Abstract
OBJECTIVE
Human β-defensin-2 (HBD-2) is a potent anti-microbial peptide that is part of the innate immune response. The purpose of this study was to determine whether HBD-2 is present in amniotic fluid and if its concentration changes with microbial invasion of the amniotic cavity (MIAC) and labor.
STUDY DESIGN
Amniotic fluid was retrieved by amniocentesis from 318 patients in the following groups: 1) mid-trimester (n=75); 2) term not in labor (n=28) and in labor (n=51); 3) preterm labor and intact membranes without MIAC who delivered at term (n=36), who delivered preterm without MIAC (n=52), and preterm labor with MIAC who delivered preterm (n=25); and 4) preterm premature rupture of membranes (PPROM) with (n=25) and without MIAC (n=26). MIAC was defined as a positive amniotic fluid culture for microorganisms. Amniotic fluid HBD-2 concentrations were determined using a sensitive and specific ELISA. Non-parametric statistics were used for analysis.
RESULTS
1) HBD-2 was detected in all amniotic fluid samples; 2) the concentration of HBD-2 did not change with gestational age from midtrimester to term (p=0.8); 3) Intra-amniotic infection was associated with a significant increase in amniotic fluid concentrations of HBD-2 in both women with preterm labor and intact membranes, and women with preterm PROM (p<0.05 for each comparison); 4) Patients with preterm labor and a negative amniotic fluid culture who delivered preterm had a higher median amniotic fluid HBD-2 concentration than those with preterm labor who delivered at term (p<0.01); and 5) Among patients with preterm labor without MIAC, those who had intra-amniotic inflammation (amniotic fluid white blood cell count >100 cell per ml) had a higher median amniotic fluid concentration of HBD-2 than those without this condition (p<0.05).
CONCLUSION
1) Amniotic fluid contains HBD-2, a natural anti-microbial peptide, and this may account for some of the anti-microbial activity of amniotic fluid; 2) Amniotic fluid HBD-2 concentrations are increased in women with MIAC, regardless of the membrane status (intact membranes or PROM); and 3) We propose that amniotic fluid HBD-2 is part of the innate immune system within the amniotic cavity.
INTRODUCTION
Intrauterine infection is a major cause of preterm labor and delivery, maternal morbidity and mortality, as well as short- and long-term neonatal morbidity.[1–4] The traditional view is that the amniotic cavity is sterile and does not contain viable microorganisms. This is presumably accomplished by the participation of components of the innate immune system, including the cervical mucus plug,[5–8] chorioamniotic membranes[9,10] and cellular components of the decidua, amnion and chorion, including neutrophils, macrophages, natural killer cells and trophoblast.[11,12]
Amniotic fluid is known to have anti-microbial properties.[13–18] Yet, a comprehensive description of all the natural anti-microbial agents present in amniotic fluid has not been achieved. Anti-microbial peptides, part of the innate limb of the immune response,[19–21] have been identified in plants, insects, and vertebrates,[19] as well as in white blood cells[22,23] and epithelial cells.[24–26] The presence of several anti-microbial peptides has been documented in amniotic fluid, including human neutrophil peptides (HNP) 1, 2 and 3, bactericidal/permeability-increasing protein (BPI), and calprotectin (MRP8/14).[27]
Defensins are anti-microbial peptides classified in three major groups: alpha (α), beta (β) and theta (θ).[28] Beta defensins include human beta defensins (HBD) 1, 2, 3 and 4.[28] Human β-defensin-2 (HBD-2) is a 41 amino acid peptide originally described in psoriatic skin lesions[29] and is expressed in the skin,[29–31] oral mucosa,[32] tracheal epithelium,[29,33–35] and renal tubular epithelial cells.[36] HBD-2 has potent antimicrobial activity against Gram-negative bacteria and, to a lesser extent, Gram-positive bacteria.[29] Moreover, HBD-2 prevents proliferation of Candida species in vitro.[29,37,38]
Accumulating evidence indicates that the anti-microbial activity of the amniotic fluid may partially be due to the presence of natural anti-microbial peptides.[27,39–42] The purpose of this study was to determine if HBD-2 is present in the amniotic fluid of women with normal pregnancies, those with preterm parturition with intact and ruptured membranes, as well as patients with and without microbial invasion of the amniotic cavity (MIAC).
MATERIAL AND METHODS
A cross-sectional study was conducted by searching our clinical database and bank of biological samples. This study included 318 women divided into four groups. Group 1 consisted of women in the mid-trimester of pregnancy (14–18 weeks; n=75) who underwent amniocentesis for genetic indications and delivered a normal neonate at term. Group 2 included normal pregnant women at term (≥ 37 weeks) not in labor (n=28) and in labor (n=51). Group 3 consisted of patients with preterm labor and intact membranes who were classified into the following categories: 1) preterm labor who delivered at term with a negative amniotic fluid culture for micro-organisms (n=36); 2) preterm labor who delivered preterm (<37 weeks) with a negative amniotic fluid culture for micro-organisms (n=52); and 3) preterm delivery with MIAC (n=25). Group 4 was comprised of women with preterm premature rupture of membranes (PROM) with (n=25) and without (n=26) MIAC. The inclusion criteria for normal pregnancy consisted of: 1) no medical, obstetrical or surgical complications; 2) absence of MIAC; 3) intact membranes; and 4) delivery of a term neonate (≥37 weeks) with a birth weight appropriate for gestational age ([AGA]; between 10th and 90th percentile).[43] Preterm labor was defined by the presence of regular uterine contractions occurring at a frequency of at least two every 10 minutes, as well as cervical changes leading to delivery before 37 completed weeks of gestation. MIAC was defined as a positive amniotic fluid culture for microorganisms, and intra-amniotic inflammation was defined as an amniotic fluid white blood cell (WBC) count ≥100 cells per ml. Preterm PROM was defined as amniorrhexis previous to the onset of spontaneous labor before 37 weeks, diagnosed with the use of vaginal pooling, ferning, or a positive nitrazine test.
The indications for transabdominal amniocentesis in patients in groups 2, 3 and 4 were for the detection of MIAC and fetal lung maturity tests. Amniotic fluid not required for clinical purposes was centrifuged at 4°C for 10 minutes to remove cellular and particulate matter, and stored at −70°C. A sample of amniotic fluid was transported to the laboratory for culture of aerobic/anaerobic bacteria and genital Mycoplasmas. An amniotic fluid WBC count and an assessment of glucose concentrations were performed in most cases. The results of these tests were used for subsequent clinical management.
All women provided written informed consent prior to the collection of amniotic fluid. The collection of samples was approved by the IRBs of both Wayne State University and the National Institute of Child Health and Human Development (NIH). These samples have been used in previous studies of amniotic fluid concentrations of other antimicrobial peptides.[27]
Human β-defensin-2 (HBD-2) immunoassays
A specific and sensitive enzyme-linked immunoassay was used to determine concentrations of HBD-2 in human amniotic fluid. Immunoassay kits for HBD-2 were obtained from the American Laboratory Products Company (ALPCO, Windham, NH). The HBD-2 immunoassay was validated for human amniotic fluid in our laboratory prior to the conduction of this study. Validation included spike and recovery experiments, which produced parallel curves indicating that amniotic fluid constituents did not interfere with antigen-antibody binding in this assay system. Briefly, amniotic fluid samples were incubated in duplicate wells of the microtiter plates that were pre-coated with polyclonal antibodies raised against HBD-2. During this incubation, the HBD-2 present in the standards or amniotic fluid samples bound to the immobilized HBD-2 antibodies (forming antigen antibody complexes). Repeated washing and aspiration removed all other unbound materials from the assay plate. This step was followed by incubation with biotinylated polyclonal HBD-2 antibodies. After washing away the excess and unbound antibody, the microtiter plate was incubated with horseradish peroxidase conjugated streptavidin solution. Following a wash to remove excess and unbound materials, a substrate solution tetramethylbenzidine (TMB) was added to the wells of the micro titer plate and color developed in proportion to the amount of HBD-2 bound in the initial step of the assay. The color development was stopped with the addition of an acid solution and the intensity of color was read at 450nm using a programmable micro titer plate spectrophotometer (Ceres 900 Micro Plate Workstation, Bio-Tek Instruments, Winooski, VT). The concentration of HBD-2 in amniotic fluid samples was determined by interpolation from individual standard curves composed of purified human HBD-2. The calculated inter- and intra-assay coefficients of variation for HBD-2 immunoassay in our laboratory were 5.11% and 5.26%, respectively. The detection limit (sensitivity) was calculated to be 0.077ng/ml.
Statistical analysis
The Shapiro-Wilk test was used to test for normal distribution of the data. Non-parametric testing was applied for comparisons, and adjustments for multiple comparisons (with a Bonferroni correction) were performed when indicated. The Pearson Chi-square test was used to test for proportions. A p-value ≤0.05 was considered statistically significant.
RESULTS
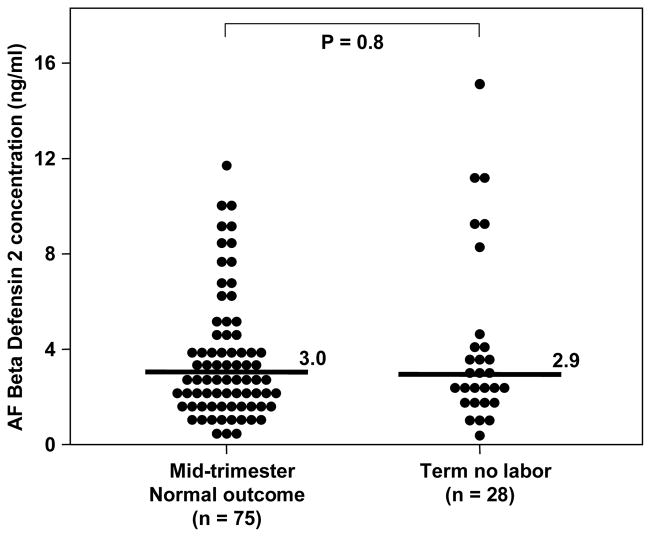
The clinical and obstetrical characteristics of the study groups are displayed in Tables I and II. Immunoreactive HBD-2 was detectable in all amniotic fluid samples (318/318). The concentration of HBD-2 in amniotic fluid did not change with gestational age [mid-trimester: median: 3 ng/ml (range: 0.04–11.8) vs. term: median: 2.9 ng/ml (range: 0.3–15.1), p=0.8; Figure 1].
Table I.
Clinical and demographic characteristics of the study population
| Normal pregnancy midgestation n=75 | Term gestation no labor n=28 | Term gestation in labor N=51 | |
|---|---|---|---|
| Maternal age (y) | 36.5 (35–38) | 29 (21.5–32) | 23 (20–27) |
| Nulliparity | 11 (14.7) | 5 (20.8) | 23 (46) |
| Gestational age at amniocentesis (wks) | 16 (16–17) | 39.5 (38.7–40) | 39.2 (38–40) |
| Gestational age at delivery (wks) | 39 (38–40) | 39.5 (38.7–40) | 39.2 (38–40) |
| Birthweight (g) | 3348 (3078.7–3623.7) | 3420 (3165–3635) | 3250 (3090–3680) |
Values are expressed as median (25–75 percentile) or number (percent)
Table II.
Clinical and demographic characteristics of the study population
| Preterm labor with MIAC | Preterm labor without MIAC | Preterm labor who delivered at term | PPROM without MIAC | PPROM with MIAC | |
|---|---|---|---|---|---|
| n=25 | n=52 | n=36 | n=25 | n=26 | |
| Maternal age (y) | 23 (20–29) | 24 (20–30) | 22.5 (20–28.7) | 23 (19.5–32.5) | 29 (22–31.5) |
| Nulliparity | 13 (52) | 20 (39.2) | 9 (25.7) | 10 (38.5) | 6 (24) |
| Gestational age at amniocentesis (wks) | 26.6 (21.5–29.9) | 27.4 (24.1–30.7) | 28.8 (26.2–30.3) | 30.7 (26.3–33) | 29 (26.8–32) |
| Gestational age at delivery (wks) | 27 (21.5–31.8) | 31 (27–35) | 38 (37–39.7) | 34 (30.7 – 34) | 30 (27.5–33.2) |
| Birthweight (g) | 1077 (450–1655) | 1588 (830–2060) | 2935 (2608.7–3196.7) | 1921 (1617.5–2419) | 1440 (1120–1872.5) |
Values are expressed as median (25–75 percentile) or number (percent)
Figure 1.
Amniotic fluid (AF) concentration of HBD-2 in women in the mid-trimester who delivered a normal neonate at term and in women at term without labor. There was no difference between the median AF concentration of HBD-2 in women in the mid-trimester and those at term without labor [median 3 ng/ml (range 0.3–11.8) vs. median 2.9 ng/ml (range 0.3–15.1), respectively; p=0.8].
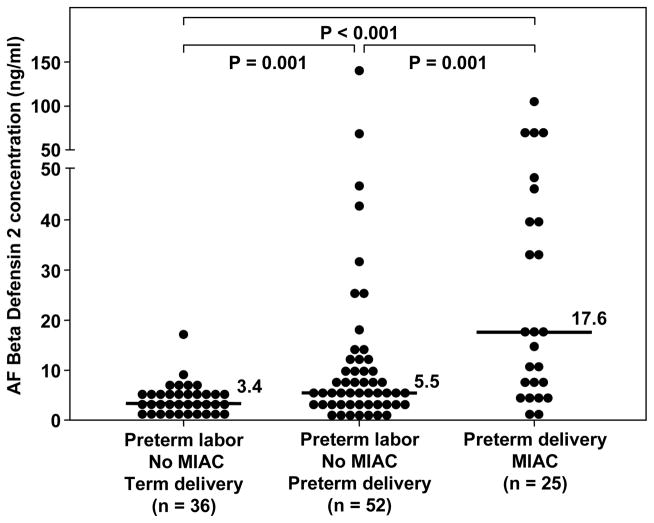
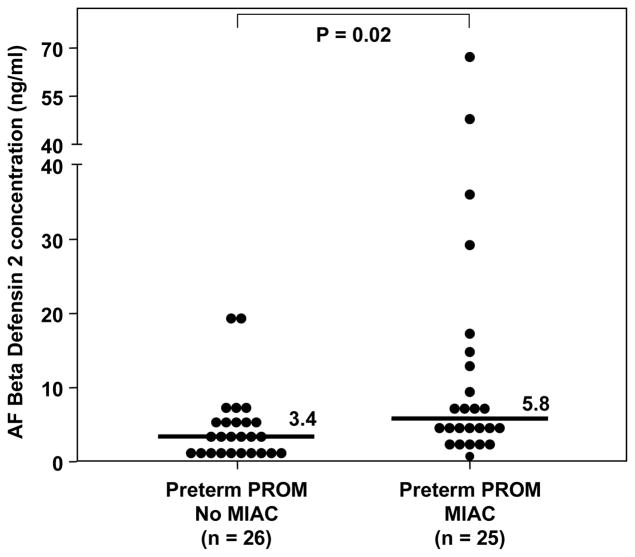
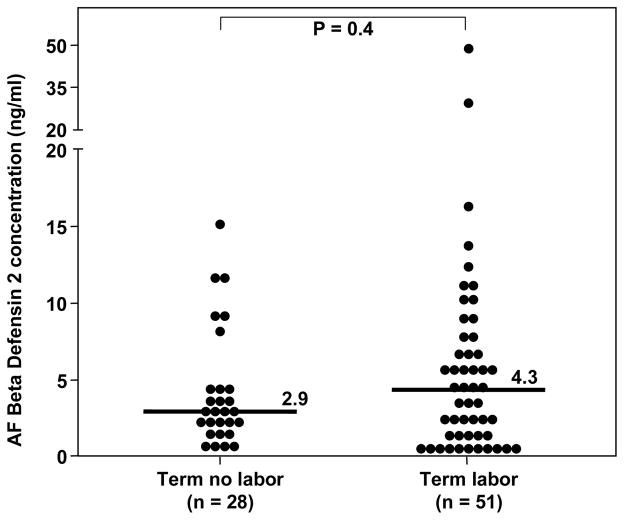
Among patients with preterm labor and intact membranes, those with MIAC had a higher amniotic fluid HBD-2 concentration than those with sterile amniotic fluid [preterm labor with MIAC, median: 17.6 ng/ml (range: 0.96–105.2) vs. preterm labor without MIAC, median: 5 ng/ml (range: 0.1–140), p<0.01; Figure 2]. Similarly, patients with preterm PROM and MIAC had a higher median amniotic fluid HBD-2 concentration than those with preterm PROM without MIAC [preterm PROM and MIAC, median: 5.8 ng/ml (range: 0.57–66.7) vs. preterm PROM without MIAC, median: 3.4 ng/ml (range: 1.04–19.6), p=0.02; Figure 3]. A sub analysis of patients with preterm labor without MIAC demonstrated that those with intra-amniotic inflammation (n=5) had a higher median amniotic fluid concentration of HBD-2 than those without intra-amniotic inflammation (n=46) [intra-amniotic inflammation, median: 26.1 ng/ml (range: 10.1–140.2) vs. no intra-amniotic inflammation, median: 5.2 ng/ml (range: 0.1–68.8), p=0.02]. No significant difference was found in the median gestational age at amniocentesis among patients with preterm labor and intact membranes, or among patients with preterm PROM (p>0.05 for each). In addition, patients with preterm labor and a negative amniotic fluid culture who delivered preterm had a higher median amniotic fluid concentration of HBD-2 than those with preterm labor who delivered at term [preterm, median: 5.5 ng/ml (range: 0.11–140.2) vs. term: median: 3.4 ng/ml (range: 0.57–17.05), p<0.01; Figure 2]. There was no significant difference in the median amniotic fluid concentration of HBD-2 between women at term in labor and those not in labor (p=0.4; see Figure 4).
Figure 2.
Amniotic fluid (AF) concentration of HBD-2 in women with preterm labor and intact membranes. The median AF concentration of HBD-2 was significantly higher in women with preterm labor who delivered preterm than in those who delivered at term [median 5.5 ng/ml, (range 0.1–140.2) vs. median 3.4 ng/ml, (range 0.5–17), respectively; p=0.001]. Similarly, the median AF concentration of HBD-2 in women with microbial invasion of the amniotic cavity (MIAC) was significantly higher than in women with preterm labor without MIAC who delivered preterm [median 17.6 ng/ml, (range 0.9–105.2) vs. median 5.5 ng/ml, (range 0.1–140.2), respectively; p=0.001]. The median AF concentration of HBD-2 was significantly higher in women with MIAC than in those with preterm labor who delivered at term [median 17.6 ng/ml, (range 0.9–105.2) vs. median 3.4 ng/ml, (range 0.5–17), respectively; p=0.004].
Figure 3.
Amniotic fluid (AF) concentration of HBD-2 in women with preterm premature rupture of membranes (PROM) with and without microbial invasion of the amniotic cavity (MIAC). The median AF concentration of HBD-2 was significantly higher in women with preterm PROM and MIAC than in those with preterm PROM without MIAC [median 5.8 ng/mL (range 0.5–66.7) vs. median 3.4 ng/mL (range 1–19.6), respectively; p=0.02].
Figure 4.
Amniotic fluid (AF) concentration of HBD-2 of normal pregnant women at term. There was no difference in the median AF HBD-2 concentration between women at term not in labor and those in labor [median: 2.9 ng/mL (range 0.3–15.1) vs. median: 4.3 ng/mL (range 0.3–48.9), respectively; p=0.4].
DISCUSSION
Principal findings of the study
1) HBD-2 is a physiological constituent of amniotic fluid; 2) the amniotic fluid concentration of HBD-2 did not change with gestational age (mid-trimester vs. term not in labor) and spontaneous labor at term; 3) patients with MIAC (with either intact or ruptured membranes) had a higher median amniotic fluid concentration of HBD-2 than those with sterile amniotic fluid; and 4) among patients with preterm labor without MIAC, those with intra-amniotic inflammation had higher median HBD-2 amniotic fluid concentrations than those without intra-amniotic inflammation.
The detection of immunoreactive HBD-2 in the amniotic fluid of women at term is novel, as our findings contrast with those recently reported by another group of investigators.[41] The difference in results may be attributed to the different immunoassay method employed in the two studies. We have used ELISA, which, in general, has a higher sensitivity than Western Blot analysis (used in the other study).
What is HBD-2?
This anti-microbial peptide was first isolated from human skin in 1997.[29] The observation that patients with psoriasis have fewer skin infections with bacteria and viruses[44] was the impetus to search for anti-microbial peptides in the skin. This was accomplished by passing psoriatic scale extracts through an Escherichia coli affinity column and purifying the bound peptides to homogeneity using High Performance Liquid Chromatography (HPLC). These peptides demonstrated antimicrobial activity in a plate assay. An amino acid sequence analysis of this peptide revealed the consensus sequence of β-defensin with homology to bovine tracheal and lingual anti-microbial β-defensins,[45], as well as human β-defensin-1.[46]
HBD-2 mRNA expression has been demonstrated in human skin,[29,33] lung and trachea,[29,33] uterus,[29] kidney[29] and salivary gland tissue.[29] Of interest is that mRNA expression has also been demonstrated in the fetal kidney.[33] In the same study, in situ hybridization demonstrated HBD-2 RNA expression in skin epithelial cells and superficial epithelium of proximal and distal airways, as well as in the secretory tubules of the submucosal glands in the respiratory tract.[33]
Anti-microbial activity of HBD-2
HBD-2 is highly effective in killing Gram-negative bacteria. Indeed, Harder et al.[29] reported that HBD-2 preparations have anti-microbial activity against E. coli, Pseudomona aeruginosa (LD90: 10 μg/ml) and Candida albicans (LD90: 25 μg/ml). Recent investigations with recombinant HBD-2 revealed that this peptide is even more potent, giving an LD50 near 100 ng/ml.[35] Bacteriostatic effects at concentrations >100 μg/ml for Staphylococcus aureus suggest that HBD-2 has a preferential effect on Gram-negative bacteria and yeast.[29] Importantly, HBD-2 also has anti-viral effects, as it can block HIV-1 replication in human oral epithelial cells.[47]
Though the precise mechanism of the anti-microbial activity of natural anti-microbial peptides is not completely understood, this activity has been attributed to: 1) formation of pores in the microorganisms’ membranes to allow leakage of microbial content; 2) activation of enzymes and pathways that degrade the bacterial cell wall; 3) disruption of the microorganisms cell wall; 4) prevention of adherence to the host epithelial cell wall; and 5) depolarization of the bacterial membrane.[19,38,48–51] The anti-microbial activity of HBD-2 has been attributed to positively charged residues that disrupt the bacterial membrane via electrostatic interactions with polar groups in the microbial membrane.[52] However, other authors have reported that electrostatic interactions alone cannot explain the fungicidal and bactericidal activity of HBD-2.[38,53]
Other biological activities of HBD-2
HBD-2 may also orchestrate the response of the innate and the adaptive immune response by recruiting immature dendritic and memory T cells to cutaneous or mucosal sites of microbial invasion.[54] In vitro experiments have demonstrated that the recruitment of immature dendritic cells by murine β-defensin-2 requires Toll-like receptor (TLR)-4.[55] HBD-2 may also enhance the inflammatory response since it is a specific chemoattractant for tumor necrosis factor (TNF)-α-treated human neutrophils.[56] On the other hand, pro-inflammatory cytokines, including interleukin (IL)-1[32,35,57–59] and TNF-α,[29,57,60,61] can induce the mRNA expression of HBD-2.
Possible origin of HBD-2 in the amniotic fluid
Though the source of HBD-2 in amniotic fluid remains unknown, it is possible that the fetal skin and epithelium of the respiratory and urinary tracts may contribute to the presence of immunoreactive HBD-2 in amniotic fluid, since these tissues constitutively express HBD-2 mRNA.[29–31,33,34,36] However, the chorion and placenta may also be additional sources of HBD-2 in amniotic fluid. Evidence in support of this view is that HBD-2 mRNA expression has been reported in chorion, villous and placental tissues.[62]
Recently, the expression of both HBD-2 and another anti-microbial peptide, LL37, was detected in human neonatal foreskin (basal layer and epidermis) by immunohistochemistry.[31] Indeed, the homologue to HBD-2 in the mouse (mBD4) has been detected in embryonic and newborn skin of mice by immunohistochemistry.[31]
HBD-2 as a effector of innate immunity in the amniotic cavity
Our findings that both MIAC and/or intra-amniotic inflammation in patients with preterm parturition (with intact or ruptured membranes) are associated with high median amniotic fluid concentrations of HBD-2 indicate that this anti-microbial peptide is part of the host response to microorganisms detected in the amniotic cavity. Elevated HBD-2 concentrations in the amniotic fluid of patients with intra-amniotic inflammation (but a negative microbial culture) may be due to infections with microorganisms not recovered by standard microbiological culture techniques, such as viruses, non-culturable microorganisms or non-viable bacteria. It is possible that natural anti-microbial peptides present in the amniotic fluid inhibit bacterial growth in vitro and make recovery and identification of microorganisms more difficult. Finally, the elevated concentration of HBD-2 in patients with negative amniotic fluid cultures may also represent an intra-amniotic inflammation elicited by a non-infectious insult.
Multiple anti-microbial peptides are present in amniotic fluid
Previous studies have documented the presence of the following anti-microbial proteins and peptides in amniotic fluid: lactoferrin,[41,63,64] lysozyme,[40,41,65–67] bactericidal/permeability increasing protein (BPI),[27] calprotectin (MRP8/14),[27] LL37,[40] and neutrophil defensins (HNP 1–3).[27,39–41] Why are there so many anti-microbial proteins and peptides in amniotic fluid? We propose that the control of microbial proliferation and destruction of such microorganisms in the amniotic cavity is required to maximize the likelihood of normal pregnancy outcome. Indeed, bacteria can gain access to the amniotic cavity by passing through intact membranes[68,69] or by transplacental passage in cases of hematogenous dissemination (bacteremia in the context of periodontal disease[70–74] or other distant infections[75]). The combination of several anti-microbial peptides enhances microbial killing. For example, there is evidence that HBD-2 can act synergistically with LL-37 to kill Group B Streptococci (GBS).[31] While LL-37 alone has a minimal bactericidal concentration (MBC) of 16 μM, and HBD-2 alone has an MBC of 8 μM against GBS, the combination of these two peptides at 4 μM each killed 100% of GBS, effectively reducing the MBC.[31] These studies were conducted in hypotonic media, which maximizes the anti-microbial action of both anti-microbial peptides. This observation is relevant since the existence of LL-37 in amniotic fluid has been reported.[40] Moreover, the minimal inhibitory concentration of HBD-2 against E. coli, P. aeruginosa and E. faecalis is reduced in the presence of lactoferrin or lysozyme.[33] Collectively, this evidence indicates that the apparent redundancy in the anti-microbial peptides and proteins in amniotic fluid is aimed at maximizing its antimicrobial activity.
In summary, our results suggest that HBD-2 may contribute to the anti-microbial activity of amniotic fluid, and that this anti-microbial peptide participates in the innate immune response against intra-amniotic infection or other insults eliciting an inflammatory response.
Acknowledgments
This research was supported by the Intramural Program of the National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Slattery MM, Morrison JJ. Preterm delivery. Lancet. 2002:1489–1497. doi: 10.1016/S0140-6736(02)11476-0. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. 2004:28–60. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathews TJ, Menacker F, MacDorman MF. Infant mortality statistics from the 2002 period: linked birth/infant death data set. Natl Vital Stat Rep. 2004:1–29. [PubMed] [Google Scholar]
- 4.Tucker J, McGuire W. Epidemiology of preterm birth. BMJ. 2004:675–678. doi: 10.1136/bmj.329.7467.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero R, Gomez R, Araneda H, Ramirez M, Cotton DB. Cervical mucus inhibits microbial growth: a host defense mechanism to prevent ascending infection in pregnant and non-pregnant women. Am J Obstet Gynecol. 1993:168. [Google Scholar]
- 6.Eggert-Kruse W, Botz I, Pohl S, Rohr G, Strowitzki T. Antimicrobial activity of human cervical mucus. Hum Reprod. 2000:778–784. doi: 10.1093/humrep/15.4.778. [DOI] [PubMed] [Google Scholar]
- 7.Hein M, Helmig RB, Schonheyder HC, Ganz T, Uldbjerg N. An in vitro study of antibacterial properties of the cervical mucus plug in pregnancy. Am J Obstet Gynecol. 2001:586–592. doi: 10.1067/mob.2001.116685. [DOI] [PubMed] [Google Scholar]
- 8.Hein M, Valore EV, Helmig RB, Uldbjerg N, Ganz T. Antimicrobial factors in the cervical mucus plug. Am J Obstet Gynecol. 2002:137–144. doi: 10.1067/mob.2002.123034. [DOI] [PubMed] [Google Scholar]
- 9.Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta. 1991:285–288. doi: 10.1016/0143-4004(91)90010-d. [DOI] [PubMed] [Google Scholar]
- 10.Kjaergaard N, Hein M, Hyttel L, Helmig RB, Schonheyder HC, Uldbjerg N, Madsen H. Antibacterial properties of human amnion and chorion in vitro. Eur J Obstet Gynecol Reprod Biol. 2001:224–229. doi: 10.1016/s0301-2115(00)00345-6. [DOI] [PubMed] [Google Scholar]
- 11.Svinarich DM, Gomez R, Romero R. Detection of human defensins in the placenta. Am J Reprod Immunol. 1997:252–255. doi: 10.1111/j.1600-0897.1997.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 12.Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat Med. 2000:589–593. doi: 10.1038/75074. [DOI] [PubMed] [Google Scholar]
- 13.Galask RP, Snyder IS. Antimicrobial factors in amniotic fluid. Am J Obstet Gynecol. 1970:59–65. doi: 10.1016/0002-9378(70)90126-2. [DOI] [PubMed] [Google Scholar]
- 14.Thadepalli H, Appleman MD, Maidman JE, Arce JJ, Davidson EC., Jr Antimicrobial effect of amniotic fluid against anaerobic bacteria. Am J Obstet Gynecol. 1977:250–254. doi: 10.1016/0002-9378(77)90463-x. [DOI] [PubMed] [Google Scholar]
- 15.Tafari N, Ross SM, Naeye RL, Galask RP, Zaar B. Failure of bacterial growth inhibition by amniotic fluid. Am J Obstet Gynecol. 1977:187–189. doi: 10.1016/0002-9378(77)90685-8. [DOI] [PubMed] [Google Scholar]
- 16.Thadepalli H, Bach VT, Davidson EC., Jr Antimicrobial effect of amniotic fluid. Obstet Gynecol. 1978:198–204. [PubMed] [Google Scholar]
- 17.Appelbaum PC, Shulman G, Chambers NL, Simon NV, Granados JL, Fairbrother PF, Naeye RL. Studies on the growth-inhibiting property of amniotic fluids from two United States population groups. Am J Obstet Gynecol. 1980:579–582. doi: 10.1016/0002-9378(80)90699-7. [DOI] [PubMed] [Google Scholar]
- 18.Thadepalli H, Gangopadhyay PK, Maidman JE. Amniotic fluid analysis for antimicrobial factors. Int J Gynaecol Obstet. 1982:65–72. doi: 10.1016/0020-7292(82)90047-9. [DOI] [PubMed] [Google Scholar]
- 19.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 20.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003:710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 21.Tosi MF. Innate immune responses to infection. J Allergy Clin Immunol. 2005:241–249. doi: 10.1016/j.jaci.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 22.Weiss J, Elsbach P, Olsson I, Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978:2664–2672. [PubMed] [Google Scholar]
- 23.Ganz T, Selsted ME, Szklarek D, Harwig SS, Daher K, Bainton DF, Lehrer RI. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones DE, Bevins CL. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992:23216–23225. [PubMed] [Google Scholar]
- 25.Zhao C, Wang I, Lehrer RI. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]
- 26.Bals R. Epithelial antimicrobial peptides in host defense against infection. Respir Res. 2000:141–150. doi: 10.1186/rr25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, Bujold E, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 28.Schneider JJ, Unholzer A, Schaller M, Schafer-Korting M, Korting HC. Human defensins. J Mol Med. 2005:587–595. doi: 10.1007/s00109-005-0657-1. [DOI] [PubMed] [Google Scholar]
- 29.Harder J, Bartels J, Christophers E, Schroder JM. A peptide antibiotic from human skin. Nature. 1997:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 30.Ali RS, Falconer A, Ikram M, Bissett CE, Cerio R, Quinn AG. Expression of the peptide antibiotics human beta defensin-1 and human beta defensin-2 in normal human skin. J Invest Dermatol. 2001:106–111. doi: 10.1046/j.0022-202x.2001.01401.x. [DOI] [PubMed] [Google Scholar]
- 31.Dorschner RA, Lin KH, Murakami M, Gallo RL. Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: innate immunity during development of the adaptive response. Pediatr Res. 2003:566–572. doi: 10.1203/01.PDR.0000057205.64451.B7. [DOI] [PubMed] [Google Scholar]
- 32.Mathews M, Jia HP, Guthmiller JM, Losh G, Graham S, Johnson GK, Tack BF, et al. Production of beta-defensin antimicrobial peptides by the oral mucosa and salivary glands. Infect Immun. 1999:2740–2745. doi: 10.1128/iai.67.6.2740-2745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, Wilson JM. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Invest. 1998:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hiratsuka T, Nakazato M, Date Y, Ashitani J, Minematsu T, Chino N, Matsukura S. Identification of human beta-defensin-2 in respiratory tract and plasma and its increase in bacterial pneumonia. Biochem Biophys Res Commun. 1998:943–947. doi: 10.1006/bbrc.1998.9239. [DOI] [PubMed] [Google Scholar]
- 35.Singh PK, Jia HP, Wiles K, Hesselberth J, Liu L, Conway BA, Greenberg EP, et al. Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci U S A. 1998:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nitschke M, Wiehl S, Baer PC, Kreft B. Bactericidal activity of renal tubular cells: the putative role of human beta-defensins. Exp Nephrol. 2002:332–337. doi: 10.1159/000065296. [DOI] [PubMed] [Google Scholar]
- 37.Joly S, Maze C, McCray PB, Jr, Guthmiller JM. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004:1024–1029. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng Z, Jiang B, Chandra J, Ghannoum M, Nelson S, Weinberg A. Human beta-defensins: differential activity against candidal species and regulation by Candida albicans. J Dent Res. 2005:445–450. doi: 10.1177/154405910508400509. [DOI] [PubMed] [Google Scholar]
- 39.Heine RP, Wiesenfeld H, Mortimer L, Greig PC. Amniotic fluid defensins: potential markers of subclinical intrauterine infection. Clin Infect Dis. 1998:513–518. doi: 10.1086/514691. [DOI] [PubMed] [Google Scholar]
- 40.Yoshio H, Tollin M, Gudmundsson GH, Lagercrantz H, Jornvall H, Marchini G, Agerberth B. Antimicrobial polypeptides of human vernix caseosa and amniotic fluid: implications for newborn innate defense. Pediatr Res. 2003:211–216. doi: 10.1203/01.PDR.0000047471.47777.B0. [DOI] [PubMed] [Google Scholar]
- 41.Akinbi HT, Narendran V, Pass AK, Markart P, Hoath SB. Host defense proteins in vernix caseosa and amniotic fluid. Am J Obstet Gynecol. 2004:2090–2096. doi: 10.1016/j.ajog.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Marinoni E, Di Iorio R, Letizia C, Villaccio B, Alberini A, Cosmi EV. Amniotic fluid concentrations of adrenomedullin in preterm labor. Obstet Gynecol. 1999:964–967. doi: 10.1016/s0029-7844(98)00551-1. [DOI] [PubMed] [Google Scholar]
- 43.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 44.Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995:982–986. doi: 10.1016/0190-9622(95)91336-x. [DOI] [PubMed] [Google Scholar]
- 45.Schonwetter BS, Stolzenberg ED, Zasloff MA. Epithelial antibiotics induced at sites of inflammation. Science. 1995:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 46.Bensch KW, Raida M, Magert HJ, Schulz-Knappe P, Forssmann WG. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 1995:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 47.Quinones-Mateu ME, Lederman MM, Feng Z, Chakraborty B, Weber J, Rangel HR, Marotta ML, et al. Human epithelial beta-defensins 2 and 3 inhibit HIV-1 replication. AIDS. 2003:F39–F48. doi: 10.1097/00002030-200311070-00001. [DOI] [PubMed] [Google Scholar]
- 48.Weinberg A, Krisanaprakornkit S, Dale BA. Epithelial antimicrobial peptides: review and significance for oral applications. Crit Rev Oral Biol Med. 1998:399–414. doi: 10.1177/10454411980090040201. [DOI] [PubMed] [Google Scholar]
- 49.Bierbaum G, Sahl HG. Induction of autolysis of staphylococci by the basic peptide antibiotics Pep 5 and nisin and their influence on the activity of autolytic enzymes. Arch Microbiol. 1985:249–254. doi: 10.1007/BF00408067. [DOI] [PubMed] [Google Scholar]
- 50.Yang L, Weiss TM, Lehrer RI, Huang HW. Crystallization of antimicrobial pores in membranes: magainin and protegrin. Biophys J. 2000:2002–2009. doi: 10.1016/S0006-3495(00)76448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westerhoff HV, Juretic D, Hendler RW, Zasloff M. Magainins and the disruption of membrane-linked free-energy transduction. Proc Natl Acad Sci U S A. 1989:6597–6601. doi: 10.1073/pnas.86.17.6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoover DM, Rajashankar KR, Blumenthal R, Puri A, Oppenheim JJ, Chertov O, Lubkowski J. The structure of human beta-defensin-2 shows evidence of higher order oligomerization. J Biol Chem. 2000:32911–32918. doi: 10.1074/jbc.M006098200. [DOI] [PubMed] [Google Scholar]
- 53.Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001:5707–5713. doi: 10.1074/jbc.M008557200. [DOI] [PubMed] [Google Scholar]
- 54.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 55.Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002:1025–1029. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 56.Niyonsaba F, Ogawa H, Nagaoka I. Human beta-defensin-2 functions as a chemotactic agent for tumour necrosis factor-alpha-treated human neutrophils. Immunology. 2004:273–281. doi: 10.1111/j.0019-2805.2004.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McDermott AM, Redfern RL, Zhang B, Pei Y, Huang L, Proske RJ. Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003:1859–1865. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu L, Roberts AA, Ganz T. By IL-1 signaling, monocyte-derived cells dramatically enhance the epidermal antimicrobial response to lipopolysaccharide. J Immunol. 2003:575–580. doi: 10.4049/jimmunol.170.1.575. [DOI] [PubMed] [Google Scholar]
- 59.Harder J, Meyer-Hoffert U, Wehkamp K, Schwichtenberg L, Schroder JM. Differential gene induction of human beta-defensins (hBD-1, -2, -3, and -4) in keratinocytes is inhibited by retinoic acid. J Invest Dermatol. 2004:522–529. doi: 10.1111/j.0022-202X.2004.23234.x. [DOI] [PubMed] [Google Scholar]
- 60.Harder J, Meyer-Hoffert U, Teran LM, Schwichtenberg L, Bartels J, Maune S, Schroder JM. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol. 2000:714–721. doi: 10.1165/ajrcmb.22.6.4023. [DOI] [PubMed] [Google Scholar]
- 61.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, Darst MA, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003:3262–3269. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 62.Feng Y, Pan X, Huang N, Feng Y, Wu Q, Wang B. The human beta-defensins expression in female genital tract and pregnancy-related tissues. Sichuan Da Xue Xue Bao Yi Xue Ban. 2003:217–219. [PubMed] [Google Scholar]
- 63.Otsuki K, Yoda A, Saito H, Mitsuhashi Y, Toma Y, Shimizu Y, Yanaihara T. Amniotic fluid lactoferrin in intrauterine infection. Placenta. 1999:175–179. doi: 10.1053/plac.1998.0368. [DOI] [PubMed] [Google Scholar]
- 64.Pacora P, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH, Romero R. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. Am J Obstet Gynecol. 2000:904–910. doi: 10.1067/mob.2000.108882. [DOI] [PubMed] [Google Scholar]
- 65.Cherry SH, Filler M, Harvey H. Lysozyme content of amniotic fluid. Am J Obstet Gynecol. 1973:639–642. doi: 10.1016/s0002-9378(15)33127-6. [DOI] [PubMed] [Google Scholar]
- 66.Ford LC, DeLange RJ, Lebherz TB. Identification of a bactericidal factor (B-lysin) in amnionic fluid at 14 and 40 weeks’ gestation. Am J Obstet Gynecol. 1977:788–792. doi: 10.1016/0002-9378(77)90259-9. [DOI] [PubMed] [Google Scholar]
- 67.Hisanaga S, Umezu T, Shimokawa H, Maesato S. Amniotic fluid lysozyme content in normal and abnormal pregnancy. Nippon Sanka Fujinka Gakkai Zasshi. 1982:541–544. [PubMed] [Google Scholar]
- 68.Evaldson G, Malmborg AS, Nord CE, Ostensson K. Bacteroides fragilis, Streptococcus intermedius and group B streptococci in ascending infection of pregnancy. An animal experimental study. Gynecol Obstet Invest. 1983:230–241. doi: 10.1159/000299415. [DOI] [PubMed] [Google Scholar]
- 69.Galask RP, Varner MW, Petzold CR, Wilbur SL. Bacterial attachment to the chorioamniotic membranes. Am J Obstet Gynecol. 1984:915–928. doi: 10.1016/0002-9378(84)90534-9. [DOI] [PubMed] [Google Scholar]
- 70.Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, McKaig R, et al. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. 1996:1103–1113. doi: 10.1902/jop.1996.67.10s.1103. [DOI] [PubMed] [Google Scholar]
- 71.Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: results of a prospective study. J Am Dent Assoc. 2001:875–880. doi: 10.14219/jada.archive.2001.0299. [DOI] [PubMed] [Google Scholar]
- 72.Madianos PN, Lieff S, Murtha AP, Boggess KA, Auten RL, Jr, Beck JD, Offenbacher S. Maternal periodontitis and prematurity. Part II: Maternal infection and fetal exposure. Ann Periodontol. 2001:175–182. doi: 10.1902/annals.2001.6.1.175. [DOI] [PubMed] [Google Scholar]
- 73.Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. BJOG. 2002:527–533. doi: 10.1111/j.1471-0528.2002.01349.x. [DOI] [PubMed] [Google Scholar]
- 74.Dortbudak O, Eberhardt R, Ulm M, Persson GR. Periodontitis, a marker of risk in pregnancy for preterm birth. J Clin Periodontol. 2005:45–52. doi: 10.1111/j.1600-051X.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- 75.Boggess KA, Madianos PN, Preisser JS, Moise KJ, Jr, Offenbacher S. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. Am J Obstet Gynecol. 2005:554–557. doi: 10.1016/j.ajog.2004.09.001. [DOI] [PubMed] [Google Scholar]