Summary
Carcinoembryonic antigen cell adhesion molecule like I (CEACAM1) is expressed on activated T cells and signals through either a long (L) cytoplasmic tail containing immune receptor tyrosine based inhibitory motifs, which provide inhibitory function, or a short (S) cytoplasmic tail with an unknown role. Previous studies on peripheral T cells show that CEACAM1-L isoforms predominate with little to no detectable CEACAM1-S isoforms in mouse and human. We show here that this was not the case in tissue resident T cells of intestines and gut associated lymphoid tissues which demonstrated predominant expression of CEACAM1-S isoforms relative to CEACAM1-L isoforms in human and mouse. This tissue resident predominance of CEACAM1-S expression was determined by the intestinal environment where it served a stimulatory function leading to the regulation of T cell subsets associated with generation of secretory IgA immunity, the regulation of mucosal commensalism, and defense of the barrier against enteropathogens.
Introduction
Carcinoembryonic antigen cell adhesion molecule 1 (CEACAM1), a member of the carcinoembryonic antigen family, is engaged in intercellular binding interactions that affect signal transduction by a number of cell surface receptors. CEACAM1 is constitutively expressed by a variety of cell types including those of endothelial, epithelial and hematopoietic origin such as B cells, polymorphonuclear leukocytes, monocytes, and dendritic cells. In addition, CEACAM1 is expressed at minimal quantities in circulating natural killer (NK) and T cells but is upregulated in these cell types following a variety of different forms of activation (Azuz-Lieberman et al., 2005; Boulton et al., 2002; Coutelier et al., 1994; Nakajima et al., 2002; Singer et al., 2002). On the cell surface, CEACAM1 can associate with and modify the function of numerous signaling receptors that are dictated by the CEACAM1 isoforms expressed. The individual CEACAM1 isoforms are generated by alternative splicing and consist of transmembrane proteins that possess a characteristic membrane distal, IgV-like domain (the N-domain) which functions in homophilic binding but differs with respect to the number of membrane proximal extracellular IgC2-like domains and the length of their cytoplasmic tail (Gray-Owen et al., 2006). Specifically, the 11 human and 4 mouse CEACAM1 splice variants encode either a short (S) cytoplasmic tail about which little is known or a long (L) cytoplasmic tail containing immune receptor tyrosine-based inhibitory motifs (ITIM). In the latter case, consistent with the presence of ITIM motifs in their cytoplasmic tail, the CEACAM1-L isoforms of mice and humans are linked to inhibition of many different signaling receptors (Chen et al., 2001; Chen et al., 2008; Kammerer et al., 1998; Pan et al., 2010). In each example, the inhibitory function of CEACAM1-L isoforms is triggered by the phosphorylation of the ITIM tyrosine residues by receptor tyrosine kinases or Src-related kinases, resulting in recruitment of the Src homology 2 (SH2) domain-containing protein-tyrosine phosphatases (SHP)-1 or -2 (Huber et al., 1999; Nagaishi et al., 2006). Thus, CEACAM1-L-mediated recruitment of these phosphatases results in the dephosphorylation of critical tyrosines contained within activating motifs associated with a variety of cell surface receptors such as the B cell receptor, epidermal growth factor receptor and T cell receptor (TCR)-CD3 complex (Abou-Rjaily et al., 2004; Boulton et al., 2002; Chen et al., 2008; Lobo et al., 2009). As such, CEACAM1-L isoforms are functionally inhibitory and are the major isoforms described in mouse and human lymphocytes including NK cells, B cells and T cells.
CEACAM1-L and CEACAM1-S isoforms are expressed at varying ratios in different cell types with recent evidence showing that such expression is under the influence of cytokines (e.g. interferon-γ), transcription factors (e.g. IRF-1) and splicing regulators (e.g. members of the heterogeneous nuclear ribonucleoprotein family) (Dery et al. 2011; Gencheva et al., 2010). Although not directly demonstrated, this is presumed to be the case for T lymphocytes as well. It is known that while mouse and human T cells predominantly express CEACAM1-L isoforms, there is some evidence that CEACAM1-S isoforms can be expressed. However, this is a controversial issue as little or no detection has been described depending upon the study (Donda et al., 2000; Singer et al., 2002). This is not an inconsequential issue as in vitro transfection studies in T cells suggest that CEACAM1 is a ‘tunable’ receptor system given that CEACAM1-S isoforms may possess co-stimulatory function that is capable of ameliorating the inhibitory signals provided by CEACAM1-L isoforms in the context of TCR-CD3 complex signaling when expressed together (Chen et al., 2004a; Chen et al., 2008). However, there is no information available on CEACAM1 isoform regulation in T cells in vivo and the physiologic function(s) that this might confer.
To this end, we have focused our attention on the regulation of CEACAM1 isoform expression and function within intestinal tissues in order to shed light on the normal physiologic roles of CEACAM1 in these compartments. We have done so given the fact that CEACAM1 can function as a microbial receptor and the gastrointestinal surface is in direct contact with large concentrations of commensal and pathogenic microbes (Kuespert et al., 2006). In addition, CEACAM1 expression is increased on the cell surface of T cells in human diseases such as Celiac disease and inflammatory bowel disease (Donda et al., 2000; Morales et al., 1999). Furthermore, in vivo ligation of CEACAM1 with CEACAM1 homophilic ligands as provided by N-domain-Fc-fusion proteins or forced expression of CEACAM1-L isoforms in T cells is able to prevent or block mucosal inflammation associated with either hapten-mediated colitis or naïve T cell transfer models of colitis (Iijima et al., 2004; Nagaishi et al., 2006). Together these studies predict an important physiological role for CEACAM1 in mucosal tissues of the gastrointestinal tract.
In this study, we have identified a key role for CEACAM1 in regulating steady-state secretory immunity in the intestines. By establishing a PCR method for quantifying mouse CEACAM1-S and CEACAM1-L variants, we found that T cells within the intestines, relative to peripheral tissues such as the spleen, not only exhibited increased overall CEACAM1 expression but also uniquely possessed a relative overabundance of CEACAM1-S relative to CEACAM1-L isoforms. Moreover, in this environment, we show that CEACAM1-S isoforms have special physiological functions which at a cellular level enhanced T cell activation independently of CEACAM1-L isoforms and were preferentially expressed in cells performing common mucosal immune functions. Specifically, we have found that CEACAM1-S acts as an individual signaling unit which promoted the induction of CD4+LAP+ regulatory T (Treg) cells, CD4+PD1+CXCR5+ follicular helper T cells (Tfh cells), and the development of IgA committed B cells within Peyer’s patches (PP). Consistent with this, T cell-associated CEACAM1-S isoforms led to enhanced mucosal IgA secretion which influenced both the composition of commensal microbiota and host defense against exposure to pathogens such as Listeria monocytogenes. In the absence of CEACAM1 expression, these barrier-protective functions were diminished. Together, these studies provide tissue-specific evidence for CEACAM1 isoform regulation linked to important physiological functions within the intestines.
Results
Intestinal T cells predominantly express CEACAM1-S isoforms while extraintestinal T cells mainly express CEACAM1-L
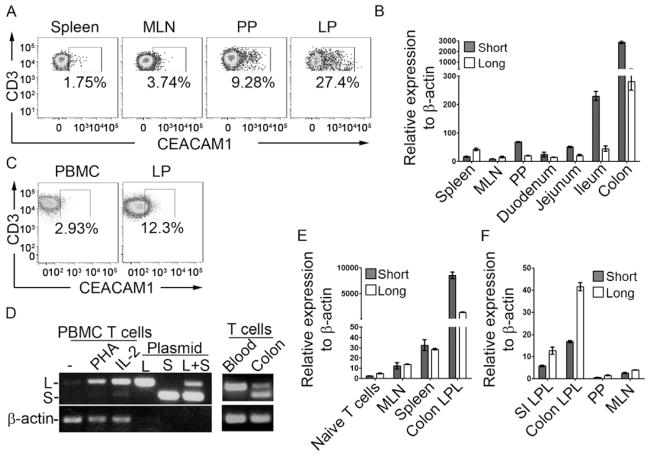
The expression of CEACAM1 isoforms in hematopoietic cells has not been systematically examined outside peripheral sites such as the spleen and blood. We therefore compared CEACAM1 expression on mouse splenic T cells to the quantities expressed on T cells from intestinal tissues under homeostatic conditions. Interestingly, the proportion of T cells that stained positive for CEACAM1 was increased on T cells obtained from colonic lamina propria (LP) and PP in comparison to that observed on T cells isolated from either spleen or mesenteric lymph nodes (MLN) as defined by staining with the CC1 monoclonal antibody (mAb), a mouse anti-mouse CEACAM1 antibody (Dveksler et al., 1993) (Figure 1A). These studies showed that T cells within intestinal mucosal tissues displayed substantially greater CEACAM1 amounts on the cell surface under steady-state conditions in comparison to those within non-mucosal sites.
Figure 1.
Intestinal T cells predominantly express CEACAM1-S isoforms. (A) Cell surface expression of CEACAM1 (CC1 antibody) on CD3+ T cells from spleen, MLN, PP or lamina propria (LP) of colon from C57BL/6 mice. (B) qPCR for transcriptional analysis of CEACAM1-L and -S in CD3+ T cells isolated from spleen, MLN, blood, PP or segments of small intestine and colon LP from WT mice. (C) Cell surface expression of CEACAM1 (5F4 antibody) on CD3+ T cells from human peripheral blood (PBMC) or colon LP. (D) Semi-quantification of the relative transcription of CEACAM1-L and -S in human CD3+ T cells isolated from peripheral blood with indicated treatment or from fresh colon LP. (E) qPCR for quantification of CEACAM1-L and -S transcription in naïve CD3+ T cells from WT mice, MLN T cells from WT mice and CD3+ T cells isolated from spleen and colon LP of Rag2−/− mice adoptively transferred with WT CD4+ naïve T cells. (F) T cells isolated from mouse LP were expanded with ConA and IL-2 in vitro for two weeks and the expression of CEACAM1-L and -S were determined by qPCR.
We next assessed the relative expression of CEACAM1 isoforms and to do so we first established a quantitative assay for analyzing expression of mouse CEACAM1-L and -S variants as currently available antibodies are not able to distinguish between these two general classes of CEACAM1 in a quantitative fashion. Two pairs of real-time PCR primers were designed to specifically quantify these in mouse T cells. The specificity and efficiency of these primers was verified using mouse (m) CEACAM1-4L (4L) and CEACAM1-4S (4S) plasmids (Figure S1A). Quantitative (q)PCR analysis of mouse primary CD3+ T cells isolated from spleen and lymph nodes (LN) demonstrated that CEACAM1-L isoforms predominated in resting peripheral T cells with increased expression upon anti-CD3 stimulation as previously described (Boulton et al., 2002; Nakajima et al., 2002) (Figure S1B). In addition, although CEACAM1-S isoforms were detected, CEACAM1-L isoforms predominated in peripheral T cells such that the CEACAM1-L:CEACAM1-S (L:S) ratio was 1.95 ± 0.34, consistent with previous studies (Singer et al., 2002).
Having validated this qPCR assay with splenic T cells, we performed a broad survey of CEACAM1 isoform expression in primary T cells isolated from several different tissues. It was noted that, unlike CD3+ T cells isolated from spleen or MLN wherein CEACAM1 expression was low and CEACAM1-L isoforms predominated over CEACAM1-S isoforms, CD3+ T cells from the intestinal LP of the duodenum, jejunum, ileum or colon and PP were observed to express an overall increase in CEACAM1 transcript abundance with significantly greater quantities of CEACAM1-S isoforms relative to CEACAM1-L isoforms. Thus, an average L:S ratio of <1.0 (PP: 0.53 ± 0.08; small intestine: 0.36 ± 0.11; and colon; 0.19 ± 0.04) was observed, which was reversed from peripheral T cells (Figure 1B). Accordingly, it was noted that the quantity and L:S ratio of CEACAM1 expression varied cephalocaudally along the axis of the gut. Specifically, the amounts of CEACAM1 and L:S ratio were more similar to spleen, MLN and peripheral blood in the proximal intestines. Furthermore, CEACAM1 expression increased and the L:S ratio progressively decreased from the distal small intestine (ileum) to the colon. As a result, LP T cells of the PP and colon contained the highest amount of CEACAM1 and the lowest L:S ratios relative to all other compartments examined (Figure 1B). This was verified by immunoblotting with the CC1 mAb, further confirming that T cells isolated from PP and colonic LP expressed higher CEACAM1 amounts relative to peripheral blood and especially CEACAM1-S isoforms which was most notable in the colonic LP (Figure S1C). These studies both confirmed the increased expression of CEACAM1 within intestinal T cells and further indicated that CEACAM1-S isoforms were not only expressed by primary T cells but established that they were, surprisingly, the major isoform within intestinal T cells.
Given these observations, we also determined whether CEACAM1-S isoforms predominated in T cells obtained from the LP of human colon. In a first group of studies, we confirmed that, in comparison to peripheral T cells, a greater proportion of T cells from the human colon expressed CEACAM1 on the cell surface as we had previously observed in mouse (Figure 1C and Figure S1D). Moreover, using previously published RT-PCR primers which amplify a 160 bp fragment from all CEACAM1-S cDNAs and a 210 bp fragment from all CEACAM1-L cDNAs from human CEACAM1 (Chen et al., 2008; Singer et al., 2002), we confirmed that CEACAM1 was barely detectable in resting peripheral blood T cells (Figure 1D). However, after activation with either phytohemagglutinin (PHA) or interleukin-2 (IL-2), CEACAM1 was induced and CEACAM1-L isoforms became the dominant type detected (Figure 1D, left). In contrast to these observations with peripheral blood T cells and similar to the findings obtained with intestinal T cells from mice, CD3+ T cells from the LP of human colon exhibited readily detectable CEACAM1 at baseline with markedly elevated quantities of CEACAM1-S relative to CEACAM1-L when compared to that observed in CD3+ T cells from peripheral blood (Figure 1D, right).
Preferential expression of CEACAM1-S over CEACAM1-L isoforms requires continuous exposure to mucosa-associated factors
Together, these studies demonstrated that intestinal T cells from both mouse and human uniquely expressed CEACAM1-S isoforms in particularly high quantities. In addition, this dominance of CEACAM1-S expression by intestinal T cells was not simply due to T cell activation since activation of peripheral blood T cells did not induce significant quantities of CEACAM1-S (Figure S1B) nor were significant quantities of CEACAM1-S detectable in T cells from the spleen or peripheral blood (Figure 1B and 1D). Given this, we hypothesized that CEACAM1 expression and especially that of CEACAM1-S isoforms in T cells was regulated by signals encountered within the intestinal microenvironment. We therefore addressed this question by adoptively transferring spleen-derived, naïve CD4+CD62L+ T cells from wild-type (WT) mice that expressed little detectable CEACAM1 expression by flow cytometry (data not shown) but with low amounts of predominantly CEACAM1-L transcripts as shown by qPCR into Rag2−/− mice and analyzed CEACAM1 isoform expression in the adoptively transferred T cells after 2 weeks, prior to the development of intestinal inflammation (Figure 1E). These studies showed that, whereas T cells isolated from the MLN and spleens of the Rag2−/− recipients expressed low-to-moderate amounts of CEACAM1 expression with an L:S ratio of approximately 1.0, the T cells which homed to the LP of the colon expressed substantially increased CEACAM1 with an L:S ratio of approximately 0.1–0.2 consistent with a significant dominance of CEACAM1-S isoforms. Thus, peripheral T cells entering the intestines expressed not only increased quantities of CEACAM1 but also a marked dominance of CEACAM1-S isoforms.
We also questioned whether these effects by intestinal milieu were imprinted and thus irreversible. To do so, we examined the expression of CEACAM1-S isoforms associated with CD3+ T cells obtained from colonic LP before and after two weeks of ex vivo culture with concanavalin-A and IL-2. These studies showed that dominant expression of CEACAM1-S isoforms was lost under such conditions (Figure 1F) and demonstrate that the L:S ratio observed within intestinal tissues is determined by, and requires continuous exposure to, the relevant mucosal factors. Considering that CEACAM1-S expression predominated in colon LP T cells from germ free Swiss Webster and C57BL/6 mice (Figure S1E and S1F), Myd88−/− (Figure S1G) and Nod2−/− (Figure S1H) mice, the factors responsible for this phenotype were neither dependent upon the presence of commensal microbiota nor microbial-induced signaling.
The alternative splicing of CEACAM1 depends upon regulatory cis-acting elements within exon 7 and flanking introns (Dery et al., 2011). Recently, the trans-acting auxiliary splicing factors heterogeneous nuclear ribonucleoprotein (hnRNP) L, hnRNP A1 and hnRNP M variants were shown to associate with exon 7 of the CEACAM1 mRNA and control splice-site recognition motifs giving rise to CEACAM1-L or CEACAM1-S isoforms. Specifically, whereas hnRNP A1 or hnRNP L promote CEACAM1-S isoform processing, hnRNP M fosters the generation of CEACAM1-L isoforms (Dery et al., 2011). We therefore examined whether the preferential expression of CEACAM1-S isoforms observed in the intestinal T cells correlated with the appropriate profile of hnRNP expression as determined by qPCR. Indeed, the expression of hnRNP A1 (Figure S1I) was higher and the expression hnRNP M (Figure S1J) lower in T cells from PP in comparison to that observed in spleen and MLN. As such, the hnRNP A1:hnRNP M ratio gradually increased in T cells isolated from spleen, MLN and PP consistent with the increased amounts of CEACAM1-S relative to CEACAM1-L in the intestine-associated tissues (Figure S1K). Together, these studies indicated that the tissue-associated microenvironment of the intestine was uniquely promoting CEACAM1 splicing which resulted in predominant CEACAM1-S expression.
CEACAM1-4S functions as an independent signaling unit in the induction of T cell activation
Although CEACAM1-L isoform expression in T cells is well-known to provide important negative regulation of TCR-CD3 complex function after ITIM phosphorylation by the src-related kinase, p56lck, and recruitment of SHP1 resulting in deposphorylation of CD3-ζ and ZAP70 (Chen et al., 2008), very little is known about CEACAM1-S function in general or in T cells in particular. Results in T cells are limited to transfected in vitro model systems and remain controversial with both stimulatory and inhibitory functions assigned to CEACAM1-S splice variants (Chen et al., 2004a; Chen et al., 2004b). Given the impossibility of generating a mouse specifically lacking the CEACAM1-S splice variant without also deleting the alternative CEACAM1-L variant, we generated CEACAM1-4S (4S) transgenic (Tg) mice, overexpressing the mouse 4S isoform specifically in T cells under the human CD2 (hCD2) promoter in order to explore the function of CEACAM1-S isoforms in T cells in vivo. The overexpression of 4S in T cells was confirmed by flow cytometry using the CC1 mAb. These studies confirmed that the T cells in the 4S Tg mice uniformly expressed increased CEACAM1 (Figure S2A) with a predominance of CEACAM1-S isoforms (Figure S2B).
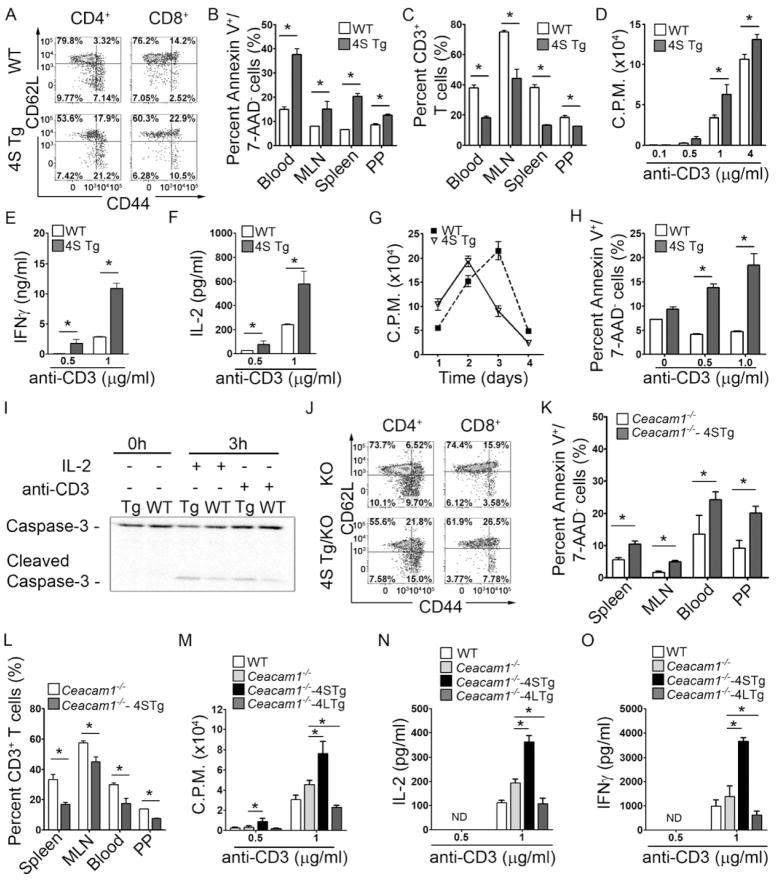
When examined by flow cytometry, 4S Tg mice exhibited greater quantities of activated CD4+ and CD8+ T cells as shown by lower percentages of CD62LhiCD44lo naïve T cells, and a higher percentage of CD62LloCD44hi or CD62LhiCD44hi cells consistent with effector memory and potentially central memory T cells (Seder et al., 2003) respectively, in spleen (Figure 2A), MLN, PP and colon LP (Figure S2C). We also noted that 4S Tg mice exhibited increased numbers of apoptotic CD3+ T cells (annexin V+7-AAD−CD3+ cells) within the peripheral blood, spleen, MLN and PP (Figure 2B) which correlated inversely with decreased quantities of CD3+ cells (Figure 2C). In spite of this, total PP and MLN cell counts were found to be very similar between WT and CEACAM1-4S Tg mice (Figure S2D) indicating that overexpression of CEACAM1-4S was selectively depleting T cells. Intracellular cytokine staining of the CD4+ T cells isolated from spleen, MLN and PP also revealed that IL-2, IL-4, IL-10, IL-17, IFN-γ and TGF-β were all increased in the T cells of 4S Tg mice when compared to that observed in WT littermates (Figure S2E). As illustrated in Figure S2F for IFNγ, CEACAM1-S induction of cytokines influenced the total CD4+ T cell population consistent with a broad activating function of the 4S isoform when highly expressed. CD3+ T cells isolated from the spleen of 4S Tg mice also exhibited a higher proliferation rate (Figure 2D) and more IFN-γ (Figure 2E) and IL-2 (Figure 2F) secretion upon anti-CD3 stimulation ex vivo in comparison to that associated with T cells from WT littermate controls. However, with longer time-periods of culture, the proliferation rate of splenic CD3+ T cells from the 4S Tg mice was observed to be lower than that detected with the WT littermates after stimulation, consistent with in vitro T cell contraction due to increased activation-induced cell death (AICD) in the presence of the 4S isoform (Figure 2G). Consistent with this, splenic 4S Tg T cells exhibited increased annexin V staining (Figure 2H) and cleavage of caspase 3 (Figure 2I) after 3 hours of anti-CD3 stimulation. These results show that the mouse 4S isoform both co-stimulated primary T cells and sensitized them to AICD, supporting previous in vitro transfection studies which ascribe activating functions to this CEACAM1 isoform in contrast to the inhibitory functions of the L isoform (Chen et al., 2004a; Chen et al., 2004b). Moreover, these observations further supported the idea that both the increased total CEACAM1 expression and the enrichment of the activating S isoform observed on T cells in intestinal tissues are associated with the effector memory T cell phenotype which predominates in the normal intestinal LP under steady-state conditions (Hurst et al., 1999).
Figure 2.
CEACAM1-4S is associated with activation of primary T cells. (A) Spleen cells were first gated on CD3+ cells, and either CD4+ or CD8+ cells, and later examined for CD44 and CD62L expression. 4S Tg mice possessed lower percentages of CD62LhiCD44low naïve T cells and higher percentages of CD62LlowCD44hi effector memory T cells when compared to that of WT mice. (B–C) Compared to WT littermates, 4S Tg mice exhibited higher percentages of CD3+ Annexin V+/7AAD− apoptotic cells (B) and lower percentages of CD3+ T cells (C) in various tissues. (n = 5 mice per group) (D–H) CD3+ T cells isolated from the spleen of 4S Tg mice and their WT littermates were cultured with anti-CD3 antibody at indicated concentrations, or 1 μg/ml anti-CD3 over the indicated time course (G). Proliferation assays were performed by [3H]-thymidine incorporation (D and G); IFNγ (E) and IL-2 (F) production were measured by ELISA; and apoptosis was determined using annexin-V and 7-AAD staining (H). (n = 5 mice per group) (I) CD3+ T cells were isolated from the spleen of 4S Tg or WT mice, and cultured with IL-2 (100 U/ml) or anti-CD3 (1 μg/ml) coated plates. Cell lysates were prepared at the indicated times, and caspase-3 and its activated form were determined using immunoblot. (J–L) Compared to Ceacam1−/− mice, Ceacam1−/−-4S Tg mice have lower percentages of CD62LhiCD44low naïve T cells and higher percentages of CD62LlowCD44hi effector memory T cells in the spleen (J), higher percentages of annexin V+/7AAD− apoptotic cells (K) and lower percentages of CD3+ T cells (L). (n = 5 mice per group) (M–O) T cells isolated from spleen of WT, Ceacam1−/−, Ceacam1−/−-4S Tg (4S Tg/KO) or Ceacam1−/−-4L Tg (4L Tg/KO) mice were cultured with anti-CD3 at the indicated concentrations and proliferation assays were performed by [3H]-thymidine incorporation (M). IL-2 (N) and IFNγ (O) production were measured by ELISA. (n=4 mice per group). All data are mean ± SEM; *, p < 0.05 (two-tailed Student’s t test), ND = not detected.
The activating function of CEACAM1-4S in T cells is observed independently of CEACAM1-L isoform expression
Because the studies we performed in 4S Tg mice were undertaken in the context of endogenous CEACAM1 expression, it was possible that the apparent activating and inhibiting functions of the 4S isoform were either the consequence of CEACAM1-mediated neutralization of CEACAM1-L inhibitory function and/or the result of a direct activating signal by CEACAM1-S. To examine this question, we generated 4S Tg mice that lacked expression of any other CEACAM1 isoforms by crossing 4S Tg mice with Ceacam1−/− mice on a C57BL/6 background (Ceacam1−/−-4S Tg). Although less than that observed in 4S Tg T cells containing endogenous amounts of CEACAM1 in all cell types, Ceacam1−/−-4S Tg mice still contained more activated T cells in vivo as revealed by lower percentages of CD4+(or CD8+) CD62LhiCD44lo naïve T cells, and higher percentages of CD4+(or CD8+) CD62LloCD44hi effector memory T cells in spleen (Figure 2J), MLN, PP and colon LP (Figure S2G) in comparison to Ceacam1−/− littermates. Ceacam1−/−-4S Tg mice also exhibited more apoptotic CD3+ T cells within the MLN, blood and PP (Figure 2K) inversely correlating with a decrease in CD3+ cells (Figure 2L). CD4+ T cells isolated from the spleens of Ceacam1−/−-4S Tg mice also proliferated more vigorously (Figure 2M) and secreted more IL-2 (Figure 2N) and IFN-γ (Figure 2O) upon anti-CD3 stimulation. Notably, splenic CD4+ T cells from Ceacam1−/− mice exhibited increased activation upon anti-CD3 stimulation that was intermediate to the anti-CD3 induced stimulation observed with WT and Ceacam1−/−-4S Tg T cells (Figure 2M–O). On the other hand, splenic T cells from mice in which only the CEACAM1-4L (4L) isoform was expressed by T cells, generated by crossing previously established 4L Tg mice (Nagaishi et al., 2006) with Ceacam1−/− mice, exhibited a blunted response to anti-CD3 stimulation which was less than that observed with WT T cells and is consistent with the inhibitory functions of this isoform (Figure 2M–O). Taken together, these studies showed that loss of dominant endogenous CEACAM1-L inhibitory function in Ceacam1−/− mice or gain of CEACAM1-S expression independently of CEACAM1-L isoforms, as observed in Ceacam1−/−-4S Tg T cells, resulted in enhanced TCR-CD3 complex signaling. In comparison, gain of CEACAM1-L isoforms independently of CEACAM1-S isoforms led to inhibition of T cell function. Thus CEACAM1-S and CEACAM1-L were able to function as independent activating and inhibitory signaling units in T cells. Moreover, the blunted effects of CEACAM1 overexpression on T cells in vivo in the absence of CEACAM1 expression elsewhere (Figures 2J–L) not only highlights the importance of homophilic interactions presumably in trans with CEACAM1 on non-T cells but also suggests a possible role for other heterophilic ligands in CEACAM1 activation.
CEACAM1-4S isoform function is associated with NFAT signaling
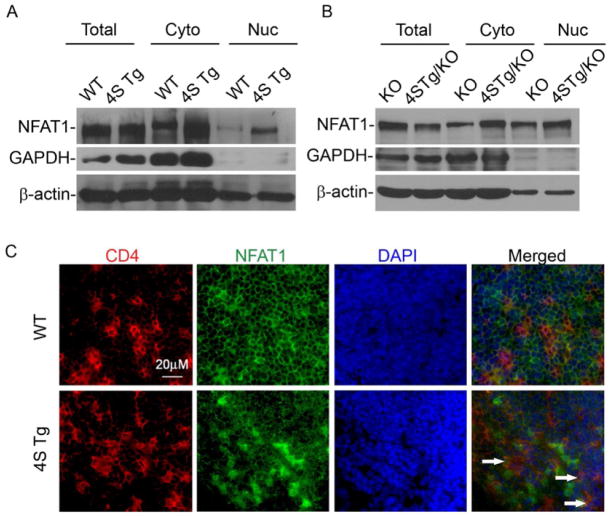
The studies described above suggested that CEACAM1-S isoforms possess direct signaling functions in T cells. However, the mechanisms by which this might occur are unknown. To clarify the possible signaling pathways involved in the functions observed as a consequence of CEACAM1-4S expression on primary T cells, we performed Affymetrix cDNA microarray analysis on primary CD4+ T cells isolated from MLN of WT or 4S Tg mice and compared their transcriptional profiles. By doing so, we identified an elevation of a number of genes associated with the Ca++- nuclear factor of activation (NFAT) signaling pathway (Hermann-Kleiter et al., 2010) (Table S1). These included cytokines such as IFN-γ, IL-2 and IL-4 and cell surface proteins such as CD40L.
We therefore assessed NFAT expression and its intracellular distribution in primary CD4+ T cells from the MLN of WT, 4S Tg, Ceacam1−/− and Ceacam1−/−-4S Tg mice. The most important finding was that CD4+ T cells from 4S Tg or Ceacam1−/−-4S Tg mice exhibited an obvious increase in the quantities of intranuclear NFAT relative to the amounts observed in the T cells from their WT or Ceacam1−/− littermate controls, respectively, as defined by immunoblotting with an NFAT1 specific antibody in the setting of similar amounts of intranuclear β-actin (Figure 3A and 3B). These biochemical studies were confirmed by the morphological examination of tissues from 4S Tg mice (Figure 3C) which revealed increased intranuclear accumulation of NFAT in CD4+ T cells within the PP of 4S Tg mice in comparison to that observed in the PP of WT littermate controls (Figure 3C). These studies confirmed the activating function of the 4S isoform and suggest that CEACAM1-S-mediated activation of T cells involves NFAT.
Figure 3.
Overexpression of CEACAM1-4S on T cells enhances the nuclear translocation of NFAT1. (A–B) Immunoblot of whole cell lysates (Total), cytosolic (Cyto) and nuclear (Nuc) extracts of splenic CD4+ T cells isolated from (A) WT and 4S Tg or (B) Ceacam1−/− and Ceacam1−/−-4S Tg (4S Tg/KO) mice with anti-NFAT1, anti-GAPDH or β-actin antibodies. (C) Frozen sections of PP from WT or 4S Tg animals were stained with anti-CD4 (red), anti-NFAT1 (green) and DAPI (blue). Areas of overlap are indicated by white arrows. Data are representative of 3 pairs of animals.
CEACAM1-4S on T cells regulates Peyer’s patches and the generation of IgA-dependent secretory immunity
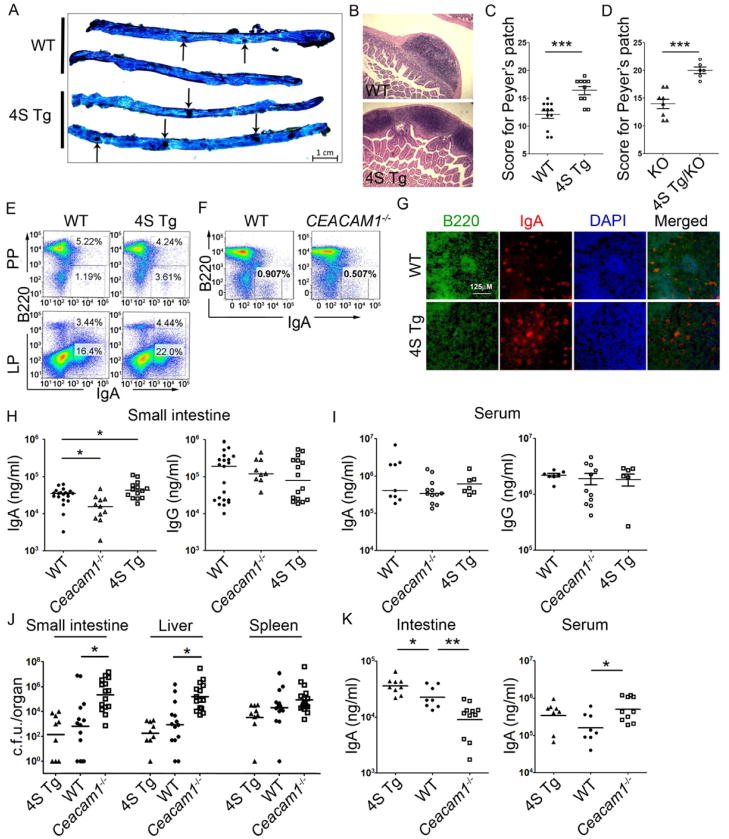
Given the relative importance observed for CEACAM1-S isoforms in T cells within intestinal tissues, including their enrichment in the S isoform and increased expression of NFAT-dependent factors such as CD40L, we next sought to determine whether the functions associated with CEACAM1-S-mediated signaling were linked to normal physiologic operations within the intestines. We first observed that, despite being present in equal numbers (Figure 4A), PP of 4S Tg small intestine were considerably larger in size than those of their WT counterparts (Figure 4B). Using a previously published scoring system which takes into account both PP frequency and size (Barreau et al., 2007), we determined that the total surface area occupied by PP of 4S Tg small intestine was substantially greater than that associated with their WT counterparts (Figure 4C). Since similar observations were made when Ceacam1−/−-4S Tg mice were compared to Ceacam1−/− littermate controls (Figure 4D), these observations provided evidence that enlarged PP size was a direct consequence of CEACAM1-S function.
Figure 4.
CEACAM1 regulates secretory immunity and mucosal defense against oral L. monocytogenes infection. (A) Representative methylene blue staining of the small intestine used to accurately quantify PP numbers. PPs are indicated by black arrows. One representative picture from each group of three is shown. (B) Hematoxylin and eosin staining shows 4S Tg mice have enlarged PP compared to that of WT mice. One representative picture from each group of eight is shown. (C and D) 4S Tg mice and Ceacam1−/−-4S Tg (4S Tg/KO) mice have significantly increased numbers and size of PP when compared to that of WT or Ceacam1−/− mice, respectively. ***, p ≤ 0.001 (two-tailed Student’s t test) (Mean, ± SEM.) (E–G) PP and LP in 4S Tg mice exhibit increased B220−IgA+ plasma blasts as assessed by flow cytometry (E) while PP of Ceacam1−/− mice possess decreased B220−IgA+ plasma blasts (F). Immunofluorescence confirmation of enrichment of B220−IgA+ in the PP from WT or 4S Tg animals for B220 (green) and IgA (red) (G). Data are representative of 3 animal pairs. (H–I) IgA and IgG concentrations were measured using ELISA and compared between WT, Ceacam1−/− and 4S Tg littermates in the small intestine (H) and serum (I). (n ≥ 12 per group) *, p < 0.05 (two-tailed Student’s t test) (Mean, ± SD) (J) L. monocytogenes colony number (c.f.u.) in the small intestine, liver and spleen of WT, Ceacam1−/− and 4S Tg mice 72 hour after oral infection with L. monocytogenes (n ≥ 9 per group). *, p < 0.05 (Mann-Whitney test). (K) IgA concentrations for intestinal washes or serum isolated from diseased mice, as measured by ELISA. (n ≥ 9 per group). *, p < 0.05; **, p < 0.01 (Mann-Whitney t test).
We therefore next examined B cell development in the PP of WT and 4S Tg mice. As shown in Figure 4E (and Table S2), we detected increased B220−IgA+ cells resembling activated plasma cells by flow cytometry in the PP and LP of 4S Tg mice relative to WT littermate controls. Consistent with this, the proportion of B220−IgA+ cells was decreased in the PP of Ceacam1−/− mice relative to their WT littermate controls (Figure 4F and data not shown). Using immunofluorescence microscopy, we confirmed the specific localization of these plasma cells in the PP of WT and 4S Tg mice (Figure 4G). In contrast, no differences were seen in the frequency of B220+IgA+ cells in either the PP or LP of WT and 4S Tg mice (Table S2). These studies showed that the PP of 4S Tg exhibited an increase in activated, IgA-committed plasma cells and that Ceacam1−/− mice demonstrated a corollary decrease in such cells, thereby confirming the physiological requirement for CEACAM1-S in establishing an adequate plasma cell distribution in the intestine.
We then examined the functional consequences of CEACAM1-4S in T cells on gut B cells by studying the immunoglobulin content of the intestinal secretions from 4S Tg and Ceacam1−/− mice. These studies revealed that the quantity of secreted IgA correlated with CEACAM1-4S expression in that lumenal IgA, but not IgG, was increased in 4S Tg mice and decreased in Ceacam1−/− mice relative to WT littermate controls (Figure 4H). Interestingly, although there were no significant differences in the serum IgA concentration observed in WT and Ceacam1−/− animals, there was a slight trend toward an increase in serum IgA, but not IgG concentrations, in 4S Tg mice compared to WT littermate controls (Figure 4I). Together, these data revealed that CEACAM1-S led to increases in both the quantity and activity of IgA+ plasma cells in intestinal tissues, enlarging the size of PP and enhancing secretory immunity.
CEACAM1 regulates mucosal defense to oral Listeria monocytogenes infection
We next determined whether the physiological role of CEACAM1 in regulating PP-mediated secretory immunity through CEACAM1-S expression extended to defense against a mucosal pathogen. In this regard, we chose to study Listeria monocytogenes, a Gram-positive intracellular pathogen, since previous studies indicate that Ceacam1−/− mice are susceptible to an intraperitoneal challenge with L. monocytogenes (Pan et al., 2010) and secretory immunity is likely to play an important role in immune defense against this bacterial pathogen (Manohar et al., 2001). We therefore examined the responses of Ceacam1−/− mice, 4S Tg mice and their WT littermates to oral infection with L. monocytogenes. 72 hours after induction of infection, the bacterial burden in both Ceacam1−/− small intestine and liver were substantially higher than that in WT and 4S Tg mice. Specifically, a 1000-fold increase in colony forming units (c.f.u.) of L. monocytogenes was detected in the small intestine of Ceacam1−/− mice and a 100-fold increase in c.f.u. of L. monocytogenes was observed in the liver of Ceacam1−/− mice compared to WT or 4S Tg mice, while no statistically significant difference was observed in the spleen for any group (Figure 4J). Conversely, the number of L. monocytogenes recovered from the small intestine, liver and spleen of 4S Tg mice was decreased relative to that observed in WT mice (Figure 4J). It is also notable that the quantity of secretory IgA detected in the small intestinal secretions of 4S Tg mice challenged with L. monocytogenes was greatly increased relative to that observed in WT mice (Figure 4K). Consistent with this observation, the unrestrained L. monocytogenes infection identified in Ceacam1−/− mice was associated with decreased secretion of intestinal IgA and increased IgA concentration in the serum of Ceacam1−/− mice in response to infection (Figure 4K). These data demonstrated that CEACAM1, and particularly CEACAM1-S, was necessary for an optimal response to mucosal challenge with a bacterial pathogen and that this was mediated through CEACAM1-dependent regulation of intestinal IgA secretion.
Overexpression or loss of CEACAM1-S on T cells alters the composition of the commensal microbiota
The studies above showed that a normal physiologic function of CEACAM1 in mucosal tissues is promoting secretory immunity in the PP and that this process is capable of enhancing protection against intestinal pathogens. Since IgA is a major regulator of the commensal microbiota (Macpherson et al., 2001), we hypothesized that forced expression of CEACAM1-S on T cells, as observed in 4S Tg mice, or loss of CEACAM1 expression, as observed in Ceacam1−/− mice, and which lead to increased and decreased IgA concentrations in the secretions, respectively, might result in demonstrable changes in the composition of the commensal microbiota in the ileum, the predominant site of the anatomic changes observed.
We therefore used quantitative culture methods to assess the composition of the commensal microbiota in the ileum from groups of age, gender and strain matched WT, 4S Tg and Ceacam1−/− mice. Culture of full-thickness bowel and of luminal contents is used as a means for evaluation of predominant species found in the gut and provides a normalized assessment of microbial biomass relative to the input mass of full thickness segments of terminal ileum (Bjorneklett et al., 1983). Culture-based studies are also leveraged to identify individual species and communities of bacteria that play an essential role in eliciting host phenotypes (Baumgart et al., 2007). Using aerobic and anaerobic culture techniques and selective and differential media, as described in Supplementary Methods, we recovered nine distinct isolates from the ileum of 4S Tg and WT mice (Table 1 and Table S3). These isolates spanned three orders, Lactobacillales, Bacillales and Bacteroidales, with 55% of isolates belonging to the genus Lactobacillus. The microbial diversity of the samples from 4S Tg mice was significantly higher than that of littermate WT controls, as assessed by multiple ecological measures of diversity (Table 1 and Table S3). In addition, certain isolates were substantially more abundant in the 4S Tg mice as compared to WT controls, including Gemella morbillorum, Lactobacillus murinus and Lactobacillus acidophilus. For Ceacam1−/− mice, we recovered ten distinct isolates spanning five orders, including those found in 4S Tg mice, and additionally the orders Clostridiales and Bifodobacteriales (Table 1 and Table S3). Microbial diversity of the samples from Ceacam1−/− mice was also considerably higher than that of controls, but only one isolate, Lactobacillus brevis, was found to be more abundant in Ceacam1−/− mice (Table 1). These studies showed that, as would be predicted by the observed increased and decreased IgA secretion (Fagarasan, 2008; Macpherson et al., 2004) in 4S Tg and Ceacam1−/− mice, respectively, the composition of the commensal microbiota was regulated by CEACAM1.
Table 1.
Alterations in the composition of the commensal microbiota in mice with overexpression of CEACAM1-4S on T cells.
| log10 c.f.u./g | Community Diversity | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Bacteroidales | Bifidobacteriales B. pseudolongum |
Lactobacillales | Bacillales G. morbillorum |
Clostridiales Eubacterium sp. |
Shannon | Simpson | ||||||||||
| P. loescheii | P. tannerae | Prevotella sp. | E. faecalis | L. leichmanii | L. murinus | L. acidophilus | L. brevis | L. curvatus | L. farcimus | L. viridescens | ||||||
| 4S Tg | 5.8 | 4.5 | 6.3 | 7.0 | 6.8 | 5.6 | 0 | 5.3 | 2.1 | 0.9 | ||||||
| 0 | 3.9 | 5.7 | 4.1 | 4.4 | 3.3 | 0 | 4.6 | 1.8 | 0.8 | |||||||
| 0 | 0 | 0 | 6.4 | 6.5 | 6.0 | 5.3 | 4.2 | 1.4 | 0.7 | |||||||
| 0 | 0 | 0 | 0 | 6.4 | 0 | 0 | 5.4 | 1.4 | 0.8 | |||||||
|
| ||||||||||||||||
| WT | 0 | 0 | 0 | 0 | 0 | 0 | 3.0 | 0 | 0.0 | 0.0 | ||||||
| 0 | 0 | 0 | 3.5 | 3.5 | 0 | 0 | 0 | 0.7 | 0.5 | |||||||
| 0 | 0 | 0 | 3.0 | 0 | 0 | 0 | 0 | 0.0 | 0.0 | |||||||
| 0 | 0 | 0 | 3.6 | 3.0 | 0 | 0 | 0 | 0.7 | 0.5 | |||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 4.6 | 0 | 0.0 | 0.0 | |||||||
|
| ||||||||||||||||
| p-val ue | 0.6 | 0.3 | 0.3 | 0.02 | 0.02 | 0.07 | 0.9 | 0.02 | 0.02 | 0.02 | ||||||
|
| ||||||||||||||||
| CC 1−/− | 0 | 0 | 4.6 | 6.8 | 6.5 | 6.8 | 6.3 | 0 | 5.6 | 1.9 | 0.9 | |||||
| 3.0 | 0 | 0 | 0 | 3.5 | 3.7 | 0 | 0 | 0 | 1.4 | 0.8 | ||||||
| 3.0 | 0 | 3.5 | 6.9 | 6.6 | 6.1 | 0 | 4.3 | 0 | 1.4 | 0.7 | ||||||
|
| ||||||||||||||||
| WT | 0 | 6.1 | 0 | 7.0 | 6.9 | 0 | 3.0 | 0 | 0 | 1.1 | 0.7 | |||||
| 0 | 0 | 0 | 6.2 | 6.0 | 0 | 0 | 0 | 0 | 1.1 | 0.7 | ||||||
| 0 | 5.3 | 0 | 6.7 | 6.7 | 0 | 0 | 0 | 0 | 1.1 | 0.7 | ||||||
| 0 | 0 | 0 | 7.3 | 6.8 | 0 | 0 | 0 | 0 | 0.7 | 0.5 | ||||||
| 0 | 0 | 0 | 4.3 | 4.4 | 0 | 4.6 | 0 | 0 | 0.7 | 0.5 | ||||||
|
| ||||||||||||||||
| p-val ue | 0.1 | 0.4 | 0.1 | 0.2 | 0.3 | 0.04 | 0.6 | 0.6 | 0.6 | 0.04 | 0.04 | |||||
Shannon and Simpson indices are measures of microbial community diversity, with larger values indicating more diverse samples. These indices take into account the abundances of species relative to one another in a sample. Numbers in bold indicate statistically significant differences.
CEACAM1 is preferentially expressed on specific subsets of circulating and tissue-resident CD4+ T cells
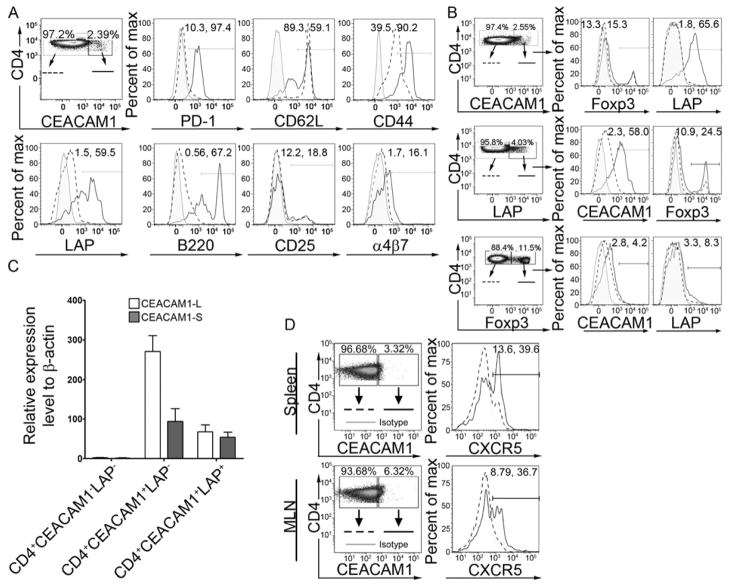
Given our observations concerning the impact of CEACAM1 on the regulation of secretory IgA and its consequences in T cell-targeted 4S Tg mice as well as the known importance of T cells to B cell development, we sought to define the subsets of CEACAM1-regulated T cells responsible for these activities. To do so, we focused our attention on defining the accessory molecules expressed by the small subset of CEACAM1+ T cells detected under physiologic conditions in spleen and MLN and which were increased in frequency in PP and LP in WT mice (see Figure 1A). We observed that CD4+CEACAM1+ T cells from the blood, MLN, PP and LP expressed higher amounts of both CD44 and CD62L than did CD4+CEACAM1− T cells (Figure 5A and S3A–C). This is consistent with CEACAM1 being associated with effector and/or central memory T cells (Boulton et al., 2002). Additionally, we noted that CD4+CEACAM1+ T cells from each of these tissue compartments expressed more α4β7-integrin than their CD4+CEACAM1− counterparts, suggesting that CEACAM1 expression on T cells, even those within the peripheral immune system, is associated with enhanced potential to home to intestinal tissues. Interestingly, we also noted that CEACAM1 positivity was associated with preferential expression of PD-1, LAP (TGFβ Latency Associated Peptide) and B220, all of which are characteristics associated with T cell subsets possessing regulatory or accessory potential in the production of IgA (Fagarasan et al., 2001; Good-Jacobson et al., 2010; Ochi et al., 2006; Oida et al., 2003).
Figure 5.
CEACAM1-4S is preferentially expressed on specific subsets of both circulating and intestinal CD4+ T cells. (A) Expression of CEACAM1 is associated with the enrichment of specific cell surface molecules. Percentages on histogram gates indicate the frequency of cells positive for the given marker in the CD4+CEACAM1− and CD4+CEACAM1+ cell populations, respectively, of MLN CD3+ T cells from WT mice. (B) CEACAM1 expression co-segregates with LAP expression and is unrelated to Foxp3 expression in CD4+ T cells from the MLN of naïve WT mice. (C) The CEACAM1-S isoform is enriched on CD4+CC1+ T cells which express LAP. T cells were isolated from pooled spleen and LN. (D) CXCR5 is preferentially enriched on CEACAM1-expressing CD4+ T cells in the spleen and MLN of WT mice. Data are representative of 5 pairs of animals. (A–C) show representative data from 3 independent experiments with n = 5–6 mice per group.
These observations led us to next focus on specific cell subsets on which the identified molecules are known to be enriched. In particular, since our data showed CEACAM1 expression to be strongly co-segregating with surface LAP on CD4+ T cells from naïve WT animals (Figures 5A and S3A–C), we carefully examined CD4+CD25−LAP+ T cells, a subset of Treg that are concentrated in intestinal tissues and provide TGF–β dependent regulatory function and, potentially, support for secretory IgA immunity (Niemir et al., 1995; Ochi et al., 2006; Oida et al., 2003). Whereas on average 43.6 ± 10.9 % of CD4+CD25−LAP+ T cells within MLN co-expressed CEACAM1, only 2.07 ± 0.8 % of CD4+CD25−LAP− cells were also CEACAM1+ under steady-state conditions in WT mice (Figure 5B). This was in stark contrast to the CEACAM1 expression in another regulatory T cell subset wherein similar low quantities of CEACAM1 were observed on CD4+Foxp3+ and CD4+Foxp3− Treg cells from the MLN (Figure 5B). We next examined the relative expression of the CEACAM1-L and -S isoforms in CD4+CEACAM1−LAP−, CD4+CEACAM1+LAP−, or CD4+CEACAM1+LAP+ cells flow-cytometrically sorted from pooled spleen and MLN of WT mice using the real-time PCR assay described above (Figures S1A–B). Consistent with our findings in the total population of peripheral CD4+ T cells, the ratio of L:S isoforms was 2.78 ± 0.07 in CD4+CEACAM1+LAP− cells, indicating a predominance of the L isoform in this group of T cells (Figure 5C). In contrast, the L:S ratio was decreased to 1.16 ± 0.27 (P < 0.05) in CD4+CEACAM1+LAP+ cells indicating an enrichment of the CEACAM1-S isoform in this regulatory cell subset (Figure 5C). No differences were observed in the L:S ratio between CD4+CEACAM1+CD25− cells (1.52 ± 0.29) and CD4+CEACAM1+CD25+ cells (1.52 ± 0.11) isolated from the spleen and LN (Figure S3D). These data demonstrated the existence of a close association between the expression of CEACAM1 and LAP.
The preferential expression of PD-1 noted on CD4+CEACAM1+ T cells further led us to investigate a subset of T cells on which this molecule is enriched. High expression of PD-1 is associated with T follicular helper (Tfh) cells which regulate the formation of long-lived plasma cells by driving the survival and selection of B cells in mature germinal centers (Good-Jacobson et al., 2010). In addition to PD-1, Tfh cells are identified by high expression of CXCR5 (Linterman et al., 2011) and upon investigation, we observed that CD4+CEACAM1+ T cells from both the spleen and MLN of WT mice are enriched in expression of this chemokine receptor compared to their CD4+CEACAM1− counterparts (Figure 5D). Thus, our data indicated that Tfh cells preferentially expressed CEACAM1 under physiological conditions. A deeper investigation of the CEACAM1 isoforms expressed by PD-1+CXCR5+ Tfh cells also revealed an enrichment of CEACAM1-S isoforms in Tfh cells from the spleen, PP and MLN (Figure S3E). Specifically, in contrast to the majority of peripheral CD4+ T cells, which predominantly express CEACAM1-L isoforms, Tfh cells expressed near equal amounts of CEACAM1-L and CEACAM1-S (Figure S3E). Collectively, these data provided evidence that high expression of CEACAM1, and in particular enrichment of CEACAM1-S, was a defining feature of certain subsets of CD4+ T cells under physiological conditions which serve a regulatory or accessory role in secretory immunity and preferentially localize to organized lymphoid structures and/or intestinal tissues.
CEACAM1-S independently regulates the expansion and function of CD4+ T cells in which it is enriched
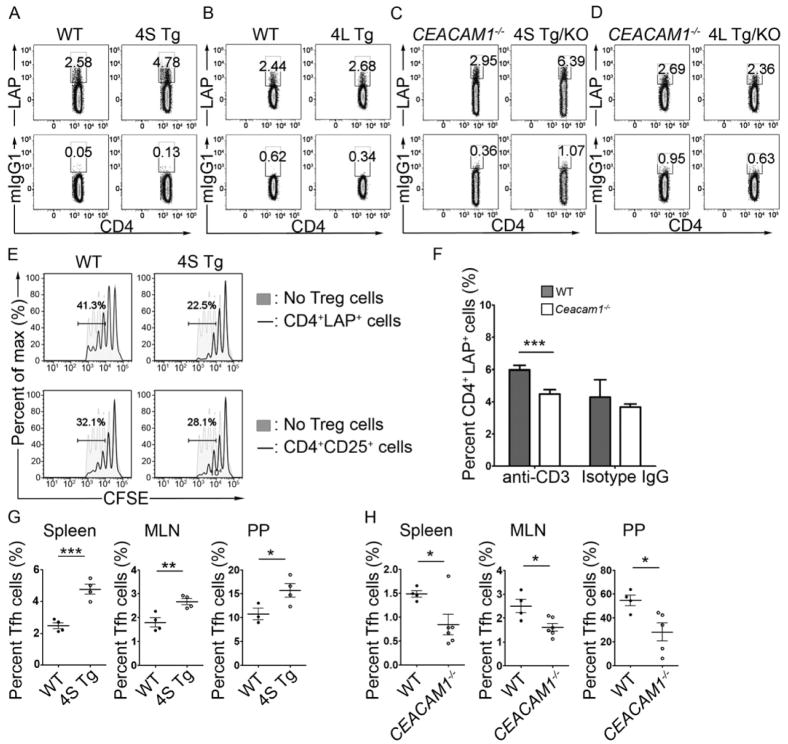
Our observations of preferential CEACAM1 expression on CD4+CD25−LAP+ and Tfh cells from WT mice led us to question the ability of CEACAM1 to regulate these cells and, further, to interrogate the relative contribution of CEACAM1-L and CEACAM1-S isoforms in this process. Utilizing the CEACAM1 transgenic mice, we first examined the frequency of CD4+CD25−LAP+ T cells in mucosal tissues under conditions of forced overexpression of the CEACAM1-4L or -4S isoforms. These data demonstrated a significant increase in the percentage of CD4+CD25−LAP+ T cells in the MLN from 4S Tg mice relative to WT controls (Figure 6A and B) and in Ceacam1−/−-4S Tg mice compared to Ceacam1−/− controls (Figure 6C and D). This increase in CD4+CD25−LAP+ cells was specifically detected under conditions of overexpression of the CEACAM1-4S isoform in both WT and Ceacam1−/− animals (Figure 6A and C) and was notably absent in CEACAM1-4L transgenic mice in which the inhibitory CEACAM1 isoform was overexpressed (Figure 6B and D). Given the detected increases in cell death in both CD4+LAP− and CD4+LAP+ populations in 4S Tg animals (Figure S4A), our finding of an expanded population of viable CD4+LAP+ T cells under conditions of CEACAM1S overexpression can best be explained not by decreased AICD amongst this population but rather by increased generation of the CD4+LAP+ T cells in the presence of forced CEACAM1-4S expression. In stark contrast, our analysis did not reveal any differences in the frequency of CD4+CD25+Foxp3+ T cells upon forced expression of CEACAM1-4S in T cells relative to that observed in WT mice (data not shown), In order to ascertain whether enrichment of activating CEACAM1-S was functionally relevant to CD4+CD25−LAP+ T cells, we examined the in vitro suppressive function of CD4+LAP+ T regulatory cells obtained from the MLN of 4S Tg mice. We found that these cells possessed enhanced regulatory activity when compared to CD4+CD25−LAP+ T cells from WT controls (Figure 6E). In contrast, no differences were observed in the regulatory potential between 4S Tg and WT CD4+CD25+ Treg cells obtained from the MLN in similar assays (Figure 6E). Taken together, these studies indicated that the relative increase of CEACAM1-4S, in the absence of any contribution from the inhibitory CEACAM1-L isoforms, specifically enhanced both the development and functional capacity of regulatory CD4+CD25−LAP+ T cells.
Figure 6.
CEACAM1-S directly enhances the generation of CD4+LAP+ and Tfh cells. (A–D) The percentage of CD4+LAP+ cells is increased in MLN of naïve 4S Tg mice (A) but not 4L Tg mice (B).Ceacam1−/−-4S Tg mice (C) but not Ceacam1−/−-4L Tg mice (D) are enriched in CD4+LAP+ T cells compared to their littermate controls. (E) Overexpression of CEACAM1-4S enhances the regulatory function of CD4+LAP+ Treg cells in vitro as shown by the decrease in proliferation of responder T cells when co-cultured with CD4+LAP+ Treg cells from pooled spleen and LN of 4S Tg mice. (F) Oral feeding of anti-CD3 antibody significantly increases the percentage of CD4+LAP+ Treg cells in the MLN of WT but not CEACAM1−/− mice. Mice were fed 5 μg anti-CD3 (or isotype control) for each of 5 consecutive days and CD4+LAP+ T cell frequency was assessed at day 7. (G–H) The percentage of CD4+PD1+CXCR5+ Tfh cells in the spleen, MLN and PP is increased 4S Tg mice (G) and decreased in Ceacam1−/− mice (H) when compared to their littermate controls. All data are representative of 3 independent experiments with n = 3–6 mice per group. All data are mean ± SEM, *, p < 0.05; **, p < 0.01, ***, p < 0.005 (two-tailed Student’s t test).
It is known that CD4+CD25−LAP+ T cells accumulate in intestinal tissues under steady-state and inflammatory conditions and that orally administered CD3-specific antibodies induce CD4+CD25–LAP+ regulatory T cells in MLN (Boirivant et al., 2008; Ochi et al., 2006). We thus made use of this approach in order to determine whether CEACAM1 is specifically involved in the induction phase of CD4+CD25–LAP+ T cell development under conditions of physiological CEACAM1 expression. While no significant differences were identified in the proportions of CD3+CD25+/−LAP+ Treg cells in spleen, MLN, PP or blood of untreated or isotype-treated Ceacam1−/− and WT mice, oral administration of anti-CD3 antibody substantially increased the percentage of CD4+CD25−LAP+ T cells in the MLN of WT but not Ceacam1−/− mice (Figure 6F). In contrast, oral administration of a CD3-specific antibody was not observed to alter the percentage of CD4+Foxp3+ T regulatory cells in the MLN of WT or Ceacam1−/− mice (Figure S4B). These studies confirmed a physiological role played by CEACAM1 in regulating the expansion of CD4+CD25−LAP+ T cells in mucosal tissues as well as in promoting their suppressive function.
Given our observation of PP enlargement in 4S Tg mice and the preferential expression of CEACAM1-S in Tfh cells in WT mice, we next examined the impact of highly abundant of CEACAM1-4S on the frequency of Tfh cells in the spleen, MLN and PP of 4S Tg mice. Flow cytometric analysis of each of these tissues revealed an enrichment not only of PD-1 itself (Figure S4C) but also of the PD-1+CXCR5+ population of Tfh cells in the 4S Tg mice compared to their WT littermate controls (Figure 6G). Importantly, CEACAM1-4S-mediated enrichment of Tfh cells occurred completely independently of the CEACAM1-4L isoform since Ceacam1−/−-4S Tg mice also contained much higher amounts of Tfh cells than their Ceacam1−/− counterparts (Figure 6H). Collectively, our data are consistent with a role for CEACAM1-S isoforms in promoting the development of two types of accessory cells (CD4+CD25−LAP+ cells and Tfh cells) which are not only enriched in organized lymphoid structures and mucosal tissues but are also known to be involved in B cell activation (Cerutti, 2008; Good-Jacobson et al.), and likely thus contribute to the ability of CEACAM1 to regulate secretory intestinal immunity.
Discussion
Here, we provide evidence for tissue and cell-type specific regulation of CEACAM1 splicing in T cells which results in the unique predominance of CEACAM1-S isoforms in intestinal resident T cells and specific subsets of T cells associated with humoral immunity. Although CEACAM1 is an activation-induced molecule on T cells, the observed tissue-specific splicing was not due to activation of the T cells per se as it was not observed when T cells were stimulated ex vivo. Adoptive transfer experiments demonstrated that CEACAM1-S dominance was gained by naïve peripheral T cells upon entry into the intestines, and was lost when mucosal T cells were removed from the intestines and re-stimulated ex vivo. This indicated that the specific mucosal milieu of the intestines promotes CEACAM1-S expression in T cells. Moreover, our results in 4S Tg mice indicated that CEACAM1-S facilitates T cell activation and the induction of secretory IgA immunity. Consistent with this, T cell subsets which bore a memory phenotype characteristic of mucosal tissues (Gibbons et al., 2011) and specific subsets of T cells such as CD4+CD25−LAP+ and Tfh cells which encourage secretory immunity displayed an enrichment upon CEACAM1-S expression and were driven by its presence. Thus, CEACAM1-S expression is promoted and maintained by the mucosal environment and facilitates T cell activation linked to the generation of cell types responsible for mucosal regulation and protection.
The mucosal factors responsible for this preferential splicing within the mucosal microenvironment of the intestines are unknown. We showed that the differential splicing of CEACAM1 occurred independently of microbial factors, as observed in GF mice, and related signaling pathways such as MyD88 and NOD2. This suggests that microbial-independent tissue-specific factors and their own related pathways regulate CEACAM1 splicing and expression in the intestines. Whether these factors include soluble (e.g. cytokines) or cell surface molecules unique to the mucosal tissues, it is clear that they regulate both the extent of CEACAM1 expression and the splicing of its mRNA. These studies also demonstrated that tissue- and cell-specific regulation of Ceacam1 alternative splicing occurs in vivo and is linked to specific immune functions.
Importantly, these studies also showed that CEACAM1-S is not a minor isoform in primary T cells as was currently thought (Gray-Owen et al., 2006). In transgenic mice with conditional expression of CEACAM1 in T cells, we found that CEACAM1-S provided unique co-stimulatory signals which promoted both AICD and an effector memory phenotype, whereas CEACAM1-L was co-inhibitory for TCR-CD3 complex signaling (Nagaishi et al., 2006). Moreover, we have now shown that CEACAM1-S in T cells can elicit this stimulation as an independent signaling unit without CEACAM1-L assistance in vivo. Through these properties, CEACAM1-S regulates specific subsets of T cells. These cell populations include CD4+LAP+ T cells and Tfh cells which are an integral component of mucosal tissues and organized lymphoid structures, respectively, and are known to regulate secretory immunity (Linterman et al., 2011). In view of CEACAM1-S enrichment in these latter cell types irrespective of their localization to peripheral or mucosal tissues as well as with our observations that the intestinal tissues facilitate CEACAM1-S expression, our studies also raise that possibility that CD4+LAP+ T cells and Tfh cells may be born in and/or modified remotely by mucosal factors.
Although little is known about CEACAM1-S signaling in T cells, in epithelial cells it is known to associate with the cytoskeleton (actin and tropomyosin), calmodulin and annexin II (Edlund et al., 1996; Kirshner et al., 2003; Schumann et al., 2001). Such intracellular associations, interestingly, are linked to the promotion of apoptosis of mammary epithelial cells during acini morphogenesis (Kirshner et al., 2003), In addition, consistent with previous transfection studies in Jurkat T cells, CEACAM1-S signaling is able to activate NFAT in vivo (Chen et al., 2004b). As shown here, this was associated with the upregulation of NFAT-dependent soluble and cell surface molecules such as PD-1 and CD40L. Thus the current studies not only demonstrated the importance of CEACAM1-S in T cells under physiological conditions but also their role in delivering independent signals that may oppose those provided by CEACAM1-L isoforms. Such observations further highlight the functional similarities between CEACAM1 and other signaling pathways that comprise highly related activating and inhibiting modules such as CD28 and CTLA-4 (Greenwald et al., 2005).
Presumably through its NFAT-related activation functions, CEACAM1-S-mediated T cell activation of B cells in the PP promoted the generation of IgA plasma blasts, the expansion of PP size and IgA secretion into the lumen. Given the decreases in IgA secretion observed in the absence of CEACAM1 expression and increased IgA secretion when CEACAM1-4S is overexpressed on T cells, CEACAM1-S in T cells emerges as an important factor in directly regulating B cell function and PP structure. PP are the essential inductive sites for regulation of antigen-specific IgA antibody responses following oral immunization, although a PP-independent pathway for mucosal IgA responses also likely exists (Macpherson et al., 2007; Cerutti, 2008; Hashizume et al., 2008). Previous show that selective TGF-β receptor type II deficiency in B cells results in loss of IgA-expressing cells in the spleen and PP (Borsutzky et al., 2004; Cazac et al., 2000). Consistent with this, CD4+CD25+ Tregs have been identified as major helper cells for IgA responses to microbial antigens such as flagellin, presumably through their secretion of TGF-β (Cong et al., 2009). As CD4+CD25−LAP+ T regulatory cells are also TGF-β-dependent (Ochi et al., 2006; Oida et al., 2003), were increased in the mucosal tissues of mice that overexpress CEACAM1-4S and inadequately expanded by T cell receptor-dependent signals in the absence of CEACAM1 expression, a plausible mechanistic explanation for the increased IgA production and TGF-β secretion observed in 4S Tg mice is that CD4+CD25−LAP+ T cells are a responsible factor. Additionally, the increased frequency of PD-1+CXCR5+ Tfh cells which we observed in the spleen, MLN and PP of 4S Tg mice is likely to provide additional stimulation to IgA-producing plasma blasts since CXCR5+ Tfh cells in B cell zones of lymphoid tissues possess significant capacity for supporting IgG and IgA production by B cells (Fazilleau et al., 2009a). The ability of Tfh cells to regulate antigen-specific B cell immunity in vivo is likely linked to its high expression of PD-1, which is NFAT dependent (Oestreich et al., 2008) and is known to deliver signals that promote long-lived plasma cell responses (Good-Jacobson et al., 2010). Thus, our studies showed that CEACAM1-S regulates secretory IgA within PP via its ability to elicit NFAT-mediated signaling which enhances the expression of cell surface molecules such as CD40L and PD-1, the expansion of CD4+CD25−LAP+ T cells and Tfh cells and the secretion of TGF-β, IL-2, IL-4 and IL-10 by CD4+ T cells; all factors which promote secretory IgA immunity (Cazac et al., 2000; Cerutti, 2008; Good-Jacobson et al., 2010; Massacand et al., 2008). As a consequence, in the absence of CEACAM1, secretory IgA is reduced.
The ability of CEACAM1 to regulate secretory IgA production is also interesting given the well-known role of CEACAM1 as a microbial receptor (Gray-Owen et al., 2006). Moreover, bacteria are well-known inducers of secretory IgA production (Macpherson et al., 2004) and IgA in turn regulates the composition of the commensal microbiota (Fagarasan, 2008). For example, IgA concentrations are very low in GF animals with normalization of these amounts within a few weeks following intestinal bacterial colonization after birth (Hapfelmeier et al., 2010). IgA-deficiency is also associated with a bloom in certain organisms in the intestinal lumen (Suzuki et al., 2004). Furthermore, secretory IgA promotes both immune exclusion and neutralization of translocated bacteria which preserves intestinal barrier integrity by preventing bacterial-induced inflammation (Boullier et al., 2009). In addition, IgA can promote bacterial colonization as shown for Helicobacter sp. which bind to the gastric epithelium via IgA but do not invade (Taylor et al., 2007). As such, IgA can contribute to the survival of certain classes of bacteria within the intestines. The latter is also consistent with the ability of secreted IgA to bind to luminal bacteria (van der Waaij et al., 1996) and in some cases inhibit their invasiveness, as observed during Salmonella typhimurium infection (Wijburg et al., 2006). Consistent with this, we observed that CEACAM1 gain-of-function in T cells and loss-of-function correlated with the ability of the host to resist the pathogenicity of L. monocytogenes infection. Ceacam1−/− and 4S Tg mice also demonstrated increased community diversity of the commensal ileal microbiota, with a predominance of Lactobacillus and Streptococcus species as compared to WT controls. This indicated that CEACAM1-S dependent effector functions of these T cells have the capacity to modulate the composition of microbial communities in the small intestine, presumably through their effects on IgA secretion. These observations not only further support a role for CEACAM1 in the generation of secretory immunity but show that it is physiologically important to the regulation and control of the intestinal microbiota and enteropathogens.
In summary, our studies have shown that in tissue resident T cells of the intestines as well as in specific subsets of T cells, not only are CEACAM1-S isoforms expressed, but they often dominate over CEACAM1-L isoforms. In these cells, CEACAM1-S provides unique signals that promote populations of T cells regulating IgA production and secretion associated with control of the commensal microbiota and resistance to enteropathogens. These studies demonstrate the important role played by CEACAM1-S isoforms in immunity.
Experimental Procedures
Animals
Wild-type (WT) C57BL/6 (B6) mice were purchased from The Jackson Laboratory. hCD2 promoter controlled CEACAM1-4S transgenic mice were generated in the of Brigham and Women’s Hospital Transgenic Core Facility by a similar strategy to that previously described (Nagaishi et al., 2006). Ceacam1−/− mice were previously described (Leung et al., 2006). Myd88−/− mice and Nod2−/− mice were kindly provided by Dr. S. Akira (Osaka, Japan) and Dr. K. S. Kobayashi (Dana-Farber Cancer Institute, Boston, MA), respectively. Swiss Webster germ-free (SWGF) mice and C57BL/6 germ-free mice (B6GF) were maintained in germ-free isolators at Taconic. All other mice were maintained under specific pathogen-free conditions at the Harvard Center for Comparative Medicine at Harvard Medical School. All mice were used between 8 and 12 weeks of age. All animal experimentation was performed in accordance with the Institutional Animal Care and Use Committee (IACUC) of Harvard Medical School which granted permission for this study.
Flow Cytometry
For CEACAM1 expression assays, cells were incubated with the CC1 antibody for 20 min followed by FITC-conjugated rat anti-mouse IgG1. For other cell surface proteins, cells were stained with the indicated fluorescence-conjugated antibodies for 20 min, washed and resuspended with 1% BSA/PBS FACS staining buffer containing DAPI (Invitrogen). Cell apoptosis assays were performed according to the Annexin V-FITC Apoptosis Detection Kit manual (BD Bioscience). For intracellular cytokine staining, cells were stimulated with ionomycin (Sigma, 2 μg/ml), PMA (Sigma, 30 ng/ml) and Golgistop (BD Bioscience) for 4 hours followed by intracellular cytokine staining using Cytofix/Cytoperm solution kit according to the manufacturer’s instructions (BD Bioscience). IgA staining was performed according to the instructions provided with the rat anti-mouse IgA antibody (C10-3). CD4+LAP+ T cells were stained for 20 min with biotinylated anti-LAP antibody (R&D Systems) followed by APC-conjugated streptavidin (BD Biosciences) as described previously (Ochi et al., 2006). Tfh cells were stained for CXCR5 and PD-1 as described previously (Linterman et al., 2011). All stained cells were analyzed on an LSRII (BD Biosciences) or a MACSQuant (Miltenyi Biotech) flow cytometer, and data were analyzed using Flowjo software.
Culture-based studies of ileal microbial community structure
Samples of terminal ileum (2 cm from the ileocecal valve) were sterilely dissected from mice, placed in cryovials and immediately snap frozen on liquid nitrogen. Samples were delivered to the Harvard Digestive Disease Center’s (HDDC) Microbiome and Gnotobiotics Core facility on dry ice and stored at −80°C until processing. Isola tion of microorganisms and determination of microorganisms is described in the Supplementary Methods.
Oral Listeria monocytogenes Infection
Gender- and age-matched groups of Ceacam1−/− mice, 4S Tg mice and WT littermates were housed under BL2 conditions prior to infection. Approximately 5×108 c.f.u. of a fourteen- to sixteen-hour culture of L. monocytogenes strain InlAm (Wollert et al., 2007) was suspended in 0.2 mL PBS. The suspension was delivered intragastrically to mice with a 21 gauge feeding needle attached to a 1 mL syringe. Animals were monitored daily and at 72 hours post infection were sacrificed and dissected for histological analysis, IgA production and determination of the number of bacteria present in the small intestine, spleen and liver. c.f.u. assays (small intestine, spleen and liver c.f.u./organ) were performed as previously described (Kaser et al., 2008).
Oral feeding of anti-CD3
Mice were fed with anti-CD3 antibody using a protocol described previously (Ochi et al., 2006). Specifically, groups of paired mice were fed anti-CD3 or IgG isotype control antibody at a dose of 5 μg/mouse/day for 5 days. After one day’s rest, mice were sacrificed at day 7 and the percentage of CD4+LAP+ regulatory T cells was determined in specific lymphoid tissues.
Suppression assays
Regulatory T cell suppression assays were performed as previously described (Fantini et al., 2007). Briefly, 1×105 freshly isolated splenic CD4+CD25− T cells were stained with CFSE and mixed with an equal number of either CD4+CD25+ or CD4+LAP+ T regulatory cells isolated via magnetic sorting (Miltenyi). T cell mixtures were then activated in the presence of soluble anti-CD3 (1 μg/ml) and 4×105 irradiated total splenocytes as antigen presenters. After 4 days of culture, CFSE dilution of the responder CD4+CD25− T cells was measured as an indication of proliferation.
Total Serum and secretion Immunoglobulin ELISA
The sera, intestinal washes or fecal extracts were collected for ELISA as previously described (Hapfelmeier et al., 2010).
Immunohistochemical analysis
PP were isolated and embedded in OCT compound (Sakura Finetechnical) and snap frozen in dry ice. Frozen sections were prepared on micro slides using a cryostat (Leica), and stored at −70°C until use. The slides were fixed in 100% aceto ne for 5 min at 4°C and air dried at room temperature. Cryostat sections were blocked with 10% normal goat serum for 1 hour at room temperature (RT). For B220 and IgA staining, the slides were stained with purified rat anti-mouse IgA antibody for 1 hour at RT, followed by incubation with Alexa Fluro 568 conjugated goat anti-rat IgG secondary antibody (Invitrogen) and FITC-conjugated anti-mouse B220 antibody (BD Bioscience) for 1 hour at RT. For NFAT staining, the slides were stained with biotin conjugated-anti-mouse CD4 antibody and rabbit anti-NFAT1 antibodies for 2 hours at RT, followed by incubation with rhodamine red-X-streptavidin (Jackson ImmunoResearch) and Alexa Fluor® 488 F(ab′)2 fragment of goat anti-rabbit IgG (H+L) (Invitrogen) for 1 hour at RT. The counterstaining was performed using DAPI (Invitrogen). The specimens were analyzed using a Nikon Eclipse Ti microscope.
Statistics
The Mann-Whitney U-test (two-tailed) was used to assess differences in log10 colony forming units (c.f.u.) in quantitative culture assays. The t-test (two-tailed) was used to assess differences between means for other data analyzed. Tests with a P-value less than 0.05 were considered statistically significant. Data are presented as mean ± SD or ± SEM., as indicated in the figure legend. Microbial diversity was assessed using two common ecological measures of diversity, Shannon information entropy (Shannon, 1997) and Simpson’s diversity index (Baumgart et al., 2007). Both measures take into account the abundances of species relative to one another in a sample.
Supplementary Material
Acknowledgments
We thank R. Maas (Division of Genetics, Brigham and Women’s Hospital) for microarray data analysis, C.G. Vinuesa (Australian National University), H. Cantor (Dana Farber Cancer Institute) and K. Smith (Addenbrooke Hospital, University of Cambridge) for guidance on analysis of Tfh cells and J. Cusick for technical assistance. LC and TN were supported by Research Fellowship Awards from the Crohn’s & Colitis Foundation of America. RSB was supported by NIH DK051362, DK044319, DK053056, DK088199, Harvard Digestive Diseases Center (NIH P30DK034854) and the High Pointe Foundation. GG was supported by the Stanley L. Robbins Memorial Research Award. JA is supported by NIH T32 DK007737. KH was supported by NIH AI059576. KB and NB were supported by the Canadian Institutes of Health Research. JS was supported by NIH CA84202. CJ was supported by NIH DK73338 and DK47700. LB was supported by the Harvard Digestive Diseases Center (NIH P30DK034854), HD061916 and the BWH Gnotobiotics and Microbiome Core. TN was supported by Japanese Ministry of Education, Culture, Sports, Science and Technology; Nihon Univ. Medical Association; Abbott Japan Research Fund; Foundation for Advancement of International Science; Takeda Science Foundation.
Footnotes
The authors declare they have no financial conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Rjaily GA, Lee SJ, May D, Al-Share QY, Deangelis AM, Ruch RJ, Neumaier M, Kalthoff H, Lin SH, Najjar SM. CEACAM1 modulates epidermal growth factor receptor--mediated cell proliferation. J Clin Invest. 2004;114:944–952. doi: 10.1172/JCI21786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuz-Lieberman N, Markel G, Mizrahi S, Gazit R, Hanna J, Achdout H, Gruda R, Katz G, Arnon TI, Battat S, et al. The involvement of NK cells in ankylosing spondylitis. Int Immunol. 2005;17:837–845. doi: 10.1093/intimm/dxh270. [DOI] [PubMed] [Google Scholar]
- Barreau F, Meinzer U, Chareyre F, Berrebi D, Niwa-Kawakita M, Dussaillant M, Foligne B, Ollendorff V, Heyman M, Bonacorsi S, et al. CARD15/NOD2 is required for Peyer’s patches homeostasis in mice. PLoS One. 2007;2:e523. doi: 10.1371/journal.pone.0000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart M, Dogan B, Rishniw M, Weitzman G, Bosworth B, Yantiss R, Orsi RH, Wiedmann M, McDonough P, Kim SG, et al. Culture independent analysis of ileal mucosa reveals a selective increase in invasive Escherichia coli of novel phylogeny relative to depletion of Clostridiales in Crohn’s disease involving the ileum. ISME J. 2007;1:403–418. doi: 10.1038/ismej.2007.52. [DOI] [PubMed] [Google Scholar]
- Bjorneklett A, Fausa O, Midtvedt T. Bacterial overgrowth in jejunal and ileal disease. Scand J Gastroenterol. 1983;18:289–298. doi: 10.3109/00365528309181596. [DOI] [PubMed] [Google Scholar]
- Boirivant M, Amendola A, Butera A, Sanchez M, Xu L, Marinaro M, Kitani A, Di Giacinto C, Strober W, Fuss IJ. A transient breach in the epithelial barrier leads to regulatory T-cell generation and resistance to experimental colitis. Gastroenterology. 2008;135:1612–1623. e1615. doi: 10.1053/j.gastro.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Borsutzky S, Cazac BB, Roes J, Guzman CA. TGF-beta receptor signaling is critical for mucosal IgA responses. J Immunol. 2004;173:3305–3309. doi: 10.4049/jimmunol.173.5.3305. [DOI] [PubMed] [Google Scholar]
- Boullier S, Tanguy M, Kadaoui KA, Caubet C, Sansonetti P, Corthesy B, Phalipon A. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J Immunol. 2009;183:5879–5885. doi: 10.4049/jimmunol.0901838. [DOI] [PubMed] [Google Scholar]
- Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat Immunol. 2002;3:229–236. doi: 10.1038/ni769. [DOI] [PubMed] [Google Scholar]
- Cazac BB, Roes J. TGF-beta receptor controls B cell responsiveness and induction of IgA in vivo. Immunity. 2000;13:443–451. doi: 10.1016/s1074-7613(00)00044-3. [DOI] [PubMed] [Google Scholar]
- Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Shively JE. The cell-cell adhesion molecule carcinoembryonic antigen-related cellular adhesion molecule 1 inhibits IL-2 production and proliferation in human T cells by association with Src homology protein-1 and down-regulates IL-2 receptor. J Immunol. 2004a;172:3544–3552. doi: 10.4049/jimmunol.172.6.3544. [DOI] [PubMed] [Google Scholar]
- Chen D, Iijima H, Nagaishi T, Nakajima A, Russell S, Raychowdhury R, Morales V, Rudd CE, Utku N, Blumberg RS. Carcinoembryonic antigen-related cellular adhesion molecule 1 isoforms alternatively inhibit and costimulate human T cell function. J Immunol. 2004b;172:3535–3543. doi: 10.4049/jimmunol.172.6.3535. [DOI] [PubMed] [Google Scholar]
- Chen T, Zimmermann W, Parker J, Chen I, Maeda A, Bolland S. Biliary glycoprotein (BGPa, CD66a, CEACAM1) mediates inhibitory signals. J Leukoc Biol. 2001;70:335–340. [PubMed] [Google Scholar]
- Chen Z, Chen L, Qiao SW, Nagaishi T, Blumberg RS. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits proximal TCR signaling by targeting ZAP-70. J Immunol. 2008;180:6085–6093. doi: 10.4049/jimmunol.180.9.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y, Wang L, Konrad A, Schoeb T, Elson CO. Curcumin induces the tolerogenic dendritic cell that promotes differentiation of intestine-protective regulatory T cells. Eur J Immunol. 2009;39:3134–3146. doi: 10.1002/eji.200939052. [DOI] [PubMed] [Google Scholar]
- Coutelier JP, Godfraind C, Dveksler GS, Wysocka M, Cardellichio CB, Noel H, Holmes KV. B lymphocyte and macrophage expression of carcinoembryonic antigen-related adhesion molecules that serve as receptors for murine coronavirus. Eur J Immunol. 1994;24:1383–1390. doi: 10.1002/eji.1830240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dery KJ, Gaur S, Gencheva M, Yen Y, Shively JE, Gaur RK. Mechanistic control of carcinoembryonic antigen-related cell adhesion molecule-1 (CEACAM1) splice isoforms by the heterogeneous nuclear ribonuclear proteins hnRNP L, hnRNP A1, and hnRNP M. J Biol Chem. 2011;286:16039–16051. doi: 10.1074/jbc.M110.204057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donda A, Mori L, Shamshiev A, Carena I, Mottet C, Heim MH, Beglinger C, Grunert F, Rochlitz C, Terracciano L, et al. Locally inducible CD66a (CEACAM1) as an amplifier of the human intestinal T cell response. Eur J Immunol. 2000;30:2593–2603. doi: 10.1002/1521-4141(200009)30:9<2593::AID-IMMU2593>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Dveksler GS, Pensiero MN, Dieffenbach CW, Cardellichio CB, Basile AA, Elia PE, Holmes KV. Mouse hepatitis virus strain A59 and blocking antireceptor monoclonal antibody bind to the N-terminal domain of cellular receptor. Proc Natl Acad Sci USA. 1993;90:1716–1720. doi: 10.1073/pnas.90.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund M, Blikstad I, Obrink B. Calmodulin binds to specific sequences in the cytoplasmic domain of C-CAM and down-regulates C-CAM self-association. J Biol Chem. 1996;271:1393–1399. doi: 10.1074/jbc.271.3.1393. [DOI] [PubMed] [Google Scholar]
- Fagarasan S. Evolution, development, mechanism and function of IgA in the gut. Curr Opin Immunol. 2008;20:170–177. doi: 10.1016/j.coi.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- Fantini MC, Dominitzki S, Rizzo A, Neurath MF, Becker C. In vitro generation of CD4+ CD25+ regulatory cells from murine naive T cells. Nat Protoc. 2007;2:1789–1794. doi: 10.1038/nprot.2007.258. [DOI] [PubMed] [Google Scholar]
- Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009a;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gencheva M, Chen CJ, Nguyen T, Shively JE. Regulation of CEACAM1 transcription in human breast epithelial cells. BMC Mol Biol. 2010;11:79. doi: 10.1186/1471-2199-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons DL, Spencer J. Mouse and human intestinal immunity: same ballpark, different players; different rules, same score. Mucosal Immunol. 2011;4:148–157. doi: 10.1038/mi.2010.85. [DOI] [PubMed] [Google Scholar]
- Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ. PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol. 2010;11:535–542. doi: 10.1038/ni.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6:433–446. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328:1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume T, Togawa A, Nochi T, Igarashi O, Kweon MN, Kiyono H, Yamamoto M. Peyer’s patches are required for intestinal immunoglobulin A responses to Salmonella spp. Infect Immun. 2008;76:927–934. doi: 10.1128/IAI.01145-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann-Kleiter N, Baier G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood. 2010;115:2989–2997. doi: 10.1182/blood-2009-10-233585. [DOI] [PubMed] [Google Scholar]
- Huber M, Izzi L, Grondin P, Houde C, Kunath T, Veillette A, Beauchemin N. The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J Biol Chem. 1999;274:335–344. doi: 10.1074/jbc.274.1.335. [DOI] [PubMed] [Google Scholar]
- Hurst SD, Cooper CJ, Sitterding SM, Choi J, Jump RL, Levine AD, Barrett TA. The differentiated state of intestinal lamina propria CD4+ T cells results in altered cytokine production, activation threshold, and costimulatory requirements. J Immunol. 1999;163:5937–5945. [PubMed] [Google Scholar]
- Iijima H, Neurath MF, Nagaishi T, Glickman JN, Nieuwenhuis EE, Nakajima A, Chen D, Fuss IJ, Utku N, Lewicki DN, et al. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J Exp Med. 2004;199:471–482. doi: 10.1084/jem.20030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer R, Hahn S, Singer BB, Luo JS, von Kleist S. Biliary glycoprotein (CD66a), a cell adhesion molecule of the immunoglobulin superfamily, on human lymphocytes: structure, expression and involvement in T cell activation. Eur J Immunol. 1998;28:3664–3674. doi: 10.1002/(SICI)1521-4141(199811)28:11<3664::AID-IMMU3664>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshner J, Schumann D, Shively JE. CEACAM1, a cell-cell adhesion molecule, directly associates with annexin II in a three-dimensional model of mammary morphogenesis. J Biol Chem. 2003;278:50338–50345. doi: 10.1074/jbc.M309115200. [DOI] [PubMed] [Google Scholar]
- Kuespert K, Pils S, Hauck CR. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol. 2006;18:565–571. doi: 10.1016/j.ceb.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung N, Turbide C, Olson M, Marcus V, Jothy S, Beauchemin N. Deletion of the carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) gene contributes to colon tumor progression in a murine model of carcinogenesis. Oncogene. 2006;25:5527–5536. doi: 10.1038/sj.onc.1209541. [DOI] [PubMed] [Google Scholar]
- Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo EO, Zhang Z, Shively JE. Pivotal advance: CEACAM1 is a negative coreceptor for the B cell receptor and promotes CD19-mediated adhesion of B cells in a PI3K-dependent manner. J Leukoc Biol. 2009;86:205–218. doi: 10.1189/jlb.0109037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Hunziker L, McCoy K, Lamarre A. IgA responses in the intestinal mucosa against pathogenic and non-pathogenic microorganisms. Microbes Infect. 2001;3:1021–1035. doi: 10.1016/s1286-4579(01)01460-5. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Slack E. The functional interactions of commensal bacteria with intestinal secretory IgA. Curr Opin Gastroenterol. 2007;23:673–678. doi: 10.1097/MOG.0b013e3282f0d012. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- Manohar M, Baumann DO, Bos NA, Cebra JJ. Gut colonization of mice with actA-negative mutant of Listeria monocytogenes can stimulate a humoral mucosal immune response. Infect Immun. 2001;69:3542–3549. doi: 10.1128/IAI.69.6.3542-3549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massacand JC, Kaiser P, Ernst B, Tardivel A, Burki K, Schneider P, Harris NL. Intestinal bacteria condition dendritic cells to promote IgA production. PLoS One. 2008;3:e2588. doi: 10.1371/journal.pone.0002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales VM, Christ A, Watt SM, Kim HS, Johnson KW, Utku N, Texieira AM, Mizoguchi A, Mizoguchi E, Russell GJ, et al. Regulation of human intestinal intraepithelial lymphocyte cytolytic function by biliary glycoprotein (CD66a) J Immunol. 1999;163:1363–1370. [PubMed] [Google Scholar]
- Nagaishi T, Pao L, Lin SH, Iijima H, Kaser A, Qiao SW, Chen Z, Glickman J, Najjar SM, Nakajima A, et al. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity. 2006;25:769–781. doi: 10.1016/j.immuni.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Iijima H, Neurath MF, Nagaishi T, Nieuwenhuis EE, Raychowdhury R, Glickman J, Blau DM, Russell S, Holmes KV, et al. Activation-induced expression of carcinoembryonic antigen-cell adhesion molecule 1 regulates mouse T lymphocyte function. J Immunol. 2002;168:1028–1035. doi: 10.4049/jimmunol.168.3.1028. [DOI] [PubMed] [Google Scholar]
- Niemir ZI, Stein H, Noronha IL, Kruger C, Andrassy K, Ritz E, Waldherr R. PDGF and TGF-[beta] contribute to the natural course of human IgA glomerulonephritis. Kidney Int. 1995;48:1530–1541. doi: 10.1038/ki.1995.443. [DOI] [PubMed] [Google Scholar]
- Ochi H, Abraham M, Ishikawa H, Frenkel D, Yang K, Basso AS, Wu H, Chen ML, Gandhi R, Miller A, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25- LAP+ T cells. Nat Med. 2006;12:627–635. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 2008;181:4832–4839. doi: 10.4049/jimmunol.181.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oida T, Zhang X, Goto M, Hachimura S, Totsuka M, Kaminogawa S, Weiner HL. CD4+CD25- T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J Immunol. 2003;170:2516–2522. doi: 10.4049/jimmunol.170.5.2516. [DOI] [PubMed] [Google Scholar]
- Pan H, Shively JE. Carcinoembryonic antigen-related cell adhesion molecule-1 regulates granulopoiesis by inhibition of granulocyte colony-stimulating factor receptor. Immunity. 2010;33:620–631. doi: 10.1016/j.immuni.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann D, Chen CJ, Kaplan B, Shively JE. Carcinoembryonic antigen cell adhesion molecule 1 directly associates with cytoskeleton proteins actin and tropomyosin. J Biol Chem. 2001;276:47421–47433. doi: 10.1074/jbc.M109110200. [DOI] [PubMed] [Google Scholar]
- Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- Shannon CE. The mathematical theory of communication 1963. MD Comput. 1997;14:306–317. [PubMed] [Google Scholar]
- Singer BB, Scheffrahn I, Heymann R, Sigmundsson K, Kammerer R, Obrink B. Carcinoembryonic antigen-related cell adhesion molecule 1 expression and signaling in human, mouse, and rat leukocytes: evidence for replacement of the short cytoplasmic domain isoform by glycosylphosphatidylinositol-linked proteins in human leukocytes. J Immunol. 2002;168:5139–5146. doi: 10.4049/jimmunol.168.10.5139. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, Fagarasan S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci USA. 2004;101:1981–1986. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JM, Ziman ME, Fong J, Solnick JV, Vajdy M. Possible correlates of long-term protection against Helicobacter pylori following systemic or combinations of mucosal and systemic immunizations. Infect Immun. 2007;75:3462–3469. doi: 10.1128/IAI.01470-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waaij LA, Limburg PC, Mesander G, van der Waaij D. In vivo IgA coating of anaerobic bacteria in human faeces. Gut. 1996;38:348–354. doi: 10.1136/gut.38.3.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijburg OL, Uren TK, Simpfendorfer K, Johansen FE, Brandtzaeg P, Strugnell RA. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J Exp Med. 2006;203:21–26. doi: 10.1084/jem.20052093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert T, Pasche B, Rochon M, Deppenmeier S, van den Heuvel J, Gruber AD, Heinz DW, Lengeling A, Schubert WD. Extending the host range of Listeria monocytogenes by rational protein design. Cell. 2007;129:891–902. doi: 10.1016/j.cell.2007.03.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.