Abstract
The genetic and physiological similarities between mice and humans have focused considerable attention on rodents as potential models of human health and disease. Together with the wealth of resources, knowledge, and technologies surrounding the mouse as a model system, these similarities have propelled this species to the forefront of biomedical research. The advent of genomic manipulation has quickly led to the creation and use of genetically engineered mice as powerful tools for cutting edge studies of human disease research, including the discovery, refinement, and utility of many currently available therapeutic regimes. In particular, the creation of genetically modified mice as models of human disease has remarkably changed our ability to understand the molecular mechanisms and cellular pathways underlying disease states. Moreover, the mouse models resulting from gene transfer technologies have been important components correlating an individual’s gene expression profile to the development of disease pathologies. The objective of this review is to provide physician-scientists with an expansive historical and logistical overview of the creation of mouse models of human disease through gene transfer technologies. Our expectation is that this will facilitate on-going disease research studies and may initiate new areas of translational research leading to enhanced patient care.
Keywords: Animal Model, Biomedical Research, Genetic Engineering, Murine, Rodent
Existing treatments for many human diseases have resulted (and/or have dramatically benefitted) from studies in animal models. Specifically, whether it be concept development (e.g., treatment of diabetes with insulin: dog (Banting 1990)), validation of mechanism of action (e.g., blockade of eosinophil proliferation in asthma patients with the humanized anti-interleukin 5 antibody - Mepolizumab®: mouse (Kung 1995) and non-human primate (Mauser 1995)), or safety/efficacy (e.g., toxicity of Penicillin for clinical use: Guinea pig (Hamre 1943)) animal models have been, and continue to be, critical to the development and/or improvement of therapeutic modalities. These models have also facilitated recent advances in genomic research. Specifically, changes in gene expression and/or protein structure/function are recognized as root causes of disease. This suggests that unique sequence variations (e.g., deletions or recombination events within the genome and/or within a chromosome(s), specific single nucleotide polymorphisms (SNPs) or indels) inherited by individuals will dictate, and ultimately be predictive of, disease outcome. The complexity of the approaches needed to define these genetic contributions to human disease often required the use of animal models either to develop original hypotheses or validate candidate cellular and/or molecular pathways. This genomic research employed many prokaryotic organisms and eukaryotic cell-based systems, including basic research studies with bacteria (e.g., Escherichia coli) and yeast (e.g., Saccharomyces cerevisiae) as well as the use of invertebrate species (e.g., Caenorhabditis elegans (Stinchcomb 1985) and Drosophila melanogaster (Rubin 1982)), clinically relevant small mammalian models (e.g., mice, Lee J. J. 1997), and pre-clinical studies in large animals (e.g., non-human primates, Mauser 1995). In particular, pioneering studies in bacteria (Escherichia coli) have led to the now omnipresent science of molecular biology or, as noted in pop culture, “recombinant DNA technology” and/or “genetic engineering”. Subsequent (and in some cases concurrent) studies of spontaneous mutations in the fruit fly (Drosophila melanogaster) confirmed and often identified fundamental mechanisms by which genetic information confers specific and heritable traits to offspring. In turn, the ever increasing ability to manipulate and characterize DNA sequences and structure quickly cleared the way for genomic manipulations in larger animal species. Historically, the ability to induce heritable genomic changes initially correlated with the sequence complexity of the organisms of interest, occurring first in more primitive organisms such as bacteria (Escherichia coli (1973)) and yeast (Saccharomyces cerevisiae (1978)) before expanding almost concurrently among invertebrate animal species (e.g., roundworms (Caenorhabditis elegans (1985)), fruit flies (Drosophila melanogaster (1982)), sea urchins (Strongylocentrotus purpuratus (1985)) as well as several vertebrate animal models, including amphibians (Xenopus laevis (1983)), fish (Danio rerio (1988)), chickens (Gallus gallus (1986)), and mice (Mus musculus (1981)). The mouse rapidly became the animal model of choice to understand human disease and translate discoveries into practical therapies because of the genetic, biochemical and physiological similarities shared among mammals, all of which diverged from one another approximately 65-75 million years ago (McKenna 1969). That is, while less complex single-celled eukaryotes and metazoans such as yeast, roundworms, fruit flies, and sea urchins share a great deal with humans at a molecular mechanistic level, these species, as well as non-mammalian vertebrates, have divergent biochemistry and physiology relative to humans. The noted differences have all but precluded the use of these model systems in many studies of human disease. This review focuses on the techniques of gene manipulation in the mouse to highlight not only the advances in studies of gene expression/function possible with these rodent models but also the increasing ability to understand human disease and translate this knowledge into novel and/or improved diagnostics and therapeutics.
Mouse Models of Human Disease - The “Macroperspective”
In vivo studies of gene expression and biochemical pathways often yield very different results relative to ex vivo experimentation using either primary cell cultures or tissue/organ explants due to the genetic and physiological complexities of a living organism. Thus, while molecular studies of human disease have benefitted tremendously from ex vivo cell culture experiments and even in vivo studies using bacteria, primitive eukaryotes, as well as invertebrate and non-mammalian vertebrate species, these ex vivo/in vivo models often inadequately represent human subjects. The shortcomings of these model systems have helped drive the use of the mouse to model human health and disease. The utility of the mouse, indeed any model system, is dependent on its ability to accurately represent human disease. As such, the mouse in many ways is an exquisite subject for clinically relevant studies:
The shared sequence complexity between mice and humans is startling. Although some physiological and immunological differences exist between mice and humans (Mestas 2004), genomic sequencing has demonstrated that of ~30,000 genes in both mice and humans only 300 (i.e., 1%) are unique to either species (Waterston 2002).
All mice in a given study are generally on the same genetic background (i.e., mouse models are either in a single inbred strain or they are backcrossed on to a genetic background as to be“congenic” for a given strain). As a consequence, all subjects are genetically identical, eliminating potential complicating factors such as genetic variation and unique gene polymorphisms (e.g., SNPs).
The inter-generational interval of mice is less than three months. Litter sizes are also large enough to permit the rapid expansion of experimental subjects into statistically relevant cohorts.
Mice in a given study have defined medical histories and all animals will be equivalently housed, experiencing identical environmental exposures.
All mice in a given study are subject to identical experimental protocols and it is possible for all subjects to undergo a complete assessment of endpoint pathologies, including measures of physiological parameters and post-mortem histopathology.
In addition to the qualities noted above, the most significant asset that the mouse offers as a model of human disease is the ability to manipulate its genome (i.e., gene expression) in a Mendelian-inheritable fashion through gene transfer technologies. Although considered by many to be common knowledge now, the historical and logistical issues surrounding the development of gene transfer technologies provide insight as to the value and usefulness of clinically relevant mouse models.
The activities of mouse fanciers in the early 1900’s, followed by Ernest Castle at the Bussey Institute and some of his students including Clarence Little (founder of The Jackson Laboratory), led to established lines of genetically identical mice (e.g., C57BL/6J and BALB/cJ) - virtual clones of one another due to generations of selective breeding (Silver 1995). Early studies of mouse genetics were based largely on spontaneous mutations that would arise in mouse breeding colonies. These mutations were then selectively bred for their noted similarities with particular human traits and/or diseases. Two examples of single gene spontaneous mutations that highlight the utility of these genetic abberations in biomedical research include select oculocutaneous pigment dilution mutations (Swank 1998) and immunocompromised strains of mice such as SCID (Severe Combined Immuno-Deficiency) (Bosma 1983). In the case of oculocutaneous pigment dilution mutations, several spontaneously occurring mutations in mice result in effects on lysosomes and dense granules of platelets. The subsequent genetic mapping and eventual cloning of at least four homologous loci in humans has since demonstrated that these genes are responsible for the etiology and pathology of the Hermansky Pudlak Syndrome of human patients (Suzuki 2001; Suzuki 2002; Zhang 2003). SCID is the result of another of these serendipitous mutations that exemplifies the value and utility of these model systems. SCID mice lack both B and T cells, leaving them unable to mount any acquired immune response and were immediately identified as a model for patients with severely compromised immunity. Moreover, studies with these mice also demonstrated that human cells/tissues were not rejected following engraftment. Consequently, SCID mice provide a means of performing xenograft studies without the complications of transplant rejection (e.g., Schatton 2008).
Despite the utility of spontaneous mutations, these events are infrequent, occurring at a rate of ~5×10−6 per locus (Stanford 2001). In addition, phenotypic characterization of early mouse breeding colonies was usually the result of visual observations by animal husbandry staff. As a consequence, many of these spontaneous mutations were detected because of readily identifiable phenotypes such as unusual behavior, changes in the texture and/or color of the animal’s coat, illness, and/or death. Thus, the reliance on the incidence of spontaneous mutations subsequently gave way to other strategies collectively known as “forward genetic” approaches. That is, the initiation of a mutagenic event(s) followed by phenotypic screens to identify mice with a desirable genetic trait. Early forward genetic approaches used radiation and chemical treatments to induce mutations. One of the chemical mutagens, ethylnitrosourea (ENU) (Russell 1979), was such an effective agent that it is still widely used in several national/international large scale mutagenesis programs (e.g., http://mutagenetix.scripps.edu). Together with the development of high-throughput biochemical, immunological, and physiology-based screens, studies of the gene expression underlying many human diseases were now possible in vivo using this mammalian model (e.g., Hoebe 2006). In addition to these early mutagenic approaches, strategies have since been developed and employed based on directed gene recombination events. These recombination events include both insertional mutagenesis events and a collection of strategies to identify genes based on their patterns of expression described as “gene-trap” approaches. Random integration mutants have been described based on retroviral DNA insertion in the mouse genome (Soriano 1986; Lois 2002), mutants resulting from DNA integration events associated with the production of transgenic mice (Perry 1995), and recombination-mediated events using transposons such as Sleeping Beauty and PiggyBac (Luo 1998; Dupuy 2001; Ding 2005). In contrast, “gene trap” approaches are not intended to generate mutants but represent a correlative strategy that relies on engineered recombination events to identify genes with provocative patterns of expression through the use of reporter genes such as $-galactosidase or Green Fluorescent Protein (GFP). Specifically, gene trap vectors typically have reporter genes that are not expressed unless the random integration event integrating the vector into the genome occurs in an intron or exon of a transcription unit. In this case, the integration event results in reporter gene expression that reflects the expression pattern of the endogenous gene or is influenced by “neighborhood effects” mediated by transcriptional regulatory elements in proximity of the integration site. Moreover, the reporter gene itself represents a molecular tag for the cloning and identification of the sequences adjoining the integration event. To date, several initiatives have used to these methodologies as part of larger strategies, including the use of both retroviral-based gene trap approaches (e.g., “ROSA” strains of mice (reviewed in Zambrowicz 1997)) and transposon-based strategies again using Sleeping Beauty or PiggyBac-based transposon vectors (see for example Wang 2008).
Gene transfer technologies in mice have also allowed for the generation of novel genetic alterations with which to develop hypothesis-driven studies testing the relevance of unique cell types and/or genetic pathways in diseases. In this “reverse genetic” approach a gene of interest is identified as a result of association with human disease and becomes the target of gene transfer studies in mice. Specifically, the abilities to introduce exogenous structural and regulatory gene sequences into the mouse genome (i.e., transgenic mice) and/or to ablate/modify the expression of specific endogenous mouse genes (i.e., knockout/knockin mice) have alone propelled this animal to the forefront of biomedical research of human health and disease pathology.
The production of Transgenic Mice: Introduction of gene sequences into the mouse genome
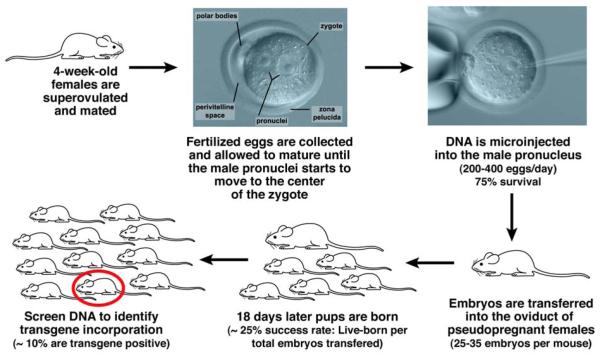
In the early 1980’s several research groups (Gordon and Ruddle (Gordon 1981), Costantini and Lacy (Costantini 1981), Harbers et al (Harbers 1981), EF Wagner et al (Wagner E. F., Stewart 1981), TC Wagner et al (Wagner T. E., Hoppe 1981)) developed the necessary strategies to introduce exogenous coding sequences of DNA into the mouse genome, conferring Mendelian inheritable traits. Specifically, gene transfer in mice became possible through the physical introduction of DNA through microinjection of defined sequences into one or both pronuclei of zygote-stage embryos. Microinjected embryos were then surgically transferred into pseudopregnant recipient females where embryonic development proceeds unperturbed, yielding newborn mice approximately 20 days following the procedure (Figure 1). In its early stages, this technique had an almost wild west “shoot from the hip” character with many mistakes and missteps (Lacy 1983). However, years of experience and the pains/joys of trial and error by numerous investigators have yielded an almost artful science by which lines of mice are created that express specific genes in both spatially and temporally defined patterns (Hogan 1994).
Figure 1. Overview of the creation of transgenic mice.
The fundamental strategy for the production of these strains of mice is ultimately straight-forward: the physical introduction of DNA into a pronucleus of a fertilized egg where it randomly integrates into a single chromosomal location within the genome. Injected eggs are subsequently returned to a surrogate mom to complete gestation with 2% of the injected eggs surviving the process and producing mice carrying the gene of interest (i.e., the transgene).
Innumerable transgenic lines of mice constitutively expressing disease-related candidate genes (“transgenes”) have been created to investigate the consequences of gene over-expression. The vast majority of these transgenic models have been constructed using bacterial (i.e., prokaryotic) plasmid vectors containing cDNA clones of gene-specific transcripts, genomic sequences encoding a gene of interest, or cDNA-genomic gene hybrids (Hogan 1994). Although now a commonplace technology, technical difficulties blocking transgene expression were initially encountered that prevented the generation of transgenic mouse lines which reproducibly displayed correct spatial and temporal specific gene expression. These difficulties included, but were not necessarily limited to, the recognition that expression constructs used in DNA microinjection could only include limited amounts of bacterial vector sequences (Lacy 1983) and that the DNA microinjection constructs must include the basic recognition sequences eukaryotes use to process nuclear RNAs to form translatable cytoplasmic mRNAs (e.g., the poly(A)-addition signal sequence and exon-intron splicing motifs (endogenous or exogenous) associated with most protein-encoding mRNAs (Palmiter 1991)). The resolution of these logistical problems has since ushered in a period of ever-expanding growth in the creation and use of transgenic lines of mice as models of human disease. The consequences of constitutive expression of growth hormone (Palmiter 1982) or various inflammatory cytokines (e.g., IL-5, IFN-(, IL-17) associated with allergic inflammation (Lee J. J. 1997; Seery 1997; Kim 2002) highlight models that have significantly impacted our understanding of human disease. In addition, other studies have assessed the mechanistic consequences of gene expression in the wrong cell and/or at the wrong time with the goals of defining a broader understanding of gene function and the biochemical/physiological consequences of dysregulated gene expression. For example, transgenic expression of candidate genes that were identified from earlier cell-based and/or patient-based studies demonstrated the feasability of inducing tumorigenesis through tissue-specific expression of oncogenes (Brinster 1984). In yet another example, this technology has also been useful in studies defining embryonic cell fate during gestation by spatial/temporal miss-expression of Hox genes (Wolgemuth 1989).
The realization that homeostatic baseline of healthy individuals and the pathologic events associated with disease are each characterized by specific patterns of enhanced and/or reduced levels of gene expression has made the value of transgenic mice to model human disease difficult to overestimate. Several specific examples highlight the significant utility of these mouse models in the understanding of patient health and disease:
The dramatic increase in our understanding of the complexities of gene expression patterns in patients has become the driving force for the identification and further characterization of mice expressing reporter genes to assess expression before during and after disease onset (e.g., (Hu-Li 2001; Keller 2009)). Additionally, transgenic animals expressing cytocidal gene products have been created to determine the consequences of ablating specific cell types (e.g., Lee James J. 2004; Perl 2011).
Although models of constitutive transgene expression in mice are informative, they inaccurately reflect what occurs in disease states. For example, the level of gene expression in transgenic mice is often quantitatively much different relative to the endogenous level observed in wild type mice. In some cases, concerns have been raised that phenotypes observed in adult animals occurred, in part, as a consequence of constitutive gene expression during embryogenesis (Ray 1997). Moreover, concerns have been raised that persistent constitutive expression of a transgene would have undesirable consequences that were not representative of the “on again-off again” pattern of gene expression associated with most diseases, including those chronic in character. As a result, strategies were developed that allowed investigators to control the expression of transgenes by simple benign inclusion of a drug or an inert small molecule. These strategies include the administration of tetracycline and/or doxycycline (Figure 2) in tetracycline/doxycycline-inducible mice (Lewandoski 2001), administration of ecdysone in steroid - inducible mice (Saez 2000), exposure to tamoxifen in ERt mice (expression of nuclear proteins only (Feil 1996)), or the induction of gene expression mediated by the administration of IPTG to inducible Lac-I mice (Cronin 2001)).
Gene regulation that occurs on the order of large chromosomal segments has also been assessed using transgenic mouse approaches with large DNA constructs containing multiple gene sequences such as BAC (Bacterial Artificial Chromosomes (≥100kb) or YAC (Yeast Artificial Chromosomes (≥500-1000kb)). The use of these vector systems is greatly facilitated by the incredibly high rates of homologous recombination possible in bacteria and yeast, permitting high-throughput recombineering strategies leading to the production of complex genetic alterations within the large DNA constructs associated with these approaches (reviewed in Giraldo 2001). The use of artificial chromosome vector strategies are highlighted by studies of the enhancer and/or chromatin-domain effects such as those associated with the locus control region (LCR) driving Th2 cytokine expression linked with allergic diseases (Lee G. R. 2003). In these studies, large genomic regions derived from BAC clones were used in transgenic strategies to define, and subsequently validate, the extent to which this LCR contributes to the regulation of Th2 cytokines levels. Studies assessing the importance of specific SNPs and or chromosomal rearrangements are currently underway and will likely make a significant impact on our understanding of disease-associated gene expression (see for example Jiang 2005).
Viral germline transgenesis represents another strategy to introduce exogenous coding sequences of DNA into the mouse genome. The use of viral vectors as a methodology was initially attempted as early as the ’70s (e.g., (Jaenisch 1976)), prior to the successes of pronuclear microinjection. However, these early investigations encountered difficulties with silencing of the transgene due to de novo DNA methylation after insertion. Thus, while germline transmission of the transgene was accomplished, expression was problematic. Lentivirus (a retrovirus family member) - mediated gene transfer has overcome this issue and provides a new and exciting method of germline gene manipulation (Lois 2002). In these studies, the gene(s) of interest is inserted into a lentiviral vector derived from the human immunodeficiency virus (HIV) which has been manipulated such that once genomic insertion takes place there is no further replication of the viral vector. Transgenic mouse production is achieved by introducing the engineered lentivirus (at the appropriate titer) by simple injection of virus into the perivitelline space (the area between the egg/zygote and its protective “shell” (i.e., zona pellucida)) of a zygote-stage embryo. Similar to traditional transgenic mouse production by DNA microinjection, virus-infected embryos are then surgically transferred into recipient females, yielding newborn mice approximately 20 days after the procedure. Relatively high rates of transgenesis have been achieved by this method (>80% of pups may be transgene positive relative to the 10-20% experienced with pronuclear injection). However, because of the labor associated with creating lentiviral transgenes and the added complexity of manipulating lentiviral vectors/virus relative to the simple manipulation of plasmid DNA, relatively few lines have been created to date with this technique. Despite these difficulties, lentiviral transgenesis presents immediate possibilities for the production of “large-animal” transgenics or new species transgenesis where such integration efficiencies are very attractive. In addition, this technology has potential application with embryos from strains of mice with which it is difficult to perform traditional pronuclear DNA microinjection (e.g., BALB/cJ). Consequently, lentiviral transgenesis is likely to play an increasingly prominent role in the future of gene manipulation (Park 2007).
As noted earlier, studies have employed transposon-mediated strategies as a means of generating transgenic mice (reviewed in Shinohara 2007). Similar to lentivirus-mediated transgenic approaches, the complexities of expression vector construction and/or transfection are generally off-set by dramatically increased efficiencies of germline integration possible with transposons relative to DNA microinjection of more classical transgenic expression constructs. Moreover, transposon-based vectors offer a greater cargo capacity and have fewer concerns over handling relative to lentiviral approaches. However, concerns over continued transposon movement exist requiring creative and somewhat complex solutions (e.g., co-injection of transposase RNA which degrades quickly after initial integration of the transposon (Wilber 2006)). In summary, the utility of these approaches is yet to be fully realized but this developing technology holds significant promise for the future of transgenesis and potentially human gene therapy (see for example Wilson 2001).
Transgenic expression of micro RNAs (miRNA) or small interfering RNAs (siRNA) have been used to modulate gene expression by the degradation of mRNA or the blockade of translation, respectively (reviewed in Metzger 2007). The gradient of lowered gene expression levels occurring in these mice has similarities to the patterns of altered gene expression that may occur, for example, in patients with unique SNPs or other defined or unknown genetic abberations (see for example http://projects.tcag.ca/variation and http://ncbi.nlm.nih.gov/projects/SNP). The allelic p53 “knock-down” series described by Hannon and colleagues represents an important example of how these “hypomorph” lines of mice provide novel models of human disease (Hemann 2003)). Expanding libraries of these miRNAs and/or siRNAs are currently available (see for example, http://mirbase.org and (Chalk 2005)) and thus, provide invaluable resources for the design and creation of potentially significant models of human disease.
Figure 2. Production of transgenic mice leading to lung-specific gene expression.
(A) Transgenic founder mice constitutively expressing a gene of interest specifically in the lung. In this example, transgenic founder mice expressing the gene of interest in Alveolar Type II cells are generated by the injection of an expression cassette utilizing a promoter derived from the gene encoding the surfactant protein C (pSPC) together with the gene of interest and an additional DNA fragment derived from the human growth hormone gene (hGH) that supplies required RNA processing motifs for transgenic gene expression, including exon-intron junctions and a poly A addition signal sequence (p(A)+). Incorporation of this transgene leads to transgenic mice constitutively expressing the gene of interest from Alveolar Type II cells of the lung. (B) Transgenic founder mice whose transgene expression is an inducible consequence of the administration of antibiotics (i.e., doxycycline and/or tetracyclin). In the example shown here, three constructs are co-injected providing the necessary components to generate mice whose transgene expression is stringently regulated. In Alveolar Type II cells, the surfactant protein C promoter (pSPC) drives expression of both an inhibitory bacterial transcription factor (tet-TS) and an activating/permissive transcription factor (tet-TAM2), each of which is capable of binding a DNA regulatory region known as tetO. In the absence of doxycycline/tetracycline the binding of the inhibitory factor (tet-TS) to the tetO region is greater than the activating/permissive factor (tet-TAM2), silencing gene expression. However, administration of doxycycline/tetracycline to mice leads to conformational changes in the transcription factors such that the affinity of the activating/permissive factor (tet-TAM2) to the tetO region is now greater than the inhibitory factor (tet-TS), inducing expression of the gene of interest.
The production of Knockout/Knockin Mice: Deleting and/or modifying endogenous mouse genes
The latter half of the 1980’s witnessed the development of a new methodology that enabled the generation of mouse models with gene-specific null mutations and/or models carrying gene-specific modifications at the endogenous chromosomal position of a given gene. From the earliest mouse model studies of human disease this need was clear and apparent. Specifically, clinical studies of patients often revealed that the genetic basis of their disease resulted from either the loss of gene function (e.g., cystic fibrosis - CFTR (Riordan 1989)), genetic changes leading to the modification of expression levels (e.g., leukemogenic diseases - c-Myc (Amanullah 2002)), or the appearance of unique activities not occurring in healthy individuals (e.g., Marfan syndrome - FBN1 (McKusick 1991)). The development of mouse models of these diseases required new strategies to generate lines of animals with identical genetic changes; logistically, the gene-specific character of these proposed changes required a shift in experimental approach. The identification of, and subsequent abilities to manipulate, pluripotent stem cells from mouse embryos (embryonic stem (ES) cells) provided such an opportunity. Landmark studies by a series of independent investigators including, but not limited to, the individuals who were eventually awarded the 2007 Nobel Prize in Physiology or Medicine for this technology (i.e., Martin Evans (Evans 1981), Oliver Smithies (Smithies 1985), and Mario Capecchi (Thomas 1986)) led to a unique strategy of generating mouse models with gene-specific changes leading to the generation of null alleles that were Mendelian-inheritable traits. This strategy, summarily known as gene targeting (and/or the generation of knockout mice), exploited the accessibility and plasticity of ES cells and experimental strategies to mediate gene-specific changes in these cells. The ability to return genetically modified ES cells to early embryos, where they contribute to the various cell lineages of the recipient embryo (including the germ line), permitted genomic changes created in cultured ES cells to be inherited by successive generations of mice (Figure 3). Early studies using ES cells to generate gene knockout strains of mice were limited to the “129” genetic background strain due to historical issues related to the the development of ES cells and the perceived ease of generating ES cell lines in this strain (reviewed in Draper 2007). This led to the subsequent wide spread use of these cell lines because of “if it isn’t broke why fix it” perspectives of various investigators. Unfortunately, it was recognized early on that these genetic manipulations often resulted in phenotypes (e.g., changes in physiologically relevant parameters) that varied as a function of the background strain (see for example Duguet 2000; Shinagawa 2003). This necessitated laborious, time consuming, and expensive backcrossing of founder animals to generate mice whose altered locus is congenic with background strains commonly used for other mouse models of human disease, typically C57BL/6J or BALB/c. More recently, the advent and now common use of ES cells derived from C57BL/6J or BALB/c offers an alternative option that allows gene knockout (and now knockin) strains of mice to be generated directly on these genetic backgrounds (reviewed in Seong 2004).
Figure 3. Production of knockout mice: Gene targeting leading to the generation of animals deficient of specific genes of interest.
Gene knockout mice are generated in a two staged process that utilizes pluripotent embryonic stem (ES) cells as a vehicle with which to translate experimental genetic manipulations into Mendelian inheritable traits in mice. (A) Using now standard “off-the-shelf” technologies a DNA targeting construct containing specific regions of homology to the gene of interest is transfected into ES cells (usually by electroporation). In the nuclei of these transfected ES cells homologous recombination events occur leading to a 1-to-1 replacement of the endogenous gene with sequence derived from the targeting construct. This process is greatly facilitated by a selection process that identifies ES cell clones that have successfully undergone a targeting event by positive (expression of a neomycin resistance (NEOR) gene) and negative (expression of the herpes simplex thymidine kinase (TK) gene) drug selection strategies while the cells are in culture. Nonetheless, the frequency of occurrence of these homologous recombination events is incredibly small. For example, the frequency of stable transfection is <0.1% and successful homologous recombination events generally occur at a frequency of 1-5% among cell clones identified by the drug selection schemes, yielding an overall frequency of success < 1 in 105 events. (B) Targeted ES cell clones containing the appropriate genetic changes that ablate expression of the gene of interest are transferred to the blastocoel cavities of 3.5 day blastocyst embryos (generally 10-15 ES cells/host embryo) and, in turn, the embryos are transferred to surrogate mothers where gestation is completed. At a frequency highly dependent on the quality and care with which the ES cells are maintained, transferred targeted ES cells contribute to embryonic development, generating ES cell-derived chimeric founder mice with varying degrees of chimerism - in this example, judged by ES cell (black strain of mice) contribution to coat color when transferred into blastocyst embryos from a white strain of mice. Commonly, one or more high chimeric founders are crossed with mice of the background strain from which the ES cells were derived to generate ES cell-derived founder mice which have inherited the targeted gene, generating a null-allele at the chosen genetic locus.
The utility of mouse models representative of genetic lesions found in human patients has far exceeded even the lofty expectations of early investigators. Studies using these models have provided a wealth of mechanistic data identifying unique cellular and molecular pathways as well as the independent and/or overlap characters of these pathways in mammals. Specifically, “Systems Biology” (the science of understanding the complex interactions in biological systems) and the era of “omics” - genomics, proteomics, metabolomics, etc. - have become paradigms to explain the etiology of many human diseases, in part, as a consequence of the generation and subsequent characterization of mice resulting from gene targeting strategies. The evolution of these gene targeting strategies has been both brisk and complex, transforming what originally was a “black box” science into an activity utilized ubiquitously in biomedical research laboratories. As noted earlier, the “first generation” mouse models that resulted from gene targeting were the so-called knockouts; that is, animals with engineered null mutations for specific genes (Figure 3). Their value to the understanding of systemic biochemistry and physiology of mammals was incalculable, exemplified by the meteoric rise in the number of published reports describing knockout lines of mice deficient for selected gene products. The generation of knockout mice lacking the Trp53 tumor suppressor gene (p53−/− mice) is a classic example of the usefulness of this technology to model human disease (Donehower 1992). Similar to observations from human cancer patients, the loss of Trp53 in knockout mice was linked to the onset and progression of many tumors (e.g., lymphomas and osteosarcomas), providing researchers with an invaluable model of this component of carcinogenesis. In summary, these models provide a direct link with events occurring in many diseases. Indeed, assessments of the knockout phenotypes for the targets of the 100 best-selling drugs show that these phenotypes correlate with the known drug efficacies (Zambrowicz 2003). It is noteworthy that while the development of new knockout mice no longer appears on the cover of major journals nearly every other month, the remaining importance and underlying need to generate/distribute new knockout lines of mice are highlighted by the creation of investigator-driven consortiums (see for example, http://genome.gov/17515708), the goals of which are to generate null alleles (i.e., knockout mice) for every gene of the mouse genome (reviewed in Rosenthal 2007). Moreover, as part of the trans-NIH initiative Knockout Mouse Program (KOMP, http://komp.org) an effort to generate knockout mice (and/or ensure the availability of targeted ES cells) corresponding to each locus of the mouse genome (Collins 2007). More importantly, this initiative has recently been significantly expanded to include the phenotyping of up to 1500 of these knockout mice with the data available to investigators as a searchable phenotype database (http://kompphenotype.org).
Gene knockout mice have also proven to be invaluable components in conjunction with transgenic lines of mice as a means of generating “humanized” mouse models as part of drug discovery strategies. The production of human antibodies from transgenic farm animals had its origins in studies utilizing such a compound knockout/transgenic mice approach (reviewed in Lonberg 2005). Specifically, two independent groups (Green 1994; Lonberg 1994) each utilized homologous recombination in ES cells to engineer similar disruptions of the endogenous mouse heavy- and 6 light-chain antibody encoding genes. In one report, Lonberg and colleagues (Lonberg 1994) then used pronuclear microinjection to introduce recombinant mini-loci into the knockout mice representing both a limited human heavy chain locus (i.e., 3 heavy chain variable(VH), 16 diversity (D), all 6 heavy-chain joining (JH) regaions, and: and (1 constant-region gene segments) as well as a limited human light chain locus containing 4 V6 segments, all 5 J6 regions, and the 6 constant segment (C6). In contrast, Green and colleagues (Green 1994) generated mice with similar human heavy and 6 light chain mini-loci via DNA transfection into ES cells using yeast protoplasts to deliver a yeast artificial chromosome (YAC)-based vector. These studies subsequently demonstrated that uniquely human humoral responses (i.e., human polyclonal antibody production) to sensitizing antigen were possible from these mice, including the establishment of hybridoma cell lines secreting fully human monoclonal IgM and IgG antibodies. In turn, this set the stage for subsequent studies in larger farm animals such as the cow (Kuroiwa, Y. et al., (2002) Nat. Biotechnol. 20, 889-894, Kuroiwa, Y. (2004) Nat. Genet. 36, 775-780) for the large-scale production of antigen/pathogen-specific human polyclonal antibodies.
Interestingly, in addition to replicating genetic deficiencies that were initially shown to be the cause of specific human diseases, knockout mice have also provided significant insights regarding the larger consequences of gene expression in mammals including humans. For example, the generation of knockout mice quickly demonstrated that some genes, previously identified only from their role in disease, were also necessary during embryogenesis (e.g., Vcam-1 (Kwee 1995) and Brca-1 (Hakem 1996)). The generation of knockout mice has also revealed that many genetic functions in mammals are redundant and the targeted loss of genes that were thought to be critical sometimes had little to no effect (e.g., Joyner 1991). Although the redundancy of gene function added complexities not predictable on first principles, this phenomenon has often revealed previously unknown and/or underappreciated molecular/cellular pathways that possibly represent new targets for therapeutic intervention. Some studies involving knockout mice have even demonstrated significant differences between mice (i.e., rodents) and humans (i.e., primates). For example, whereas the loss of the CFTR gene in humans is specifically linked to the pulmonary pathophysiological changes of cystic fibrosis (Riordan 1989), mice carrying a similar null mutation display virtually no lung phenotype (Snouwaert 1992). Such studies, while frustrating and seemingly unproductive with regard to modeling human diseases, serve as insightful investigations that attest to the complexity of mammalian biochemistry/physiology and a cautionary note to investigators using mice to model human disease.
The ability to generate null alleles for specific genes clearly provided investigators with an invaluable tool with which to define the role(s) of individual loci. However, strategies based on this technology had a very important shortcoming: Gene ablation occurred in all cells of the mouse and was present even in zygote stage embryos. That is, whereas gene ablation in many knockout mice had specific and easily defined consequences, knockout mice resulting from the ablation of genes that were expressed in multiple cell lineages often had complex phenotypes. An example of this is the observed perturbations and/or the loss of multiple hematopoeitic lineages as a consequence of eliminating the expression of a single transcription factor, Gata-1 (Pevny 1991). In addition, these complex phenotypes may arise because of an embryonic lethality associated with the loss of disease-important genes that are also critical to the successful completion of embryogenesis; as noted above representative examples include VCAM-1: “4 integrin cell adhesive interactions in leukocytes (Springer 1995) as well as extraembryonic lineages (Kwee 1995) and the functions mediated by brca-1 in both mammary tissue (Xu 1999) and early embryos (Hakem 1996). Thus, the embryonic lethal consequence of losing either of these genes limits the usefulness of the respective knockout mice as disease models that by necessity require using adult animals. These complex phenotypes are also often particularly difficult to understand because in some cases the molecular and cellular pathways are not fully understood and in other cases it is difficult to ascribe specific phenotypic events, including which cells are affected, to the loss of a given gene.
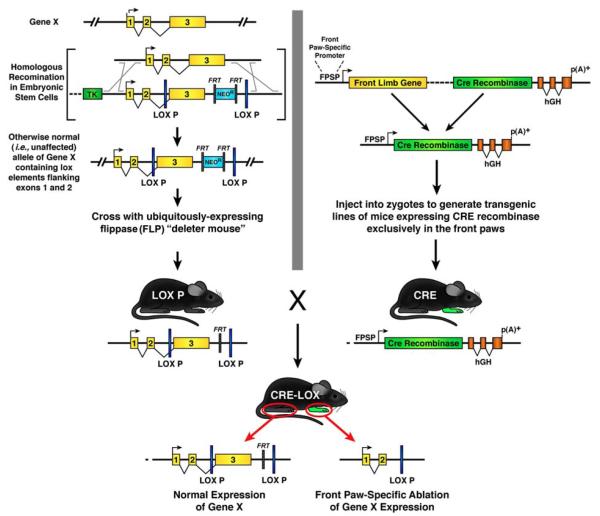
The need to address complex phenotypes gave rise to another generation of knockout mice, the so-called “conditional and/or inducible knockouts” (Figure 4). This experimental strategy capitalizes on the characterization of mechanisms (reviewed in Lewandoski 2001) by which either bacterial phage mediate recombination gains access to the bacterial genome (Cre-LOX mediated recombination) or repetitive transposable sequence elements mediate recombination to move within the genomes of simple eukaryotes such as yeast (FLP-FRT mediated recombination). In these organisms, site-specific genetic recombination is achieved by the recognition of small (25-50bp) repetitive sequence elements, LOX P or FRT, by the cognate recombinases Cre or FLP, respectively. The basic strategy associated with the creation of inducible knockout mice is elegantly simple and capitalizes on the absence of these recombination pathways in mammals. ES cell manipulations are used to create a line of mice with recombination sequence elements flanking either the entire gene of interest or functionally important exons of that gene. In parallel, a traditional transgenic line of mice is generated expressing the cognate recombinase with a previously characterized cell-specific promoter. These two lines of mice are crossed (i.e., bred) to generate cell lineage specific knockout mice in which genetic recombination and, in turn, gene ablation occurs only in the cells that express the necessary recombinase. Although this strategy is more complex than a global gene knockout (i..e, in all cells of the mouse), gene ablation in these lines of mice is limited to specific cell populations. Thus, knockout mice with formerly complex compound phenotypes could be re-engineered to create lines of animals in which the loss of gene function occurs only in a specific cell type. The development of a mouse model to investigate the role(s) of the human BRCA-1 gene in breast cancer is a relevant example of the utility of this technology to model human disease. Specifically, the loss of brca-1 in knockout mice had embryonic lethal consequences preventing the use of these mice in breast cancer studies (Hakem 1996). However, by limiting the gene deficiency only to mammary gland tissue using a Cre-LOX strategy, investigators were able to generate mouse models to study breast cancer in adults by overcoming the embryonic lethality associated with the original knockout line of mice (Xu 1999). Interestingly, the bottleneck associated with these approaches is often not the availability of lines of mice with genes (and/or exons) flanked by recombination sites (so-called “floxed mice” when it involves Cre-Lox strategies) but the availability of mice expressing recombinases in a unique cell type(s). Nonetheless, consortia efforts to generate both “floxed mice” (e.g., http://jaxmice.jax.org/list/sprs_creRT.html#xprs1804) and cell-specific Cre-expressing (i.e., “Cre-driver”) lines of animals (e.g., http://informatics.jax.org/recombinase.shtml) are now beginning to eliminate this strategic gap.
Figure 4. Development of conditional (inducible) knock out mice with temporal, cell, and/or tissue specific ablation of gene expression.
To restrict the loss of gene expression in knockout mice in time or location with the body, a binary system of unique strains of mice are developed that utilizes both ES cell-derived genetic manipulations and traditional transgenic technologies. One strain is developed (LOX P) through ES cell manipulations leading to a homologous recombination event that inserts small repetitive sequence elements which are the recognition sequences of DNA recombination enzymes. One set of these repetitive sequence elements (FRT) flank the drug selection markers, facilitating the removal of these markers by breeding the ES cell-derived mouse with a transgenic animal expressing the DNA recombinase recognizing the FRT sequence elements (flippase (FLP)) either ubiquitously (i.e., in all cells) or in cells of the germline. Another set of repetitive sequence elements (LOX P) flank an important sequence region of a gene necessary for expression, including, but not limited to, protein encoding exons, RNA processing motifs, or cis-regulatory elements. In this example, the LOX P elements flank exon 3 of Gene X. The second strain of mice (CRE) necessary for this binary system is a transgenic line expressing the DNA recombinase that recognizes the LOX P repetitive elements (i.e., Cre recombinase) in a temporal and/or spatially restricted pattern. In this example, a constitutive transgenic line of mice is established expressing the Cre recombinase only in the paws of the front limbs of the animals using a “front paw-specific promoter”. When the binary system is complete and the LOX P mouse is crossed with the CRE line of mice the resulting offspring (CRE-LOX) will express Cre recombinase in the front paws and in doing so mediate the deletion of exon 3 of Gene X through a Cre-mediated DNA recombination event between the LOX P sequence elements. Thus, in CRE-LOX mice, Gene X is expressed normally in all tissues of the body with the exception of the front paws where the induced genetic ablation of exon 3 renders a null allele of Gene X in this region. It is noteworthy that in addition to spatially restricted knockouts, gene ablation can be restricted temporally through the selective generation of a transgenic Cre recombinase mouse using a promoter that mediates Cre expression at a unique time (e.g., at a specific point during gestation) or under inducible control (e.g., Ert-Cre).
Traditional genetic studies (regardless of the organism under study) exploited mutations that resulted in both loss of function as well as gain of function. In humans, these latter mutations have often proved invaluable because, in addition to identifying genes of interest, they also provided specific mechanistic insights not possible by gene ablation. The current generation of models developed by gene targeting strategies attempts to create similar gain of function mutations in mice by engineering specific sequence changes as opposed to gene deletion (so called “knockin models”). These knockin lines of mice did not result from the development of new technologies/methods but instead arose from a more sophisticated use of existing strategies employed in earlier gene targeting efforts (Figure 5). Specifically, gene targeting vectors previously designed to mediate the deletion of gene sequences (gene knockouts) were now crafted to mediate the one-for-one replacement of sequences so that defined base sequence changes could be engineered into the genetic locus of interest. These experimental strategies also took advantage of the methodologies used to create conditional knockout mice by using site directed recombination as a means of erasing evidence of genetic manipulations other than the targeted sequence changes of interest. Despite representing the most difficult (and expensive) strategy to develop a gene targeted mouse, these knockin models are very informative, allowing for the introduction of specific genetic aberrations based on earlier in vitro as well as in vivo studies. Moreover, these strategies allow very targeted and limited changes, often replicating the genetic aberrations occurring in patients. Thus, these “next-generation” knockin models have become the “workhorses” of hypothesis-driven studies whose goals are to define gene function and to integrate these definitions into broader models with which to explain organismal biochemistry and physiology; in many ways representing significant leaps forward in our understanding of gene function and its role in both health and disease (relevant examples reviewed in Roebroek 2003).
Figure 5. Production of knock-in mice: The generation of animals expressing unique genetic alleles of a given gene of interest.
Gene knock-in mice similar to their earlier knockout counterparts are generated in a two staged process that utilizes pluripotent embryonic stem (ES) cells as a vehicle with which to translate experimental genetic manipulations into Mendelian inheritable traits in mice. In these strategies, the DNA targeting construct not only contains specific regions of gene homology but also has a uniquely engineered mutation or sequence change such that the 1-to-1 replacement of the endogenous gene with sequence derived from the targeting construct following transfection into ES cells yields an allele in the genome of these cells containing this new sequence variant. In the example here, exon 2 is replaced with a modified version of this sequence information through this gene knock-in process. As noted earlier, targeted ES cell clones containing the appropriate genetic changes are transferred to the blastocoel cavities of 3.5 day blastocyst embryos. In turn, the embryos are transferred to surrogate mothers where gestation is completed generating ES cell-derived founder mice which have inherited the new sequence variant (i.e., the knock-in mutation), generating a gain-of-function allele at this chosen genetic locus.
The development of genetically engineered mouse models of increasing complexity to replicate the unique patterns of gene expression occurring in many human diseases
Investigators trying to understand human disease understandably focused initially on pathologies whose causal origins were simple and direct; for example, investigations of diseases with single gene origins. However, it soon became apparent that this was a limited strategic approach as the origins of most human diseases are rooted in elaborate cellular and genetic pathways involving multiple genes and/or complex patterns of gene expression. As a result, increasingly complex mouse models were required to replicate these genetic aberrations (see for example Nguyen 2007; Roes 2007; Vignjevic 2007; Wamhoff 2007). The development of compound transgenic/knockin conditional mice highlight this approach through the production of animal models by combining transgenic and ES cell-based strategies.“Knockout-first gene trap” mice represent an early attempt to modulate complex patterns of gene expression using this strategy (Testa 2004). This variation of the “gene trap” approach that was described earlier is one of the primary technologies employed by the International Knockout Mouse Consortium (www.knockoutmouse.org). Knockin lines of mice at a given locus are generated by concurrently flanking sequences of importance (e.g., an exon) with LoxP sites and introducing an FRT flanked reporter cassette containing both splice acceptor sites and a polyadenylation sequence into an intron of the target gene. Thus, before an exon is excised by Cre-mediated recombination, the gene of interest is already effectively knocked out, encoding a truncated protein which may be traced via the reporter gene incorporated into the knockin allele. The investigator is then strategically left with a choice of simply leaving the reporter gene in place (resulting in a constitutive knockout line of mice - “knockout-first”) or removal of the reporter cassette leaving the LoxP flanked exon in place which may be subsequently deleted in a specific cell population or at a unique point in time. In some instances splicing events may bypass the reporter cassette leaving some level of gene expression resulting in a hypomorphic allele. The value here is the possibility of using the reporter cassette deliberately as a means of creating reduced levels of gene expression, having utility in situations where deletion of the target gene would otherwise result in an embryonic lethality or where reduced expression levels are informative.
Given the specificity and otherwise unique character of the pattern of gene expression occurring in human diseases (both spatial and temporal), it was also only a matter of time before enterprising investigators would develop equally complex strategies to replicate these unique gene expression patterns in mice. For example, compound transgenic/knockin conditional models have been developed to inducibly express genes and/or to create conditional gene fusions. The potential of inducible gene expression in these models is exemplified by the “floxed-stop” cassette approach (see for example Wu 2010)). In this approach, a knockin line of mice is created with an exon containing a stop codon flanked by loxP recombination sequences in direct sequence orientation. This knockin allele generates an mRNA whose translation generates a non-functional truncated product (effectively creating a gene knockout). However, following a cross with a specific Cre-driver transgenic line and the induced excision of the stop codon, cell-specific expression of the knockin locus now occurs. A further refinement of this strategy by crossing the knockin line of mice with an inducible Cre-driver transgenic line allows for both the spatial and, more importantly, the temporal-specific expression of sequences in these compound transgenic/knockin lines of mice. In addition, mouse models have been created using a similar, but modified, approach by engineering an exon of the knockin locus in the opposite direction of transcription that is also flanked with LoxP sites that are in an indirect sequence orientation (reviewed in Branda 2004). In this case, the open reading frame of the knockin locus is disrupted by the exonic sequences in the wrong direction, thus effectively creating a knockout allele. However, when crossed with a specfic Cre-driver line of mice, recombinase expression now promotes the inversion of the sequences between the indirect-oriented LoxP sites, thus restoring the open reading frame (and in turn promoting gene expression). An even more recent variation of this strategy used a LoxP-mismatched Cre/Lox cassette to yield stochastic gene expression that led to the random and progressive death of specific cell types. These investigators used this strategy to generate mouse models of denigrative diseases such as diabetes, wound healing, and progressive deafness (Masato 2011).
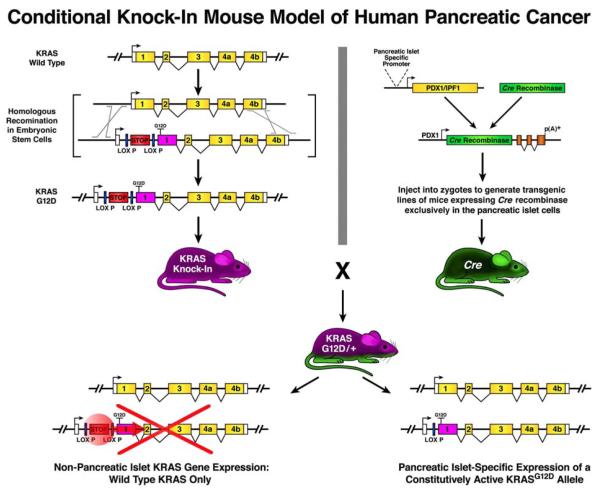
Some of the more sophisticated uses of compound transgenic/knockin conditional lines of mice are found in studies of cancer where investigators are required to replicate both the cell specific character and temporal spontaneity of neoplastic events. The recently developed mouse models of tumor-specific chromosomal transloctions (Forster 2005), BRCA1-associated breast cancer (Drost 2009), and preinvasive/invasive ductal pancreatic cancer described by David Tuveson and Tyler Jacks (Hingorani 2003) highlight both the utility and elegant complexities of these compound conditional lines of mice to replicate human disease. Specifically, these compound conditional models utilize mulitple unique strains of mice, none of which alone results in neoplastic transformation. The details of the Tuveson/Jacks pancreatic cancer model are particularly instructive and characterize the complexity of this experimental strategy and these compound conditional mouse models (Figure 6). The first of the two mouse strains comprising the pancreatic cancer model is a Kras knockin line of mice with an engineered mutation (KrasG12D) promoting constitutive activation but whose expression is prevented by a LoxP flanked stop codon. The second line of mice is a transgenic animal that utilizes the pancreatic glandular epithelial-specific promoter from the Pdx1 gene to drive cell-specific Cre recombinase expression. While neither of these mouse strains develops pancreatic cancer, offspring from crosses of these two strains of mice (Pdx1-CreTg/−/KRASG12D/+) replicate the full spectrum of human pancreatic cancer, developing spontaneous grade 2 and 3 pancreatic intraepithelial neoplasias (PanIN-2, -3) by 5 months of age with some lesions progressing to fully invasive adenocarcinoma with metastasis to distant organs. This approach thus achieved several important milestones toward the generation of a useful mouse model replicating human pancreatic cancer in mice: (i) Neoplastic transformation replicating human disease through the expression of a mutation commonly found in human pancreatic patients (Gene knockin/ES cell based technology); (ii) Expression of this oncogenic mutation was achieved exclusively in the targeted pancreatic islet cells (transgenic mouse production); and (iii) The compound character of the model allowed for the maintenance/breeding of mice independent of cancer development (Cre-lox based inducible gene expression).
Figure 6. Compound transgenic/conditional knockin mouse model of human pancreatic cancer.
The complexity of neoplastic transformation occurring in pancreatic cancer was replicated in a mouse model through a unique binary approach breeding of a knockin animal capable of inducible expression of a constitutively active oncogene with a cell-specific Cre-expressing transgenic mouse. The first component of this compound model is a knockin line of mice (KRAS/Knock-In) generated using ES cells. These mice harbor an inducible allele of KRAS that was constitutively active. Specifically, the engineered KRAS locus in these knockin mice was created by inserting a genetic element upstream of a exon 1 that inhibit transcription/translation flanked by functional LoxP sites (LoxP-STOP-LoxP sequence element). In addition, the open reading frame of this KRAS allele was modified with a G to A transition in codon 12 of the open reading frame to produce a constitutively active variate of KRAS (KrasG12D) commonly found in human pancreatic ductal adenocarcinoma. The second engineered mouse of this compound model is a transgenic line (PDX-Cre) expressing the Cre recombinase exclusively in pancreatic islet cells through the use of a promoter fragment from the PDX-1 gene. Each of the component line of mice are maintained as “stand alone” strains with neither developing pancreatic cancer due to the expression of only wild type KRAS. However, offspring resulting from a cross of these mice (Pdx1-CreTg/−/KRASG12D/+) recapitulate the full spectrum of human pancreatic intraepithelial neoplasias (PanINs); some of these lesions even becoming invasive and metastatic.
CONCLUSION/SUMMARY
The development and characterization of increasingly complex mouse models have had profound effects on discoveries pertinent to gene function in mammalian systems. As we move forward, the expectation is that these insights will have ever increasing translational benefits for human patients. The robust understanding of mouse genetics, immunology, biochemistry, and physiology, and gene transfer technologies have now catapulted these rodent models to the forefront of preclinical studies, including the definition/validation of cellular and molecular mechanisms of disease, the creation of novel therapies, and the refinement of existing therapeutic modalities (Figure 7). Moreover, the delivery of health care in general is becoming increasingly dependent on a variety of diagnostic assays assessing an individual’s “genomic fingerprint” (e.g., the identification of unique SNPs) as well as a patient’s cellular/tissue/organ-specific pattern of gene expression. The role of mouse models of human disease will thus become ever more critical; the validation/development of diagnostic assays and patient targeted therapeutic modalities represent just two examples. It is noteworthy that a number of the gene transfer technologies first pioneered in the mouse have also made profound indirect contributions to human health and welfare. For example, the gene transfer technologies developed in mice, including the cloning of potentially important animal models (reviewed in Thomson 2004), are now being used to genetically manipulate larger animal species (e.g., domestic livestock) which may have an increasingly vital role in our food supply. In addition, the application of gene transfer technologies and assisted reproductive strategies in these larger animals has led to the development of important and often critical new sources for the production of pharmaceuticals. Ironically, during the last 10,000 years mice have been taking advantage of human shelter and food supplies. Furthermore, they have been the source of many human diseases that have led to untold death and suffering. Nonetheless, in the last 30 years the development and use of gene transfer technologies have allowed mice to shed the mantle of pest and assume the role of our greatest ally in the fight to cure human diseases.
Figure 7. The utility of genetically-modified mouse models in biomedical research.
Gene transfer technologies have been, and continue to be, very productive components of a larger approach with which to generate clinically relevant mouse models of human disease that are integral to our understanding of health and the development of new/novel therapeutic modalities.
ACKNOWLEDGMENTS
The authors wish to thank the members of Lee Laboratories as well as colleagues in the research community for insightful discussions and critical comments during the preparation of this review. Moreover, we also wish to acknowledge the tireless efforts of the Mayo Clinic Arizona medical graphic artist Marv Ruona and Joseph Esposito of Research Library Services. In addition, we wish to express our gratitude to the Lee Laboratories administrative staff (Linda Mardel and Charlie Kern), without whom we could not function as an integrated group. This effort was supported by the Mayo Foundation for Medical Education and Research and grants from the National Institutes of Health to JJ Lee (HL065228 and K26-RR019709) and NA Lee (HL058723) as well as a grant from the American Heart Association to JJ Lee (0855703G). Support for A Doyle is provided by a Mayo Clinic Sidney Luckman Family Predoctoral Fellowship.
Glossary/Definition of Terms
| blastocyst embryo | A day 3.5 embryo with 64-128 cells comprised of essentially two cell types: trophectoderm cells contributing to extra-embryonic membranes/tissues and inner cell mass cells (ICM) that lead to the embryo proper. In the mouse, these embryos characteristically have a diamond ring-shape appearance with a hollowed-out cavity (blastocoel) as a consequence of hydrostatic pressure generated by the trophectoderm cells |
| cDNA clone | Double stranded DNA copy of a messenger RNA (mRNA) encoding a specific gene |
|
conditional and/or inducible knockouts |
Mice generated by the genetic manipulation of embryonic stem (ES) cells such that a specific genetic locus has been altered by the addition of recombinase recognition elements flanking the gene sequence, or region of importance, to facilitate its deletion from the genome (i.e., the generation of a null allele) in the cells of one or more defined lineages |
| congenic strain of mice | Strains of mice that are genetically identical except for a limited genetic region or locus. For instance, a homozygous strain of mouse can be said to be congenic to another strain of mice at a particular locus following 12 or more successive backcrosses to this new strain. Selection of offspring based on specific genetic markers at each successive generation can speed this process such that a congenic strain of mice is produced in as little as 5 backcross generations |
| DNA methylation | The chemical modification of genomic DNA occurring in many eukaryotes (particularly mammals) in which methyl (CH3) groups are added to cytosine bases as a mechanism of inducing heritable changes in gene function without a change in DNA sequence (i.e., epigenetic changes). Generally, methylation is usually associated with turning-off (i.e., silencing) gene expression |
|
Embryonic Stem cell (ES cell) |
A pluripotent undifferentiated cell derived from the inner cell mass (ICM) of a blastocyst embryo that can give rise to cells comprising any embryonic lineage, and therefore any tissue/organ, with the exception of the extra-embryonic lineages of the developing fetus |
|
exon-intron splicing motifs |
Sequence elements in nuclear RNA representing the boundaries of gene segments that will be included (exons) or excluded (introns) during RNA processing and the production of mature mRNAs exported to the cytoplasm for translation into protein |
| expression cassette | A DNA construct that contains the necessary transcriptional (i.e., promoter) and RNA processing motifs (i.e., exon-intron splicing and poly(A) addition recognition sites) to allow expression of a protein encoding sequence in a transgenic mouse |
| gain of function mutation | A genetic mutation that confers new and/or enhanced activity(ies) to a given gene |
| genome | The total genetic information contained within a single set of chromosomes (eukaryotes) or in the heritably transferred genetic information associated with bacteria, phages, or eukaryotic DNA/RNA viruses |
| germ line | The lineage of embryonic and/or adult cells devoted to the production of eggs (female) or sperm (male) needed for sexual reproduction |
| hypomorph | Engineered mouse that has reduced levels of gene expression relative to the level of expression observed in a wild type mouse |
| indel | Genetic mutation leading to a co-localized insertion/deletion resulting in the net gain or loss in nucleotides. A microindel is similarily defined as an indel that results in the net gain or loss of 1 to 50 nucleotides |
| Knockin (KI) Mice | Mice generated by the genetic manipulation of embryonic stem (ES) cells such that a specific genetic locus has been altered either by the one-forone substitution of DNA sequence information or by the addition of sequence information not found in the endogenous genetic locus |
| Knockout (KO) Mice | Mice generated by the genetic manipulation of embryonic stem (ES) cells such that a specific genetic locus is targeted and rendered non-functional either by the insertion of irrelevant DNA sequence information to disrupt the expression of the encoding locus or by the deletion of DNA sequence information from the targeted locus |
|
loss of function (i.e., null) mutation |
A genetic mutation that results in either the complete loss (null) or greatly diminished activity(ies) of a given gene |
| metazoan | Any of the animals belonging to the subkingdom Metazoa, having a body made up of differentiated cells arranged in tissues and organs. All multicellular animals besides sponges are metazoans |
| orthologue | A gene in different organisms that have diveraged as a consequence of speciation and not DNA gene duplication (i.e., paralogue) paralogue A gene in different organisms that have diveraged as a consequence of DNA gene duplication and not speciation (i.e., orthologue) |
|
poly(A)-addition signal sequence |
Genomic DNA sequence motif that promotes cleavage of the primary nuclear RNA transcript and the subsequent extra-genomic addition of adenine (A)-containing nucleotides to this cleaved RNA |
| promoter | cis-acting DNA sequence of a gene that independently (i.e., even when removed from the protein encoding DNA sequences of that gene) is able to “promote” gene expression through the binding of available transcription factors |
| pronuclei | The haploid nuclei of a sperm and egg present prior to fusion and the formation of the single nucleus of the zygote (i.e., single-cell stage of embryonic development including the time between fertilization and prior to the initial cleavage into a two cell-stage embryo) |
|
pseudopregnant-recipient females |
A female mouse following copulation with a vasectomized (or sterile) male in which many of the characteristics of pregnancy are present (without an accompanying fetus), allowing the introduction and subsequent implanation of embryos following adoptive transfer into the reproductive tract |
| sequence complexity | The total length of unique (i.e., diverse non-repetitive) sequence information in a given population or genome |
| SNPs |
Single Nucleotide Polymorphism(s) - unique nucleotide change(s) in a DNA sequence associated with a given gene or genetic locus that have the potential to change and/or modify gene expression |
| Systems Biology | A gestalt view of life science which attempts to define the functional aspects of a cell and/or organism through studies of interactions between the individual components of a given biological system |
| transgene | The gene(s) and/or DNA sequence information integrated into the genome following introduction into an embryo usually, but not exclusively, by physically introducing the DNA by microinjection |
| transgenic mice | Strains of mice generated by the introduction of exogenous genes or DNA sequences (transgenes) which typically integrate as a single chromosomal insertion event that becomes a heritable Mendelian trait |
|
vector and/or plasmid sequences |
The DNA sequence elements (usually of prokaryotic origin) that allow the propagation and production of the genes and/or DNA sequences of interest in preparation for transgenic mouse production |
| xenograft | A surgical graft of cells, tissues, and/or organs from an animal species of one genus to an animal species of another genus |
Footnotes
Copyright transfer is subject to applicable Mayo terms located on the following page: http://www.mayo.edu/copyright/.
REFERENCES
- Amanullah A, Liebermann DA, Hoffman B. Deregulated c-myc prematurely recruits both type i and ii cd95/fas apoptotic pathways associated with terminal myeloid differentiation. Oncogene. 2002;21:1600–1610. doi: 10.1038/sj.onc.1205231. [DOI] [PubMed] [Google Scholar]
- Banting FG, Best CH. Pancreatic extracts.1922. J Lab Clin Med. 1990;115:254–272. [PubMed] [Google Scholar]
- Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004;6:7–28. doi: 10.1016/s1534-5807(03)00399-x. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Chen HY, Messing A, van Dyke T, Levine AJ, Palmiter RD. Transgenic mice harboring sv40 t-antigen genes develop characteristic brain tumors. Cell. 1984;37:367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalk AM, Warfinge RE, Georgii-Hemming P, Sonnhammer EL. Sirnadb: A database of sirna sequences. Nucleic Acids Res. 2005;33:D131–134. doi: 10.1093/nar/gki136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins FS, Rossant J, Wurst W. A mouse for all reasons. Cell. 2007;128:9–13. doi: 10.1016/j.cell.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Costantini F, Lacy E. Introduction of a rabbit beta-globin gene into the mouse germ line. Nature. 1981;294:92–94. doi: 10.1038/294092a0. [DOI] [PubMed] [Google Scholar]
- Cronin CA, Gluba W, Scrable H. The lac operator-repressor system is functional in the mouse. Genes Dev. 2001;15:1506–1517. doi: 10.1101/gad.892001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Wu X, Li G, Han M, Zhuang Y, Xu T. Efficient transposition of the piggybac (pb) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr., Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Draper JS, Nagy A. Improved embryonic stem cell technologies. Handb Exp Pharmacol. 2007:107–128. doi: 10.1007/978-3-540-35109-2_5. [DOI] [PubMed] [Google Scholar]
- Drost RM, Jonkers J. Preclinical mouse models for brca1-associated breast cancer. Br J Cancer. 2009;101:1651–1657. doi: 10.1038/sj.bjc.6605350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguet A, Biyah K, Minshall E, Gomes R, Wang CG, Taoudi-Benchekroun M, Bates JH, Eidelman DH. Bronchial responsiveness among inbred mouse strains. Role of airway smooth-muscle shortening velocity. Am J Respir Crit Care Med. 2000;161:839–848. doi: 10.1164/ajrccm.161.3.9906054. [DOI] [PubMed] [Google Scholar]
- Dupuy AJ, Fritz S, Largaespada DA. Transposition and gene disruption in the male germline of the mouse. Genesis. 2001;30:82–88. doi: 10.1002/gene.1037. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Feil R, Brocard J, Mascrez B, LeMeur M, Metzger D, Chambon P. Ligand-activated site-specific recombination in mice. Proc Natl Acad Sci U S A. 1996;93:10887–10890. doi: 10.1073/pnas.93.20.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A, Pannell R, Drynan LF, Codrington R, Daser A, Metzler M, Lobato MN, Rabbitts TH. The invertor knock-in conditional chromosomal translocation mimic. Nat Methods. 2005;2:27–30. doi: 10.1038/nmeth727. [DOI] [PubMed] [Google Scholar]
- Giraldo P, Montoliu L. Size matters: Use of yacs, bacs and pacs in transgenic animals. Transgenic Res. 2001;10:83–103. doi: 10.1023/a:1008918913249. [DOI] [PubMed] [Google Scholar]
- Gordon JW, Ruddle FH. Integration and stable germ line transmission of genes injected into mouse pronuclei. Science. 1981;214:1244–1246. doi: 10.1126/science.6272397. [DOI] [PubMed] [Google Scholar]
- Green LL, Hardy MC, Maynard-Currie CE, Tsuda H, Louie DM, Mendez MJ, Abderrahim H, Noguchi M, Smith DH, Zeng Y, et al. Antigen-specific human monoclonal antibodies from mice engineered with human ig heavy and light chain yacs. Nat Genet. 1994;7:13–21. doi: 10.1038/ng0594-13. [DOI] [PubMed] [Google Scholar]
- Hakem R, de la Pompa JL, Sirard C, Mo R, Woo M, Hakem A, Wakeham A, Potter J, Reitmair A, Billia F, Firpo E, Hui CC, Roberts J, Rossant J, Mak TW. The tumor suppressor gene brca1 is required for embryonic cellular proliferation in the mouse. Cell. 1996;85:1009–1023. doi: 10.1016/s0092-8674(00)81302-1. [DOI] [PubMed] [Google Scholar]
- Hamre DM, Rake GMB, McKee CM, MacPhillamy HB. The toxicity of penicillin as prepared for clinical use. Am J Med Sci. 1943;206:642–652. [Google Scholar]
- Harbers K, Jahner D, Jaenisch R. Microinjection of cloned retroviral genomes into mouse zygotes: Integration and expression in the animal. Nature. 1981;293:540–542. doi: 10.1038/293540a0. [DOI] [PubMed] [Google Scholar]
- Hemann MT, Fridman JS, Zilfou JT, Hernando E, Paddison PJ, Cordon-Cardo C, Hannon GJ, Lowe SW. An epi-allelic series of p53 hypomorphs created by stable rnai produces distinct tumor phenotypes in vivo. Nat Genet. 2003;33:396–400. doi: 10.1038/ng1091. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Jiang Z, Tabeta K, Du X, Georgel P, Crozat K, Beutler B. Genetic analysis of innate immunity. Adv Immunol. 2006;91:175–226. doi: 10.1016/S0065-2776(06)91005-0. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo. A laboratory manual. Cold Spring Harbor Laboratory Press; 1994. Plainview. [Google Scholar]
- Hu-Li J, Pannetier C, Guo L, Lohning M, Gu H, Watson C, Assenmacher M, Radbruch A, Paul WE. Regulation of expression of il-4 alleles. Analysis using a chimeric gfp/il-4 gene. Immunity. 2001;14:1–11. doi: 10.1016/s1074-7613(01)00084-x. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration and mendelian transmission of the exogenous moloney leukemia virus. Proc Natl Acad Sci U S A. 1976;73:1260–1264. doi: 10.1073/pnas.73.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Dalton TP, Jin L, Wang B, Tsuneoka Y, Shertzer HG, Deka R, Nebert DW. Toward the evaluation of function in genetic variability: Characterizing human snp frequencies and establishing bac-transgenic mice carrying the human cyp1a1_cyp1a2 locus. Hum Mutat. 2005;25:196–206. doi: 10.1002/humu.20134. [DOI] [PubMed] [Google Scholar]
- Joyner AL, Herrup K, Auerbach BA, Davis CA, Rossant J. Subtle cerebellar phenotype in mice homozygous for a targeted deletion of the en-2 homeobox. Science. 1991;251:1239–1243. doi: 10.1126/science.1672471. [DOI] [PubMed] [Google Scholar]
- Keller AF, Gravel M, Kriz J. Live imaging of amyotrophic lateral sclerosis pathogenesis: Disease onset is characterized by marked induction of gfap in schwann cells. Glia. 2009;57:1130–1142. doi: 10.1002/glia.20836. [DOI] [PubMed] [Google Scholar]
- Kim MR, Manoukian R, Yeh R, Silbiger SM, Danilenko DM, Scully S, Sun J, DeRose ML, Stolina M, Chang D, Van GY, Clarkin K, Nguyen HQ, Yu YB, Jing S, Senaldi G, Elliott G, Medlock ES. Transgenic overexpression of human il-17e aresults in eosinophilia, b-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100:2330–2340. doi: 10.1182/blood-2002-01-0012. [DOI] [PubMed] [Google Scholar]
- Kung TT, Stelts DM, Zurcher JA, Adams GK, Egan RW, Kreutner W, Watnick AS, Jones H, Chapman RW. Involvement of il-5 in a murine model of allergic pulmonary inflammation - prophylactic and therapeutic effect of an anti-il-5 antibody. Am J Respir Cell Mol Biol. 1995;13:360–365. doi: 10.1165/ajrcmb.13.3.7654390. [DOI] [PubMed] [Google Scholar]
- Kwee L, Baldwin HS, Shen HM, Stewart CL, Buck C, Buck CA, Labow MA. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (vcam-1) deficient mice. Development. 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- Lacy E, Roberts S, Evans EP, Burtenshaw MD, Costantini FD. A foreign beta-globin gene in transgenic mice: Integration at abnormal chromosomal positions and expression in inappropriate tissues. Cell. 1983;34:343–358. doi: 10.1016/0092-8674(83)90369-0. [DOI] [PubMed] [Google Scholar]
- Lee GR, Fields PE, Griffin TJ, Flavell RA. Regulation of the th2 cytokine locus by a locus control region. Immunity. 2003;19:145–153. doi: 10.1016/s1074-7613(03)00179-1. [DOI] [PubMed] [Google Scholar]
- Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, Leikauf GD, Lee NA. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- Lewandoski M. Conditional control of gene expression in the mouse. Nat Rev Genet. 2001;2:743–755. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Lonberg N, Taylor LD, Harding FA, Trounstine M, Higgins KM, Schramm SR, Kuo CC, Mashayekh R, Wymore K, McCabe JG, et al. Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature. 1994;368:856–859. doi: 10.1038/368856a0. [DOI] [PubMed] [Google Scholar]
- Lonberg N. Human antibodies from transgenic animals. Nat Biotechnol. 2005;23:1117–1125. doi: 10.1038/nbt1135. [DOI] [PubMed] [Google Scholar]
- Luo G, Ivics Z, Izsvak Z, Bradley A. Chromosomal transposition of a tc1/mariner-like element in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 1998;95:10769–10773. doi: 10.1073/pnas.95.18.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masato F, Tokano H, Fujoka KS, Okano H, Edge ASB. Gernerating mouse models of degenerative diseases using cre/lox-mediated in vivo mosaic cellablation. The Journal of Clnical Investigatation. 2011;121:2462–2469. doi: 10.1172/JCI45081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauser PJ, Pitman AM, Fernandez X, Foran SK, Adams Gr, Kreutner W, Egan RW, Chapman RW. Effects of an antibody to interleukin-5 in a monkey model of asthma. Am J Respir Crit Care Med. 1995;152:467–472. doi: 10.1164/ajrccm.152.2.7633694. [DOI] [PubMed] [Google Scholar]
- McKenna MG. The origin and early differentiation of therian mammals. Ann NY Acad Sci. 1969;167:217–240. [Google Scholar]
- McKusick VA. The defect in marfan syndrome. 1991;352:279–281. doi: 10.1038/352279a0. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: Differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Metzger D, Chambon P. Contribution of targeted conditional somatic mutagenesis to deciphering retinoid x receptor functions and to generating mouse models of human diseases. Handb Exp Pharmacol. 2007:511–524. doi: 10.1007/978-3-540-35109-2_21. [DOI] [PubMed] [Google Scholar]
- Nguyen DC, Sainte-Marie Y, Jaisser F. Animal models in cardiovascular diseases: New insights from conditional models. Handb Exp Pharmacol. 2007:377–405. doi: 10.1007/978-3-540-35109-2_16. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Brinster RL, Hammer RE, Trumbauer ME, Rosenfeld MG, Birnberg NC, Evans RM. Dramatic growth of mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. Nature. 1982;300:611–615. doi: 10.1038/300611a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Sandgren EP, Avarbock MR, Allen DD, Brinster RL. Heterologous introns can enhance expression of transgenes in mice. Proc Natl Acad Sci U S A. 1991;88:478–482. doi: 10.1073/pnas.88.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park F. Lentiviral vectors: Are they the future of animal transgenesis? Physiol Genomics. 2007;31:159–173. doi: 10.1152/physiolgenomics.00069.2007. [DOI] [PubMed] [Google Scholar]
- Perl AK, Riethmacher D, Whitsett JA. Conditional depletion of airway progenitor cells induces peribronchiolar fibrosis. Am J Respir Crit Care Med. 2011;183:511–521. doi: 10.1164/rccm.201005-0744OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry WL, Vasicek TJ, Lee JJ, Rossi JM, Zeng L, Zhang T, Tilghman SM, Costantini F. Phenotypic and molecular analysis of a transgenic insertional allele of the mouse fused locus. Genetics. 1995;141:321–332. doi: 10.1093/genetics/141.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L, Simon MC, Robertson E, Klein WH, Tsai SF, D’Agati V, Orkin SH, Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor gata-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Ray P, Tang W, Wang P, Homer R, Kuhn C, 3rd, Flavell RA, Elias JA. Regulated overexpression of interleukin 11 in the lung. Use to dissociate development-dependent and -independent phenotypes. J Clin Invest. 1997;100:2501–2511. doi: 10.1172/JCI119792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. Erratum in: Science 1989 Sep 1029;1245(4925):1437. [DOI] [PubMed] [Google Scholar]
- Roebroek AJM, Wu X, Bram RJ. Chapter 10 knockin approaches. In: Hofker MH, Deursen Jv, editors. Transgenic mouse: Methods and protocols. Humana Press; Totowa, NJ: 2003. pp. xiiipp. 3741pp. 3187–3200. [Google Scholar]
- Roes J. Conditional mutagenesis reveals immunological functions of widely expressed genes: Activation thresholds, homeostatic mechanisms and disease models. Handb Exp Pharmacol. 2007:289–314. doi: 10.1007/978-3-540-35109-2_12. [DOI] [PubMed] [Google Scholar]
- Rosenthal N, Brown S. The mouse ascending: Perspectives for human-disease models. Nat Cell Biol. 2007;9:993–999. doi: 10.1038/ncb437. [DOI] [PubMed] [Google Scholar]
- Rubin GM, Spradling AC. Genetic transformation of drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Russell WL, Kelly EM, Hunsicker PR, Bangham JW, Maddux SC, Phipps EL. Specific-locus test shows ethylnitrosourea to be the most potent mutagen in the mouse. Proc Natl Acad Sci U S A. 1979;76:5818–5819. doi: 10.1073/pnas.76.11.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez E, Nelson MC, Eshelman B, Banayo E, Koder A, Cho GJ, Evans RM. Identification of ligands and coligands for the ecdysone-regulated gene switch. Proc Natl Acad Sci U S A. 2000;97:14512–14517. doi: 10.1073/pnas.260499497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH, Frank MH. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seery JP, Carroll JM, Cattell V, Watt FM. Antinuclear autoantibodies and lupus nephritis in transgenic mice expressing interferon gamma in the epidermis. J Exp Med. 1997;186:1451–1459. doi: 10.1084/jem.186.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong E, Saunders TL, Stewart CL, Burmeister M. To knockout in 129 or in c57bl/6: That is the question. Trends Genet. 2004;20:59–62. doi: 10.1016/j.tig.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Shinagawa K, Kojima M. Mouse model of airway remodeling: Strain differences. Am J Respir Crit Care Med. 2003;168:959–967. doi: 10.1164/rccm.200210-1188OC. [DOI] [PubMed] [Google Scholar]
- Shinohara ET, Kaminski JM, Segal DJ, Pelczar P, Kolhe R, Ryan T, Coates CJ, Fraser MJ, Handler AM, Yanagimachi R, Moisyadi S. Active integration: New strategies for transgenesis. Transgenic Res. 2007;16:333–339. doi: 10.1007/s11248-007-9077-z. [DOI] [PubMed] [Google Scholar]
- Silver LM. Mouse genetics: Concepts and applications. Oxford University Press; New York: 1995. [Google Scholar]
- Smithies O, Gregg RG, Boggs SS, Koralewski MA, Kucherlapati RS. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985;317:230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, Koller BH. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- Soriano P, Cone RD, Mulligan RC, Jaenisch R. Tissue-specific and ectopic expression of genes introduced into transgenic mice by retroviruses. Science. 1986;234:1409–1413. doi: 10.1126/science.3024318. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [review] [280 refs] [DOI] [PubMed] [Google Scholar]
- Stanford WL, Cohn JB, Cordes SP. Gene-trap mutagenesis: Past, present and beyond. Nat Rev Genet. 2001;2:756–768. doi: 10.1038/35093548. [DOI] [PubMed] [Google Scholar]
- Stinchcomb DT, Shaw JE, Carr SH, Hirsh D. Extrachromosomal DNA transformation of caenorhabditis elegans. Mol Cell Biol. 1985;5:3484–3496. doi: 10.1128/mcb.5.12.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Li W, Zhang Q, Novak EK, Sviderskaya EV, Wilson A, Bennett DC, Roe BA, Swank RT, Spritz RA. The gene mutated in cocoa mice, carrying a defect of organelle biogenesis, is a homologue of the human hermansky-pudlak syndrome-3 gene. Genomics. 2001;78:30–37. doi: 10.1006/geno.2001.6644. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Li W, Zhang Q, Karim A, Novak EK, Sviderskaya EV, Hill SP, Bennett DC, Levin AV, Nieuwenhuis HK, Fong CT, Castellan C, Miterski B, Swank RT, Spritz RA. Hermansky-pudlak syndrome is caused by mutations in hps4, the human homolog of the mouse light-ear gene. Nat Genet. 2002;30:321–324. doi: 10.1038/ng835. [DOI] [PubMed] [Google Scholar]
- Swank RT, Novak EK, McGarry MP, Rusiniak ME, Feng L. Mouse models of hermansky pudlak syndrome: A review. Pigment Cell Res. 1998;11:60–80. doi: 10.1111/j.1600-0749.1998.tb00713.x. [DOI] [PubMed] [Google Scholar]
- Testa G, Schaft J, van der Hoeven F, Glaser S, Anastassiadis K, Zhang Y, Hermann T, Stremmel W, Stewart AF. A reliable lacz expression reporter cassette for multipurpose, knockout-first alleles. Genesis. 2004;38:151–158. doi: 10.1002/gene.20012. [DOI] [PubMed] [Google Scholar]
- Thomas KR, Folger KR, Capecchi MR. High frequency targeting of genes to specific sites in the mammalian genome. Cell. 1986;44:419–428. doi: 10.1016/0092-8674(86)90463-0. [DOI] [PubMed] [Google Scholar]
- Thomson AJ, McWhir J. Biomedical and agricultural applications of animal transgenesis. Mol Biotechnol. 2004;27:231–244. doi: 10.1385/MB:27:3:231. [DOI] [PubMed] [Google Scholar]
- Vignjevic D, Fre S, Louvard D, Robine S. Conditional mouse models of cancer. Handb Exp Pharmacol. 2007:263–287. doi: 10.1007/978-3-540-35109-2_11. [DOI] [PubMed] [Google Scholar]
- Wagner EF, Stewart TA, Mintz B. The human beta-globin gene and a functional viral thymidine kinase gene in developing mice. Proc Natl Acad Sci U S A. 1981;78:5016–5020. doi: 10.1073/pnas.78.8.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner TE, Hoppe PC, Jollick JD, Scholl DR, Hodinka RL, Gault JB. Microinjection of a rabbit beta-globin gene into zygotes and its subsequent expression in adult mice and their offspring. Proc Natl Acad Sci U S A. 1981;78:6376–6380. doi: 10.1073/pnas.78.10.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamhoff BR, Sinha S, Owens GK. Conditional mouse models to study developmental and pathophysiological gene function in muscle. Handb Exp Pharmacol. 2007:441–468. doi: 10.1007/978-3-540-35109-2_18. [DOI] [PubMed] [Google Scholar]
- Wang W, Lin C, Lu D, Ning Z, Cox T, Melvin D, Wang X, Bradley A, Liu P. Chromosomal transposition of piggybac in mouse embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:9290–9295. doi: 10.1073/pnas.0801017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterston RH, Lindblad-Toh K. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Wilber A, Frandsen JL, Geurts JL, Largaespada DA, Hackett PB, McIvor RS. Rna as a source of transposase for sleeping beauty-mediated gene insertion and expression in somatic cells and tissues. Mol Ther. 2006;13:625–630. doi: 10.1016/j.ymthe.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Wilson MH, Coates CJ, George AL. Piggybac transposon-mediated gene transfer in human cells. 2001;15:139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- Wolgemuth DJ, Behringer RR, Mostoller MP, Brinster RL, Palmiter RD. Transgenic mice overexpressing the mouse homoeobox-containing gene hox-1.4 exhibit abnormal gut development. Nature. 1989;337:464–467. doi: 10.1038/337464a0. [DOI] [PubMed] [Google Scholar]
- Wu SP, Lee DK, Demayo FJ, Tsai SY, Tsai MJ. Generation of es cells for conditional expression of nuclear receptors and coregulators in vivo. Mol Endocrinol. 2010;24:1297–1304. doi: 10.1210/me.2010-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng CX. Conditional mutation of brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat Genet. 1999;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- Zambrowicz BP, Imamoto A, Fiering S, Herzenberg LA, Kerr WG, Soriano P. Disruption of overlapping transcripts in the rosa beta geo 26 gene trap strain leads to widespread expression of beta-galactosidase in mouse embryos and hematopoietic cells. Proc Natl Acad Sci U S A. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrowicz BP, Sands AT. Knockouts model the 100 best-selling drugs--will they model the next 100? Nat Rev Drug Discov. 2003;2:38–51. doi: 10.1038/nrd987. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Zhao B, Li W, Oiso N, Novak EK, Rusiniak ME, Gautam R, Chintala S, O’Brien EP, Zhang Y, Roe BA, Elliott RW, Eicher EM, Liang P, Kratz C, Legius E, Spritz RA, O’Sullivan TN, Copeland NG, Jenkins NA, Swank RT. Ru2 and ru encode mouse orthologs of the genes mutated in human hermansky-pudlak syndrome types 5 and 6. Nat Genet. 2003;33:145–153. doi: 10.1038/ng1087. [DOI] [PubMed] [Google Scholar]