Abstract
Age, sex, and gonadal hormones have profound effects on ischemic stroke outcomes, although how these factors impact basic stroke pathophysiology remains unclear. There is a plethora of inconsistent data reported throughout the literature, primarily due to differences in the species examined, the timing and methods used to evaluate injury, the models used, and confusion regarding differences in stroke incidence as seen in clinical populations versus effects on acute neuroprotection or neurorepair in experimental stroke models. Sex and gonadal hormone exposure have considerable independent impact on stroke outcome, but these factors also interact with each other, and the contribution of each differs throughout the lifespan. The contribution of sex and hormones to experimental stroke will be the focus of this review. Recent advances and our current understanding of age, sex, and hormone interactions in ischemic stroke with a focus on inflammation will be discussed.
1. Introduction
Stroke is a major cause of mortality and the leading cause of long-term disability in the USA. Ischemic stroke accounts for 87% of all strokes [1, 2]. To date only one FDA approved therapy is available for patients with acute ischemic stroke, the thrombolytic tissue plasminogen activator (tPA) [3]. Unfortunately, despite our best efforts, only a small number of patients are eligible for tPA therapy. Thrombolytic therapy, although extremely efficacious, has a very short time window of treatment, ranging to 3 hours in the USA to 4.5 hours in Europe [4] after which decreasing efficacy and increased risk of hemorrhagic complications occur. Basic scientists have identified numerous potential “neuroprotective” agents that reduce stroke injury in experimental models, but attempts to bring these therapies into the clinic has met with limited success. Numerous promising agents have failed to show protective effects in clinical trials [5]. This has led to the questions 1) are we utilizing the most appropriate animal models in our preclinical studies? and 2) are we designing our clinical trials with guidance from emerging experimental data? These concepts must be considered if we hope to develop efficacious neuroprotective candidates.
Young, male animals are the invariable favorite for use by most stroke researchers in experimental studies. However, clinical stroke is a disease that mainly affects the elderly [1, 2]. In fact, age is the most important independent risk factor for stroke [6] with stroke rates doubling every decade after the age of 55 [1]. The aged brain undergoes numerous neurochemical and physiological changes over the lifespan that change the responsiveness to a variety of therapies compared with young brains [7]. Acetylcholinesterase (AChE) and Na+K+ATPase activity in synaptosomes decreased with age resulting in neuronal vulnerability changes to excitotoxic insults [8]. Simvastatin, a hypolipidemic drug for the treatment of dyslipidemia, fully restored short- and long-term memory in adult, but not in aged mice of Alzheimer’s disease model [9]. Sex differences are also seen in the epidemiology of ischemic stroke [10]. In neonates, male sex is a risk factor for poor outcome, whereas increased incidence and morbidity is seen in elderly females. Childhood ischemic stroke appears to be more common in boys regardless of age, stroke subtype, or history of trauma [11, 12]. Elderly women not only have higher stroke incidence than age-matched men, but also have poorer recovery, higher morbidity and mortality once a stroke occurs [13-17]. These clinical data remind us that age and sex are important impact factors which should be fully taken into account in experimental stroke studies. This review discusses age- and sex-related differences in stroke phenotypes and the possible underlying mechanisms, aiming to shed light on the discrepancies between experimental and clinical data of stroke studies.
2. Impact of age and sex on normal brains
2.1 Aging effects on the brain
Both global and regionally specific changes in brain tissue volume occur with aging [18] (Table 1). Studies with magnetic resonance imaging (MRI) revealed that in the elderly white matter volume loss predominates over that seen in the gray matter [19-21]. The frontal lobes show the greatest decline in the volume with age (approximately 12%), followed by the temporal lobe (9%) and the occipital and parietal lobes which only showed modest change [22]. Apart from the age related changes in the brain volume, neurochemical and physiological changes also occur with aging [7] (Table 1). Cerebral blood-flow (CBF) is regionally reduced with aging in the fronto- and temporocortical area and in the subcortical region, and age negatively correlates with perfusion in both the left and right fronto-cortical regions [23]. Recent studies showed that in aging tissues from female and male animals, markers of oxidative stress increase due to decreased activity of antioxidant enzymes. In addition, proteolysis increases due to decreased activity of aminotransferase [24, 25]. Significant alterations in neurotransmitters and enzyme activity are seen with advancing age in the non-demented elderly. These include well documented changes in levels of choline acetyltransferase, and an increase in vasoactive intestinal peptide immunoreactivity [26]. Age-related changes are also seen by the relatively robust decrease in brain nutritional factors, including carbohydrates, proteins, and fat [27], and by the loss of key enzymes of the respiratory chain involving cytochrome oxidase and succinic dehydrogenase [28].
Table 1.
Impact of aging on brain anatomy and physiology.
| Parameters | Changes with aging | Species | Refs | |
|---|---|---|---|---|
| Tissue Volume | Amount of CSF | Increase | Human | [189, 190] |
| Overall brain volumes | Decrease | Human | [189, 190] | |
| White matter | Decrease | Human | [19-21] | |
| Gray matter | Small decrease | Human | [19-21] | |
| Frontal lobe | Decrease | Human | [22] | |
| Temporal lobe | Decrease | Human | [22] | |
| Occipital and parietal lobe | Decrease | Human | [22] | |
| Hippocampus | Decrease | Human | [191] | |
|
| ||||
|
Physiology and
Neurochemistry |
Oxidative stress | Increase | Rat | [24, 25] |
| Glutathione peroxidase and NADPH generation |
Increase | Rat | [192] | |
| Glucose-6-phosphate dehydrogenase activity |
Increase | Rat | [193] | |
| Malic enzyme | Increase | Rat | [193] | |
| Vasoactive intestinal peptide immunoreactivity |
Increase | Human | [26] | |
| CBF | Decrease | Dog | [23] | |
| ATP citrate-lyase | Decrease | Rat | [193] | |
| Acetyl Co-A carboxylase | Decrease | Rat | [193] | |
| Fatty acid synthetase | Decrease | Rat | [193] | |
| Carbohydrates | Decrease | Human | [27] | |
| Tryptophan and tyrosine | Decrease | Human | [27] | |
| Unsaturated fatty acids | Decrease | Human | [27] | |
| GABA | Decrease | Rat | [194] | |
2.2 Sex differences in the brain
Numerous sex differences in human brain structure have been described demonstrating that the brain is a sexually dimorphic organ (Table 2). In vivo imaging and postmortem studies report that the cerebrum is larger in men than women by 8-10% [29-33], a finding that is not wholly attributed to body size. Regionally specific sex differences relative to size of cerebrum have also been reported. Goldstein et al [34] reported that sexual dimorphisms of adult brain volumes were more evident in the cortex, with women having larger volumes, relative to cerebrum size, particularly in frontal and medial paralimbic cortices. Men had larger volumes, relative to cerebrum size, in the frontomedial cortex, the amygdala and hypothalamus. Other studies showed that relative to cerebrum size, women have larger volumes in cortical gray matter [35], in regions associated with language functions, e.g. Broca’s area [36], in the hippocampus, caudate, and thalamic nuclei [29, 37, 38] than men. In contrast, men have been found to have larger volumes, relative to cerebrum size, in hypothalamus [39-41], paracingulate gyrus [42], and greater cerebrospinal fluid [35, 43, 44].
Table 2.
Sex differences in brain anatomy and physiology.
| Parameters | Women(F) vs.Men(M) |
Species | Refs | |
|---|---|---|---|---|
| Tissue Volume | Gray matter | F>M | Human | [35] |
| Broca’s area,hippocampus,caudate and thalamic nuclei |
F>M | [36] | ||
| Frontal and medial paralimbic cortices | F>M | Human | [34] | |
| Frontomedial cortex,amygdala and hypothalamus |
F<M | Human | [34] | |
| Amount of CSF | F<M | Human | [35, 43, 44] | |
| Cerebrum size | F<M | Human | [29-33] | |
| White matter | F<M | Human | [35] | |
| Hypothalamus,paracingulate gyrus | F<M | Human | [39-42] | |
| Number of neurons | Inconsistency | Human | [32, 36, 195, 196] |
|
|
| ||||
|
Physiology and
Neurochemistry |
Global CBF | F>M | Human | [54, 55] |
| 5-HT transporter availability | F>M | Human | [45] | |
| Dopamine release | F>M | Human | [46] | |
| Cortical GABA level | F>M | Human | [49] | |
| Cortical muscarinic acetylcholine receptors |
F>M | Human | [48] | |
| Cortical mu-opioid binding | F>M | Human | [51] | |
| N-acetyl aspartate | F>M | Human | [197] | |
| Receptor affinity of glucocorticoids | F<M | Human | [198] | |
| Glutathione/reduced glutathione ratio |
F<M | SHR rat | [199] | |
| Cerebral metabolic rate of glucose utilization |
Inconsistent | Human | [60, 200-203] | |
Normal sexual dimorphism exists not only in the brain structure, but also in brain function and neurochemistry (Table 2). Compared with men, healthy women have higher 5-HT transporter availability in the striatum, diencephalon and brainstem [45]. Greater dopamine release in the right globus pallidus and inferior frontal gyrus was seen in females than males [46]. Sex differences were even reported in some receptor systems, such as cholinergic system [47, 48], GABAergic system [49, 50], and opioid system [51, 52]. Sex steroids exert significant impacts on neurotransmission and cerebral blood flow (CBF). For example, women received combined estrodiol and progestorone administration had higher 5-HT(2A)R binding potential throughout the cerebral cortex relative to baseline [45]. Estrogens inhibit the dopaminergic supersensitivity induced by neuroleptics [53]. In premenopausal women, the menstrual cycle has a significant impact on physiological and neurochemical parameters in the brain. Accumulating data showed that women have higher global cerebral blood flow (CBF) compared with men during rest [54, 55] and cognitive activity [55-59]; consistent with this, cerebral metabolic rate of glucose utilization tends to be higher in women versus men [60]. Corresponding to high plasmic concentration of 17β-estrodial (E2), increased CBF was found in women between days 10 and 15 of the cycle followed by an established elevation in the luteal phase [61, 62]. The increased CBF is caused mainly by a decrease in vascular resistance in the brain, presumably due to the direct dilating effect of estrogen on cerebral vessels [63, 64]. Fluctuating hormone levels during the menstrual cycle also have acute effects on brain glucose metabolism [65] and D2 dopamine receptor density[66]. These studies highlighted the importance that studies on sex differences in the brain should consider, or if necessary, control the effects of sex steroids.
3. Effect of Age on Ischemic Stroke
3.1 Clinical data
Aging is not only the single most important independent risk factor for the incidence and the prevalence of stroke [67], but also a significant predictor of outcome independent of stroke severity, etiology, efficacy of thrombolysis, sex, other vascular risk factors, and stroke complications [68, 69]. For each successive 10 years after the age of 55, stroke rates more than double in both men and women [1]. Neurologists also see constant reminders of the fact that older patients do not do as well after a stroke compared to younger counterparts indicating that stroke in older patients has different characteristics than that seen in the young. A clinical investigation revealed that in the old, stroke outcome data such as 30-day case fatality rate and disability are far poorer than for young patients, and hospital management is often less active in this age group [70]. The increased mortality seen in older individuals may not be related to the stroke per se, but rather an effect of aging and advanced co-morbid diseases. Results from mixed-sex clinical studies showed stroke severity and location did not differ between young and old stroke patients, but the latter seem more vulnerable to infectious complications, such as pneumonia and urinary tract infection [71]. This mirrors what we have shown in our studies [72, 73]: aging female mice had larger infarct and males had paradoxically smaller strokes, but both of them had higher mortality than young mice.
3.2 Experimental data
Only a few experimental studies exist in the literature that have examined aging animals and have led to somewhat inconsistent results. The studies during the recent two decades have been summarized in Table 3. Intriguingly, experiments performed on animals of different sexes yielded different results. Studies using females consistently showed aging females have worsened stroke outcomes than their young counterparts regardless of strains and model types employed [72, 74-76], except those in which ovariectomized(Ovx) females [77, 78] were used, that exhibited no difference in outcomes most likely due to effects of acute ovary removal. However, studies using male animals have yielded very inconsistent data, with 5 studies in which aging males have larger strokes [79-83], 6 studies that demonstrated smaller infarcts in aging males [72, 73, 84-87], and 3 studies that found equivalent infarct size between young and aging animals [88-90]. Male animals gain more body weight than females over the life span [72, 91, 92], which may complicate surgical procedures used in experimental studies and thus leading to larger variability in histological stroke outcomes in different labs using different stroke models. Further studies on stroke in aging animal are needed to determine the effect of aging on stroke, including detailed assessment of both histological and behavioral phenotypes. In spite of these disagreements on the histological effects of aging on infarct volume, invariably significantly higher mortality rates and more severe neurological impairments were found in the older animals, which is consistent with clinical data [70, 71] and data from our own lab which showed both aging male and female mice have exacerbated neurological deficits compared to young animals independent of infarct volumes [72].
Table 3.
Effect of aging on experimental ischemia.
| Age |
Aging effect on infarct volume |
|||||
|---|---|---|---|---|---|---|
| Strain/Species | Sex | Young | Aging | Model | Refs | |
| F344 rats | M | 2-3 m | 26-28 m | pMCAO | 24h =; 7d ↑ | [79] |
| Wistar rats | M | 4 m | 20,27m | 100 min tMCAO | 7d ↑ | [80] |
| SD rats | M | 3 m | 6,12,18 m | 20 min tMCAO | 2w ↑ | [81] |
| C57BL6 mice | M | 4 w,4 m | 11,18 m | 60 min tMCAO | 1,3d =; 7d ↑ | [82] |
| Wistar rats | M | 4 m | 22-24 m | pMCAO | 1 m ↓ | [84] |
| C57BL6 mice | M | 2 m | 9,15,24 m | 30 min tMCAO | 2h, 2d, 7d ↓ | [85] |
| C57BL6 mice | M | 4 m | 9,20 m | pMCAO | 24h ↓ | [86] |
| C57BL6 mice | M | 2-3 m | 15-16 m | 90 min tMCAO | 24h ↓ | [72] |
| C57BL6 mice | M | 3 m | 15-16 m | 90 min tMCAO | 24h ↓ | [73] |
| C57BL6 mice | M | 2-3 m | 16-18 m | 91 min tMCAO | 24h,30d ↓ | [87] |
| F344 rats | M | 3 m | 24 m | 60 min tMCAO | 3d = | [88] |
| SD rats | M | 3-4 m | 22-24 m | 60 min tMCAO | 24h,28d = | [89] |
| SD rats | M | 3-4 m | 22-24 m | 60 min tMCAO | 1w = | [90] |
| SD rats | F | 3m | 9,18 m | 2h tMCAO | 24h ↑ | [83] |
| SD rats | F | 3-4 m | 18-20 m | 2h tMCAO | 24h ↑ | [74] |
| SD rats | F | 3-4 m | 18-20 m | 2h tMCAO | 24h ↑ | [75] |
| SD rats | F | 3-4 m | 17-18 m | 2h tMCAO | 24h ↑ | [76] |
| C57BL6 mice | F | 2-3 m | 15-16 m | 90 min tMCAO | 24h ↑ | [72] |
| Wistar rats | Ovx F | 3 m | 15 m | 60 min tMCAO | 7w = | [77] |
| SD rats | Ovx F | 3-4 m | 9-12 m | pMCAO | 24h = | [78] |
M,male; F, female; Ovx, ovariectomy; min, minute; h, hour; d, day; w, week; m, month; p, permanent; t, transient; =, no change; ↑, increase; ↓, decrease.
In an attempt to understand the strikingly different stroke phenotypes seen in animals of different ages, our own laboratory recently explored several specific neurochemical markers closely related to ischemia and revealed interesting results. Phosphorylated adenosine monophosphate-activated protein kinase (pAMPK), an evolutionary conserved energy sensor sensitive to changes in cellular AMP/ATP ratio [93], exhibits a muted response to stroke in aged brain, perhaps due to the fact that aging independently increased baseline brain pAMPK levels [87]. Compared to young mice, aging (15-16 months) C57BL6 mice had decreased expression of the Na+-K+-Cl− co-transporter (NKCC) in the brain after stroke, and concurrently, stroke-induced edema formation is also significantly less robust, suggesting NKCC expression and edema formation are age dependent after ischemic stroke [73]. Another recent study reported that concentrations of brain-derived neurotrophic factor (BDNF) and bFGF were significantly lower in both the cortex and striatum of old mice compared with young and middle-aged mice after stroke [94]. Neuron-specific intermediate filaments, neurofilaments (NFs) that play critical roles in establishment of new synaptic contacts with other nerve cells, also exhibited significantly lower level of gene expression in the brain of aged rats compared with young animals after ischemia [95]. Although limited data are currently available about aging effect on neurochemical markers in the ischemic brain, all the studies suggested that aging down-regulates the activity of biochemical mediators that respond to ischemia. One notable exception is that the N- and C-terminal amyloid precursor protein beta was more rapidly increased in both the peri-infarct area and the infarct core in aging compared to that of young brains after stroke [96]. All these data seem to support the hypothesis that stroke outcomes are exacerbated with aging; however, the inconsistent histological outcomes imply that the molecular signaling pathways underlying ischemic infarction with aging are quite complex and need further investigation.
4. Effect of Hormones on Ischemic Stroke
4.1 Estrogen and stroke
Clinically women have lower age-adjusted stroke incidence than men [97]; and numerous experimental studies have also shown that young adult female animals are guarded from stroke relative to their counterpart males [98]. This “male-sensitive” phenomenon in stroke has been attributed to the protective effect of ovarian hormone exposure [99]. Observational clinical studies have demonstrated the potential protective effects of circulating ovarian hormones in coronary heart disease (CHD) [100], vascular disease [100-102] and stroke [103]. Experimental studies also showed young male animals have larger infarct size after induced strokes compared to young females, and E2 treatment at physiological relevant concentrations reduces infarction after stroke in ovariectomized female animals [72] as well as in males [104, 105], even when given after stroke [106]. All these studies indicate that estrogens have protective effect in stroke, at least in young adults.
Estrogen’s neuroprotective effects on ischemic stroke are regulated by multiple signaling pathways [107]. These include genomic effects mediated by estrogen-responsive elements (EAE) leading to enhanced transcription, in addition to non-genomic effects executed by rapid activation of second messenger cascades [99]. Several estrogen receptors (ER) such as ERα, ERβ, or ERx, play important roles in mediating these effects. E2 modulates the expression of a variety of genes in the ischemic brain, including those that influence the balance between cell death and cell survival such as the Bcl-2 family of genes [108] and caspases [109]. Through ERs, E2 can also exert anti-inflammatory actions by inhibiting nuclear factor (NF)-κB, a transcription factor that regulates expression of many pro-inflammatory molecules [110]. E2 is a vasodilator and promotes blood flow in the brain recovering from an acute insult [111], a non-genomic effect induced by ERα-mediated activation of tyrosone kinase-MAPK and Akt/protein kinase B signaling, leading to increases in endothelial nitric oxide synthase (eNOS) activity [99, 112]. In addition, at pharmacological concentrations (the micromolar range) E2 can act as directly as an anti-oxidant [113, 114]. E2 has also been reported to enhance both angiogenesis [115] and neurogenesis [116] following ischemic damage.
4.2 Hormone replacement therapy (HRT) in aging
Recent studies revealed that aging not only affects the brain response to ischemic insults, but also alters the neuroprotective effects of estrogen on the ischemic brain [117-119]. Although numerous experimental studies have demonstrated that E2 has neuroprotective effects on ischemic injury in young animals, two well-known clinical trials of HRT in aged women, Women’s Health Initiative (WHI) and Women Estrogen Stroke Trial (WEST), have concluded that E2 increases the incidence of stroke and can lead to enhanced damage and increased rates of fatal stroke [120, 121]. The trials have triggered an ongoing debate in the literature as to how the beneficial effects of E2 seen in pre-clinical studies could be translated into a feasible and effective clinical application. A hypothesis of the importance of the timing of initiation of replacement therapy has therefore been put forward that suggests that ERT should be initiated immediately after menopause in women to achieve its protective effect [122]. In both the WHI and WEST trials the participants were well beyond menopause when they received ERT which might explain why detrimental effects occurred. Utilizing young ovariectomized (Ovx) mice, ERT was given to female mice either immediately after Ovx or delayed for 10 weeks. It was found that only the immediate E2 treatment group exhibited beneficial effect of ERT which was secondary to a profound decrease in stroke-induced inflammation [119]. In a more recent study designed to mimic the clinical population that is a target for ERT [117], aged female mice (17months; an age post natural gonadal senescence in mice) were examined. ERT initiated at 17 months (chronic ERT) led to improved infarct damage when the animals were subjected to stroke 3 months later, at 20 months of age. However ERT given to mice of 20 months (acute ERT), for only two weeks prior to the onset of ischemia, was no longer protective and exacerbated tissue damage and markers of inflammation. Intriguingly an independent effect of sex was seen in this model as aged males benefited from both chronic and acute ERT, regardless of the timing and duration of administration. Of note, differentiated levels of NF-κB translocation after stroke were seen between chronic and acute ERT groups [117]. These studies suggest that timing effect of E2 therapy may be mediated by different inflammatory responses following ischemia. One recent study indicated that persistently elevated levels of ERβ2 may be the molecular basis for the diminished effectiveness of ERT in late post-menopausal women [118] and this remains to be investigated in pre-clinical models.
4.3 Androgens and stroke
Although male sex is an acknowledged risk factor for stroke, data from experimental studies of cerebral ischemia testing effects of androgens are surprisingly few and contradictory [123]. Hawk et al [124] found that administration of testosterone led to increased infarct size after MCAO in male rats. In young adult animals, castration significantly reduces ischaemic injury. Supplementation with testosterone or its non-aromatisable metabolite dihydrotestosterone (DHT) restored infarct volumes to levels seen in intact males [123]. However other studies reported that testosterone or DHT treatment reduces the number of pyknotic cells in the dentate gyrus after adrenalectomy [125]; similar protective effects of testosterone was also found in in vitro studies of oxidative stress, β-amyloid toxicity, and serum deprivation [126-129]. How androgens impact on sex differences in ischemic injury remains elusive.
Interestingly the effect of androgens on ischemia seems to mirror that seen with E2, and show a strong age dependent effect. Clinical studies have reported that high testosterone levels are associated with an increased risk of thromboembolic events in pediatric populations [130], but that low circulating testosterone levels are associated with higher stroke incidence and poorer functional outcomes in elderly men [131, 132]. However, despite the fact that low testosterone levels are associated with increased stroke incidence in older males [131-133], attempts to replace testosterone led to an increase in vascular risk in elderly men [134]. This mirrors what was seen in the WEST and WHI trials with ERT in aging women, suggesting gonadal hormones have important and distinct effects on the vasculature in aging populations. Further studies in the aging vasculature are needed if we hope to optimize any potential neuroprotective or vasculoprotective effects of hormone replacement therapy.
Sex differences in ischemic sensitivity over the life span
5.1 From neonates to young adults
Both clinical and experimental data indicate that stroke is sexually dimorphic throughout the life span. A male predominance in childhood ischemic stroke has been seen in multiple centers worldwide [11, 135-138]. One recent study revealed that boys comprise a significantly higher proportion (57-63%) of both arterial ischemic stroke (AIS) and cerebral sinovenous thrombosis [11]; another center reported similar findings, a higher number of males among children with AIS (63.8 in boys vs. 36.2% in girls), with the exception of transient ischemic attack (42.9 in boys vs. 57.1% in girls) [138]. This male “ischemia-sensitive” phenotype persists after stratification by ischemic stroke subtype or by other etiologies [11]. Risk-taking behaviors may partly explain a gender disparity in childhood stroke; however, the increased prevalence seen in males persisted when cases of ischemia due to trauma were excluded [11, 139, 140]. The male predominance in childhood ischemic stroke continues throughout the neonatal period into adolescence [11, 137], and then switches to a female predominance until the age of 30 [137, 141, 142]. This phenomenon is associated with the wide use of oral contraceptives, frequency of migraine, and reproductive activity in young females of this age group and may also occur in conjunction with complex, poorly understood, genetic and environmental interactions [143]. After the age of 30, male predominance again occurs [141, 142], which may be secondary to the protective effect of estrogens in women [99]. These clinical reports have been recapitulated in experimental studies, as PND 3-11 male mice show more brain volume loss than age matched females after experimentally induced hypoxic ischemic encephalopathy (HIE) [144]. Importantly for translation, these sex differences extend to the response to therapies designed to protect the injured brain. A study performed in a model of HIE in P7 rats showed that hypothermia protects females, but not males, from histological damage and sensorimotor deficits [145].
5.2 Aging populations
Evidence of sexual dimorphism in aging populations also exists. As women age, stroke rates increase and surpass that of men, coincident with diminished circulating levels of E2 and progesterone [97, 98]. Despite the higher overall lifetime incidence of stroke in men, aged women have more severe strokes, poorer recovery and greater long-term disability [14-17] compared to age-matched men. There is even a persistent sex disparity in midlife stroke prevalence in the United States [146]. According to the National Health and Nutrition Examination Surveys (NHANES) 1999-2004, women aged 45 to 54 years were more than twice as likely to have had a stroke than men [147]. The trend of female predominance in stroke in midlife extended as the NHANES 2005-2006 reported women aged 35-64 years had three times the odds of prior stroke compared with men, and the significant difference was also in group of 45-54 years [146]. The exact reasons behind this persistent sex difference are difficult to decipher precisely; however, obesity, the hormonal and inflammatory milieu in midlife and middle aged women may conceivably play important roles [146]. So far very few experimental studies have been performed to mimic this clinical phenomenon. The only available data is from our own lab, which showed either aged (20 months) or middle aged (15-16 months) female mice had larger infarcts than their male counterparts after induced ischemia, and this was related to more robust inflammatory responses seen in females [72, 117]. More research is required to understand sex differences in the pathophysiological mechanisms of stroke in aging populations.
5. Effects of age, sex, and hormones on inflammatory responses following stroke
6.1 Effect of age and sex on inflammatory responses
Inflammatory processes have a fundamental role in the pathophysiology of ischemic injury. Brain ischemia is a powerful stimulus that triggers a series of events that lead to vasodilatation, increased permeability of local blood vessels, and mobilization and infiltration of circulating leukocytes, a process modulated by many inflammatory cell adhesion molecules and cytokines [148-151]. Inflammatory responses following ischemia have different characteristics depending on age and sex, and are also regulated by hormone levels. Recent studies revealed that ischemia induced inflammatory responses are compromised in the aged brain, probably due to the immunosenescence that occurs with aging [152]. For example, the response of pro-inflammatory cytokines (TNF and IL-1β) and the level of chemokines (Mip-1α and MCP-1) were strongly diminished in the aged post-ischemic brain tissue, and IL-6 showed the strongest age-dependent decrease in its post-ischemic expression profile [85]. Neutrophils isolated from elderly individuals exhibit attenuated chemotaxis, oxidant release, and phagocytosis, and it has been suggested that these deficiencies are related to an age-associated increase in glucocorticoid production and oxidative stress [153]. Aging also has a detrimental effect on progenitor populations, for example oligodendrocyte progenitor cells (OPCs) showed more proliferative activity and process branching in young brains compared to aged after induced ischemia [154]. Interestingly evidence of immunosenescence has been primarily obtained from males [155, 156]; aged females appear to exhibit a more robust inflammatory response than young females. One recent study [157] reported that astrocyte-conditioned media from middle-aged female astrocytes induced greater migration of peripheral blood monocyte cells and neural progenitor cells, and expressed higher levels of the chemoattractant macrophage inflammatory protein-1 (MIP-1) compared with young female astrocytes; however, no age-related impairment was observed in astrocyte function in males. An enhanced immunological response in aged females is also seen in other organ systems. The pulmonary inflammatory response is greater in aged female mice (18-20 months) which showed a six-fold higher neutrophil infiltration and three-fold higher level of myeloperoxidase activity in the lung compared to young females upon lipopolysaccharide (LPS) exposure [158].
Biological sex has a distinct impact on many inflammatory pathways. One example is the pro-inflammatory signaling pathway mediated by NF-κB. NF-κB is a transcription factor that regulates the expression of multiple inflammatory molecules, such as IL-6, tumor necrosis factor-α (TNF-α), mitogen-activated protein kinase 1 (MAPK-1), etc. [159, 160] all of which are activated following ischemic insults. Female fibroblasts cultured without E2 exhibited higher level of NF-κB than male cells after hypoxia [161]. P2 female rats had evidence of high levels of NF-κB in the anteroventral periventricular nucleus (AVPV), whereas NF-κB signaling was repressed in male neonates, forming the basis of a new model of sex differentiation of the AVPV that may apply to the development of other sexually dimorphic nuclei [162]. Aged female mice (20 months old) had significantly higher NF-κB expression than their counterpart males when subjected to 90 minute MCAO and corresponding elevations in serum levels of inflammatory markers regulated by NF-κB were also seen [72]. Intriguingly the up-regulated activity of NF-κB signaling correlated with exacerbated stroke injuries in aging females [72, 117], suggesting NF-κB is a key protein in mediating sex differences in ischemic injury. The sexual dimorphism in NF-κB pro-inflammatory signaling may be related to specific X-chromosome linked genes (see below).
6.2 Effects of hormones on inflammatory responses
The effects of hormones on the inflammatory responses are complicated and no conclusive studies currently available. In general, estrogens have anti-inflammatory effects in the brain [163]; however, this effect is modified by multiple other factors, such as 1) the immune stimulus; 2) the cell type involved during different phases of the disease; 3) timing and duration of hormone exposure; 4) the concentration of estrogens; 5) the variability in expression of ERs, etc. [164]. The protective, anti-inflammatory effect of E2 on cerebral blood vessels that is observed in young adults may be attenuated in aged animals, which exhibit a greater overall cerebrovascular response to inflammatory stimuli [165]. However, estrogen’s anti-inflammatory effect in aged animals seem to be demarcated by both biologic sex and timing of administration, as discussed earlier [117]. ERT suppressed plasma level of IL-6, TNF-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-4, and IL-5 in MCAO mice if given 10 weeks after Ovx, but not if administered immediately after Ovx [119]. Data regarding of progesterone or testosterone effects on inflammatory responses after stroke are virtually absent from the literature. However, several studies reported that either progesterone or testosterone augmented cerebrovascular inflammation in young adult rats induced by intraperitoneal LPS injection, opposite to 17β-estradiol’s suppressive effect [165, 166]. Nevertheless, one recent study indicates that testosterone may exert anti-inflammatory effects by reducing TNF-α expression in macrophages obtained from healthy older men and postmenopausal women [167]. Most likely the effects of progesterone or testosterone are also modified by age, sex, and timing of administration, etc.
6. X-chromosome contribution to ischemic sensitivity
As at both neonatal and post-menopausal ages, hormone levels are relatively equivalent between the sexes, sex differences in ischemic sensitivity seen at both ends of the age spectrum may be influenced by biologic sex (XX vs. XY) in addition to the hormonal milieu [168-171]. Males and females differ in sex chromosome compliment (XY vs. XX) and X chromosome imbalance is tolerated because of dosage compensation by X-chromosome inactivation (XCI) [172-175]. As a result of XCI, both males and females are functionally monosomic for most X-linked genes. A recent study from this lab confirmed X-chromosome dosage has no effect on the degree of cerebral infarction after experimental stroke in female mice [176]. However, non-random XCI [177] or genes escaping from XCI with aging may be associated with X-linked diseases [178, 179] and may lead to the imbalance of X-linked genes expression between sexes. Several X-chromosome linked genes have been recently recognized as contributors to disease. These genes regulate several molecular pathways, including that of NFκB [180-182]. Three important X-linked genes have been identified in the toll-like receptor (TLR) signaling family [182] and are intimately involved in the immune response via activation of NF-κB inflammatory signaling [182-184]. These include interleukin 1 receptor-associated kinase 1 (IRAK-1), NF-κB essential modulator (NEMO), and Bruton tyrosine kinase (BTK). However, no literature is available as to how these three X-linked proteins are involved in stroke. To add to this complexity, NF-κB may also have anti-apoptotic functions by inhibiting JNK and caspase activity, a process primarily mediated via up-regulation of another X-chromosome gene, X-linked inhibitor of apoptosis protein (XIAP) [185-187] (Fig. 1). It is presently unclear how the seemingly paradoxical NF-κB pro-inflammatory and anti-apoptotic signaling can affect ischemic insults; however, the intimate interaction between NF-κB and X-linked genes makes it possible that the balance could be perturbed or dysregulated due to the instability in XCI with aging [177, 188]. Emerging data have suggested that different cell death pathways predominate respectively in male and female neurons subjected to ischemic insult [10]; nevertheless, whether the ischemic sexual dimorphism is mediated by chromosomal complements remains undetermined.
Figure 1.
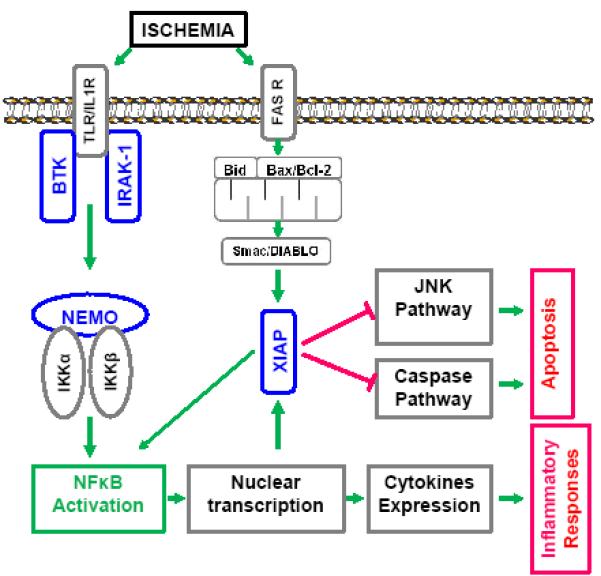
Diagram of proposed mechanisms underlying the dual roles of NF-κB signaling in ischemic stroke. Mediated by three X-chromosome linked genes (BTK, IRAK-1, and NEMO) upon ischemic insults, NF-κB translocates into the nucleus and regulates the expression of pro-inflammatory cytokines. NF-κB also interacts with XIAP to exert inhibitory effect on JNK and caspase mediated apoptosis.
In summary, multiple factors impact the pathophysiological changes that occur in ischemic stroke throughout the lifespan. Age, sex, and hormone exposure are three independent yet interacting parameters that mediate the response to ischemic stroke (Fig.2). Different mechanisms may underlie age and sex specific responses to cerebral ischemia. Due to the small amount of literature available, further investigation is needed to fully address the complicated relationship between age, sex, and hormones in the setting of stroke. Given that most experimental stroke studies are performed in young male animals, it seems an important priority for pre-clinical researchers to examine these interactions. Hopefully this will lead to identification of novel targets and to the development of therapies to treat this devastating disease.
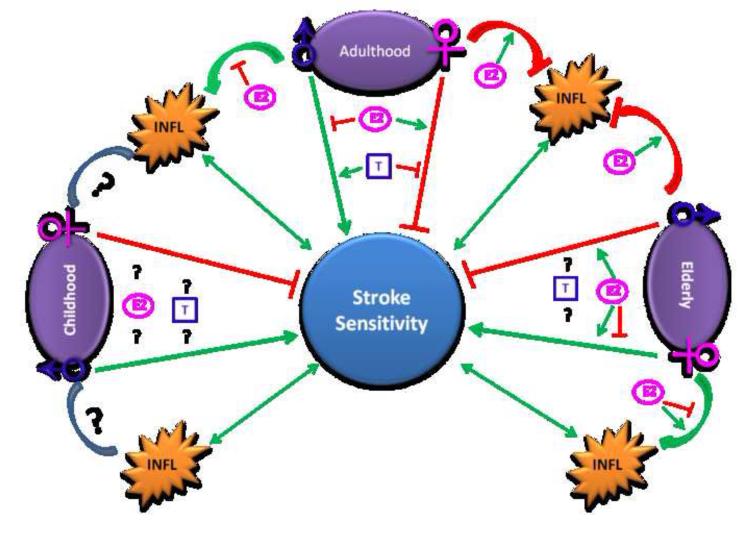
Figure 2.
Schematic summary of effects of age, sex, and hormones on stroke sensitivity. Green colors stand for stimulating effects; red colors stand for inhibitory effects. INFL: inflammation; E2: estrogen; T: testosterone.
Highlights.
Age, sex, and gonadal hormones impact stroke outcomes
Literature on the effects of age and sex on stroke outcome is inconsistent
Sex and gonadal hormone exposure have a major impact on stroke outcome
The contribution of sex and hormone varies throughout the lifespan
Age and hormones are major factors in the inflammatory response to stroke
Acknowledgements
This work was supported by the NIH/NINDS (grants NS050505 and NS055215 to LDM), and by AHA (grant 12SDG9030000 to FL)
Abbreviations
- AChE
Acetylcholinesterase
- AIF
apoptosis inducing factor
- AMP
adenosine monophosphate
- ATP
adenosine-5′-triphosphate
- AVPV
anteroventral periventricular nucleus
- BDNF
brain-derived neurotrophic factor
- bFGF
basic fibroblast growth factor
- BTK
Bruton tyrosine kinase
- CBF
cerebral blood flow
- CSF
cerebrospinal fluid
- CHD
coronary heart disease
- DAPI
4′,6-diamidino-2-phenylindole
- DHT
dihydrotestosterone
- eNOS
endothelial nitric oxide synthase
- E2
17β-estrodial
- ERT
estrogen replacement therapy
- ER
estrogen receptor
- ERE
estrogen-responsive element
- GABA
γ-Aminobutyric acid
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HIE
hypoxic-ischemic encephalopathy
- HRT
hormone replacement therapy
- 5-HT
5-hydroxytryptamine
- IL
interleukin
- IRAK-1
interleukin 1 receptor-associated kinase 1
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MIP-1
macrophage inflammatory protein-1
- MCP-1
monocyte chemotactic protein-1
- MCAO
middle cerebral artery occlusion
- MRI
magnetic resonance imaging
- MAPK-1
mitogen-activated protein kinase 1
- NADPH
nicotinamide adenine dinucleotide phosphate Neuronal Nucleus protein (NeuN)
- NHANES
National Health and Nutrition Examination Surveys
- NKCC
Na+-K+-Cl− co-transporter
- NFs
neurofilaments
- NF-κB
nuclear factor-κB
- NEMO
NF-κB essential modulator
- OPC
oligodendrocyte progenitor cell
- Ovx
ovariectomy
- pAMPK
phosphorylated adenosine monophosphate-activated protein kinase
- PARP
poly (ADP-ribose) polymerase
- TNF
tumor necrosis factor
- tPA
thrombolytic tissue plasminogen activator
- WHI
Women’s Health Initiative
- WEST
Women Estrogen Stroke Trial
- XCI
X-chromosome inactivation
- XIAP
X-linked inhibitor apoptosis protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rojas JI, Zurru MC, Romano M, Patrucco L, Cristiano E. Acute ischemic stroke and transient ischemic attack in the very old--risk factor profile and stroke subtype between patients older than 80 years and patients aged less than 80 years. Eur J Neurol. 2007;14(8):895–899. doi: 10.1111/j.1468-1331.2007.01841.x. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart Disease and Stroke Statistics--2012 Update: A Report From the American Heart Association. Circulation. 2011;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maestre-Moreno JF, Fernandez-Perez MD, Arnaiz-Urrutia C, Minguez A, Navarrete-Navarro P, Martinez-Bosch J. Thrombolysis in stroke: inappropriate consideration of the ‘window period’ as the time available. Rev Neurol. 2005;40(5):274–278. [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 5.Ginsberg MD. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology. 2008;55(3):363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117(4):e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 7.Anyanwu EC. Neurochemical changes in the aging process: implications in medication in the elderly. ScientificWorldJournal. 2007;7:1603–1610. doi: 10.1100/tsw.2007.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantha AK, Moorthy K, Cowsik SM, Baquer NZ. Membrane associated functions of neurokinin B (NKB) on Abeta (25-35) induced toxicity in aging rat brain synaptosomes. Biogerontology. 2006;7(1):19–33. doi: 10.1007/s10522-005-6044-z. [DOI] [PubMed] [Google Scholar]
- 9.Tong XK, Lecrux C, Hamel E. Age-dependent rescue by simvastatin of Alzheimer’s disease cerebrovascular and memory deficits. J Neurosci. 2012;32(14):4705–4715. doi: 10.1523/JNEUROSCI.0169-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turtzo LC, McCullough LD. Sex-specific responses to stroke. Future Neurol. 2010;5(1):47–59. doi: 10.2217/fnl.09.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golomb MR, Fullerton HJ, Nowak-Gottl U, Deveber G. Male predominance in childhood ischemic stroke: findings from the international pediatric stroke study. Stroke. 2009;40(1):52–57. doi: 10.1161/STROKEAHA.108.521203. [DOI] [PubMed] [Google Scholar]
- 12.Cheong JL, Cowan FM. Neonatal arterial ischaemic stroke: obstetric issues. Semin Fetal Neonatal Med. 2009;14(5):267–271. doi: 10.1016/j.siny.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Glader EL, Stegmayr B, Norrving B, Terent A, Hulter-Asberg K, Wester PO, Asplund K. Sex differences in management and outcome after stroke: a Swedish national perspective. Stroke. 2003;34(8):1970–1975. doi: 10.1161/01.STR.0000083534.81284.C5. [DOI] [PubMed] [Google Scholar]
- 14.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40(4):1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 15.Niewada M, Kobayashi A, Sandercock PA, Kaminski B, Czlonkowska A. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. 2005;24(3):123–128. doi: 10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda M, Kanda T, Kamide N, Akutsu T, Sakai F. Gender differences in long-term functional outcome after first-ever ischemic stroke. Intern Med. 2009;48(12):967–973. doi: 10.2169/internalmedicine.48.1757. [DOI] [PubMed] [Google Scholar]
- 17.Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003;34(7):1581–1585. doi: 10.1161/01.STR.0000078562.82918.F6. [DOI] [PubMed] [Google Scholar]
- 18.Killiany RJ, Meier DS, Guttmann CR. Image processing: global and regional changes with age. Top Magn Reson Imaging. 2004;15(6):349–353. doi: 10.1097/01.rmr.0000175131.63152.53. [DOI] [PubMed] [Google Scholar]
- 19.Double KL, Halliday GM, Kril JJ, Harasty JA, Cullen K, Brooks WS, Creasey H, Broe GA. Topography of brain atrophy during normal aging and Alzheimer’s disease. Neurobiol Aging. 1996;17(4):513–521. doi: 10.1016/0197-4580(96)00005-x. [DOI] [PubMed] [Google Scholar]
- 20.Guttmann CR, Jolesz FA, Kikinis R, Killiany RJ, Moss MB, Sandor T, Albert MS. White matter changes with normal aging. Neurology. 1998;50(4):972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- 21.Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Bryan RN, Zonderman AB. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10(5):464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- 22.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26(4):491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Peremans K, Audenaert K, Blanckaert P, Jacobs F, Coopman F, Verschooten F, Van Bree H, Van Heeringen C, Mertens J, Slegers G, et al. Effects of aging on brain perfusion and serotonin-2A receptor binding in the normal canine brain measured with single photon emission tomography. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(7-8):1393–1404. doi: 10.1016/s0278-5846(02)00306-8. [DOI] [PubMed] [Google Scholar]
- 24.Sinha N, Baquer NZ, Sharma D. Anti-lipidperoxidative role of exogenous dehydroepiendrosterone (DHEA) administration in normal ageing rat brain. Indian J Exp Biol. 2005;43(5):420–424. [PubMed] [Google Scholar]
- 25.Bala K, Tripathy BC, Sharma D. Neuroprotective and anti-ageing effects of curcumin in aged rat brain regions. Biogerontology. 2006;7(2):81–89. doi: 10.1007/s10522-006-6495-x. [DOI] [PubMed] [Google Scholar]
- 26.Perry EK, Blessed G, Tomlinson BE, Perry RH, Crow TJ, Cross AJ, Dockray GJ, Dimaline R, Arregui A. Neurochemical activities in human temporal lobe related to aging and Alzheimer-type changes. Neurobiol Aging. 1981;2(4):251–256. doi: 10.1016/0197-4580(81)90032-4. [DOI] [PubMed] [Google Scholar]
- 27.Solfrizzi V, Colacicco AM, D’Introno A, Capurso C, Parigi AD, Capurso SA, Torres F, Capurso A, Panza F. Macronutrients, aluminium from drinking water and foods, and other metals in cognitive decline and dementia. J Alzheimers Dis. 2006;10(2-3):303–330. doi: 10.3233/jad-2006-102-314. [DOI] [PubMed] [Google Scholar]
- 28.Bertoni-Freddari C, Mocchegiani E, Malavolta M, Casoli T, Di Stefano G, Fattoretti P. Synaptic and mitochondrial physiopathologic changes in the aging nervous system and the role of zinc ion homeostasis. Mech Ageing Dev. 2006;127(6):590–596. doi: 10.1016/j.mad.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 29.Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr. The young adult human brain: an MRI-based morphometric analysis. Cereb Cortex. 1994;4(4):344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- 30.Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112(Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- 31.Passe TJ, Rajagopalan P, Tupler LA, Byrum CE, MacFall JR, Krishnan KR. Age and sex effects on brain morphology. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21(8):1231–1237. doi: 10.1016/s0278-5846(97)00160-7. [DOI] [PubMed] [Google Scholar]
- 32.Rabinowicz T, Dean DE, Petetot JM, de Courten-Myers GM. Gender differences in the human cerebral cortex: more neurons in males; more processes in females. J Child Neurol. 1999;14(2):98–107. doi: 10.1177/088307389901400207. [DOI] [PubMed] [Google Scholar]
- 33.Nopoulos P, Flaum M, O’Leary D, Andreasen NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000;98(1):1–13. doi: 10.1016/s0925-4927(99)00044-x. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr., Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11(6):490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- 35.Gur RC, Turetsky BI, Matsui M, Yan M, Bilker W, Hughett P, Gur RE. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J Neurosci. 1999;19(10):4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harasty J, Double KL, Halliday GM, Kril JJ, McRitchie DA. Language-associated cortical regions are proportionally larger in the female brain. Arch Neurol. 1997;54(2):171–176. doi: 10.1001/archneur.1997.00550140045011. [DOI] [PubMed] [Google Scholar]
- 37.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, et al. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 38.Murphy DG, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, Szczepanik J, Schapiro MB, Grady CL, Horwitz B, et al. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996;53(7):585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- 39.Swaab DF, Fliers E. A sexually dimorphic nucleus in the human brain. Science. 1985;228(4703):1112–1115. doi: 10.1126/science.3992248. [DOI] [PubMed] [Google Scholar]
- 40.Allen LS, Hines M, Shryne JE, Gorski RA. Two sexually dimorphic cell groups in the human brain. J Neurosci. 1989;9(2):497–506. doi: 10.1523/JNEUROSCI.09-02-00497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou JN, Hofman MA, Gooren LJ, Swaab DF. A sex difference in the human brain and its relation to transsexuality. Nature. 1995;378(6552):68–70. doi: 10.1038/378068a0. [DOI] [PubMed] [Google Scholar]
- 42.Paus T, Otaky N, Caramanos Z, MacDonald D, Zijdenbos A, D’Avirro D, Gutmans D, Holmes C, Tomaiuolo F, Evans AC. In vivo morphometry of the intrasulcal gray matter in the human cingulate, paracingulate, and superior-rostral sulci: hemispheric asymmetries, gender differences and probability maps. J Comp Neurol. 1996;376(4):664–673. doi: 10.1002/(SICI)1096-9861(19961223)376:4<664::AID-CNE12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 43.Agartz I, Saaf J, Wahlund LO, Wetterberg L. Quantitative estimations of cerebrospinal fluid spaces and brain regions in healthy controls using computer-assisted tissue classification of magnetic resonance images: relation to age and sex. Magn Reson Imaging. 1992;10(2):217–226. doi: 10.1016/0730-725x(92)90482-f. [DOI] [PubMed] [Google Scholar]
- 44.Kaye JA, DeCarli C, Luxenberg JS, Rapoport SI. The significance of age-related enlargement of the cerebral ventricles in healthy men and women measured by quantitative computed X-ray tomography. J Am Geriatr Soc. 1992;40(3):225–231. doi: 10.1111/j.1532-5415.1992.tb02073.x. [DOI] [PubMed] [Google Scholar]
- 45.Ortiz J, Artigas F, Gelpi E. Serotonergic status in human blood. Life Sci. 1988;43(12):983–990. doi: 10.1016/0024-3205(88)90543-7. [DOI] [PubMed] [Google Scholar]
- 46.Riccardi P, Zald D, Li R, Park S, Ansari MS, Dawant B, Anderson S, Woodward N, Schmidt D, Baldwin R, et al. Sex differences in amphetamine-induced displacement of [(18)F]fallypride in striatal and extrastriatal regions: a PET study. Am J Psychiatry. 2006;163(9):1639–1641. doi: 10.1176/ajp.2006.163.9.1639. [DOI] [PubMed] [Google Scholar]
- 47.Smith YR, Minoshima S, Kuhl DE, Zubieta JK. Effects of long-term hormone therapy on cholinergic synaptic concentrations in healthy postmenopausal women. J Clin Endocrinol Metab. 2001;86(2):679–684. doi: 10.1210/jcem.86.2.7222. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida T, Kuwabara Y, Sasaki M, Fukumura T, Ichimiya A, Takita M, Ogomori K, Ichiya Y, Masuda K. Sex-related differences in the muscarinic acetylcholinergic receptor in the healthy human brain--a positron emission tomography study. Ann Nucl Med. 2000;14(2):97–101. doi: 10.1007/BF02988587. [DOI] [PubMed] [Google Scholar]
- 49.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, Berman RM, Charney DS, Krystal JH. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56(11):1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 50.Epperson CN, Haga K, Mason GF, Sellers E, Gueorguieva R, Zhang W, Weiss E, Rothman DL, Krystal JH. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59(9):851–858. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- 51.Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry. 1999;156(6):842–848. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- 52.Smith YR, Zubieta JK, del Carmen MG, Dannals RF, Ravert HT, Zacur HA, Frost JJ. Brain opioid receptor measurements by positron emission tomography in normal cycling women: relationship to luteinizing hormone pulsatility and gonadal steroid hormones. J Clin Endocrinol Metab. 1998;83(12):4498–4505. doi: 10.1210/jcem.83.12.5351. [DOI] [PubMed] [Google Scholar]
- 53.Fields JZ, Gordon JH. Estrogen inhibits the dopaminergic supersensitivity induced by neuroleptics. Life Sci. 1982;30(3):229–234. doi: 10.1016/0024-3205(82)90503-3. [DOI] [PubMed] [Google Scholar]
- 54.Devous MD, Sr., Stokely EM, Chehabi HH, Bonte FJ. Normal distribution of regional cerebral blood flow measured by dynamic single-photon emission tomography. J Cereb Blood Flow Metab. 1986;6(1):95–104. doi: 10.1038/jcbfm.1986.12. [DOI] [PubMed] [Google Scholar]
- 55.Gur RC, Gur RE, Obrist WD, Hungerbuhler JP, Younkin D, Rosen AD, Skolnick BE, Reivich M. Sex and handedness differences in cerebral blood flow during rest and cognitive activity. Science. 1982;217(4560):659–661. doi: 10.1126/science.7089587. [DOI] [PubMed] [Google Scholar]
- 56.Jones K, Johnson KA, Becker JA, Spiers PA, Albert MS, Holman BL. Use of singular value decomposition to characterize age and gender differences in SPECT cerebral perfusion. J Nucl Med. 1998;39(6):965–973. [PubMed] [Google Scholar]
- 57.Slosman DO, Chicherio C, Ludwig C, Genton L, de Ribaupierre S, Hans D, Pichard C, Mayer E, Annoni JM, de Ribaupierre A. (133)Xe SPECT cerebral blood flow study in a healthy population: determination of T-scores. J Nucl Med. 2001;42(6):864–870. [PubMed] [Google Scholar]
- 58.Esposito G, Van Horn JD, Weinberger DR, Berman KF. Gender differences in cerebral blood flow as a function of cognitive state with PET. J Nucl Med. 1996;37(4):559–564. [PubMed] [Google Scholar]
- 59.Podreka I, Baumgartner C, Suess E, Muller C, Brucke T, Lang W, Holzner F, Steiner M, Deecke L. Quantification of regional cerebral blood flow with IMP-SPECT. Reproducibility and clinical relevance of flow values. Stroke. 1989;20(2):183–191. doi: 10.1161/01.str.20.2.183. [DOI] [PubMed] [Google Scholar]
- 60.Baxter LR, Jr., Mazziotta JC, Phelps ME, Selin CE, Guze BH, Fairbanks L. Cerebral glucose metabolic rates in normal human females versus normal males. Psychiatry Res. 1987;21(3):237–245. doi: 10.1016/0165-1781(87)90028-x. [DOI] [PubMed] [Google Scholar]
- 61.Krejza J, Huba M, Mariak Z, Owlasiuk M. Measurement of cerebral blood flow volume in healthy adults using color duplex sonography. Stroke. 2000;31(8):2026–2028. doi: 10.1161/01.str.31.8.2026. [DOI] [PubMed] [Google Scholar]
- 62.Brackley KJ, Ramsay MM, Broughton Pipkin F, Rubin PC. The effect of the menstrual cycle on human cerebral blood flow: studies using Doppler ultrasound. Ultrasound Obstet Gynecol. 1999;14(1):52–57. doi: 10.1046/j.1469-0705.1999.14010052.x. [DOI] [PubMed] [Google Scholar]
- 63.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340(23):1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 64.Gangar KF, Vyas S, Whitehead M, Crook D, Meire H, Campbell S. Pulsatility index in internal carotid artery in relation to transdermal oestradiol and time since menopause. Lancet. 1991;338(8771):839–842. doi: 10.1016/0140-6736(91)91500-t. [DOI] [PubMed] [Google Scholar]
- 65.Reiman EM, Armstrong SM, Matt KS, Mattox JH. The application of positron emission tomography to the study of the normal menstrual cycle. Hum Reprod. 1996;11(12):2799–2805. doi: 10.1093/oxfordjournals.humrep.a019214. [DOI] [PubMed] [Google Scholar]
- 66.Wong DF, Broussolle EP, Wand G, Villemagne V, Dannals RF, Links JM, Zacur HA, Harris J, Naidu S, Braestrup C, et al. In vivo measurement of dopamine receptors in human brain by positron emission tomography. Age and sex differences. Ann N Y Acad Sci. 1988;515:203–214. doi: 10.1111/j.1749-6632.1988.tb32986.x. [DOI] [PubMed] [Google Scholar]
- 67.Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27(3):373–380. [PubMed] [Google Scholar]
- 68.Moulin T, Tatu L, Vuillier F, Berger E, Chavot D, Rumbach L. Role of a stroke data bank in evaluating cerebral infarction subtypes: patterns and outcome of 1,776 consecutive patients from the Besancon stroke registry. Cerebrovasc Dis. 2000;10(4):261–271. doi: 10.1159/000016068. [DOI] [PubMed] [Google Scholar]
- 69.Knoflach M, Matosevic B, Rucker M, Furtner M, Mair A, Wille G, Zangerle A, Werner P, Ferrari J, Schmidauer C, et al. Functional recovery after ischemic stroke--a matter of age: data from the Austrian Stroke Unit Registry. Neurology. 78(4):279–285. doi: 10.1212/WNL.0b013e31824367ab. [DOI] [PubMed] [Google Scholar]
- 70.Orlandi G, Gelli A, Fanucchi S, Tognoni G, Acerbi G, Murri L. Prevalence of stroke and transient ischaemic attack in the elderly population of an Italian rural community. Eur J Epidemiol. 2003;18(9):879–882. doi: 10.1023/a:1025639203283. [DOI] [PubMed] [Google Scholar]
- 71.Fromm A, Waje-Andreassen U, Thomassen L, Naess H. Comparison between Ischemic Stroke Patients <50 Years and >/=50 Years Admitted to a Single Centre: The Bergen Stroke Study. Stroke Res Treat. 2011;2011:183256. doi: 10.4061/2011/183256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29(4):792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu F, Akella P, Benashski SE, Xu Y, McCullough LD. Expression of Na-K-Cl cotransporter and edema formation are age dependent after ischemic stroke. Exp Neurol. 2010;224(2):356–361. doi: 10.1016/j.expneurol.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan Z, Li X, Kelly KA, Rosen CL, Huber JD. Plasminogen activator inhibitor type 1 derived peptide, EEIIMD, diminishes cortical infarct but fails to improve neurological function in aged rats following middle cerebral artery occlusion. Brain Res. 2009;1281:84–90. doi: 10.1016/j.brainres.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kelly KA, Li X, Tan Z, VanGilder RL, Rosen CL, Huber JD. NOX2 inhibition with apocynin worsens stroke outcome in aged rats. Brain Res. 2009;1292:165–172. doi: 10.1016/j.brainres.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DiNapoli VA, Huber JD, Houser K, Li X, Rosen CL. Early disruptions of the blood-brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiol Aging. 2008;29(5):753–764. doi: 10.1016/j.neurobiolaging.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Darsalia V, Heldmann U, Lindvall O, Kokaia Z. Stroke-induced neurogenesis in aged brain. Stroke. 2005;36(8):1790–1795. doi: 10.1161/01.STR.0000173151.36031.be. [DOI] [PubMed] [Google Scholar]
- 78.Dubal DB, Wise PM. Neuroprotective effects of estradiol in middle-aged female rats. Endocrinology. 2001;142(1):43–48. doi: 10.1210/endo.142.1.7911. [DOI] [PubMed] [Google Scholar]
- 79.Kharlamov A, Kharlamov E, Armstrong DM. Age-dependent increase in infarct volume following photochemically induced cerebral infarction: putative role of astroglia. J Gerontol A Biol Sci Med Sci. 2000;55(3):B135–141. doi: 10.1093/gerona/55.3.b135. discussion B142-133. [DOI] [PubMed] [Google Scholar]
- 80.Sutherland GR, Dix GA, Auer RN. Effect of age in rodent models of focal and forebrain ischemia. Stroke. 1996;27(9):1663–1667. doi: 10.1161/01.str.27.9.1663. discussion 1668. [DOI] [PubMed] [Google Scholar]
- 81.Chen Y, Sun FY. Age-related decrease of striatal neurogenesis is associated with apoptosis of neural precursors and newborn neurons in rat brain after ischemia. Brain Res. 2007;1166:9–19. doi: 10.1016/j.brainres.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 82.Li N, Kong X, Ye R, Yang Q, Han J, Xiong L. Age-related differences in experimental stroke: possible involvement of mitochondrial dysfunction and oxidative damage. Rejuvenation Res. 2011;14(3):261–273. doi: 10.1089/rej.2010.1115. [DOI] [PubMed] [Google Scholar]
- 83.Rosen CL, Dinapoli VA, Nagamine T, Crocco T. Influence of age on stroke outcome following transient focal ischemia. J Neurosurg. 2005;103(4):687–694. doi: 10.3171/jns.2005.103.4.0687. [DOI] [PubMed] [Google Scholar]
- 84.Shapira S, Sapir M, Wengier A, Grauer E, Kadar T. Aging has a complex effect on a rat model of ischemic stroke. Brain Res. 2002;925(2):148–158. doi: 10.1016/s0006-8993(01)03270-x. [DOI] [PubMed] [Google Scholar]
- 85.Sieber MW, Claus RA, Witte OW, Frahm C. Attenuated inflammatory response in aged mice brains following stroke. PLoS One. 2011;6(10):e26288. doi: 10.1371/journal.pone.0026288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Correa F, Gauberti M, Parcq J, Macrez R, Hommet Y, Obiang P, Hernangomez M, Montagne A, Liot G, Guaza C, et al. Tissue plasminogen activator prevents white matter damage following stroke. J Exp Med. 2011;208(6):1229–1242. doi: 10.1084/jem.20101880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu F, Benashski SE, Persky R, Xu Y, Li J, McCullough LD. Age-related changes in AMP-activated protein kinase after stroke. Age (Dordr) 2011 doi: 10.1007/s11357-011-9214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Won SJ, Xie L, Kim SH, Tang H, Wang Y, Mao X, Banwait S, Jin K. Influence of age on the response to fibroblast growth factor-2 treatment in a rat model of stroke. Brain Res. 2006;1123(1):237–244. doi: 10.1016/j.brainres.2006.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang RY, Wang PS, Yang YR. Effect of age in rats following middle cerebral artery occlusion. Gerontology. 2003;49(1):27–32. doi: 10.1159/000066505. [DOI] [PubMed] [Google Scholar]
- 90.Wang RY, Yu SM, Yang YR. Treadmill training effects in different age groups following middle cerebral artery occlusion in rats. Gerontology. 2005;51(3):161–165. doi: 10.1159/000083987. [DOI] [PubMed] [Google Scholar]
- 91.Anisimov VN, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Egormin PA, Yurova MV, Rosenfeld SV, Semenchenko AV, Kovalenko IG, et al. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging (Albany NY) 2010;2(12):945–958. doi: 10.18632/aging.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bowen H, Mitchell TD, Harris RB. Method of leptin dosing, strain, and group housing influence leptin sensitivity in high-fat-fed weanling mice. Am J Physiol Regul Integr Comp Physiol. 2003;284(1):R87–100. doi: 10.1152/ajpregu.00431.2002. [DOI] [PubMed] [Google Scholar]
- 93.Li J, McCullough LD. Effects of AMP-activated protein kinase in cerebral ischemia. J Cereb Blood Flow Metab. 2010;30(3):480–492. doi: 10.1038/jcbfm.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67(1):41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Schroeder E, Vogelgesang S, Popa-Wagner A, Kessler C. Neurofilament expression in the rat brain after cerebral infarction: effect of age. Neurobiol Aging. 2003;24(1):135–145. doi: 10.1016/s0197-4580(02)00063-5. [DOI] [PubMed] [Google Scholar]
- 96.Badan I, Dinca I, Buchhold B, Suofu Y, Walker L, Gratz M, Platt D, Kessler CH, Popa-Wagner A. Accelerated accumulation of N- and C-terminal beta APP fragments and delayed recovery of microtubule-associated protein 1B expression following stroke in aged rats. Eur J Neurosci. 2004;19(8):2270–2280. doi: 10.1111/j.0953-816X.2004.03323.x. [DOI] [PubMed] [Google Scholar]
- 97.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Manwani B, McCullough LD. Sexual dimorphism in ischemic stroke: lessons from the laboratory. Womens Health (Lond Engl) 2011;7(3):319–339. doi: 10.2217/whe.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends Endocrinol Metab. 2003;14(5):228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 100.Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. Jama. 1991;265(14):1861–1867. [PubMed] [Google Scholar]
- 101.Ho JY, Chen MJ, Sheu WH, Yi YC, Tsai AC, Guu HF, Ho ES. Differential effects of oral conjugated equine estrogen and transdermal estrogen on atherosclerotic vascular disease risk markers and endothelial function in healthy postmenopausal women. Hum Reprod. 2006;21(10):2715–2720. doi: 10.1093/humrep/del245. [DOI] [PubMed] [Google Scholar]
- 102.Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. Faseb J. 1996;10(5):615–624. [PubMed] [Google Scholar]
- 103.Haberman S, Capildeo R, Rose FC. Sex differences in the incidence of cerebrovascular disease. JEpidemiol Community Health. 1981;35(1):45–50. doi: 10.1136/jech.35.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murphy SJ, McCullough LD, Smith JM. Stroke in the female: role of biological sex and estrogen. Ilar J. 2004;45(2):147–159. doi: 10.1093/ilar.45.2.147. [DOI] [PubMed] [Google Scholar]
- 105.Simpkins JW, Yang SH, Wen Y, Singh M. Estrogens, progestins, menopause and neurodegeneration: basic and clinical studies. Cell Mol Life Sci. 2005;62(3):271–280. doi: 10.1007/s00018-004-4382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Postischemic estrogen reduces hypoperfusion and secondary ischemia after experimental stroke. Stroke. 2001;32(3):796–802. doi: 10.1161/01.str.32.3.796. [DOI] [PubMed] [Google Scholar]
- 107.Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30(2):201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci. 1999;19(15):6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rau SW, Dubal DB, Bottner M, Gerhold LM, Wise PM. Estradiol attenuates programmed cell death after stroke-like injury. J Neurosci. 2003;23(36):11420–11426. doi: 10.1523/JNEUROSCI.23-36-11420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vegeto E, Ghisletti S, Meda C, Etteri S, Belcredito S, Maggi A. Regulation of the lipopolysaccharide signal transduction pathway by 17beta-estradiol in macrophage cells. J Steroid Biochem Mol Biol. 2004;91(1-2):59–66. doi: 10.1016/j.jsbmb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 111.Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20(4):631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 112.Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23(5):665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- 113.Amantea D, Russo R, Bagetta G, Corasaniti MT. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacol Res. 2005;52(2):119–132. doi: 10.1016/j.phrs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 114.Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc Natl Acad Sci U S A. 1999;96(16):8867–8872. doi: 10.1073/pnas.96.16.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Soares R, Guo S, Russo J, Schmitt F. Role of the estrogen antagonist ICI 182,780 in vessel assembly and apoptosis of endothelial cells. Ultrastruct Pathol. 2003;27(1):33–39. doi: 10.1080/01913120309946. [DOI] [PubMed] [Google Scholar]
- 116.Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98(4):1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu F, Benashski SE, Xu Y, Siegel M, McCullough LD. Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. J Neuroendocrinol. 2012;24(2):319–330. doi: 10.1111/j.1365-2826.2011.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang JM, Hou X, Adeosun S, Hill R, Henry S, Paul I, Irwin RW, Ou XM, Bigler S, Stockmeier C, et al. A Dominant Negative ERbeta Splice Variant Determines the Effectiveness of Early or Late Estrogen Therapy after Ovariectomy in Rats. PLoS One. 2012;7(3):e33493. doi: 10.1371/journal.pone.0033493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007;104(14):6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 121.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345(17):1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 122.Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol. 2007;19(2):77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- 123.Liu M, Dziennis S, Hurn PD, Alkayed NJ. Mechanisms of gender-linked ischemic brain injury. Restor Neurol Neurosci. 2009;27(3):163–179. doi: 10.3233/RNN-2009-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hawk T, Zhang YQ, Rajakumar G, Day AL, Simpkins JW. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 1998;796(1-2):296–298. doi: 10.1016/s0006-8993(98)00327-8. [DOI] [PubMed] [Google Scholar]
- 125.Frye CA, McCormick CM. Androgens are neuroprotective in the dentate gyrus of adrenalectomized female rats. Stress. 2000;3(3):185–194. doi: 10.3109/10253890009001122. [DOI] [PubMed] [Google Scholar]
- 126.Ahlbom E, Grandison L, Bonfoco E, Zhivotovsky B, Ceccatelli S. Androgen treatment of neonatal rats decreases susceptibility of cerebellar granule neurons to oxidative stress in vitro. Eur J Neurosci. 1999;11(4):1285–1291. doi: 10.1046/j.1460-9568.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- 127.Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892(2):255–262. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- 128.Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919(1):160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- 129.Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77(5):1319–1326. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- 130.Normann S, de Veber G, Fobker M, Langer C, Kenet G, Bernard TJ, Fiedler B, Strater R, Goldenberg NA, Nowak-Gottl U. Role of endogenous testosterone concentration in pediatric stroke. Ann Neurol. 2009;66(6):754–758. doi: 10.1002/ana.21840. [DOI] [PubMed] [Google Scholar]
- 131.Yeap BB, Hyde Z, Almeida OP, Norman PE, Chubb SA, Jamrozik K, Flicker L, Hankey GJ. Lower testosterone levels predict incident stroke and transient ischemic attack in older men. J Clin Endocrinol Metab. 2009;94(7):2353–2359. doi: 10.1210/jc.2008-2416. [DOI] [PubMed] [Google Scholar]
- 132.Hollander M, Koudstaal PJ, Bots ML, Grobbee DE, Hofman A, Breteler MM. Incidence, risk, and case fatality of first ever stroke in the elderly population. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2003;74(3):317–321. doi: 10.1136/jnnp.74.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vannucci SJ, Hurn PD. Gender differences in pediatric stroke: is elevated testosterone a risk factor for boys? Ann Neurol. 2009;66(6):713–714. doi: 10.1002/ana.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Muwakkit SA, Majdalani M, Hourani R, Mahfouz RA, Otrock ZK, Bilalian C, Chan AK, Abboud M, Mikati MA. Inherited thrombophilia in childhood arterial stroke: data from Lebanon. Pediatr Neurol. 2011;45(3):155–158. doi: 10.1016/j.pediatrneurol.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 136.Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109(1):116–123. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 137.Ogeng’o JA, Olabu BO, Mburu AN, Sinkeet SR. Pediatric stroke in an African country. J Pediatr Neurosci. 2010;5(1):22–24. doi: 10.4103/1817-1745.66676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lenicek Krleza J, Duranovic V, Lujic L, Coen Herak D, Mejaski-Bosnjak V, Nakic M, Zadro R. The burden of paediatric stroke and cerebrovascular disorders in Croatia. Int J Stroke. 2009;4(5):390–394. doi: 10.1111/j.1747-4949.2009.00321.x. [DOI] [PubMed] [Google Scholar]
- 139.Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: ethnic and gender disparities. Neurology. 2003;61(2):189–194. doi: 10.1212/01.wnl.0000078894.79866.95. [DOI] [PubMed] [Google Scholar]
- 140.Fullerton HJ, Johnston SC, Smith WS. Arterial dissection and stroke in children. Neurology. 2001;57(7):1155–1160. doi: 10.1212/wnl.57.7.1155. [DOI] [PubMed] [Google Scholar]
- 141.Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, Kaste M, Tatlisumak T. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke. 2009;40(4):1195–1203. doi: 10.1161/STROKEAHA.108.529883. [DOI] [PubMed] [Google Scholar]
- 142.Spengos K, Vemmos KN. Female predominance at very young ages and other similarities between Finnish and Greek young ischemic stroke patients. Stroke. 2009;40(7):e491. doi: 10.1161/STROKEAHA.109.555961. reply e492. [DOI] [PubMed] [Google Scholar]
- 143.Bousser MG. Estrogens, migraine, and stroke. Stroke. 2004;35(11 Suppl 1):2652–2656. doi: 10.1161/01.STR.0000143223.25843.36. [DOI] [PubMed] [Google Scholar]
- 144.Mayoral SR, Omar G, Penn AA. Sex differences in a hypoxia model of preterm brain damage. Pediatr Res. 2009;66(3):248–253. doi: 10.1203/PDR.0b013e3181b1bc34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bona E, Hagberg H, Loberg EM, Bagenholm R. Thoresen M: Protective effects of moderate hypothermia after neonatal hypoxia-ischemia: short- and long-term outcome. Pediatr Res. 1998;43(6):738–745. doi: 10.1203/00006450-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 146.Towfighi A, Markovic D, Ovbiagele B. Persistent sex disparity in midlife stroke prevalence in the United States. Cerebrovasc Dis. 2011;31(4):322–328. doi: 10.1159/000321503. [DOI] [PubMed] [Google Scholar]
- 147.Towfighi A, Saver JL, Engelhardt R, Ovbiagele B. A midlife stroke surge among women in the United States. Neurology. 2007;69(20):1898–1904. doi: 10.1212/01.wnl.0000268491.89956.c2. [DOI] [PubMed] [Google Scholar]
- 148.Ishikawa M, Cooper D, Arumugam TV, Zhang JH, Nanda A, Granger DN. Platelet-leukocyte-endothelial cell interactions after middle cerebral artery occlusion and reperfusion. J Cereb Blood Flow Metab. 2004;24(8):907–915. doi: 10.1097/01.WCB.0000132690.96836.7F. [DOI] [PubMed] [Google Scholar]
- 149.Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26(5):248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 150.Han HS, Yenari MA. Cellular targets of brain inflammation in stroke. Curr Opin Investig Drugs. 2003;4(5):522–529. [PubMed] [Google Scholar]
- 151.Seymour JF, Pro B, Fuller LM, Manning JT, Hagemeister FB, Romaguera J, Rodriguez MA, Ha CS, Smith TL, Ayala A, et al. Long-term follow-up of a prospective study of combined modality therapy for stage I-II indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2003;21(11):2115–2122. doi: 10.1200/JCO.2003.07.111. [DOI] [PubMed] [Google Scholar]
- 152.Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, Lord JM, Shaw AC. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30(7):325–333. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Harris NR, Rumbaut RE. Age-related responses of the microcirculation to ischemia-reperfusion and inflammation. Pathophysiology. 2001;8(1):1–10. doi: 10.1016/s0928-4680(01)00064-5. [DOI] [PubMed] [Google Scholar]
- 154.Ohta K, Iwai M, Sato K, Omori N, Nagano I, Shoji M, Abe K. Dissociative increase of oligodendrocyte progenitor cells between young and aged rats after transient cerebral ischemia. Neurosci Lett. 2003;335(3):159–162. doi: 10.1016/s0304-3940(02)01177-1. [DOI] [PubMed] [Google Scholar]
- 155.Park Y, Kim Y, Stanley D. Cellular immunosenescence in adult male crickets, Gryllus assimilis. Arch Insect Biochem Physiol. 2011;76(4):185–194. doi: 10.1002/arch.20394. [DOI] [PubMed] [Google Scholar]
- 156.Gimenez-Llort L, Arranz L, Mate I, De la Fuente M. Gender-specific neuroimmunoendocrine aging in a triple-transgenic 3xTg-AD mouse model for Alzheimer’s disease and its relation with longevity. Neuroimmunomodulation. 2008;15(4-6):331–343. doi: 10.1159/000156475. [DOI] [PubMed] [Google Scholar]
- 157.Lewis DK, Thomas KT, Selvamani A, Sohrabji F. Age-related severity of focal ischemia in female rats is associated with impaired astrocyte function. Neurobiol Aging. 2012;33(6):1123, e1121–1116. doi: 10.1016/j.neurobiolaging.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gomez CR, Hirano S, Cutro BT, Birjandi S, Baila H, Nomellini V, Kovacs EJ. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med. 2007;35(1):246–251. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- 159.Altinoz MA, Korkmaz R. NF-kappaB, macrophage migration inhibitory factor and cyclooxygenase-inhibitions as likely mechanisms behind the acetaminophen- and NSAID-prevention of the ovarian cancer. Neoplasma. 2004;51(4):239–247. [PubMed] [Google Scholar]
- 160.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184(1-2):53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhao X, Eghbali-Webb M. Gender-related differences in basal and hypoxia-induced activation of signal transduction pathways controlling cell cycle progression and apoptosis, in cardiac fibroblasts. Endocrine. 2002;18(2):137–145. doi: 10.1385/ENDO:18:2:137. [DOI] [PubMed] [Google Scholar]
- 162.Krishnan S, Intlekofer KA, Aggison LK, Petersen SL. Central role of TRAF-interacting protein in a new model of brain sexual differentiation. Proc Natl Acad Sci U S A. 2009;106(39):16692–16697. doi: 10.1073/pnas.0906293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ritzel RM, Capozzi LA, McCullough LD. Sex, stroke, and inflammation: The potential for estrogen-mediated immunoprotection in stroke. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 165.Sunday L, Osuna C, Krause DN, Duckles SP. Age alters cerebrovascular inflammation and effects of estrogen. Am J Physiol Heart Circ Physiol. 2007;292(5):H2333–2340. doi: 10.1152/ajpheart.01057.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Razmara A, Krause DN, Duckles SP. Testosterone augments endotoxin-mediated cerebrovascular inflammation in male rats. Am J Physiol Heart Circ Physiol. 2005;289(5):H1843–1850. doi: 10.1152/ajpheart.00465.2005. [DOI] [PubMed] [Google Scholar]
- 167.Corcoran MP, Meydani M, Lichtenstein AH, Schaefer EJ, Dillard A, Lamon-Fava S. Sex hormone modulation of proinflammatory cytokine and C-reactive protein expression in macrophages from older men and postmenopausal women. J Endocrinol. 2010;206(2):217–224. doi: 10.1677/JOE-10-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hagberg H, Wilson MA, Matsushita H, Zhu C, Lange M, Gustavsson M, Poitras MF, Dawson TM, Dawson VL, Northington F, et al. PARP-1 gene disruption in mice preferentially protects males from perinatal brain injury. J Neurochem. 2004;90(5):1068–1075. doi: 10.1111/j.1471-4159.2004.02547.x. [DOI] [PubMed] [Google Scholar]
- 169.McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25(4):502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- 170.Yuan M, Siegel C, Zeng Z, Li J, Liu F, McCullough LD. Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke. Exp Neurol. 2009;217(1):210–218. doi: 10.1016/j.expneurol.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Renolleau S, Fau S, Goyenvalle C, Joly LM, Chauvier D, Jacotot E, Mariani J, Charriaut-Marlangue C. Specific caspase inhibitor Q-VD-OPh prevents neonatal stroke in P7 rat: a role for gender. J Neurochem. 2007;100(4):1062–1071. doi: 10.1111/j.1471-4159.2006.04269.x. [DOI] [PubMed] [Google Scholar]
- 172.Heard E, Disteche CM. Dosage compensation in mammals: fine-tuning the expression of the X chromosome. Genes Dev. 2006;20(14):1848–1867. doi: 10.1101/gad.1422906. [DOI] [PubMed] [Google Scholar]
- 173.Goto T, Monk M. Regulation of X-chromosome inactivation in development in mice and humans. Microbiol Mol Biol Rev. 1998;62(2):362–378. doi: 10.1128/mmbr.62.2.362-378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Latham KE. X chromosome imprinting and inactivation in preimplantation mammalian embryos. Trends Genet. 2005;21(2):120–127. doi: 10.1016/j.tig.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 175.Prothero KE, Stahl JM, Carrel L. Dosage compensation and gene expression on the mammalian X chromosome: one plus one does not always equal two. Chromosome Res. 2009;17(5):637–648. doi: 10.1007/s10577-009-9063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Turtzo LC, Siegel C, McCullough LD. X chromosome dosage and the response to cerebral ischemia. J Neurosci. 2011;31(37):13255–13259. doi: 10.1523/JNEUROSCI.0621-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hatakeyama C, Anderson CL, Beever CL, Penaherrera MS, Brown CJ, Robinson WP. The dynamics of X-inactivation skewing as women age. Clin Genet. 2004;66(4):327–332. doi: 10.1111/j.1399-0004.2004.00310.x. [DOI] [PubMed] [Google Scholar]
- 178.Cookson W, Liang L, Abecasis G, Moffatt M, Lathrop M. Mapping complex disease traits with global gene expression. Nat Rev Genet. 2009;10(3):184–194. doi: 10.1038/nrg2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Morleo M, Franco B. Dosage compensation of the mammalian X chromosome influences the phenotypic variability of X-linked dominant male-lethal disorders. J Med Genet. 2008;45(7):401–408. doi: 10.1136/jmg.2008.058305. [DOI] [PubMed] [Google Scholar]
- 180.Xu J, Disteche CM. Sex differences in brain expression of X- and Y-linked genes. Brain Res. 2006;1126(1):50–55. doi: 10.1016/j.brainres.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 181.Talebizadeh Z, Simon SD, Butler MG. X chromosome gene expression in human tissues: male and female comparisons. Genomics. 2006;88(6):675–681. doi: 10.1016/j.ygeno.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Spolarics Z. The X-files of inflammation: cellular mosaicism of X-linked polymorphic genes and the female advantage in the host response to injury and infection. Shock. 2007;27(6):597–604. doi: 10.1097/SHK.0b013e31802e40bd. [DOI] [PubMed] [Google Scholar]
- 183.Wuerzberger-Davis SM, Nakamura Y, Seufzer BJ, Miyamoto S. NF-kappaB activation by combinations of NEMO SUMOylation and ATM activation stresses in the absence of DNA damage. Oncogene. 2007;26(5):641–651. doi: 10.1038/sj.onc.1209815. [DOI] [PubMed] [Google Scholar]
- 184.Mabb AM, Wuerzberger-Davis SM, Miyamoto S. PIASy mediates NEMO sumoylation and NF-kappaB activation in response to genotoxic stress. Nat Cell Biol. 2006;8(9):986–993. doi: 10.1038/ncb1458. [DOI] [PubMed] [Google Scholar]
- 185.Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414(6861):313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- 186.Kucharczak J, Simmons MJ, Fan Y, Gelinas C. To be, or not to be: NF-kappaB is the answer--role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene. 2003;22(56):8961–8982. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- 187.Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998;188(1):211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Smrt RD, Pfeiffer RL, Zhao X. Age-dependent expression of MeCP2 in a heterozygous mosaic mouse model. Hum Mol Genet. 2011;20(9):1834–1843. doi: 10.1093/hmg/ddr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51(9):874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 190.Matsumae M, Kikinis R, Morocz IA, Lorenzo AV, Sandor T, Albert MS, Black PM, Jolesz FA. Age-related changes in intracranial compartment volumes in normal adults assessed by magnetic resonance imaging. J Neurosurg. 1996;84(6):982–991. doi: 10.3171/jns.1996.84.6.0982. [DOI] [PubMed] [Google Scholar]
- 191.Raz N. Aging of the brain and its impact on cognitive performance: integration of structural and functional findings. In: Craik FIM, Salthouse TA, editors. Handbook of Aging and Cognition. Erlbaum; Mahwah, NJ: 2000. [Google Scholar]
- 192.Hothersall JS, El-Hassan A, McLean P, Greenbaum AL. Age-related changes in enzymes of rat brain. 2. Redox systems linked to NADP and glutathione. Enzyme. 1981;26(5):271–276. doi: 10.1159/000459190. [DOI] [PubMed] [Google Scholar]
- 193.Baquer NZ, Taha A, Kumar P, McLean P, Cowsik SM, Kale RK, Singh R, Sharma D. A metabolic and functional overview of brain aging linked to neurological disorders. Biogerontology. 2009;10(4):377–413. doi: 10.1007/s10522-009-9226-2. [DOI] [PubMed] [Google Scholar]
- 194.Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, Naritoku DK. Age-related changes in GABA(A) receptor subunit composition and function in rat auditory system. Neuroscience. 1999;93(1):307–312. doi: 10.1016/s0306-4522(99)00121-9. [DOI] [PubMed] [Google Scholar]
- 195.Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384(2):312–320. [PubMed] [Google Scholar]
- 196.Witelson SF, Glezer II, Kigar DL. Women have greater density of neurons in posterior temporal cortex. J Neurosci. 1995;15(5 Pt 1):3418–3428. doi: 10.1523/JNEUROSCI.15-05-03418.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Grachev ID, Apkarian AV. Anxiety in healthy humans is associated with orbital frontal chemistry. Mol Psychiatry. 2000;5(5):482–488. doi: 10.1038/sj.mp.4000778. [DOI] [PubMed] [Google Scholar]
- 198.Turner BB, Weaver DA. Sexual dimorphism of glucocorticoid binding in rat brain. Brain Res. 1985;343(1):16–23. doi: 10.1016/0006-8993(85)91153-9. [DOI] [PubMed] [Google Scholar]
- 199.Ren J. Influence of gender on oxidative stress, lipid peroxidation, protein damage and apoptosis in hearts and brains from spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2007;34(5-6):432–438. doi: 10.1111/j.1440-1681.2007.04591.x. [DOI] [PubMed] [Google Scholar]
- 200.Andreason PJ, Zametkin AJ, Guo AC, Baldwin P, Cohen RM. Gender-related differences in regional cerebral glucose metabolism in normal volunteers. Psychiatry Res. 1994;51(2):175–183. doi: 10.1016/0165-1781(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 201.Hatazawa J, Brooks RA, Di Chiro G, Campbell G. Global cerebral glucose utilization is independent of brain size: a PET Study. J Comput Assist Tomogr. 1987;11(4):571–576. doi: 10.1097/00004728-198707000-00002. [DOI] [PubMed] [Google Scholar]
- 202.Azari NP, Rapoport SI, Grady CL, DeCarli C, Haxby JV, Schapiro MB, Horwitz B. Gender differences in correlations of cerebral glucose metabolic rates in young normal adults. Brain Res. 1992;574(1-2):198–208. doi: 10.1016/0006-8993(92)90817-s. [DOI] [PubMed] [Google Scholar]
- 203.Kuhl DE, Metter EJ, Riege WH, Phelps ME. Effects of human aging on patterns of local cerebral glucose utilization determined by the [18F]fluorodeoxyglucose method. J Cereb Blood Flow Metab. 1982;2(2):163–171. doi: 10.1038/jcbfm.1982.15. [DOI] [PubMed] [Google Scholar]