition and event-related fMRI protocol optimized for the investigation of brain activation in response to gustatory stimuli. J Neurosci Methods. 2007;159:98–107. doi: 10.1016/j.jneumeth.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage. 2009;44(3):1008–1021. doi: 10.1016/j.neuroimage.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R, Davidson TM, Murphy C, Gilbert PE, Chen M. Clinical evaluation and symptoms of chemosensory impairment: one thoughts consecutive cases from the Nasal Dysfunction Clinic in San Diego. Am J Rhinol. 2006;20:101–108. [PubMed] [Google Scholar]
- Hamann SB, Eli TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci. 1999;2:289–303. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hill SW, McCutcheon NB. Contributions of obesity, gender, hunger, food preference and body size to bite size, bite speed and rate of eating. Appetite. 1984;5:73–83. doi: 10.1016/s0195-6663(84)80026-4. [DOI] [PubMed] [Google Scholar]
- Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Agras WS. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychol Bull. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- Jacobson A, Green E, Murphy C. Age-related functional changes in gustatory and reward processing regions: an fMRI study. Neuroimage. 2010;53(2):602–610. doi: 10.1016/j.neuroimage.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W, Yurgelun-Todd D. Sex differences in cerebral responses to images of high versus low-calorie food. Neuroreport. 2010;21:354–358. doi: 10.1097/WNR.0b013e32833774f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelsas E, Bjornstrom C, Gotestam KG. Prevalence of eating disorders in female and male adolescents (14-15 years) Eat Behav. 2004;5(1):13–25. doi: 10.1016/S1471-0153(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Klein S, Smolka MN, Wrase J, Grusser SM, Mann K, Braus DF, Heinz A. The influence of gender and emotional valence of visual cues on FMRI activation in humans. Pharmacopsychiatry. 2003;36(Suppl 3):S191–194. doi: 10.1055/s-2003-45129. [DOI] [PubMed] [Google Scholar]
- Koch K, Pauly K, Kleermann T, Seiferth NY, Reske M, Backes V, Stocker T, Shah NJ, Amunts K, Kricher T, Schneider F, Hable U. Gender differences in the cognitive control of emotion: An fMRI study. Neuropsychologia. 2007:2744–2754. doi: 10.1016/j.neuropsychologia.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Kringelback ML, de Arajo IET, Rolls ET. Taste-related activity in the human dorsolateral prefrontal cortex. Neuroimage. 2004;21:781–789. doi: 10.1016/j.neuroimage.2003.09.063. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O'Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13(10):1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Kuchinke L, Jacobs AM, Grubich C, Vo ML, Conrad M, Herrmann M. Incidental effects of emotional valence in single word processing: An fMRI study. NeuroImage. 28:1022–1032. doi: 10.1016/j.neuroimage.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Laeng B, Berridge KC, Butter CM. Pleasantness of a sweet taste during hunger and satiety: effects of gender and sweet tooth. Appetite. 1993;21(3):247–254. doi: 10.1006/appe.1993.1043. [DOI] [PubMed] [Google Scholar]
- Lenard NR, Berthoud HR. Central and eripheral regulation of food intake and physical activity: pathways and genes. Obesity. 2008;16(3):1–26. doi: 10.1038/oby.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem M, Katz-Levin T, Schulkin J. Calcium taste preference and sensitivity in humans: I, gender comparisons. Physiol Behav. 2003;78:403–407. doi: 10.1016/s0031-9384(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Levy LM, Henkin RI, Hutter A, Lin CS, Martins D, Schellinger D. Functional MRI of human olfaction. J Comput Assist Tomogr. 1997;21(6):849–856. doi: 10.1097/00004728-199711000-00002. [DOI] [PubMed] [Google Scholar]
- McBurney DH, Pfaffmann C. Gustatory adaptation to saliva and sodium chloride. J Exp Psychol. 1963;65:523–529. [Google Scholar]
- McKinley MJ, Denton DA, Oldfield BJ, De Oliveira, Mathai ML. Water intake and the neural correlates of the consciousness of thirst. Semin Nephrol. 2006;26(3):249–257. doi: 10.1016/j.semnephrol.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Mori D, Pliner PL. Eating lightly and the self-presentation of femininity. J Pers Soc Psychol. 1987;53:693–702. doi: 10.1037//0022-3514.53.4.693. [DOI] [PubMed] [Google Scholar]
- Murphy C, Gilmore MM, Seery CS, Salmon DP, Lasker BR. Olfactory thresholds are associated with degree of dementia in Alzheimer's disease. Neurobiol Aging. 1990;11(4):465–469. doi: 10.1016/0197-4580(90)90014-q. [DOI] [PubMed] [Google Scholar]
- O'Doherty J. Reward representations and reward-related learning in the human brain: insights from human neuroimaging. Curr Opin Neurobiol. 2004;14(6):769–76. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85(3):1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- Ostovich JM, Rozin P. Body image across three generation of Americans: inter-family correlations, gender differences, and generation differences. Eat Weight Disord. 2004;9(3):186–193. doi: 10.1007/BF03325065. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laruent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2004;30(5):263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro V, Gray JA, et al. Neural response to facial and vocal expressions of fear and disgust. Proc R Soc Lond B Biol Sci. 1998;265:1809–1817. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Markowitsch HJ, Fink GR. Gender differences in the functional neuroanatomy of emotional episodic autobiographical memory. Hum Brain Mapp. 2005;24:313–32. doi: 10.1002/hbm.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard TC, Hamilton RB, Morse JR, Norgen R. Projections of thalamic gustatory and lingual areas inthe monkey, Macaca fascicularis. J Comp Neurol. 1989;244(2):213–228. doi: 10.1002/cne.902440208. [DOI] [PubMed] [Google Scholar]
- Pritchard TC, Hamilton RB, Norgen R. Neural coding of gustatory information in the thalamus of macaca mulatta. Journal Neurophysiol. 1989;61:1–14. doi: 10.1152/jn.1989.61.1.1. [DOI] [PubMed] [Google Scholar]
- Pukall CF, Strigo IA, Binik YM, Amsel R, Khalife S, Bushnell MC. Neural correlates of painful genital touch in women with vulvar vestibulitis syndrome. Pain. 2005;115:118–127. doi: 10.1016/j.pain.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan DJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 7:278–85. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Robin O, Rousmans S, Dittmar A, Vernet-Maury E. Gender influence on emotional responses to primary tastes. Physiol Behav. 2003;78(3):385–393. doi: 10.1016/s0031-9384(02)00981-2. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Fedoroff IC, Guthrie JF. Gender differences in eating behavior and body weight regulation. Health Psychol. 1991;10(2):133–142. doi: 10.1037//0278-6133.10.2.133. [DOI] [PubMed] [Google Scholar]
- Rolls BJ, Rolls ET, Rowe EA, Sweeney K. Sensory specific satiety in man. Physiological Behavior. 1980;27:137–42. doi: 10.1016/0031-9384(81)90310-3. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: From affect to decision-making. Prog Neurobiol. 2008;86(3):216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kringelbach ML, de Araujo IET. Different representations of pleasant and unpleasant odours in the human brain. Eur J Neurosci. 2003;18:695–703. doi: 10.1046/j.1460-9568.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Yaxley S, Sienkiewicz ZJ. Gustatory responses of single neurons in the orbitofrontal cortex of the macaque monkey. J Neurophysiol. 1990;64:1055–1066. doi: 10.1152/jn.1990.64.4.1055. [DOI] [PubMed] [Google Scholar]
- Royet JP, Plailly J, Delon-Martin C, Kareken DA, Segebarth C. fMRI of emotional responses to odors: influence of hedonic valence and judgment, handedness, and gender. Neuroimage. 2003;20(2):713–728. doi: 10.1016/S1053-8119(03)00388-4. [DOI] [PubMed] [Google Scholar]
- Rozin P, Trachtenberg S, Cohen AB. Stability of body image and body image dissatisfaction in American college students over about the last 15 years. Appetite. 2001;37(3):245–248. doi: 10.1006/appe.2001.0426. [DOI] [PubMed] [Google Scholar]
- Savic I, Berglund H, Lindstrom P. Brain response to putative pheromones in homosexual men. PNAS. 2005;102(20):7366–7361. doi: 10.1073/pnas.0407998102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Stark R, Walter B, Vaitl D. Gender differences in the processing of disgust- and fear-inducing pictures: an fMRI study. Neuroreport. 2005;16(3):277–280. doi: 10.1097/00001756-200502280-00015. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Harris GJ, Tien AY, Peng L, Lee S, Pearlson GD. Structural differences in the cerebral cortex of healthy female and male subjects: a magnetic resonance imaging study. Psychiatry Res. 1995;61:129–135. doi: 10.1016/0925-4927(95)02634-a. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Kessler C, Salloum JB, Posse S. Gender differences in regional cerebral activity during sadness. Hum Brain Mapp. 2000;9(4):226–238. doi: 10.1002/(SICI)1097-0193(200004)9:4<226::AID-HBM4>3.0.CO;2-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TR, Karadi Z, Oomura Y, Nishino H, Plata-Salaman CR, Lenard L, et al. Gustatory neural coding in the amygdala of the alert macaque monkey. J Neurophysiol. 1993;69(6):1810–20. doi: 10.1152/jn.1993.69.6.1810. [DOI] [PubMed] [Google Scholar]
- Scott TR, Yan J, Rolls ET. Brain mechanisms of satiety and taste in macaques. Neurobiology. 1995;3:281–92. [PubMed] [Google Scholar]
- Scott TR, Yaxley S, Sienkiewicz ZJ, Rolls ET. Gustatory responses in the frontal opercular cortex of the alert cnynomolgus monkey. J Neurophysiol. 1986;56:876–890. doi: 10.1152/jn.1986.56.3.876. [DOI] [PubMed] [Google Scholar]
- Shibasaki H. Central mechanisms of pain perception. Suppl Clin Neurophysiol. 2004;57:39–49. doi: 10.1016/s1567-424x(09)70341-1. [DOI] [PubMed] [Google Scholar]
- Shigemune Y, Abe N, Suzuki M, Ueno A, Mori E, Tashiro M, Fujii T, et al. Effects of emotion and reward motivation on neural correlates of episodic memory encoding: A PET study. Neurosciences Res. 2010;67:72–79. doi: 10.1016/j.neures.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Shirao N, Okamoto Y, Mantani T, Okamoto Y, Yamawaki S. Gender differences in brain activity generated by unpleasant word stimuli concerning body image: an fMRI study. Br J Psychiatry. 2005;186:48–53. doi: 10.1192/bjp.186.1.48. [DOI] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124(Pt 9):1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- Smeets PA, de Graaf C, Stafleu A, van Osch MJ, Nievelstein RA, van der Grond J. Effect of satiety on brain activation during chocolate taking in men and women. Am J Clin Nutr. 2006;83:1297–305. doi: 10.1093/ajcn/83.6.1297. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Toga AW, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by Taq1A A1 Allele. Science. 2008;322:449–52. doi: 10.1126/science.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Cox JE, Cook EW, Weller RE. Motivational state modulates the hedonic value of food images differently in men and women. Appetite. 2007;48(2):139–144. doi: 10.1016/j.appet.2006.07.079. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Kwok K, Brot MD, Marck BT, Matsumoto AM, Donahue BA, Palmiter RD. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Ravussin E, et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A. 1999;96(8):4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touryan SR, Johnson MK, Mitchell KJ, Farb N, Cunningham WA, Raye CL. The influence of self-regulatory focus on encoding of, and memory for, emotional words. Soc Neurosci. 2007;2:14–27. doi: 10.1080/17470910601046829. [DOI] [PubMed] [Google Scholar]
- Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: effects of fasting and gender. Behav Brain Res. 2006;169(1):111–119. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Veldhuijzen DS, Greenspan JD, Kim JH, Lenz FA. Altered pain and thermal sensation in subjects with isolated parietal and insular cortical lesions. Eur J Pain. 2010;14(5):1–11. doi: 10.1016/j.ejpain.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol. 2002;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Fowler JS, et al. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Fowler JS, et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci. 2009;106:1249–54. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous inference for fMRI data. Biophysics Research Institute, Medical College of Wisconsin; 1997. [Google Scholar]
- Wardle J, Haase AM, Steptoe A, Nillapun M, Jonwutiwes K, Bellisle F. Gender differences in food choice: the contribution of health beliefs and dieting. Ann Behav Med. 2004;27(2):107–116. doi: 10.1207/s15324796abm2702_5. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Callese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Woodside DB, Garfinkel PE, Lin E, Goering P, Kaplan AS, Goldbloom DS, Kennedy SH. Comparisons of men with full or partial eating disorders, men without eating disorders, and women with eating disorders in the community. Am J Psychiatry. 2001;158(4):570–574. doi: 10.1176/appi.ajp.158.4.570. [DOI] [PubMed] [Google Scholar]
- Wrase J, Klein S, Gruesser SM, Hermann D, Flor H, Mann K, Heinz A, et al. Gender differences in the processing of standardized emotional visual stimuli in humans: a functional magnetic resonance imaging study. Neurosci Lett. 2003;348(1):41–45. doi: 10.1016/s0304-3940(03)00565-2. [DOI] [PubMed] [Google Scholar]
- Yousem DM, Maldjian JA, Siddiqi F, Hummel T, Alsop DC, Geckle RJ, Doty RL, et al. Gender effects on odor-stimulated functional magnetic resonance imaging. Brain Res. 1999;818(2):480–487. doi: 10.1016/s0006-8993(98)01276-1. [DOI] [PubMed] [Google Scholar]
- Zald DH, Hagen MC, Pardo JV. Neural correlates of tasting concentrated quinine and sugar solutions. J Neurophysiol. 2002;87(2):1068–1075. doi: 10.1152/jn.00358.2001. [DOI] [PubMed] [Google Scholar]
- Zylan KD. Gender differences in the reasons given for meal termination. Appetite. 1996;26(1):37–44. doi: 10.1006/appe.1996.0003. [DOI] [PubMed] [Google Scholar]