Abstract
Purpose
Miller Fisher syndrome (MFS) is a rare immune-mediated neuropathy that commonly presents with diplopia following the acute onset of complete bilateral external ophthalmoplegia. Ophthalmoplegia is often accompanied by other neurological deficits such as ataxia and areflexia that characterize MFS. Although MFS is a clinical diagnosis, serological confirmation is possible by identifying the anti-GQ1b antibody found in a majority of affected patients. We report a patient with MFS who presented with clinical signs suggestive of ocular myasthenia gravis, but in whom the correct diagnosis was made on the basis of serological testing for the anti-GQ1b antibody.
Case Report
An 81-year-old white man presented with an acute onset of diplopia following a mild gastrointestinal illness. Clinical examination revealed complete bilateral external ophthalmoplegia and left-sided ptosis. He developed more marked bilateral ptosis, left greater than right, with prolonged attempted upgaze. He was also noted to have a Cogan’s lid twitch. Same day evaluation by a neuro-ophthalmologist revealed mild left-sided facial and bilateral orbicularis oculi weakness. He had no limb ataxia, but exhibited a slightly wide-based gait with difficulty walking heel-to-toe. A provisional diagnosis of ocular myasthenia gravis was made and anticholinesterase inhibitor therapy was initiated. However, his symptoms did not improve and serological testing was positive for the anti-GQ1b IgG antibody, supporting a diagnosis of MFS.
Conclusions
Although the predominant ophthalmic feature of MFS is complete bilateral external ophthalmoplegia, it should be recognized that MFS has variable associations with lid and pupillary dysfunction. Such confounding neuro-ophthalmic features require a thorough history, neurological examination, neuroimaging, and serological testing for the anti-GQ1b antibody to arrive at a diagnosis of MFS.
Keywords: Miller Fisher syndrome, anti-ganglioside antibody, ophthalmoplegia, diplopia, cranial neuropathies, multiple, myasthenia gravis, ocular
Miller Fisher syndrome (MFS), a variant of Guillain-Barré syndrome (GBS), is a rare immune-mediated neuropathy that typically manifests with the triad of acute ophthalmoplegia, ataxia, and areflexia.1 MFS arises from an antecedent infectious event that initiates the process of molecular mimicry, whereby a humoral immunological attack occurs against both the infectious agent and similar host GQ1b gangliosides found on peripheral and cranial nerves. Serological confirmation of MFS is possible during the acute phase of the disease by identifying the anti-GQ1b IgG antibody found in over 90% of affected patients.2 Because other neurological conditions can present with similar clinical findings, the anti-GQ1b antibody is useful for confirming MFS. We report a patient with MFS who presented with clinical signs suggestive of ocular myasthenia gravis, but in whom the correct diagnosis was made on the basis of serological testing for the anti-GQ1b antibody. Patients with MFS whose clinical features mimic ocular myasthenia gravis have rarely been reported3 and we aim to further characterize this unique presentation of MFS in a singular case.
CASE REPORT
Ophthalmic Evaluation
An 81-year-old white man presented to the clinic wearing a right eye patch following the acute onset of constant binocular diagonal diplopia, first noticed while gardening three weeks prior to examination. In addition, the patient had noted fluctuating ptosis that was worse on the left. The patient reported poor balance, but attributed this to his lack of stereopsis from the eye patch. He denied difficulty with speech, chewing, or swallowing, and sensory symptoms (numbness or paresthesias). He reported shortness of breath that pre-dated the onset of the diplopia and ptosis by months and was unchanged recently. Additionally, he recalled having a gastrointestinal illness of three days duration, one week prior to the onset of diplopia, but denied any other recent illness or vaccinations.
The patient’s ocular history was significant for dry macular degeneration. He denied previous episodes of diplopia or ptosis. His medical history was significant for coronary artery disease, hypothyroidism, dyslipidemia, hypertension, renal artery stenosis, pleural effusion, and a history of rheumatic fever when he was 20 years old. His medications included aspirin, hydralazine, atenolol, levothyroxine, albuterol, and tiotropium.
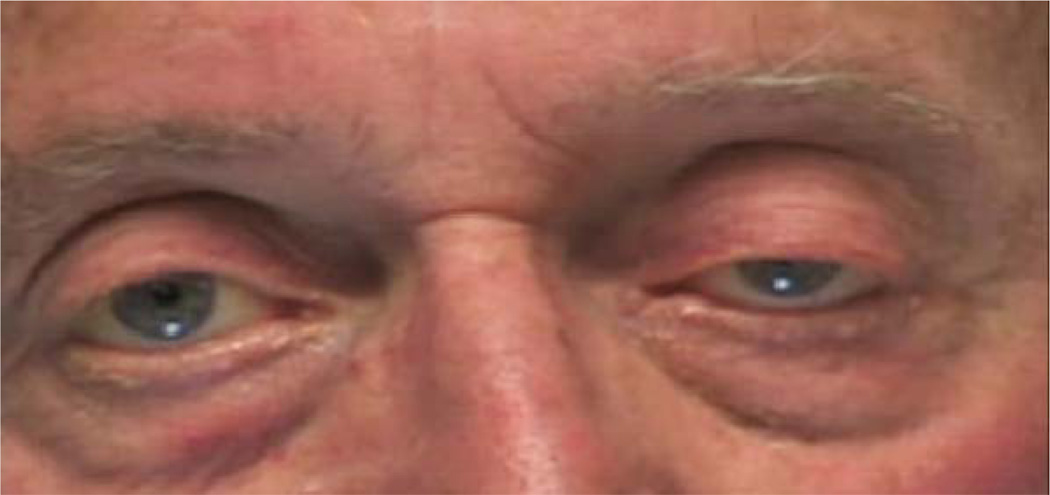
On ophthalmic examination, visual acuity with spectacle correction was 20/60 in the right eye and 20/50 in the left eye, without improvement using a pinhole. Manifest refraction was performed and the refractive error was measured to be +2.00 −0.75 × 090 in the right eye and +2.00 −1.50 × 090 in the left eye, without improvement of visual acuity in either eye. The pupils were equal and round, with a brisk reaction to light and no relative afferent pupillary defect. Evaluation of ductions revealed complete bilateral external ophthalmoplegia that could not be overcome with the vestibulo-ocular reflex. There was a primary position exotropia on Hirschberg testing. External examination revealed left-sided ptosis. He developed more marked bilateral ptosis, left greater than right, with prolonged attempted upgaze (see Figure 1). He was also noted to have a Cogan’s lid twitch bilaterally; Cogan’s lid twitch sign is characterized by transient upper eyelid overshooting during gaze refixations from down to straight ahead and is thought to be specific for ocular myasthenia gravis.4 He exhibited normal blinking and there was no lagophthalmos. Both eyes appeared mildly proptotic, but exophthalmometry was within normal limits. Intraocular pressures were 18mmHg in each eye. The dilated fundus examination findings were only significant for bilateral macular drusen, which accounted for his reduced visual acuity.
Figure 1.
The patient developed marked bilateral ptosis, left greater than right, following prolonged attempted upgaze.
Same day evaluation by a neuro-ophthalmologist revealed mild left-sided facial and bilateral orbicularis oculi weakness. Upper limb reflexes were barely present, but his lower limb reflexes were easily elicited. He had no limb ataxia, but exhibited a slightly wide-based gait with difficulty walking heel-to-toe. A provisional diagnosis of ocular myasthenia gravis was made based on the clinical findings. Although he complained of chronic shortness of breath, bulbar involvement was not suspected as the respiratory symptoms were unchanged since the onset of diplopia. Acetylcholine receptor antibodies were not detected; note that approximately 50% of patients with ocular myasthenia gravis will be seronegative.5 However, an ice pack test showed a mild short-lived improvement in his left-sided ptosis, which was felt to support the provisional diagnosis. Thyroid eye disease was considered based on the mild proptosis, but magnetic resonance imaging (MRI) of the orbits did not show enlargement of the extraocular muscle bellies. Additionally, a central nervous system lesion was not detected on MRI. Accordingly, the patient was started on oral pyridostigmine 30mg t.i.d. and instructed to follow up with the neuro-ophthalmologist in one month. At the one month follow-up examination, he reported little improvement with pyridostigmine, which prompted serological testing for the anti-GQ1b IgG antibody. Two weeks later, he started to show spontaneous improvement and at about this time, serological test results were reported to be positive for the anti-GQ1b antibody, supporting a diagnosis of MFS. MRI with contrast did not demonstrate cranial nerve enhancement, which may be present in MFS.6 He was managed conservatively, without further medical intervention or investigations, and there was a gradual spontaneous improvement in his ductions over several months. However, there was a residual right eye abduction deficit with 6 prism diopters of esotropia for which prism glasses were successfully prescribed.
DISCUSSION
Miller Fisher syndrome is typically characterized by a triad of neurological signs: ophthalmoplegia, ataxia, and areflexia. MFS is considered a variant of GBS, accounting for 5 to 10% of GBS cases7, but unlike GBS it often presents with symptoms of diplopia.8,9 The annual incidence of GBS is 1–2 cases/100,000 population, whereas MFS has a much lower annual incidence of 0.09 cases/100,000 population.10 The incidence of MFS varies with geographic location, being much more common in Japan than in the United States.6,8 MFS is more common in men and affects people of all ages, with the median age of onset being in the 5th decade.8
The onset of MFS is likely triggered by an antecedent illness that sets the stage for molecular mimicry between the GQ1b ganglioside on cranial and peripheral nerves, and molecularly similar lipo-oligosaccharides on the surface of the infectious agent.11 The majority of patients report having an antecedent upper respiratory tract or gastrointestinal illness.9 Other evidence of an antecedent infection as the impetus for an immune response against host nerve gangliosides includes serological isolation of infectious agents during the acute phase of the disease, such as Campylobacter jejuni, Haemophilus influenzae, Mycoplasma pneumoniae, and cytomegalovirus.12
The clinical course of MFS is self-limiting and is similar to an acute phase primary immune response, in which a humoral response is initiated with subsequent nadir and then spontaneous recovery.1 The median time from the infection onset to development of neurological symptoms is approximately 10 days.9
Once molecular mimicry is established, an inflammatory autoimmune response occurs and disrupts both peripheral and cranial nerve function, leading to the characteristic neurological findings. Among the clinical variants of the GBS disease spectrum, each has a predilection for autoantibody formation against different glycolipids within the ganglioside family, such as GQ1b, GM1, GM2, GD1a, and GD1b.13,14 The GQ1b ganglioside complex has been identified as the glycolipid that is most often involved in cases of MFS. In fact, serological testing for the anti-GQ1b antibody is positive in over 90% of patients with MFS and is not present in normal subjects.2,15 Occasional patients with MFS will have autoantibodies to other ganglioside antigens (e.g., GM1, GM2, GD1a, or GD1b) and, thus, a ganglioside antibody panel could be obtained to screen for these when the clinical suspicion for MFS is high and GQ1b autoantibodies are not detected. GQ1b autoantibodies may also be present in GBS patients with ophthalmoplegia, further supporting a link between MFS and GBS.14 Although the GQ1b ganglioside is found on both peripheral and cranial nerves, it is most concentrated on cranial nerves III, IV, and VI, accounting for the high incidence of ophthalmoplegia.2,8 Ataxia, characterized by unsteady gait with inability to walk heel-to-toe, is thought to originate from cerebellar involvement, although this is not proven.6,8 Ataxia is often a presenting symptom of MFS.8 Areflexia or hyporeflexia, seen as a loss of deep tendon reflexes, is thought to manifest from peripheral nerve dysfunction of the lower motor neurons.8,15 While areflexia is a consistent feature of GBS, it is not always present in patients with MFS.8 In general, areflexia does not affect the patient’s activities of daily living.16
The anti-GQ1b antibody is not unique to MFS and can be present in other conditions that make up the anti-GQ1b antibody syndrome. The neuroimmunologic diseases that comprise the anti-GQ1b antibody syndrome include MFS, GBS with ophthalmoplegia, Bickerstaff’s brainstem encephalitis, and acute ophthalmoparesis without ataxia.14 The common feature of the anti-GQ1b antibody syndrome is a humoral response against the GQ1b ganglioside that leads to dysfunction of cranial nerves, explaining why ophthalmoplegia is a manifestation of all conditions in the anti-GQ1b antibody syndrome. There is, however, variable involvement of the peripheral and central nervous systems that accounts for the distinguishing phenotypic features of these conditions.6 Patients with GBS can develop ophthalmoplegia, but it does not usually occur until after they have developed extremity and respiratory paralysis. Bickerstaff’s brainstem encephalitis has the same clinical features as MFS (ophthalmoplegia and ataxia), as well as impaired consciousness (e.g., coma) and pyramidal tract dysfunction (e.g., hyperreflexia or pathological reflexes).7,14 Acute ophthalmoparesis without ataxia is characterized by a rapid onset of ophthalmoplegia (most often bilateral) without ataxia or areflexia, but a positive anti-GQ1b antibody.17
The most common presenting symptom of MFS is diplopia, which arises due to the acute onset of external ophthalmoplegia.8,9 The external ophthalmoplegia can be unilateral or bilateral and complete or incomplete. The ocular motor deficit can be consistent with isolated or combined involvement of cranial nerves III, IV, and VI.8,18 However, the most common finding is complete bilateral external ophthalmoplegia.6,8 Supranuclear ocular motor disorders can occasionally be seen in MFS, and include internuclear ophthalmoplegia and vertical gaze palsy.9,18 Patients may also exhibit pupillary abnormalities (internal ophthalmoplegia) and abnormal lid function. Pupillary abnormalities can include mydriasis, anisocoria, and a sluggish direct response to light.8 Ptosis, if present, is often partial and can be unilateral or bilateral.9 Other lid abnormalities reported include lid retraction, upper lid jerks, and lid nystagmus.19 Facial nerve involvement, which occurs in approximately 30% of patients, may result in orbicularis oculi weakness and, consequently, lagophthalmos.20 Although the afferent visual pathways are not involved in MFS6, patients with lagophthalmos can develop decreased vision due to exposure keratopathy and, thus, should be prescribed prophylactic ocular lubrication.
Differential Diagnosis
Although complete bilateral external ophthalmoplegia is a rare cause of diplopia, there are multiple pathologic entities that can produce this finding. The rapid onset of ophthalmoplegia can help to distinguish MFS from conditions that progress chronically, such as mitochondrial myopathies, oculopharyngeal dystrophy, myotonic dystrophy, thyroid eye disease, and some cases of ocular myasthenia gravis. In a review of 31 patients with the acute onset of complete bilateral external ophthalmoplegia, MFS was found to be the underlying etiology in the majority of cases.21 Less common causes include GBS, midbrain infarction, Wernicke’s encephalopathy, and ocular myasthenia gravis. The differential diagnosis of Miller Fisher syndrome includes other polyneuropathies, brainstem lesions, neuromuscular junction disorders, and cavernous sinus or orbital lesions (see Table 1).21
Table 1.
Differential diagnosis of Miller Fisher syndrome.
| Disorder | Extraocular motility abnormalities | Lid abnormalities | Pupil abnormalities | Other abnormalities | Prevalence of anti- GQ1b antibody8 |
|---|---|---|---|---|---|
| Miller Fisher syndrome | Complete bilateral external ophthalmoplegia* Isolated cranial nerve palsy (III, IV, VI) Multiple cranial nerve palsy (III, IV, VI) Appearance of gaze palsy Appearance of internuclear ophthalmoplegia |
Ptosis Lid jerk Lid nystagmus Lid retraction Lagophthalmos |
Mydriasis Anisocoria |
Ataxia* Areflexia* Facial weakness |
>90% |
| Guillain-Barré syndrome | Complete bilateral external ophthalmoplegia Isolated cranial nerve palsy (III, IV, VI) Multiple cranial nerve palsy (III, IV, VI) |
Ptosis Lagophthalmos |
Mydriasis Anisocoria |
Flaccid paralysis* Bulbar weakness* Sensory loss* Back pain |
Up to 83% (in patients with ophthalmoplegia) |
| Bickerstaff’s brainstem encephalitis | Complete bilateral external ophthalmoplegia* | Ptosis | Mydriasis Anisocoria |
Ataxia* Pyramidal weakness* Altered level of consciousness* Sensory loss |
Up to 68% |
| Acute ophthalmoparesis without ataxia | Complete bilateral external ophthalmoplegia* Isolated cranial nerve palsy (III, IV, VI) Multiple cranial nerve palsy (III, IV, VI) Appearance of gaze palsy |
Ptosis | Mydriasis Anisocoria |
100% (required for diagnosis) | |
| Ocular myasthenia gravis | Complete bilateral external ophthalmoplegia Isolated cranial nerve palsy (III, IV, VI) Multiple cranial nerve palsy (III, IV, VI) Appearance of gaze palsy Appearance of internuclear ophthalmoplegia Appearance of skew deviation Nystagmus |
Ptosis (fatigable) Cogan’s lid twitch |
None | Bulbar weakness (in generalized) Proximal limb weakness (in generalized) |
0% |
| Brainstem stroke | Complete bilateral external ophthalmoplegia Internuclear ophthalmoplegia Gaze palsy Nystagmus Multiple cranial nerve palsy (III, IV, VI) |
Ptosis | Mydriasis Anisocoria |
Pyramidal weakness Bulbar weakness Sensory loss Ataxia |
0% |
| Pituitary apoplexy | Complete bilateral external ophthalmoplegia Isolated cranial nerve palsy (III, IV, VI) Multiple cranial nerve palsy (III, IV, VI) |
Ptosis | Mydriasis Anisocoria Relative afferent pupillary defect |
Headache Vision loss Altered level of consciousness |
0% |
| Botulism | Complete bilateral external ophthalmoplegia Nystagmus |
Ptosis | Mydriasis/tonic pupil Anisocoria |
Flaccid paralysis Bulbar weakness Sphincter disturbance |
0% |
| Wernicke’s encephalopathy | Complete bilateral external ophthalmoplegia Isolated cranial nerve palsy (typically VI) Upbeat nystagmus |
Ptosis (rarely) | None | Ataxia Confusion Vision loss (rarely) |
0% |
| Anticonvulsant intoxication | Partial or complete bilateral external ophthalmoplegia Gaze-evoked and vertical nystagmus Impaired smooth pursuit |
Ptosis (rarely) | None | Ataxia Altered level of consciousness |
0% |
Key phenotypic feature
Ocular myasthenia gravis, an autoimmune disease that disrupts extraocular muscle function through antigenic blocking of acetylcholine receptors at the neuromuscular junction, is capable of producing a wide range of neuro-ophthalmic deficits.22 MFS and ocular myasthenia gravis are capable of producing similar neurological signs and symptoms that may confound the preliminary diagnosis. Both may present acutely with symptoms of diplopia. Additionally, both conditions can produce external ophthalmoplegia, variable and asymmetric ptosis, a Cogan’s lid twitch, orbicularis oculi weakness, and bulbar weakness. The diplopia and ptosis often become worse as the day goes on in patients with ocular myasthenia gravis, whereas they usually do not worsen in patients with MFS, because the ophthalmoplegia is typically bilateral and complete. Clinical findings suggesting ocular myasthenia gravis may result from abnormal neuromuscular transmission in patients with MFS. Evidence for involvement of the neuromuscular junction in MFS is based on the presence of structural changes at the neuromuscular junction and electrophysiologic changes consistent with abnormal neuromuscular transmission.23–26 Indeed, recent studies have demonstrated that the GQ1b antibody binds close to the neuromuscular junction in human extraocular muscles.27 Experimental models suggest that injury to the neuromuscular junction occurs through activation of complement, which results in the formation and deposition of membrane attack complexes (C5b-9) in presynaptic motor nerve terminals.23,28
Clinical signs suggestive of ocular myasthenia gravis in our patient included external ophthalmoplegia, variable ptosis, orbicularis oculi weakness, and a Cogan’s lid twitch. Although our patient had normal pupil responses, the presence of pupil abnormalities can further differentiate these conditions, as ocular myasthenia gravis does not cause pupil abnormalities.3 The diagnosis of MFS in our patient was best supported by the presence of the anti-GQ1b antibody in his serum and by the course of spontaneous improvement without treatment.
Any presentation with acute-onset of complete external ophthalmoplegia should prompt a careful neuro-ophthalmic evaluation by the eye care provider. However, such patients always require an urgent neurologic consultation, to evaluate for other neurological symptoms and signs. Dependent upon the neurological findings, further investigations may include brain MRI, lumbar puncture, nerve conduction studies, and serological testing for acetylcholine receptor and GQ1b antibodies.
Prognosis and Treatment
The clinical course of MFS is self-limiting and neurological deficits resolve in weeks to months, starting as early as 2 weeks following the onset of symptoms.8,16 After 1 year, recovery from ophthalmoplegia and ataxia is usually complete, with only a small percentage having residual neurological deficits.16 MFS is usually a monophasic illness, but can rarely be recurrent.8 Although treatment with intravenous immunogloblulin and plasmapheresis has been proposed, no clinical trials have been performed to confirm a beneficial effect.7 Inhibition of complement activation, using the monoclonal antibody eculizumab, has recently been shown to minimize neuromuscular junction disruption from anti-GQ1b antibodies in a murine model, but is yet to be evaluated in patients with MFS.28
CONCLUSIONS
MFS can rarely present with signs suggestive of ocular myasthenia gravis. In such cases, the presence of the anti-GQ1b antibody can help to establish the correct diagnosis.
ACKNOWLEDGMENTS
Dr. Leigh is supported by the Department of Veterans Affairs, National Eye Institute grant R01-EY06717, and the Armington Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dr. Anthony presented a poster entitled, “Miller Fisher Syndrome Presenting with Complete External Ophthalmoplegia”, at the American Academy of Optometry meeting in San Francisco, 2010.
REFERENCES
- 1.Willison HJ, O'Hanlon GM. The immunopathogenesis of Miller Fisher syndrome. J Neuroimmunol. 1999;100:3–12. doi: 10.1016/s0165-5728(99)00213-1. [DOI] [PubMed] [Google Scholar]
- 2.Boylu EE, Togrol RE, Senol MG, Ozdag MF, Saracoglu M. Role of anti-GQ1B antibody in differential diagnosis of acute ophthalmoparesis. Neuropsychiatr Dis Treat. 2010;6:119–122. doi: 10.2147/ndt.s8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverstein MP, Zimnowodzki S, Rucker JC. Neuromuscular junction dysfunction in Miller Fisher syndrome. Semin Ophthalmol. 2008;23:211–213. doi: 10.1080/08820530802049996. [DOI] [PubMed] [Google Scholar]
- 4.Singman EL, Matta NS, Silbert DI. Use of the Cogan lid twitch to identify myasthenia gravis. J Neuroophthalmol. 2011;31:239–240. doi: 10.1097/WNO.0b013e3182224b92. [DOI] [PubMed] [Google Scholar]
- 5.Jacob S, Viegas S, Leite MI, Webster R, Cossins J, Kennett R, Hilton-Jones D, Morgan BP, Vincent A. Presence and pathogenic relevance of antibodies to clustered acetylcholine receptor in ocular and generalized myasthenia gravis. Arch Neurol. 2012:1–8. doi: 10.1001/archneurol.2012.437. [DOI] [PubMed] [Google Scholar]
- 6.Saul RF. Neuro-ophthalmology and the Anti-GQ1b antibody syndromes. Curr Neurol Neurosci Rep. 2009;9:379–383. doi: 10.1007/s11910-009-0055-0. [DOI] [PubMed] [Google Scholar]
- 7.Overell JR, Hsieh ST, Odaka M, Yuki N, Willison HJ. Treatment for Fisher syndrome, Bickerstaff's brainstem encephalitis and related disorders. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD004761.pub2. CD004761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyder LA, Rismondo V, Miller NR. The Fisher variant of Guillain-Barre syndrome (Fisher syndrome) J Neuroophthalmol. 2009;29:312–324. doi: 10.1097/WNO.0b013e3181c2514b. [DOI] [PubMed] [Google Scholar]
- 9.Berlit P, Rakicky J. The Miller Fisher syndrome. Review of the literature. J Clin Neuroophthalmol. 1992;12:57–63. [PubMed] [Google Scholar]
- 10.Yuki N. Infectious origins of, and molecular mimicry in, Guillain-Barre and Fisher syndromes. Lancet Infect Dis. 2001;1:29–37. doi: 10.1016/S1473-3099(01)00019-6. [DOI] [PubMed] [Google Scholar]
- 11.Farrugia ME, Vincent A. Autoimmune mediated neuromuscular junction defects. Curr Opin Neurol. 2010;23:489–495. doi: 10.1097/WCO.0b013e32833cc968. [DOI] [PubMed] [Google Scholar]
- 12.Koga M, Gilbert M, Li J, Koike S, Takahashi M, Furukawa K, Hirata K, Yuki N. Antecedent infections in Fisher syndrome: a common pathogenesis of molecular mimicry. Neurology. 2005;64:1605–1611. doi: 10.1212/01.WNL.0000160399.08456.7C. [DOI] [PubMed] [Google Scholar]
- 13.Willison HJ, Plomp JJ. Anti-ganglioside antibodies and the presynaptic motor nerve terminal. Ann N Y Acad Sci. 2008;1132:114–123. doi: 10.1196/annals.1405.010. [DOI] [PubMed] [Google Scholar]
- 14.Odaka M, Yuki N, Hirata K. Anti-GQ1b IgG antibody syndrome: clinical and immunological range. J Neurol Neurosurg Psychiatry. 2001;70:50–55. doi: 10.1136/jnnp.70.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo YL. Clinical and immunological spectrum of the Miller Fisher syndrome. Muscle Nerve. 2007;36:615–627. doi: 10.1002/mus.20835. [DOI] [PubMed] [Google Scholar]
- 16.Mori M, Kuwabara S, Fukutake T, Hattori T. Intravenous immunoglobulin therapy for Miller Fisher syndrome. Neurology. 2007;68:1144–1146. doi: 10.1212/01.wnl.0000258673.31824.61. [DOI] [PubMed] [Google Scholar]
- 17.Lee SH, Lim GH, Kim JS, Oh SY, Kim JK, Cha JK, Yun CH, Kang JK, Lee H, Song HK, Chung KC. Acute ophthalmoplegia (without ataxia) associated with anti-GQ1b antibody. Neurology. 2008;71:426–429. doi: 10.1212/01.wnl.0000324266.95814.74. [DOI] [PubMed] [Google Scholar]
- 18.Leigh RJ, Zee D. The Neurology of Eye Movements. 4th ed. Oxford: Oxford University Press; 2006. [Google Scholar]
- 19.Glaser J. Neuro-ophthalmologic examination: general considerations and special techniques. In: Tasman W, Jaeger EA, editors. Duane's Ophthalmology. online ed. Philadelphia: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 20.Mori M, Kuwabara S, Fukutake T, Yuki N, Hattori T. Clinical features and prognosis of Miller Fisher syndrome. Neurology. 2001;56:1104–1106. doi: 10.1212/wnl.56.8.1104. [DOI] [PubMed] [Google Scholar]
- 21.Keane JR. Bilateral ocular paralysis: analysis of 31 inpatients. Arch Neurol. 2007;64:178–180. doi: 10.1001/archneur.64.2.178. [DOI] [PubMed] [Google Scholar]
- 22.Haines SR, Thurtell MJ. Treatment of ocular myasthenia gravis. Curr Treat Options Neurol. 2012;14:103–112. doi: 10.1007/s11940-011-0151-8. [DOI] [PubMed] [Google Scholar]
- 23.Halstead SK, O'Hanlon GM, Humphreys PD, Morrison DB, Morgan BP, Todd AJ, Plomp JJ, Willison HJ. Anti-disialoside antibodies kill perisynaptic Schwann cells and damage motor nerve terminals via membrane attack complex in a murine model of neuropathy. Brain. 2004;127:2109–2123. doi: 10.1093/brain/awh231. [DOI] [PubMed] [Google Scholar]
- 24.Lo YL, Chan LL, Pan A, Ratnagopal P. Acute ophthalmoparesis in the anti-GQ1b antibody syndrome: electrophysiological evidence of neuromuscular transmission defect in the orbicularis oculi. J Neurol Neurosurg Psychiatry. 2004;75:436–440. doi: 10.1136/jnnp.2003.023630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo YL, Leoh TH, Dan YF, Lim LL, Seah A, Fook-Chong S, Ratnagopal P. Presynaptic neuromuscular transmission defect in the Miller Fisher syndrome. Neurology. 2006;66:148–149. doi: 10.1212/01.wnl.0000191400.77080.21. [DOI] [PubMed] [Google Scholar]
- 26.Lange DJ, DeAngelis T, Sivak MA. Single-fiber electromyography shows terminal axon dysfunction in Miller Fisher syndrome: a case report. Muscle Nerve. 2006;34:232–234. doi: 10.1002/mus.20544. [DOI] [PubMed] [Google Scholar]
- 27.Liu JX, Willison HJ, Pedrosa-Domellof F. Immunolocalization of GQ1b and related gangliosides in human extraocular neuromuscular junctions and muscle spindles. Invest Ophthalmol Vis Sci. 2009;50:3226–3232. doi: 10.1167/iovs.08-3333. [DOI] [PubMed] [Google Scholar]
- 28.Halstead SK, Zitman FM, Humphreys PD, Greenshields K, Verschuuren JJ, Jacobs BC, Rother RP, Plomp JJ, Willison HJ. Eculizumab prevents anti-ganglioside antibody-mediated neuropathy in a murine model. Brain. 2008;131:1197–1208. doi: 10.1093/brain/awm316. [DOI] [PubMed] [Google Scholar]