Abstract
Bone marrow-derived mononuclear cells (MNCs) enhance recovery in rodent stroke models. Since stroke activates the bone marrow, there may be biological differences of autologous MNCs derived poststroke compared with the prestroke setting. We analyzed MNCs harvested from the same Long Evans rats 1 day before and 1 day after ischemic stroke or sham stroke. Stroke was induced by suture occlusion of the middle cerebral artery for 90 min. MNCs were characterized by flow cytometry to identify differences in the percentages of different cell subpopulations. MNCs were also placed in culture and cytokines were measured in the media. In separate experiments, Long Evans rats received 24 h after stroke an intracarotid injection of saline or autologous MNCs, prepared from the same animal, either 1 day before or 1 day after stroke. The rats were then followed on the cylinder and corner tests for 28 days. In poststroke MNCs compared with prestroke MNCs, there was a significant reduction in T and mesenchymal stem cells and a significant increase in CD34+ and natural killer cells. Postsham MNCs showed an elevation in CD11b and CD45R cells compared with presham MNCs. The concentrations of IL-10, IL-6, MCP-1, vascular endothelial growth factor (VEGF), and tumor necrosis factor-α were significantly increased in poststroke MNCs compared with prestroke MNCs. Postsham MNCs showed a decrease in VEGF. Poststroke MNCs in comparison with prestroke MNCs led to a greater recovery on neurological testing and reduced lesion size. Autologous MNCs exert different biological responses when derived from the poststroke setting compared with normal animals.
Introduction
Cell-based therapy has emerged as a new approach to reduce neurological deficits and enhance recovery after stroke [1]. Bone marrow mononuclear cells (MNCs) are composed of diverse cell populations and are particularly attractive as a cell therapy because they permit rapid bone marrow harvest and separation for autologous transplantation. Several studies have reported bone marrow-derived MNCs enhance recovery in rodent models of stroke. In these studies, MNCs were prepared from the same animal (autologous) before stroke [2,3] or after stroke [4]. Other rodent stroke studies used MNCs that were derived from donor animals [5]. Since the brain regulates the bone marrow through defined neural pathways [6] and stroke activates the bone marrow [7], there may be biological differences of MNCs derived from the poststroke setting compared with MNCs derived from healthy donors or from autologous sources before stroke. Indeed, priming of cells prior to transplantation may enhance their therapeutic effects when transplanted in a brain injury/neurodegenerative model [8,9]. As such, we hypothesized that autologous MNCs derived from the poststroke setting compared with MNCs derived from the prestroke setting exert different biological behaviors with respect to their ability to exert cytoprotective effects and promote recovery after stroke. It is also important to stress that this question pertaining to bone marrow preconditioning may have broader clinical relevance as autologous MNCs derived from patients are being tested in clinical trials not only after stroke but also after traumatic brain injury and myocardial infarction.
Methods
Animals and groups
One hundred ten adult male Long Evans rats at 300–320 g were used: Six rats were either excluded because of failure to occlude the middle cerebral artery (MCA) or because of mortality within 20 h of occlusion. There was no mortality attributed to intra-arterial delivery of cells. All animals were double housed with free access to food and water. Subjects were maintained on a standard 12:12 h light/dark cycle. All outcome assessments and data analysis were completed blinded to treatment groups. All procedures were approved by the UT-Houston Health Science Center Animal Welfare Committee.
MCA occlusion
Focal ischemia of 90 min duration in male Long Evans rats was induced by suture occlusion of the middle cerebral artery (MCAo) as described everywhere [10]. In brief, animals were anesthetized with 2% isoflurane in a mixture of N2O/O2 (70%/30%). A 3-0 nylon monofilament with a heated blunt tip was introduced through the external carotid artery (ECA) and advanced to the beginning of the left MCA. Blood pressure and blood gas were recorded. The temperature of the temporalis muscle was monitored/controlled at 36.5±0.5°C using a feed-forward temperature controller. Cerebral perfusion was monitored with a laser Doppler flow-meter placed over the ischemic area. For the sham procedure, Long Evans rats underwent all procedures for suture MCAo except the suture was not advanced into the carotid artery.
Bone marrow harvest
We perform bone marrow harvest in rats without causing limb impairment [4]. The rats were anesthetized with isoflurane. An incision was made through the skin to the medial aspect of the tibia. The periosteum was removed and the surgeon drilled a 1.25×1.25 mm burr hole extending into the medullary cavity. A 20 gauge hypodermic needle was inserted into the medullary cavity and connected to a heparinized syringe. Bone marrow (1–1.5 mL) was aspirated while rotating and moving the needle back and forth. The medullary cavity was flushed with saline and the content aspirated. The burr hole was sealed with bone wax and the skin closed with a nylon suture.
Delivery route
For intra-arterial delivery, under a surgical microscope, the left common carotid artery (CCA), left ECA, and the left internal carotid artery (ICA) were isolated via a midline incision. An aneurysm clip ligation of the CCA and ICA was performed. The ECA was ligated with a nylon suture and nicked at a 45° angle. A modified PE10 catheter was inserted into the ECA and advanced so that the tip sat into the ICA. The ligature was tightened around the stump of the ECA until hemostasis was achieved. The ICA was unclipped and marrow cells or saline infused slowly over 5 min. The catheter was then flushed with saline. The catheter was withdrawn and the ligature was tightened around the stump of the ECA. The skin incision was closed with a nylon suture.
Bone marrow cell processing
The cells from the bone marrow aspirate were triturated, centrifuged, and washed in phosphate-buffered saline (PBS)+0.5% bovine serum albumin (BSA). Cells were then suspended in Media 199 and counted using a hemocytometer and coulter counter. The cell suspension was added on top of 20 mL Ficoll-Paque PLUS in a 50 mL conical vial and then centrifuged. The MNCs were collected, washed with PBS+0.5% (BSA), and then counted. Cells were then suspended in sterile, cold PBS at the desired concentration before intra-arterial (IA) injection. For animals that underwent bone marrow harvest 24 h before stroke, MNCs were isolated as described and placed in 90% fetal bovine serum (FBS)+10% dimethyl sulfoxide (DMSO) and then stored at −80°C. Viability >95% was confirmed before re-suspension in cold PBS followed by IA injection. MNCs that were first stored and thawed were also confirmed to have greater than 95% viability prior to IA infusion.
Stroke experiments and autologous MNC injection
Twenty-four hours after MCAo, rats received an intracarotid injection of saline or 10 million MNCs, prepared from the same animal, either 1 day before (prestroke MNCs) or 1 day after stroke (poststroke MNCs). Animals were randomly assigned to 1 of 3 groups. The first group of animals underwent MCAo and then 22 h later underwent a bone marrow harvest and then 2 h later received back purified MNCs via carotid injection. The second group of animals underwent MCAo and 22 h later underwent bone marrow needle injection and marrow flushing but marrow was not harvested; they then received saline via a carotid injection at 24 h after MCA occlusion. This control group was designed based on the idea that if cells were removed from the bone marrow, they would not be returned; therefore, instead, we elected to insert the needle into the bone marrow without harvest. The third group of animals underwent bone marrow harvest 24 h before stroke. The MNCs were extracted and then frozen in 90% FBS+10% DMSO and stored at −80°C. Twenty-four hours later, these animals underwent MCAo and then, another 24 h later after stroke, received an IA injection of their own thawed MNCs.
Asymmetry in the use of forelimbs for postural support (cylinder test)
Animals were placed into a plexiglas cylinder and their behavior observed for forelimb-use asymmetry during vertical movements along the wall of the cylinder. The final score=(nonimpaired forelimb movement−impaired forelimb movement)/(nonimpaired forelimb movement+impaired forelimb movement+both movement) as previously described in the rat [11]. A total of 20 movements were recorded up to a maximum of 10-min.
Asymmetry-corner test
In the home cage, an animal was placed between 2 angled boards. When entering deep into the corner, both sides of the vibrissae are stimulated together. The animal rears forward and upward, then turns back to face the open end. Twenty trials were performed for each rat and the percentage of left turns versus right turns was calculated. Only turns involving full rearing along either board were recorded.
Infarct volume
Animals (n=6 per group) underwent MCA occlusion and either received pre- or poststroke autologous MNCs or saline as described above. At 7 days after stroke, animals were sacrificed and decapitated. The brains were quickly removed, cut into 2 mm sections, and stained with 2,3,5 triphenyltetrazolium chloride (TTC). The infarct volume was calculated from the difference between the volume of contralateral cortex and the volume of the TTC-stained portion (nonischemic) of ipsilateral cortex of each rat. This indirect measure corrects the total infarct volume for edema.
Assessment of transplanted MNCs
To track the transplanted cells in the brain, Q dot nanocrystals, a green fluorescent maker (525 nm), was used prior to infusion as described previously [12]. MNCs were harvested and labeled with Q-dot according to a published protocol [13]. Ischemic stroke and cell administration were performed as described above such that animals either received prestroke or poststroke MNCs (n=3 per group). At 24 h after IA infusion, rats were deeply anesthetized with chloral hydrate, perfused with saline, and perfusion-fixed with 4% paraformaldehyde in PBS. The brains were removed and cut into 10-μm-thick coronal sections at the level of the infarct in the frontal and parietal cortex. Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and then Q-dot labeled cells were visualized by fluorescence microscopy. Total number of labeled cells was then counted according to our previously published study [12].
Serum inflammatory cytokines
Cytokine levels for IL-1β, IL-6, interferon-γ (IFN-γ), and MCP-1 were measured on a multiplex device (Magpix) 3 days after stroke in animals treated with an IA infusion of prestroke MNCs, poststroke MNCs, or saline (N=6 per group). A separate group of animals underwent needle insertion of the bone marrow 1 day before sham surgery and then 1 day after needle insertion of the contralateral limb (N=6).
Flow cytometry of MNCs
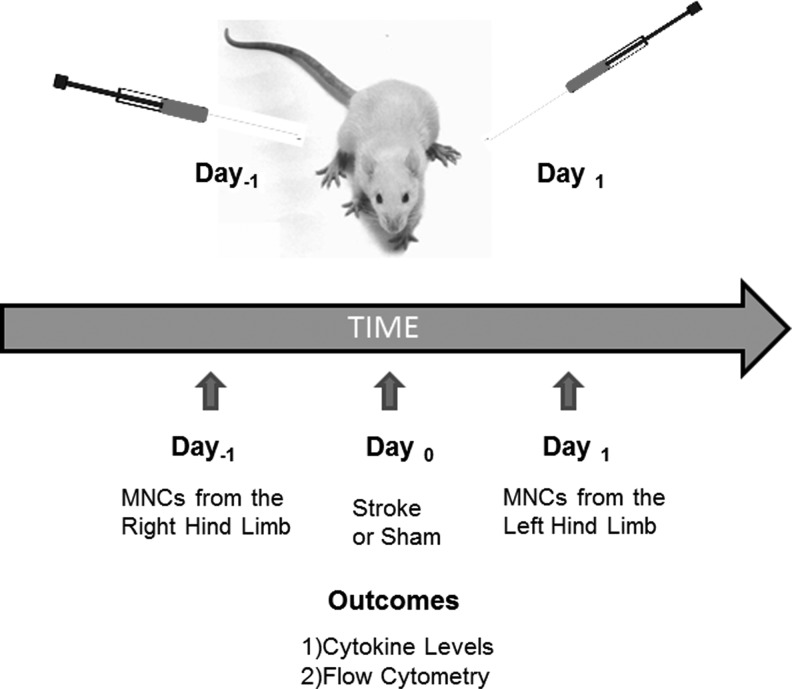
To reduce variability in cell components between individual animals (eg, caused by possible differences in previous exposure to pathogen-associated molecular patterns), we analyzed MNCs harvested from the same animals prior to and after the stroke. Specifically, rats underwent bone marrow aspiration from the right hind limb and MNCs were isolated. Twenty-four hours later, the same rats underwent MCA suture occlusion or sham procedure (N=6 per group). At 24 h after stroke, bone marrow was aspirated but this time from the left hind limb, and MNCs were isolated (Fig. 1). One milliliter bone marrow was aspirated and 1.7±0.23×107 MNCs were isolated each time from the animals (n=12 samples total). We performed flow cytometry to characterize the MNC population using a previously published protocol [14]. Briefly, 1×106 MNCs were suspended in 100 μL staining buffer (Hank's Balanced Salt Solution +2% FBS), then were incubated with anti-rat antibodies specific for CD3-FITC, CD8-PE, CD4-PE, CD45-FITC, CD90-PE, CD29-APC (1:100; Biolegend); CD11b-FITC, CD11b/c-FITC, CD45R-PE, CD71-PE, CD161a-PE (1:100; BD Bioscience); CD34-PE (1:5; Santa Cruz Biotechnology). The mice immunoglobulin G isotypes for phycoerythrobilin (PE), fluorescein isothiocyanate (FIYC), and allophycocyanin (APC) per antibody were tested as controls, respectively. Stained cell were collected using Gallios Flow Cytometer (Beckman Coulter) and analyzed with Kaluza software (Beckman Coulter).
FIG. 1.
Schematic showing the extraction of MNCs from the same rat 1 day before and 1 day after stroke or sham procedure. MNCs either underwent immunophenotyping or were measured for the secretion of various cytokines as shown in Figs. 2 and 3. MNC, mononuclear cells.
Cytokine analysis of MNCs
We followed the same experimental design as the experiments above on flow cytometry. Rats underwent bone marrow aspiration from the right hind limb and MNCs were isolated and harvested for cytokine production. Animals recovered from anesthesia and 24 h later underwent MCA suture occlusion or sham surgery. Twenty-four hours later, bone marrow was aspirated from the left hind limb, MNCs were isolated, placed in culture for 24 h, and cytokines were measured from the culture media (N=5 per group and samples were measured in triplicate from the same animal; Fig. 1). Cytokines were measured on a multiplex device (Millipore) using commercially available kits for IL-10, IL-4, IL-12, IL-17, vascular endothelial growth factor (VEGF), granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-1β, tumor necrosis factor (TNF-α), IL-6, IFN-γ, IP-10, and MCP-1. The estimation of cytokines was performed as per manufacturer's recommendations.
Effects of bone marrow harvest
To determine if a bone marrow needle insertion might change the cellular composition of the bone marrow or the cytokines produced by MNCs in the contralateral limb, bone marrow was aspirated from the right hind limb and then 48 h later drawn from the left hind limb. MNCs were isolated from both preparations and analyzed as described above for flow cytometry and cytokine analysis (N=6).
Fresh versus frozen
Because our experiments compare cells that are frozen and stored versus fresh cells, we investigated potential differences in stored versus fresh MNCs. MNCs were harvested from bone marrow and isolated with Ficoll gradient. In the frozen cell group, MNCs were placed in 90% FBS+10% DMSO and stored at −80°C for 48 h, then thawed for further study. This group was compared against MNCs freshly prepared from the same animals. We then followed the same procedures described above for flow cytometry and cytokine assays. To confirm that freezing MNCs had no effect on functional activity of the transplanted MNCs in vivo, we completed additional experiments in which animals at 24 h after stroke were treated either with prestroke MNCs as described above or poststroke MNCs by intracarotid infusion. Identical to prestroke MNCs, poststroke MNCs were placed in 90% FBS+10% DMSO and stored at −80°C. Poststroke MNCs were stored for 3 h and then were thawed and administered IA at 27 h after stroke. Serum inflammatory cytokines were then assessed at 3 days and infarct volume was assessed at 7 days after stroke (N=6 per group).
Statistical analysis
Data are presented as mean±standard deviation. Cytokine and MNC subpopulation analysis was performed using the Student's t-test and corrected for multiple comparisons. Repeated measures 2-way analysis of variances (ANOVA) and the Bonferroni post-test were used for comparison among groups at different days after stroke in the cylinder test. Repeated measures 2-way ANOVA on ranks with pair-wise comparison (Student-Newman-Keuls) tests were used for the corner test. Statistical significance was set at P<0.05 level.
Results
Flow cytometry of post versus preischemic MNCs
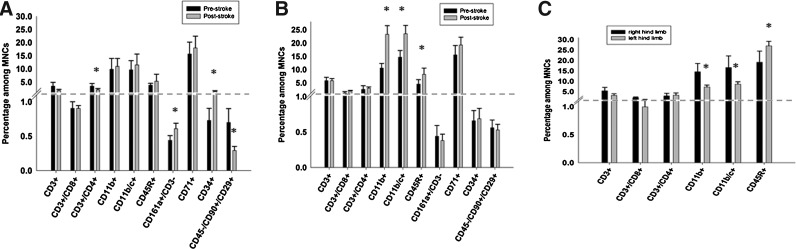
We investigated whether there are differences in cell subpopulations within MNCs harvested from animals 1 day before stroke versus 1 day after stroke (Fig. 2A). First, there was no significant difference in the number of viable cells for MNCs derived from prestroke versus poststroke animals (98.76%±1.02% vs. 98.21%±1.17%, respectively, P=0.99). Second, there was a significant reduction in CD3+/CD4+ cells (3.45%±1.06% vs. 2.09%±0.48%, P=0.038) and CD45−/CD90+/CD29+ cells (0.70%±0.20% vs. 0.29%±0.06%, P=0.015). Third, there was a significant increase in CD34+ cells (0.73%±0.17% vs. 1.43%±0.27%, P=0.012) and CD161a+/CD3− cells (0.44%±0.7% vs. 0.61%±0.08%, P=0.032) in post MCAo rats. In parallel experiments, we studied the differences in immunophenotypes of cell subpopulations within MNCs in animals 1 day before and 1 day after sham procedure. There was no significant difference in the number of viable MNCs derived from presham versus postsham animals (97.82%±1.53% vs. 98.03%±1.19%, respectively, P=0.99). Postsham MNCs showed an elevation in CD11b and CD45R cells compared with presham MNCs (10.62%±1.75% vs. 23.29%±3.24%, P=0.003, respectively). However, in contrast to pre versus poststroke MNCs, there were no differences in the CD34+ and the CD161a+/CD3− cell populations (Fig. 2B). To determine if the changes observed in different cell populations might be due to the previous harvesting of the cells in the contralateral limb, we compared the immunophenotypes of MNCs from the right hind limb and 48 h later from the left hind limb and found that the MNCs from the second harvest showed a reduction in CD11b and an elevation in CD45R compared to the first harvest (Fig. 2C).
FIG. 2.
Characterization by flow cytometry of the different cell populations within MNCs derived from the same animals before and then after a stroke (A) or sham surgery (B). MNCs were harvested from animals 1 day before stroke or sham and then again 1 day after stroke or sham. N=6 per group. *P<0.05 compared with prestroke group. (C) MNCs were harvested from 1 hind limb (right) and then again 2 days later from the other hind limb (left). N=6 per group. *P<0.05 compared with the right hind limb.
Cytokine differences of post versus preischemic MNCs
Since we found differences in MNC subpopulations before and after stroke, we hypothesized that various bioactive factors synthesized by MNCs may change depending on the pre versus poststroke setting. We therefore measured the amount of cytokines released from MNCs derived from the pre- and poststroke setting in the same animal. The concentrations of IL-10, IL-6, MCP-1, VEGF, and TNF-α were significantly increased in the media of MNCs derived from poststroke rats as compared with prestroke rats (Table 1). We then addressed the question to what extent does surgery with anesthesia (not stroke itself) contribute to the increased production of cytokines. We studied a group of animals that first underwent bone marrow aspiration and sham stroke. MNCs were analyzed for cytokine production 1 day before and 1 day after the sham procedure. Interestingly, only the secretion of VEGF was found to significantly change and had decreased in animals after sham (Table 1).
Table 1.
Cytokine Levels in the Media of Cultured Mononuclear Cells Derived from Animals that Underwent Stroke or Sham
| pg/mL | GM-CSF | IL-4 | IL-1b | IL-6 | IL-10 | IL-12(p70) | IFN-γ | IL-17 | MCP-1 | IP-10 | VEGF | TNF-α |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stroke group | ||||||||||||
| Prestroke | N/A | N/A | N/A | 17.59±2.45 | 36.52±7.16 | N/A | N/A | N/A | 575.5±243.14 | N/A | 34.29±9.48 | 35.43±12.40 |
| Poststroke | N/A | N/A | 18.54±2.41 | 143.78±43.93 | 324.2±140.06 | N/A | N/A | N/A | 2385.2±1279.18 | N/A | 80.06±38.99 | 147.13±51.33 |
| Sham group | ||||||||||||
| Prestroke | N/A | N/A | N/A | 16.32±5.40 | 37.64±4.00 | N/A | N/A | N/A | 509.2±187.00 | N/A | 38.47±22.16 | 30.25±5.26 |
| Poststroke | N/A | N/A | N/A | 18.61±9.95 | 39.96±12.57 | N/A | N/A | N/A | 576.17±233.60 | N/A | 14.63±3.61 | 32.3±7.21 |
The first section of the table refers to cytokine levels measured from MNCs harvested 1 day before ischemic stroke (prestroke) and 1 day after stroke (poststroke) in the same animals (N=5 per group). The second section of the table refers to cytokine levels measured from MNCs harvested 1 day before sham stroke procedure (prestroke) and 1 day after sham in the same animals (poststroke). N=5 per group.
GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor; MNC, mononuclear cells.
Postischemic MNCs lead to better outcomes compared with preischemic MNC
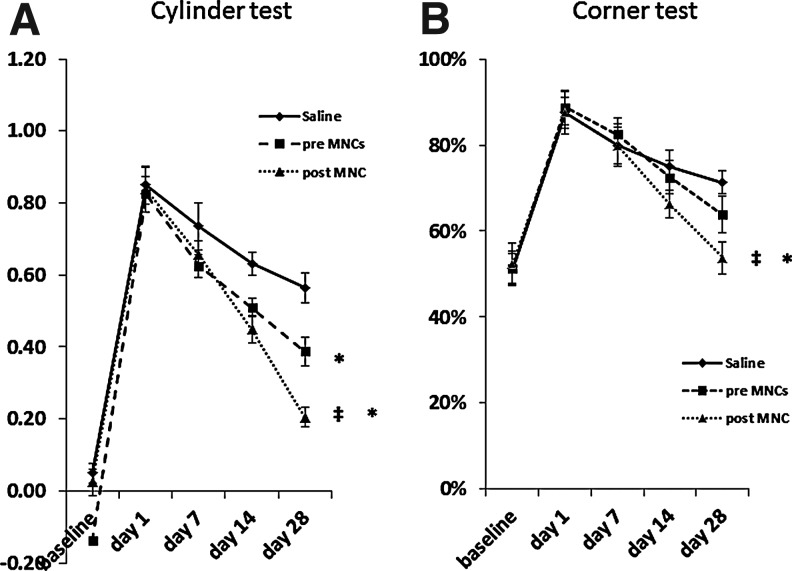
Since poststroke MNCs release modified amounts of bioactive factors, some of which may be beneficial while others detrimental to stroke outcome, we then examined differences in the effects of poststroke MNCs versus prestroke MNCs on functional behavior after stroke. Animals treated with MNCs compared with saline showed significantly greater reduction in deficits on the cylinder test by 28 days after stroke (Fig. 3A). Animals that received MNCs derived after stroke improved to a greater extent regarding fore limb asymmetry on the cylinder test (P<0.05) compared with animals that received MNCs prepared before stroke (Fig. 3A). On the corner test, rats treated with MNCs derived from animals after stroke displayed better recovery compared with saline or prestroke MNC-treated rats (P<0.05; Fig. 3B).
FIG. 3.
Line diagrams illustrating improvement in rats treated with an intra-arterial administration of MNCs 24 h after stroke. Animals were assigned to 3 treatment groups: saline, prestroke MNCs, or poststroke MNCs. All animals were evaluated on the cylinder (A) and corner (B) tests up to 28 days after stroke. Data are mean±SD; N=8 per group for the cylinder test and median±SD for the corner test. *P<0.05 compared with saline controls; ‡compared with prestroke MNCs. SD, standard deviation.
Infarct volume
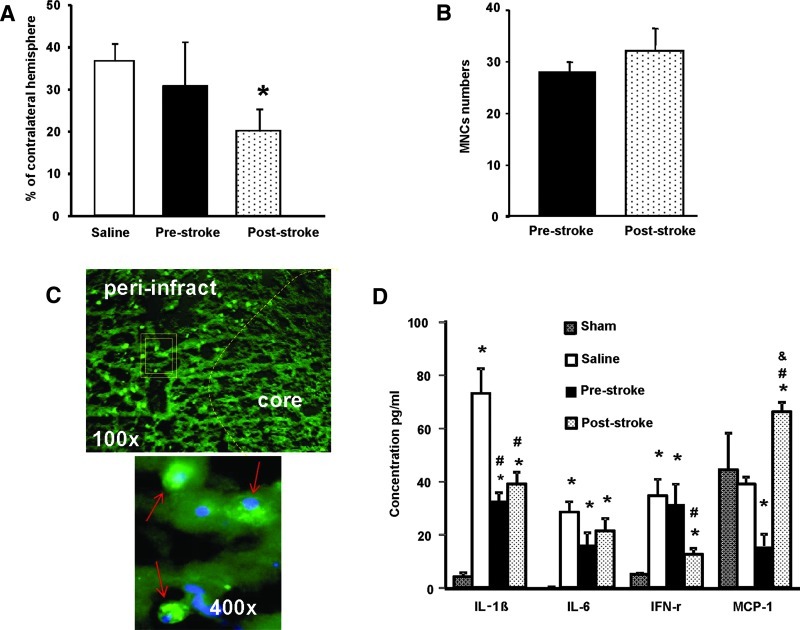
Since poststroke MNCs enhance recovery to a greater extent than prestroke MNCs and cytoprotection of peri-infarct tissue may be one of the possible mechanisms [4], we also examined differences in the effects of poststroke MNCs on tissue protection. Rats treated with poststroke MNCs showed a significant reduction in infarction size when compared with saline control animals at 7 days after stroke (P<0.05). Lesion size was not significantly reduced in animals treated with prestroke autologous MNCs compared with saline (Fig. 4A).
FIG. 4.
(A) A bar graph exhibiting significantly (P<0.05) reduced infarct volume in rats treated with an IA infusion of poststroke MNCs as compared with saline-treated control animals. Prestroke MNCs did not lead to a reduction in infarct volume. Data are mean±SD. N=6 per group.*P<0.05 compared with saline controls. (B) Twenty-four hours after ischemic stroke, Long Evans rats received an intracarotid injection of pre- or poststroke MNCs that were labeled with Q tracker nanocrystals prior to infusion. Labeled cells were quantified in the peri-infarct area at 24 h after IA infusion of MNCs. N=3 per group. Data are mean±SD. (C) Representative fluorescence microscopic images: the upper panel shows Q-dot labeled MNCs (green) in the peri-infarct region at 1 day after IA infusion (under low magnification); the lower panel under high magnification represents the outlined area in the upper panel and shows MNCs containing Q-dot nanocrystals (green). These cells are stained with the nuclear dye, 4′,6-diamidino-2-phenylindole (DAPI, blue). (D) A bar graph exhibiting serum cytokine measurements from animals treated with pre- or poststroke MNCs administered at 24 h after stroke. Cytokines were measured at 3 days after stroke. *P<0.05, compared with sham group; #P<0.05, compared with saline treatment; &P<0.05, compared with poststroke MNCs. Data are mean±SD. N=6 per group. Color images available online at www.liebertpub.com/scd
MNC biodistribution in the brain
The difference in lesion size between pre- and poststroke MNCs might be due to differences in their delivery to the brain. However, at 24 h after IA infusion, we found no differences in the amount of labeled cells in the peri-infarct area in animals treated either with prestroke or poststroke MNCs (Fig. 4B, C).
Serum inflammatory cytokines
Another possible mechanism to account for the differences in pre versus poststroke MNCs may be their effects on poststroke inflammation. To address this question, serum inflammatory cytokines were measured at 3 days after stroke in animals treated with pre- or poststroke MNCs or saline. Overall, poststroke MNCs reduced serum levels of IL-1β and IFN-γ but prestroke MNCs only reduced IL-1β. MCP-1 levels were significantly higher in animals treated with poststroke MNCs, whereas MCP-1 levels were lower in animals treated with prestroke MNCs compared with saline controls (Fig. 4D).
Fresh versus frozen
There were no significant differences in the proportions of different cell types within MNCs or the secretion of cytokines between fresh versus frozen cells (data not shown). The data suggest that freezing and then thawing does not affect MNCs with respect to these specific measurements. Similarly, freezing poststroke MNCs in cryopreservation media did not change the effects of MNCs modulating serum inflammatory cytokines or reducing infarct volume at 7 days after stroke (data not shown).
Discussion
The application of MNCs from the bone marrow as a potential new therapy for acute neurological disorders carries unique advantages for the autologous approach because of rapid isolation and re-infusion without the need for cell culture. Most studies showing efficacy have used either allogenic or syngeneic MNCs in animal stroke models or autologous MNCs extracted from animals before stroke [3]. However, clinical trials are using autologous MNCs derived from patients after stroke; thus, poststroke MNCs are more clinically relevant. We have found that autologous MNCs derived from animals 24 h after stroke also improve outcome [4,12]. Bone marrow MNCs are a heterogenous group of cells composed of lymphoid, myeloid, and stem cell populations [including both hematopoietic and mesenchymal stem cells (MSC)]. We hypothesized that cells within the bone marrow mononuclear fraction after a brain injury such as stroke may change quantitatively and qualitatively compared with bone marrow MNCs from healthy animals. We addressed this question by comparing MNCs derived from the same animals before and after stroke. This is an important issue as a recent study has found that stroke influences leukocyte responses within the bone marrow [7] and it is known that the central nervous system (CNS) controls the autonomic regulation of the bone marrow [6].
We first found that stroke may change the profile of different subpopulations of cells within MNCs. Poststroke MNCs showed downregulation of CD4+ T cells, an upregulation of CD161+/CD3− natural killer (NK) cells, and also an upregulation of CD34+ cells representing immature hematopoietic cells. The increase in hematopoietic stem cells might be due to the postischemic activation of nerve fibers innervating the bone marrow, which release hematopoiesis-stimulating neuropeptides; at 24 h, the first cells after stroke to show increased numbers would be the long- and short-term repopulating hematopoietic stem cells (CD34+). Interestingly, we also found a significant reduction in the number of CD45−/CD29+/CD90+ MSCs in poststroke MNCs. Since MSCs are well known to home to sites of injury mediated by SDF-1α signaling, their decrease in poststroke MNCs likely alludes to their exit from the bone marrow to aid the ischemic region. The quantitative changes observed in these cell types are likely caused by the stroke because the surgical procedure associated with the stroke did not cause the same phenotypic changes in the MNCs. However, we did find that the sham group induced other changes such as an elevation in CD11b myeloid cells which, according to the data, were not likely due to the invasive procedure of a needle insertion in the bone marrow
The changes within certain subpopulations of cells may change the profile of bioactive factors released by MNCs. We explored a range of bioactive factors released by MNCs because they may influence processes mediating neurological recovery after stroke by secreting factors that directly affect neurons (and other cells) or reduce inflammation [15]. We found that the secretion of various cytokines (IL-10, IL-6, MCP-1, VEGF, and TNF-α, and IL-1β) is increased in poststroke MNCs compared with prestroke MNCs. These cytokines reflect a range of different functions including anti-apoptotic, pro-angiogenic, pro-neurogenic, and modifiers of inflammatory responses. In addition, the increase in MCP-1 is further evidence suggesting that MSCs are migrating out of the bone marrow after stroke. However, to what extent does surgery and anesthesia and not the stroke itself contribute to the increased production of cytokines is unclear. These questions were addressed in the present study by comparing cytokines levels between MNCs harvested from animals 1 day before and 1 day after sham stroke surgery. We did find that needle aspiration of the bone marrow along with a sham stroke procedure under anesthesia led to a decrease in VEGF secretion but we found no changes in a broad range of other cytokines, suggesting that the majority of cytokines we studied are not affected by the combination of a bone marrow aspiration and sham stroke surgery.
The differential responses of MNCs in their secretion of different cytokines raise the question whether they affect recovery from stroke differently depending on whether they are derived from animals before or after the stroke. We found that poststroke MNCs improve neurological outcome to a greater extent than prestroke MNCs. In prior studies, we and others [4,5] have identified that cytoprotection of the peri-infarct area may be an important process underlying the therapeutic effects of MNCs in the rodent stroke model. In this study, we found that poststroke MNCs reduce infarct volume in contrast with prestroke MNCs, which further supports the hypothesis that MNCs exert cytoprotective effects on infarct maturation after stroke. We further found that MNCs compared with saline can reduce certain pro-inflammatory cytokines such as IL-1β while poststroke MNCs, but not prestroke MNCs, can also reduce IFN-γ; in addition, poststroke MNCs upregulate MCP-1, whereas prestroke MNCs decrease MCP-1. These findings raise the possibility that poststroke MNCs may modulate the inflammatory response differently compared with prestroke MNCs. It is known that intra-arterially administered MNCs migrate to the peri-infarct area [4] where they may be able to exert direct effects within the injured brain but in this study we found equal numbers in the peri-infarct area of labeled MNCs whether they were derived from the pre- or poststroke setting; it is therefore unlikely that the differential effects of poststroke versus prestroke MNCs are due to differences in MNC biodistribution in the brain.
Poststroke MNCs compared with prestroke MNCs may enhance recovery by producing various beneficial cytokines involved in a broad range of processes associated with the pathogenesis and recovery mechanisms of stroke. However, the increased secretion of TNFα and IL-1β in MNCs harvested from poststroke rats would indicate that these cells may also augment pro-inflammatory responses as well. Whether IL-1β and TNFα-mediated pro-inflammatory responses after stroke are beneficial or harmful remain controversial. In addition, the pro-inflammatory pathways might be counterbalanced by increased production of anti-inflammatory cytokines such as IL-10. It has also been shown that mesenchymal stromal cells, derived from rats 2 days after stroke, reduce neurological deficits to a greater extent compared with MSCs derived from normal rats [16]. This study along with the present report support the hypothesis that ischemic stroke modulates the cytokine profile of cells within the bone marrow.
It remains unclear which cell populations within MNCs are critical to enhance stroke recovery. Of note, CD34+ hematopoetic progenitors cells generate multiple cytokines involved in neurogenesis and neuroprotection such as IGF-1 [17] and they enhance neurogenesis and angiogenesis [18,19], which recently have been shown to be upregulated by MNCs in the rodent stroke model [20]. Our results suggest that the increase in CD34+ cells might have contributed to the elevation of certain cytokines in the poststroke MNCs. NK cells were also increased in poststroke MNCs; their role in the inflammatory stage of ischemic stroke is not well known. While NK cells show detrimental effects in a range of medical disorders, reduced activity of NK cells is associated with an increased risk for infections after stroke [21]. NK cells produce IL-10, which is known to be secreted by MNCs in culture [15], is increased in the postischemic brain of animals treated with MNCs [4], and our data show that IL-10 is upregulated in poststroke MNCs. These results raise the possibility that NK cells may be one of the main cell populations that secrete IL-10 within the MNC fraction and may contribute to enhanced stroke recovery resulting from MNC administration.
One of the major limitations of this study is that we have used young rats, whereas stroke occurs predominantly in aged individuals who may have compromised bone marrow quality and quantity. Hence, further studies using older animals may provide better insight in understanding the clinical effectiveness of autologous MNCs as a potential stroke treatment. In addition, it is possible that cytokine secretion and changes in the immunophenotypes of MNCs may not be important mechanisms relevant to the recovery effects of MNCs.
In conclusion, the present study provides evidence that autologous MNCs exert different biological responses when derived from the poststroke setting compared with normal animals. Autologous treatment with poststroke MNCs could represent a better approach than using prestroke MNCs to enhance recovery following ischemic injury to the brain. Further, we provide new data that ischemic stroke modulates the cytokine profile of MNCs within the bone marrow, which may result from changes in specific cell subpopulations within MNCs. CD34+ cells and NK cells may have contributed to the better recovery observed with poststroke MNCs. However, identifying which cell populations within MNCs mediate the beneficial effects of the mononuclear fraction of bone marrow requires further study.
Acknowledgment
This research work was supported in part by grants from AHA 0475008N, Howard Hughes Medical Institute (SIS), NIH R21 NS064316, and R-01 NS071127.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Stem Cell Therapies as an Emerging Paradigm in Stroke Participants. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–515. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- 2.Iihoshi S. Honmou O. Houkin K. Hashi K. Kocsis JD. A therapeutic window for intravenous administration of autologous bone marrow after cerebral ischemia in adult rats. Brain Res. 2004;1007:1–9. doi: 10.1016/j.brainres.2003.09.084. [DOI] [PubMed] [Google Scholar]
- 3.Baker AH. Sica V. Work LM. Williams-Ignarro S. de Nigris F. Lerman LO. Casamassimi A. Lanza A. Schiano C, et al. Brain protection using autologous bone marrow cell, metalloproteinase inhibitors, and metabolic treatment in cerebral ischemia. Proc Natl Acad Sci U S A. 2007;104:3597–3602. doi: 10.1073/pnas.0611112104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenneman M. Sharma S. Harting M. Strong R. Cox CS., Jr. Aronowski J. Grotta JC. Savitz SI. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30:140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giraldi-Guimaraes A. Rezende-Lima M. Bruno FP. Mendez-Otero R. Treatment with bone marrow mononuclear cells induces functional recovery and decreases neurodegeneration after sensorimotor cortical ischemia in rats. Brain Res. 2009;1266:108–120. doi: 10.1016/j.brainres.2009.01.062. [DOI] [PubMed] [Google Scholar]
- 6.Denes A. Boldogkoi Z. Uhereczky G. Hornyak A. Rusvai M. Palkovits M. Kovacs KJ. Central autonomic control of the bone marrow: multisynaptic tract tracing by recombinant pseudorabies virus. Neuroscience. 2005;134:947–963. doi: 10.1016/j.neuroscience.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 7.Denes A. McColl BW. Leow-Dyke SF. Chapman KZ. Humphreys NE. Grencis RK. Allan SM. Rothwell NJ. Experimental stroke-induced changes in the bone marrow reveal complex regulation of leukocyte responses. J Cereb Blood Flow Metab. 2011;31:1036–1050. doi: 10.1038/jcbfm.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borlongan CV. Su TP. Wang Y. Treatment with delta opioid peptide enhances in vitro and in vivo survival of rat dopaminergic neurons. Neuroreport. 2000;11:923–926. doi: 10.1097/00001756-200004070-00005. [DOI] [PubMed] [Google Scholar]
- 9.Theus MH. Wei L. Cui L. Francis K. Hu X. Keogh C. Yu SP. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210:656–670. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 10.Longa EZ. Weinstein PR. Carlson S. Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 11.Schallert T. Fleming SM. Leasure JL. Tillerson JL. Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 12.Yang B. Strong R. Sharma S. Brenneman M. Mallikarjunarao K. Xi X. Grotta JC. Aronowski J. Savitz SI. Therapeutic time window and dose response of autologous bone marrow mononuclear cells for ischemic stroke. J Neurosci Res. 2011;89:833–839. doi: 10.1002/jnr.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen AB. Kelly DJ. Schuldt AJ. Lu J. Potapova IA. Doronin SV. Robichaud KJ. Robinson RB. Rosen MR, et al. Finding fluorescent needles in the cardiac haystack: tracking human mesenchymal stem cells labeled with quantum dots for quantitative in vivo three-dimensional fluorescence analysis. Stem Cells. 2007;25:2128–2138. doi: 10.1634/stemcells.2006-0722. [DOI] [PubMed] [Google Scholar]
- 14.Harting M. Jimenez F. Pati S. Baumgartner J. Cox C., Jr Immunophenotype characterization of rat mesenchymal stromal cells. Cytotherapy. 2008;10:243–253. doi: 10.1080/14653240801950000. [DOI] [PubMed] [Google Scholar]
- 15.Sharma S. Yang B. Strong R. Xi X. Brenneman M. Grotta JC. Aronowski J. Savitz SI. Bone marrow mononuclear cells protect neurons and modulate microglia in cell culture models of ischemic stroke. J Neurosci Res. 2010;88:2869–2876. doi: 10.1002/jnr.22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zacharek A. Shehadah A. Chen J. Cui X. Roberts C. Lu M. Chopp M. Comparison of bone marrow stromal cells derived from stroke and normal rats for stroke treatment. Stroke. 2010;41:524–530. doi: 10.1161/STROKEAHA.109.568881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majka M. Janowska-Wieczorek A. Ratajczak J. Ehrenman K. Pietrzkowski Z. Kowalska MA. Gewirtz AM. Emerson SG. Ratajczak MZ. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97:3075–3085. doi: 10.1182/blood.v97.10.3075. [DOI] [PubMed] [Google Scholar]
- 18.Taguchi A. Soma T. Tanaka H. Kanda T. Nishimura H. Yoshikawa H. Tsukamoto Y. Iso H. Fujimori Y, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shyu WC. Lin SZ. Chiang MF. Su CY. Li H. Intracerebral peripheral blood stem cell (CD34+) implantation induces neuroplasticity by enhancing beta1 integrin-mediated angiogenesis in chronic stroke rats. J Neurosci. 2006;26:3444–3453. doi: 10.1523/JNEUROSCI.5165-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakano-Doi A. Nakagomi T. Fujikawa M. Nakagomi N. Kubo S. Lu S. Yoshikawa H. Soma T. Taguchi A. Matsuyama T. Bone marrow mononuclear cells promote proliferation of endogenous neural stem cells through vascular niches after cerebral infarction. Stem Cells. 2010;28:1292–1302. doi: 10.1002/stem.454. [DOI] [PubMed] [Google Scholar]
- 21.Peterfalvi A. Molnar T. Banati M. Pusch G. Miko E. Bogar L. Pal J. Szereday L. Illes Z. Impaired function of innate T lymphocytes and NK cells in the acute phase of ischemic stroke. Cerebrovasc Dis. 2009;28:490–498. doi: 10.1159/000236527. [DOI] [PubMed] [Google Scholar]