Abstract
The endometrium is a dynamic tissue that undergoes repeated rounds of regeneration in each reproductive (estrous or menstrual) cycle. We have previously shown that bone marrow (BM)-derived stem cells engraft the endometrium in rodents and humans; however, it is not known if these cells contribute physiologically to uterine cyclic regeneration or alternatively are primarily involved in uterine repair in response to injury. Here we performed male-to-female BM transplant and tested the ability of uterine injury to recruit BM-derived cells to endometrium in the presence and absence of sex steroids. Uterine ischemia/reperfusion injury resulted in an ∼2-fold increase in BM-derived stem cell recruitment to the endometrium. The effect was independent of sex steroids or the existence of an estrous cycle. BM-derived mesenchymal stem cells (MSCs) are involved in uterine repair after injury, but not the cyclic regeneration of the endometrium in the estrous/menstrual cycle. Granulocyte-colony stimulating factor (G-CSF) is used to increase BM mobilization for transplant and has been proposed as a means of mobilizing stem cells to the uterus. Here G-CSF treatment led to decreased BM engraftment of the uterus after injury, likely by favoring mobilization of hematopoietic stem cells over the MSCs. G-CSF is unlikely to be of benefit in repair of uterine injury in humans. Taken together, we demonstrate that ischemic injury drives BM MSC engraftment of the uterus, independent of estrous cycle, sex steroids, or G-CSF.
Introduction
Bone marrow-derived stem cells (BMDSCs) have been shown to travel to distant organs and contribute to tissue repair and regeneration [1]. BMDSCs have been detected in both human and mouse uterine endometrium, suggesting that BMDSCs are potential endometrial progenitors that may serve as a source of reparative cells for the reproductive tract [2,3]. Regeneration of the endometrium in each reproductive cycle is essential for the continued survival of most mammalian species. Under systemic hormonal changes, namely, the cyclic increase in the serum level of estradiol, endometrium is regenerated in each estrous cycle. Resident progenitor stem cells likely give rise to the cyclic endometrial regeneration seen in each cycle [4,5]. Nonresident multipotent stem cells also migrate to the endometrium from bone marrow (BM) and perhaps other sources; these cells may aid in uterine repair through release of cytokines and other mechanisms; however, they may also give rise to a group of progenitor cells that may ultimately undergo clonal expansion and become committed to specific types of differentiated cells [2,3]. The factors that promote stem cell migration and recruit these cells to endometrium have not been characterized. Systemic cyclic changes in sex steroid hormones have been hypothesized to influence the stem cell migration to uterine endometrium. An enhanced engraftment of endometrium by BMDSCs is also likely to occur after endometrial injury or an inflammatory insult.
BMDSCs include hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs). Both of these cell types represent an important source of cells for the repair of damaged tissues. However, one of main unresolved questions is which subpopulation of BMDSCs promotes the repair of this tissue. Both HSCs and MSCs are involved in response to injury of some cell types, including glial cells and neurons [6,7]. In other tissues, only the MSC subpopulation plays a role in the repair of tissue damage [8,9]. Alternatively, the HSC subpopulation alone has been shown to be involved in the repair of other tissues, such as vascular endothelial cells [10–12]. Granulocyte-colony stimulating factor (G-CSF) is the principal cytokine regulating granulopoiesis. G-CSF is a potent cytokine that causes HSC mobilization; due to this characteristic, it is routinely used to obtain stem cells for human stem cell transplantation [13]. G-CSF enhances BM stem cell mobilization and tissue repair during acute myocardial infarction, suggesting a role for HSCs in this process [14–17]. The role of G-CSF in uterine repair has not been investigated.
We hypothesized that the cyclic regeneration of endometrium driven by the estrous cycle and sex steroids would involve recruitment of BMDSCs to the uterus. Similarly, we predicted that uterine injury would recruit BMDSCs to the uterus and that BM mobilization with G-CSF would also help to maximize recruitment of these cells. While injury in most tissues recruits BM stem cells that aid in repair of the endogenous cell populations, long-term engraftment is less well characterized. Here we examined the role of ischemia/reperfusion injury, estrous cyclicity, and G-CSF in the recruitment and long-term engraftment of BMDSCs to the uterus. We found that, among those factors tested, only injury led to increased stem cell recruitment.
Materials and Methods
Animals
C57BL/6 male and female mice were obtained from Charles River Laboratories and maintained in the Animal Facility of Yale University School of Medicine. Mice are housed 4–5 per cage in a room with a 12-h light/dark cycle (7:00 am–7:00 pm) with ad libitum access to food and water. Male mice were used as BM donors for transplantation to female mice. All animals were treated under an approved Yale University institutional animal care and use committee protocol.
BM cell isolation and transplantation
Donor BM was flushed from the femurs, tibias, and humeri of 8-week-old male mice with cold sterile phosphate-buffered saline (PBS). The marrow suspension was filtered through sterile 70-μM Nitex mesh (Becton, Dickinson and Company). Recipient female mice (7 weeks old) were administered intraperitoneal injections of busulfan (Sigma) and cyclophosphamide monohydrate (Sigma) for 7 days in row, following an established protocol [18]. About 1×107 unfractionated BM cells were transplanted by internal jugular vein injection after myeloablation [18,19].
Ischemia-reperfusion uterine injury model
One week after the BM transplant, a uterine ischemia-reperfusion injury model was created. A mid-abdominal incision was made under sterile conditions, followed by clamping the lower uterine horn and uterine artery using atraumatic vascular clips for 30 min [20]. The uterus was then re-perfused by release of the clips. In the control group, identical surgical incisions were created; however, the uterus was not clamped. To assess long-term engraftment, uteri were collected 8 months after BM transplantation and evaluated by Y chromosome fluorescent in situ hybridization (FISH).
Ovarian transplant to restore the estrous cycle
In a separate experiment, 1 week after BM transplant, ischemia/reperfusion injury was created as described above. Ovarian transplantation was performed 1 week after ischemia/reperfusion injury. Survival ovariectomy was performed on donor female mice (6-week-old). The ovary was removed from the ovarian bursa, placed in a sterile 35-mm dish containing warm sterile PBS, and minced into 1-mm sections. A skin incision on the shoulder of recipient was made and followed by blunt dissection of a 1-cm subcutaneous pouch. The minced ovarian tissue was implanted into the pouch within 30 min of collection. The skin incision was then closed with surgical suture. In the control group, identical surgical incisions were created; however, no ovarian tissue was transplanted. One donor ovary was implanted into each recipient mouse. The resumption of estrous cycles was determined 1 month after the ovarian transplant and throughout the course of the experiment (ie, through month 8) by serial vaginal cytology [21]. Uteri were collected 8 months after BM transplantation and evaluated by Y chromosome FISH. Estradiol levels were measured at the time of uterine harvest. Transplanted ovaries were also collected and stained with hematoxylin and eosin (H&E). Photomicrographs were taken using an Olympus BX41 microscope.
G-CSF and interleukin-1 treatment
One week after BM transplant, ischemia/reperfusion injury was created as described above. Mice then received either 200 μg/kg G-CSF (Neupogen/Filgrastim) kindly supplied by Dr. Diane Krause (Yale University) or 10 μg/kg recombinant human interleukin-1 beta (IL-1β) (RD) per day for 3 days through intraperitoneal injection [22,23]. In the control group, saline was injected through intraperitoneal injection for 3 days. Uteri were collected 8 months after BM transplantation and evaluated by Y chromosome FISH.
Y FISH and immunofluorescence
As previously described [3], Y FISH stain was performed on Formalin-fixed paraffin-embedded specimens tissue were cut into serial 3-μm-thick sections, placed on coated slides, and deparaffinized through a series of xylene and ethanol washes. For Y FISH, CD45, and cytokeratin analysis, sections were incubated in Retrievagen A solution (BD Biosciences) for 30 min at 100°C and then 20 min at room temperature. Y FISH was performed using a digoxigenin-labeled Y chromosome probe and anti-digoxigenin-rhodamine antibody (Roche Diagnostics). The digoxigenin-labeled murine Y probe was kindly supplied by Dr. Diane Krause (Yale University). After Y FISH, slides were incubated simultaneously with both 1:20 rat anti-mouse CD45 (BD Biosciences) and 1:100 rat anti-mouse F4/80 (eBioscience, Inc.) at 4°C overnight followed by 1:500 anti-rat-Alexa 488 (Molecular Probes) for 1 h at 37°C. Alternatively, slides were incubated with 1:100 anti-cytokeratin (Z0622; Dako) or 1:30 anti-vimentin (Cell Signaling) or 1:50 anti-ERα (Santa Cruz Biotechnology) or 1:200 anti-von willebrand factor (vWF) (Abcam) at 4°C overnight, followed by 1:500 anti-rabbit-Alexa 647 (Molecular Probes) for 1 h at 37°C. All slides were coverslipped using Vectashield/4, 6-diamidino-2-phenylindole (Vector Laboratories). Negative and positive controls for Y chromosome consisted of sections of uterus from female-to-female BM transplants and testis, respectively. Negative and positive control tissues were processed simultaneously in each staining run. For each cell type, at least 500,000 cells were counted per animal.
Tissue analysis, cell counts, and image capture
BM-derived epithelial and stromal cells were counted by systematically examining the slides under ×40 magnification using an Olympus BX-51 microscope (Olympus). Areas of endometrium were randomly chosen and full-thickness counted. Multiple random slides of uterine sections from each mouse uterus were stained for use. Photomicrographs were taken using an Olympus BX51 fluorescence microscope. Images were captured with a digital camera, using IPLab software (BD Biosciences, www.bdbiosciences.com). Images were obtained using appropriate excitation and emission filter sets for 4′,6′-diamidino-2-phenylindole (nuclei), rhodamine (Y chromosome), fluorescein isothiocyanate (CD45, F4/80), and Cy5 (cytokeratin and vimiten).
Results
BMDSCs engraft the uterus
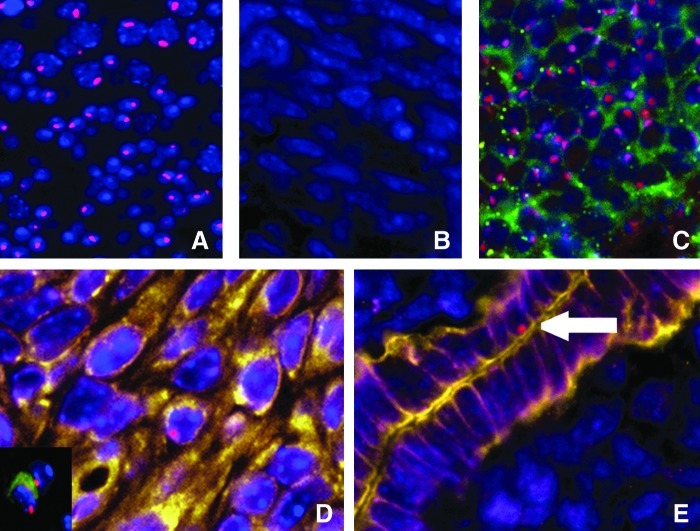
In the testis (positive control) ∼85% of the cells were positive for Y chromosome (Fig. 1A). No cells showed evidence of a Y chromosome in those mice that did not undergo transplant (Fig. 1B). CD45 is a pan-leukocyte marker used here to distinguish endometrial cells from transient leukocytes present in the endometrium. F4/80, a macrophage-specific marker, was used simultaneously. Staining for Y chromosome and CD45 in the male spleen is shown as a positive control (Fig. 1C). To investigate whether BMDSCs engraft to endometrium, we assayed the uterus for cells that were Y chromosome positive, but CD45 and F4/80 negative (Fig. 1D). Vimentin and cytokeratin are markers of differentiated stromal and epithelial cells, respectively. To investigate whether BMDSCs that engraft endometrium are capable of stromal or epithelial cells differentiation, we assayed the uterus for colocalization of Y chromosome, vimentin, or cytokertain, and absent vWF, CD45, and F4/80 immunoreactivity (Fig. 1D, E). About 500,000 cells were counted from each animal. Both stromal and epithelial cells were identified that were of BM origin.
FIG. 1.
Y chromosome, vimentin/cytokeratin, and CD45 immunofluorescence. (A) Y chromosome signal (red) in the testis shown as positive control. (B) Absence of Y chromosome signal in the endometrium of mice without BM transplanted shown as a negative control. (C) Y chromosome signal in male spleen tissue demonstrating expression of CD45 (pan leukocyte marker: green). (D) Y chromosome-positive, vimentin-positive (yellow), and vWF, CD45-negative cell, demonstrating a BM-derived endometrial stromal cell in the endometrium. The insert shows a Y chromosome-positive, CD45-positive cell indicative of a BM-derived leukocyte in the endometrium. Leukocytes are distinguished from endometrial cells and do not appear in the subsequent data. (E) Arrow indicates Y chromosome-positive, cytokeratin-positive (yellow), and CD45-negative cell, demonstrating a BM-derived endometrial epithelial cell. DAPI is shown in blue. Original magnification, ×400. BM, bone marrow; DAPI, 4′,6′-diamidino-2-phenylindole.
Ischemia/reperfusion injury promotes migration of BMDSCs to uterine endometrium
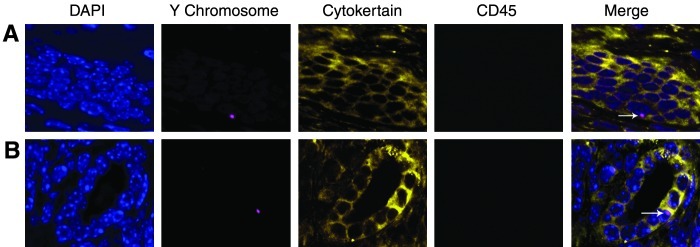
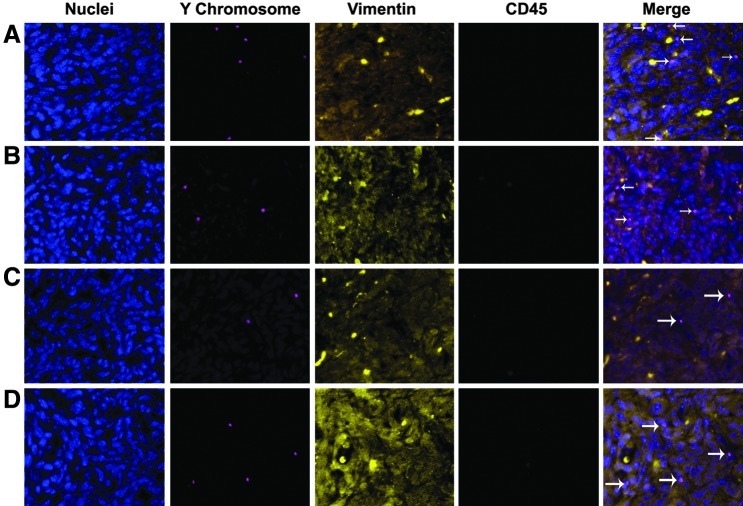
To investigate the influence of the endometrial injury on migration of BMDSCs to uterine endometrium, ischemia/reperfusion injury was induced 1 week after the BM transplant. BM-derived epithelial cells are identified by Y FISH, cytokertain, and CD45 immunofluorescence staining as shown in Fig. 2. BM-derived stromal cells are identified by vimentin, Y FISH, and CD45 immunofluorescence staining as shown in Fig. 3. vWF was absent assuring that these were not endothelial cells. In the control group (n=5), an average of 22 and 13 Y chromosome positive signals per 100,000 cells were detected in stromal and epithethial cells, respectively. In the injury group (n=5), an average of 42 and 14 Y chromosome-positive signals per 100,000 cells were detected in stromal and epithethial cells, respectively (P<0.001 control vs. injury in stromal but not epithelial cells). As shown in Table 1, the ischemia/reperfusion injury resulted in recruitment of approximately 2-fold more stem cells to the endomertrial stroma.
FIG. 2.
Ischemia/reperfusion injury does not alter BM-derived epithelial cell engraftment. (A) Y chromosome-positive (red), cytokeratin-positive (yellow), and CD45-negative cell (green), demonstrating a BM-derived endometrial epithelial cell in the Ischemia/reperfusion injury uteri. (B) Y chromosome-positive, cytokeratin-positive, and CD45-negative cell, demonstrating a BM-derived endometrial epithelial cell in the noninjured uteri (arrow). Nuclei stained by DAPI are shown in blue. Original magnification, ×400.
FIG. 3.
Ischemia/reperfusion increased engraftment of BM-derived stromal cells, while G-CSF treatment reduces engraftment. Immunofluorescence staining of Y chromosome demonstrates male donor-derived BM cells localized in the endometrium of female recipients. Immunofluorescence staining of CD45 distinguishes endometrial cells from leukocytes. Immunofluorescence staining of vimentin indicates differentiated stromal cells. (A) Y chromosome-positive (red), vimentin-positive (yellow), and CD45-negative cell (green), demonstrating a BM-derived endometrial stromal cell in the Ischemia/reperfusion injured uteri. (B) Y chromosome-positive, cytokeratin-positive, and CD45-negative cell, demonstrating a BM-derived endometrial stromal cell in the non-injured uteri. (C) Y chromosome-positive, vimentin-positive (yellow), and CD45-negative cell, demonstrating a BM-derived endometrial stromal cell in the uteri of G-CSF treatment group. (D) Y chromosome-positive, cytokeratin-positive, and CD45-negative cell, demonstrating a BM-derived endometrial stromal cell in the uteri of control group. Arrow indicates bone marrow derived cell. Nuclei stained by DAPI are shown in blue. Original magnification, ×200. G-CSF, granulocyte-colony stimulating factor.
Table 1.
Ischemia/Reperfusion Injury
| Stromal cells N/100,000 (±SEM) | Epithelium cells N/100,000 (±SEM) | |
|---|---|---|
| Ischemia/reperfusion (n=5) | 42±2.72a | 14±1.05 |
| Control (n=5) | 22±0.32 | 13±0.43 |
P<0.001.
SEM, standard error of the means.
The estrous cycle has no influence on migration of BMDSCs to uterine endometrium
The chemotherapy used to ablate the recipient BM before donor transplantation typically inactivates the ovaries. To investigate the influence of the estrous cycle on migration of BMDSCs to uterine endometrium, ovarian transplantation was performed to restore the estrous cycle after the ischemia/reperfusion injury. Controls underwent the same surgical procedure but did not receive ovarian tissue. The estrous cycle of each mouse was confirmed 1 month after the surgery, as determined by vaginal smear cytology over 4 consecutive weeks and monthly until the time of sacrifice. Vaginal cytology in mice after ovarian transplant is shown in Fig. 4. Transplanted ovaries were collected and stained by H&E stain and active ovarian folliculogenesis was observed (shown in Fig. 4E). In the group transplanted with the ovaries (n=5), each mouse had a 4–5-day cycle with each of 4 stages: proestrous, estrus, metaestrous, and diestrous. The control group (n=5) did not cycle and there were very few vaginal cells visible. No cyclic cellular changes were observed and leukocytes were continuously present. The changes in vaginal cytology persisted for the duration of the study. Further, the mice that did not receive ovary transplant had undetectable estradiol levels, while the transplanted group had a mean estradiol level of 89±38 pg/mL measured in the proestrus phase. Estadiol production persisted until the time of sacrifice. Uteri were collected 8 months after BM transplantation and evaluated by Y chromosome FISH. Both stromal and epithelial cells expressed estrogen receptor (data not shown).
FIG. 4.
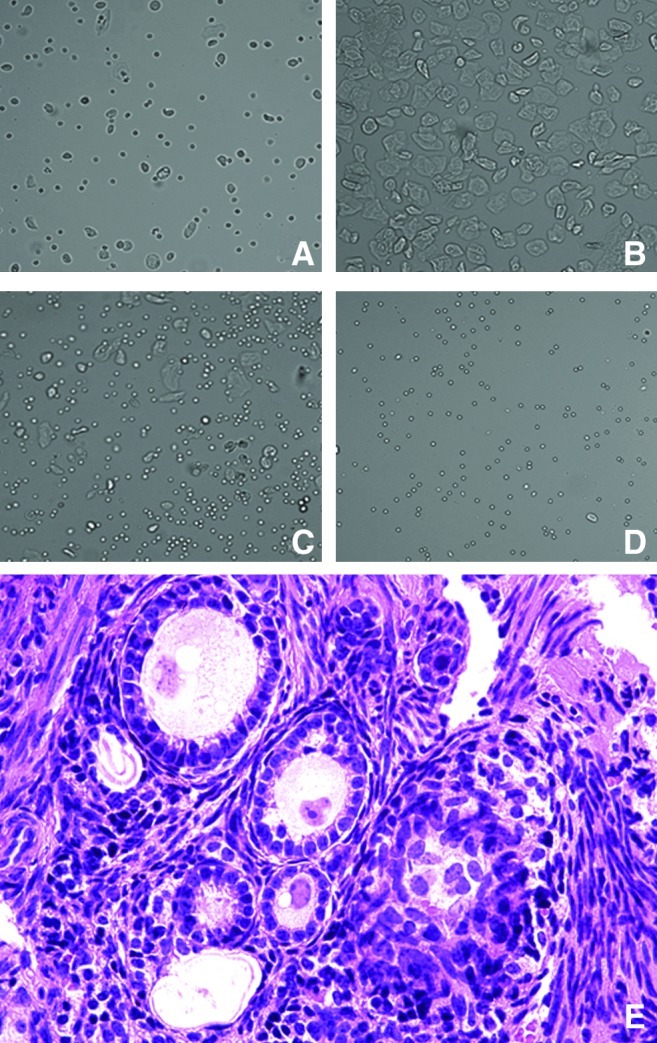
Vaginal cytology and ovarian transplant histology. Vaginal cytology shown in (A–D). (A) Pro-estrus, predominantly consisting of nucleated epithelial cells; (B) estrus, with anucleated cornified cells; (C) metestrus, consisting of the 3 types of cell, leukocytes, cornified, and nucleated epithelial cells; and (D) diestrus, consisting predominantly of leucocytes. Original magnification, ×200. (E) Transplanted ovarian tissue was stained using hematoxylin and eosin and ovarian follicles were observed in all stages of folliculogenesis. Original magnification, ×400. Color images available online at www.liebertpub.com/scd
As shown in Table 2, we detected an average 35 Y bearing stromal cells and 14 epithelial cells per 100,000 cells in the control group versus 30 Y bearing stromal cells and 13 epithelial cells per 100,000 in the group with a restored estrous cycle. These differences were not statistically significant.
Table 2.
Factors Influencing Bone Marrow-Derived Stem Cells Migration
| Stromal cells N/100,000 (±SEM) | Epithelium cells N/100,000 (±SEM) | |
|---|---|---|
| Ovary transplant (n=5) | 30±3.37 | 13±0.89 |
| G-CSF (n=5) | 20±1.35a | 14±0.22 |
| IL-1β (n=5) | 34±1.66 | 13±0.91 |
| Control (n=5) | 35±0.46 | 14±1.48 |
P<0.001.
G-CSF, granulocyte-colony stimulating factor; IL-β, interleukin-1 beta.
G-CSF inhibits migration of BM-derived stem cells to endometrium
G-CSF is a potent cytokine that causes HSC mobilization. To determine if G-CSF would increase migration of BMDSCs to the uterine endometrium, mice were treated with G-CSF or vehicle control after ischemia/reperfusion injury. The G-CSF-treated mice showed increased neutrophil mobilization after G-CSF administration and generally appeared healthier than controls. Long-term engraftment was determined after 8 months. Y FISH, vimentin immunofluorescence staining is shown in Fig. 3. As shown in Table 2, we detected an average of 20 Y chromosome-positive and CD45 and F4/80-negative cells per 100,000 stromal cells in the G-CSF treatment group versus 35 per 100,000 in controls (P<0.001). G-CSF treatment resulted in recruitment of significantly fewer stem cells to the uterus. However, it did not influence the number of the Y chromosome and cytokeratin-positive CD45 and F4/80-negative endometrial epithelium cells. To determine if an inflammatory cytokine could mediate the BMDSC recruitment to the uterus, we also used systemic administration of an inflammatory cytokine in the same manner as was used to administer G-CSF. IL-1β treatment did not alter engraftment of BMDCs in the uterus. We detected an average of 34 per 100,000 of the Y bearing stromal cells and 13 of the Y bearing epithelial cells in the IL-1β treatment group (P>0.05).
Discussion
The uterine endometrium in mammals undergoes renewal in each menstrual cycle. The progenitor cells residing in the basalis layer of endometrium are thought to serve as a source of endometrial regeneration. The presence of endometrial epithelial and stromal colonies with high proliferative potential provides evidence for the existence of putative endometrial stem cells [4,24,25]. We have previously demonstrated that bone BMDSCs engraft the murine and human endometrium [1,3]. Both stromal and epithelial cells can be derived from cells of BM origin. A study using CD45/Cre-Z/EG also showed that BM CD45 progeny cells enter the uterus and differentiate into epithelial cells, demonstrating that BM cells are unlikely to have fused with uterine cells [26]. In our previous experiments, CD45 was not detected in BM-derived stromal and epithelial cells in endometrium, which suggests that CD45 progeny loose this marker, as BM stem cells are incorporated into endometrium. However, the function of BMDSCs in endometrium and the factors influencing the BMDSCs engraftment are still not clear.
In other organs, small numbers of BM-derived stem cells are recruited in response to injury where they have been demonstrated to enhance tissue repair, but do not necessarily fully differentiate [27–29]. Similarly, in the uterus, short-term incorporation of BMDSCs likely contributes to tissue repair; however, it is unknown if these cells permanently engraft the uterus. Here, we evaluated the ability of uterine ischemia/reperfusion injury to influence BMDSC engraftment of endometrium 8 months after treatment. We demonstrated that almost 2-fold more BMDSCs were recruited to the uterine stroma after ischemia/reperfusion injury than in the control group. However, there is no significant change in the number of epithelial cells; this may indicate that the stroma is the main target of injury. BMDSCs have vast regenerative capacity.
Cells obtained from male BM are incorporated into the endometrium and give rise to cells that appear to be differentiated endometrial epithelial and stromal cells; these cells express appropriate markers of functionally differentiated endometrial cells. However, we did not observe large-scale clonal expansion and cell replacement, suggesting that the ability of MSCs to be site-specific producers of trophic factors likely is the major mechanism by which they repair the uterus after injury. BMDSCs show long-term engraftment; however, in our model they do not give rise to large numbers of uterine cells, despite the long-term nature of this study in mice. In humans who have undergone BM transplant, we previously reported several cases of large-scale cell replacement by BM-derived cells [2]. It is unclear if these cells are recruited to the uterus in response to signals not yet characterized or if the cells observed here may be capable of clonal expansion if exposed to appropriate signals.
The proliferation and development of endometrium is regulated by hormonal stimuli. Ovarian estrogen and progesterone drive endometrial growth, differentiation, and apoptosis [30,31]. The chemotherapy used before BM transplantation typically renders these mice sterile and compromises sex steroid production as demonstrated by vaginal cytology; therefore, the endometrium of transplanted mice may be subject to decreased turnover compared with that typical of untreated mice. To investigate the effects of systemic hormonal changes on migration of BMDSCs to uterine endometrium, we restored the estrous cycle in chemotherapy-treated mice and evaluated the recruitment of the BMDSCs to injured uterus. Systemic hormonal changes had no effect on migration of BMDSCs to the injured uterus. In mice, complete endometrial repair can occur in the absence of estrogen, which suggests that estrogen is not essential for endometrial restoration and only continued growth during the estrus cycle [32]. Our results also suggest that systemic hormone changes in the estrous cycle may play a key role in physiological cyclic endometrial renewal; however, sex steroids have no effect on endometrial repair mediated by stem cells. Locally emitted injury signals may be more important in mobilization of BMDSCs to injured tissues than hormonal signals. The BM-derived stem cells are unlikely, at least in this model, to serve as a source of the progenitor cells that cyclically repopulate the endometrium. Cyclic regeneration of the endometrial epithelium in each estrous cycle does not depend on BMDSCs; rather, this process likely relies primarily on endogenous progenitor cells.
BMDSCs include both hematopoietic and mesenchymal lineages. G-CSF mobilizes HSCs from the BM into the peripheral blood, an effect clinically utilized for peripheral blood stem cell transplantation. Previous studies have shown that G-CSF is effective in treating cardiac damage induced by ischemia reperfusion and cerebral ischemia [33,34]. However, G-CSF does not improve in all tissues or types of injury [35]. While G-CSF may acutely mobilize cells, here we evaluated the ability of G-CSF to mobilize BMDSCs that remain engrafted in the uterus after ischemia/reperfusion injury. Here G-CSF reduced BM cell migration to endometrium after ischemic injury. This suggests the existence of a distinct population of BM-derived stem cells that behave differently from HSCs, consisting predominantly of MSCs, and are responsible for uterine repair. G-CSF peripheral stem cell mobilization may favor recruitment of HSCs over MSCs that comprise the uterine stem cells. Current transplantation using G-CSF-mobilized peripheral stem cells is unlikely to be useful for uterine regeneration, and may actually reduce the success of treatments to repair the uterus.
IL-1β is a common mediator of the inflammation process. It induces the secretion of endothelial factors promoting the proliferation and differentiation of myeloid precursors and recovery of hemopoietic precursors. Systemic administration IL-1β did not alter uterine engraftment by BMDSCs. The signals that recruit stem cells to the injured uterus are still under investigation.
Taken together, our results suggest that MSCs of BM origin are recruited to the endometrium in response to injury. BMDSC migration to the endometrium is unlikely to play a role in cyclic endometrial regeneration during the estrous/menstrual cycle. Estrous cyclicity did not affect the engraftment of these cells. Local injury signals likely play a more important role in mobilization of BMDSCs to injured tissues. G-CSF mobilizes HSCs from the BM into the peripheral blood, an effect clinically utilized for peripheral blood stem cell transplantation. However, G-CSF prevented BM cells' migration to endometrium after ischemic injury. Uterine defects that limit endometrial growth, including common conditions such as Asherman's syndrome, can lead to infertility; current treatment options are limited. Knowledge of BM-derived MSC behavior may present new therapeutic strategies for uterine regeneration and repair.
Acknowledgments
The authors thank Dr. Diane Krause for providing the Y chromosome probe and use of the Olympus BX-51 microscope. Funding: NIH U54 HD052668.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Petersen BE. Bowen WC. Patrene KD. Mars WM. Sullivan AK. Murase N. Boggs SS. Greenberger JS. Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 2.Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. J Am Med Assoc. 2004;292:81–85. doi: 10.1001/jama.292.1.81. [DOI] [PubMed] [Google Scholar]
- 3.Du H. Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25:2082–2086. doi: 10.1634/stemcells.2006-0828. [DOI] [PubMed] [Google Scholar]
- 4.Chan RW. Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells. 2006;24:1529–1538. doi: 10.1634/stemcells.2005-0411. [DOI] [PubMed] [Google Scholar]
- 5.Cervelló I. Martínez-Conejero JA. Horcajadas JA. Pellicer A. Simón C. Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum Reprod. 2007;22:45–51. doi: 10.1093/humrep/del332. [DOI] [PubMed] [Google Scholar]
- 6.Koshizuka S. Okada S. Okawa A. Koda M. Murasawa M. Hashimoto M. Kamada T. Yoshinaga K. Murakami M. Moriya H. Yamazaki M. Transplanted hematopoietic stem cells from bone marrow differentiate into neural lineage cells and promote functional recovery after spinal cord injury in mice. J Neuropathol Exp Neurol. 2004;63:64–72. doi: 10.1093/jnen/63.1.64. [DOI] [PubMed] [Google Scholar]
- 7.Sigurjonsson OE. Perreault M-C. Egeland T. Glover JC. Adult human hematopoietic stem cells produce neurons efficiently in the regenerating chicken embryo spinal cord. Proc Natl Acad Sci. 2005;102:5227–5232. doi: 10.1073/pnas.0501029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rojas M. Xu J. Woods CR. Mora AL. Spears W. Roman J. Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krause DS. Bone marrow-derived cells and stem cells in lung repair. Proc Am Thorac Soc. 2008;5:323–327. doi: 10.1513/pats.200712-169DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivan-Loukianova E. Awad OA. Stepanovic V. Bickenbach J. Schatteman GC. CD34 blood cells accelerate vascularization and healing of diabetic mouse skin wounds. J Vasc Res. 2003;40:368–377. doi: 10.1159/000072701. [DOI] [PubMed] [Google Scholar]
- 11.Grant MB. May WS. Caballero S. Brown GA. Guthrie SM. Mames RN. Byrne BJ. Vaught T. Spoerri PE. Peck AB. Scott EW. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 12.Chan-Ling T. Baxter L. Afzal A. Sengupta N. Caballero S. Rosinova E. Grant MB. Hematopoietic stem cells provide repair functions after laser-induced Bruch's membrane rupture model of choroidal neovascularization. Am J Pathol. 2006;168:1031–1044. doi: 10.2353/ajpath.2006.050697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Link DC. Mechanisms of granulocyte colony-stimulating factor-induced hematopoietic progenitor-cell mobilization. Semin Hematol. 2000;37(Suppl 2):25–32. doi: 10.1016/s0037-1963(00)90086-6. [DOI] [PubMed] [Google Scholar]
- 14.Orlic D. Kajstura J. Chimenti S. Bodine DM. Leri A. Anversa P. Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transplant. 2003;7:86–88. doi: 10.1034/j.1399-3046.7.s3.13.x. [DOI] [PubMed] [Google Scholar]
- 15.Minatoguchi S. Takemura G. Chen X. Wang N. Uno Y. Koda M. Arai M. Misao Y. Lu C, et al. Acceleration of the healing process and myocardial regeneration may be important as a mechanism of improvement of cardiac function and remodeling by postinfarction granulocyte colony-stimulating factor treatment. Circulation. 2004;109:2572–2580. doi: 10.1161/01.CIR.0000129770.93985.3E. [DOI] [PubMed] [Google Scholar]
- 16.Valgimigli M. Rigolin GM. Cittanti C. Malagutti P. Curello S. Percoco G. Bugli AM. Della Porta M. Bragotti LZ, et al. Use of granulocyte-colony stimulating factor during acute myocardial infarction to enhance bone marrow stem cell mobilization in humans: clinical and angiographic safety profile. Eur Heart J. 2005;26:1838–1845. doi: 10.1093/eurheartj/ehi289. [DOI] [PubMed] [Google Scholar]
- 17.Christopher MJ. Link DC. Granulocyte colony-stimulating factor induces osteoblast apoptosis and inhibits osteoblast differentiation. J Bone Miner Res. 2008;23:1765–1774. doi: 10.1359/JBMR.080612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HJ. Selesniemi K. Niikura Y. Niikura T. Klein R. Dombkowski DM. Tilly JL. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol. 2007;25:3198–3204. doi: 10.1200/JCO.2006.10.3028. [DOI] [PubMed] [Google Scholar]
- 19.Abbott JD. Huang Y. Liu D. Hickey R. Krause DS. Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 20.Okazaki M. Matsuyama T. Kohno T. Shindo H. Koji T. Morimoto Y. Ishimaru T. Induction of epithelial cell apoptosis in the uterus by a mouse uterine ischemia-reperfusion model: possible involvement of tumor necrosis factor-alpha. Biol Reprod. 2005;72:1282–1288. doi: 10.1095/biolreprod.104.035840. [DOI] [PubMed] [Google Scholar]
- 21.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4:Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwasaki M. Adachi Y. Minamino K. Suzuki Y. Zhang Y. Okigaki M. Nakano K. Koike Y. Wang J, et al. Mobilization of bone marrow cells by G-CSF rescues mice from cisplatin-induced renal failure, and M-CSF enhances the effects of G-CSF. J Am Soc Nephrol. 2005;16:658–666. doi: 10.1681/ASN.2004010067. [DOI] [PubMed] [Google Scholar]
- 23.Gatti S. Beck J. Fantuzzi G. Bartfai T. Dinarello CA. Effect of interleukin-18 on mouse core body temperature. Am J Physiol Regul Integr Comp Physiol. 2002;282:R702–R709. doi: 10.1152/ajpregu.00393.2001. [DOI] [PubMed] [Google Scholar]
- 24.Chan RW. Schwab KE. Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70:1738–1750. doi: 10.1095/biolreprod.103.024109. [DOI] [PubMed] [Google Scholar]
- 25.Schwab KE. Chan RW. Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril. 2005;84(Suppl 2):1124–1130. doi: 10.1016/j.fertnstert.2005.02.056. [DOI] [PubMed] [Google Scholar]
- 26.Bratincsák A. Brownstein MJ. Cassiani-Ingoni R. Pastorino S. Szalayova I. Tóth ZE. Key S. Németh K. Pickel J. Mezey E. CD45-positive blood cells give rise to uterine epithelial cells in mice. Stem Cells. 2007;25:2820–2826. doi: 10.1634/stemcells.2007-0301. [DOI] [PubMed] [Google Scholar]
- 27.Alison MR. Poulsom R. Jeffery R. Dhillon AP. Quaglia A. Jacob J. Novelli M. Prentice G. Williamson J. Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. doi: 10.1038/35018642. [DOI] [PubMed] [Google Scholar]
- 28.Orlic D. Kajstura J. Chimenti S. Jakoniuk I. Anderson SM. Li B. Pickel J. McKay R. Nadal-Ginard B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 29.Poulsom R. Forbes SJ. Hodivala-Dilke K. Ryan E. Wyles S. Navaratnarasah S. Jeffery R. Hunt T. Alison M, et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- 30.Narkar M. Kholkute S. Chitlange S. Nandedkar T. Expression of steroid hormone receptors, proliferation and apoptotic markers in primate endometrium. Mol Cell Endocrinol. 2006;246:107–113. doi: 10.1016/j.mce.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 31.Brosens JJ. Gellersen B. Death or survival—progesterone-dependent cell fate decisions in the human endometrial stroma. J Mol Endocrinol. 2006;36:389–398. doi: 10.1677/jme.1.02060. [DOI] [PubMed] [Google Scholar]
- 32.Kaitu'u-Lino TJ. Sluka P. Foo CF. Stanton PG. Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction. 2007;133:1169–1179. doi: 10.1530/REP-06-0385. [DOI] [PubMed] [Google Scholar]
- 33.Lu CZ. Xiao BG. Neuroprotection of G-CSF in cerebral ischemia. Front Biosci. 2007;12:2869–2875. doi: 10.2741/2278. [DOI] [PubMed] [Google Scholar]
- 34.Ieishi K. Nomura M. Kawano T. Fujimoto S. Ikefuji H. Noda Y. Nishikado A. Ito S. The effect of G-CSF in a myocardial ischemia reperfusion model rat. J Med Invest. 2007;54:177–183. doi: 10.2152/jmi.54.177. [DOI] [PubMed] [Google Scholar]
- 35.Schlager GW. Griesmaier E. Wegleiter K. Neubauer V. Urbanek M. Kiechl-Kohlendorfer U. Felderhoff-Mueser U. Keller M. Systemic G-CSF treatment does not improve long-term outcomes after neonatal hypoxic-ischaemic brain injury. Exp Neurol. 2011;230:67–74. doi: 10.1016/j.expneurol.2010.11.021. [DOI] [PubMed] [Google Scholar]