Abstract
Due to their favorable intrinsic features, including engraftment, differentiation, and immunomodulatory potential, adult mesenchymal stem cells (MSCs) have been proposed for therapeutic in utero intervention. Further improvement of such attributes for particular diseases might merely be achieved by ex vivo MSC genetic engineering previous to transplantation. Here, we evaluated for the first time the feasibility, biodistribution, long-term engraftment, and transgenic enhanced green fluorescent protein (EGFP) expression of genetically engineered human adipose tissue-derived MSCs (EGFP+-ASCs) after intra-amniotic xenotransplantation at E17 of gestation into our validated pregnant rabbit model. Overall, the procedure was safe (86.4% survival rate; absence of anatomical defects). Stable, low-level engraftment of EGFP+-ASCs was confirmed by assessing the presence of the pWT-EGFP lentiviral provirus in the young transplanted rabbit tissues. Accordingly, similar frequencies of provirus-positive animals were found at both 8 weeks (60%) and 16 weeks (66.7%) after in utero intervention. The presence of EGFP+-ASCs was more frequent in respiratory epithelia (lung and trachea), according to the route of administration. However, we were unable to detect EGFP expression, neither by real-time polymerase chain reaction nor by immunohistochemistry, in the provirus-positive tissues, suggesting EGFP transgene silencing mediated by epigenetic events. Moreover, we noticed lack of both host cellular immune responses against xenogeneic ASCs and humoral immune responses against transgenic EGFP. Therefore, the fetal microchimerism achieved by the EGFP+-ASCs in the young rabbit hosts indicates induction of donor-specific tolerance after fetal rabbit xenotransplantation, which should boost postnatal transplantation for the early treatment/prevention of many devastating congenital disorders.
Introduction
To date, only a few genetic disorders have been treated in utero with some success. However, the proof of principle for the early treatment of inherited diseases by prenatal gene therapy has been extensively demonstrated in several animal models [1]. Despite the great potential of prenatal gene therapy, safety problems particularly associated with the use of integrative vectors (risk of insertional mutagenesis, germ-line transmission, or abnormal organogenesis [2–4]) highlighted that new approaches are necessary to circumvent and/or minimize these adverse events before fetal gene therapy could be accepted for clinical application. An encouraging alternative to improve the efficacy of prenatal therapy is the use of stem cells as therapeutic agents, either unmodified or genetically engineered using viral or nonviral vectors.
In utero stem cell administration would share some of the advantages of prenatal gene therapy: (i) the early fetus presents a relative immature immune system, providing a favorable environment for allogeneic or xenogeneic grafts; (ii) the small size of the fetus facilitates increased donor-to-host-cell ratio; and (iii) prenatal administration may prevent many genetic disorders before a definitive organ damage has occurred. In addition, fetal cell therapy has also some advantages per se such as the possibility that stem cells could engraft at several stem cell niches, increasing their expansion; thanks to the high rate of proliferative and migratory events that occur during ontogeny [5]. Finally, whether a combination of cell and gene therapy with integrative vectors would be necessary, the risk associated with insertional mutagenesis might be minimized by performing ex vivo clonal analysis before cell expansion and administration, selecting those stem cell clones in which the vector has been inserted into favorable loci.
At present, prenatal hematopoietic stem cell (HSC) transplantation has been the most developed application for fetal stem cell therapy. Numerous groups have demonstrated diverse degrees of engraftment after in utero HSC administration in animal models. However, significant engraftment has been only documented when cell transplantation was made on stem cell-deficient recipients, where there is a competitive advantage for donor HSCs [6]. Interestingly, studies carried out by Zanjani and col. demonstrated that cotransplantation of mesenchymal stem cells (MSCs) enhanced the engraftment of HSCs after fetal administration [7], pointing out the adjuvant potential of MSCs.
MSCs are multipotent nonhematopoietic cells, easily isolated and expanded ex vivo from a great variety of sources and species, with the capability to differentiate into a variety of cell types of mesenchymal origin such as adipocytes, chondrocytes, osteocytes, and tenocytes, and into other cell types such as myocytes, astrocytes, and neurons [8]. Furthermore, MSCs present low inherent immunogenicity [9]. The main functions described for MSCs include hematopoiesis regulation, apoptosis prevention, cellular repair, and the control of immune–inflammatory reactions [10]. All these attributes make MSCs particularly appealing in tissue repair and regenerative medicine. Therefore, special efforts are being focused in the development of preclinical in utero MSC transplantation studies. In fact, several reports have confirmed that fetal administration of MSCs resulted in correct engraftment and differentiation of transplanted cells [11–13].
To advance toward clinical application, we have focused our efforts elucidating the capacity of MSCs as cell vehicles in prenatal studies, after ex vivo transduction with integrative vectors. We have recently evaluated the overall safety, biodistribution, engraftment capacity, and transgene expression of enhanced green fluorescent protein (EGFP)-expressing rabbit fetal liver MSCs (EGFP+-fl-MSCs) after allogeneic fetal transplantation into the pregnant rabbit model [14]. We highlighted the safety of fl-MSCs for fetal transplantation in the pregnant rabbit model, the efficiency, and persistence of fl-MSC engraftment and transgene expression in fetal organs, and the absence of immune reactions against both allogeneic fl-MSCs and transgenic EGFP after fetal transplantation.
Based on our results and once established the pregnant rabbit as a reliable middle-size animal model to evaluate prenatal stem cell approaches, we decided to evaluate the capability of a widely characterized MSC type with higher ethical and clinical relevance: adult human adipose tissue-derived MSCs (ASCs), transduced with an integrative vector for in utero combined gene and cell therapy through intra-amniotic administration. This approach might become particularly appropriate for the treatment of congenital respiratory diseases.
Materials and Methods
Isolation and characterization of human ASCs
According to the Institutional Ethics Committee guidelines, ASCs were isolated from discarded human adipose tissue obtained from a healthy adult female undergoing elective lipoaspiration, as recently described [15]. Briefly, the raw lipoaspirate was washed extensively with an equal volume of phosphate-buffered saline (PBS) (Invitrogen) to remove blood cells, local anesthetic, and cellular debris. The washed tissue was then resuspended in PBS containing 0.075% type II collagenase (Invitrogen) and incubated at 37°C for 1 h. The enzymatic activity was neutralized by adding an equal volume of the Dulbecco's modified Eagle's medium (DMEM; Invitrogen) containing 10% fetal bovine serum (FBS). The resulting suspension was filtered through a 70-μm nylon filter and centrifuged at 1200 g for 10 min. The pellet was resuspended in an α-MEM (Invitrogen) containing 10% FBS, 1% penicillin/streptomycin, 1% l-glutamine, and 2.5 μg/mL amphotericin (all from Invitrogen), and seeded overnight in culture flasks (Corning, Inc.) at 37°C in a 5% CO2 atmosphere.
Next day, the cell monolayer was washed once more with PBS to remove nonadherent cells and cellular debris and replaced with a fresh medium. Finally, the ASCs were amplified for 10–12 days (passage 0) until 75%–90% confluent, substituting the culture medium every 3–4 days, and were passaged periodically by detachment [0.25% trypsin-1 mM EDTA in PBS (Invitrogen)] after achieving a subconfluent monolayer.
The isolated ASCs were further characterized by cell surface marker expression and differentiation capability [15].
Human ASC transduction and sorting
The replication-defective self-inactivating HIV-1-derived lentiviral vector pWPT-EGFP [16], expressing the EGFP, was produced and employed to generate the EGFP+-ASC population.
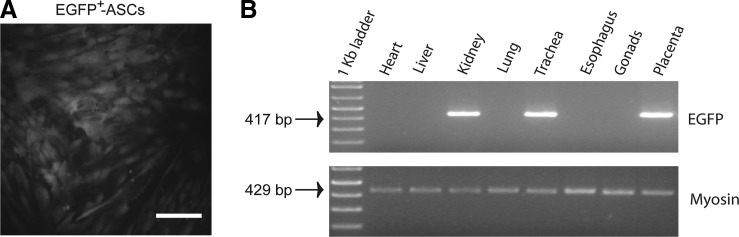
ASCs (passage 1) were transduced by spinoculation. About 5×105 ASCs and 5×104 viral vector particles (MOI=0.1) were cocultured on a 6-well cell culture plate (Corning Inc.) with 2 mL/well α-MEM complete medium plus 8 μg/mL of polybrene (Sigma), and centrifuged at 1000 g for 90 min at 32°C. Next day, the cell culture supernatant was replaced by a fresh medium, and the cells were cultured for another 24 h. The resulting EGFP+ fluorescent ASCs (Fig. 1A) were excited at 488 nm, sorted through a 530/40-band pass filter, recovered as bulk populations of 1.5×105 cells using a MoFlo® jet-in-air high-speed sorter (Beckman Coulter), and amplified in vitro to obtain enough transduced cells for in vivo administration.
FIG. 1.
Long-term assessment of xenogeneic EGFP+-ASC engraftment into young rabbit tissues after fetal transplantation. (A) Efficient lentiviral vector-mediated transduction of adipose tissue-derived mesenchymal stem cells (ASCs). Lentiviral vector-transduced ASCs were visualized by enhanced green fluorescent protein (EGFP) fluorescence (magnification: 400×; scale bar=25 μm). (B) Polymerase chain reaction-mediated detection of integrated, EGFP+-ASC-derived, pWPT-EGFP provirus into genomic DNA isolated from a representative EGFP+-ASC-treated young rabbit at 16 weeks after transplantation. EGFP-specific primers amplified a 417-bp fragment. Lane 1, 1 kb DNA ladder; lanes 2–9 analysis performed in the indicated tissues.
Animals and transplantation procedures
Timed pregnant White New Zealand rabbits (13–15 days of gestation) were housed for 1 week before surgery. The housing was quiet, at constant room temperature and humidity. Laparotomies were performed at 17 days of gestation (E17) (gestation=31–32 days), as described previously [16]. The fetus was located and fixed by external palpation of the semitransparent uterine wall, and intra-amniotic injections were performed using 26-gauge needles directly in the amniotic fluid of the fetal sac in view of the common anatomical landmarks of the E16–18 embryo [16].
Approximately 20 min before the intervention, the EGFP+-ASCs were trypsinized and resuspended in PBS. Fetuses received a unique dose of 2×105 EGFP+-ASCs in a volume of 100 μL/fetus. Control fetuses were injected with an identical volume of PBS. No more than 50% of the fetuses were treated per uterine horn (the vaginal-end sacs were always excluded). Whenever possible, injection of adjacent fetuses was avoided. Once the injections were completed, the uterus was returned to the abdominal cavity and the abdomen was closed in layers. Does were kept under a warming blanket until awake and active, and received subcutaneous injections of meloxicam (Metacam; Abbott Laboratories) (0.4 mL per rabbit/24 h) over 48 h as postoperative analgesia.
All procedures were conducted in compliance with applicable regulations and guidelines, and were reviewed and approved by the Animal Care Committee.
Tissue collection and processing
On day 31 of gestation (14 days after intervention), pregnant rabbits were sacrificed with a euthanizing dose of pentobarbital (150 mg/kg, intravenous). Samples of gonads and uterus from the does and placenta from the pups were frozen for DNA extraction. Experimental pups were retrieved by visual identification after caesarean section, reanimated, and weighted. The pups were marked with a subcutaneous microchip (AVID Microchip ID Systems) for further identification, kept in an incubator C100 (Air Shields) at 32°C for ∼1 h, and finally allocated to foster rabbits. At the end of study (8 or 16 weeks after intervention), the rabbits were sacrificed with a euthanizing dose of pentobarbital, as described above, and tissue samples were harvested (gonads, kidney, liver, heart, lung, trachea, and esophagus for 16-week-old pups). A sample fraction was quickly frozen in liquid nitrogen for DNA extraction, and the remaining fraction was fixed overnight in formalin and embedded in paraffin for immunohistochemical evaluation.
DNA isolation and polymerase chain reaction analysis
Genomic DNA was obtained from does and fetal tissues by standard phenol–chloroform extraction and isopropanol precipitation. Briefly, samples were washed twice in 1× SSC and 10 mM EDTA and incubated overnight at 56°C in a sterile lysis buffer [10 mM Tris-Cl, pH 10.5; 1 mM EDTA; 0.15 mM NaCl; 0.5% SDS; 0.3 mg/sample proteinase K (Roche)] before phenol extraction. Vector presence in the harvested organs was determined by polymerase chain reaction (PCR) amplification of a provirus-specific fragment carried out in a 25-μL volume containing 50 ng of genomic DNA, 100 μM dNTPs, 2.5 μL 10× Biotaq DNA polymerase buffer, 1.50 mM MgCl2, 0.15 μL Biotaq DNA polymerase (5 U/μL) (Bioline), and 20 pmol of the following primers: WPT-EGFP-F (5′-ACC CCG ACC ACA TGA AGC AGC-3′) and WPT-EGFP-R (5′-CGT TGG GGT CTT TGC TCA GGG-3′), localized within the EGFP sequence, in a GeneAmp® PCR system 2400 DNA thermocycler (Perkin-Elmer). A control PCR reaction containing no DNA to monitor for contamination of PCR reagents and a positive control-containing vector DNA were included in each PCR analysis. Amplification conditions involved an initial 10-min denaturation step at 94°C, followed by 42 cycles (94°C, 1 min; 60°C, 30 s; and 72°C, 45 s), and a final 7-min extension at 72°C, producing a 417-base-pair DNA fragment. Rabbit skeletal muscle myosin heavy-chain gene (MHC) primers, RMMHC-F (5′-AAAGAAGATGGATGTTGAGGC-3′), and RMMHC-R (5′-TCACCGTCACTTTCCCTGCT-3′), were used to amplify a 429-base-pair-specific DNA fragment as described previously [17], which served as a control for genomic DNA isolation and loading. All samples were evaluated through 2% agarose gel electrophoresis.
Immunohistochemistry
Formalin-fixed tissues were dehydrated with an ethanol gradient, cut, and embedded in paraffin. Sliced 5-μm sections were mounted on poly-l-lysine-coated glass slides. Before immunohistochemistry, sections were deparaffinized and rehydrated. Immunogenic retrieval was performed, incubating the slides in 10 mM citrate buffer pH 6.0 for 20 min in a microwave oven. After performing several TBS-T (TBS plus 0.3% Triton X-100) washes, tissue sections were blocked by 2-h incubation in 10% FBS diluted in a washing buffer [TBS plus 1% bovine serum albumin (BSA) and 0.2% Tween 20] at room temperature by gentle agitation. Further, TBS-T-washed sections were incubated overnight at 4°C in a humid chamber with a biotinylated goat polyclonal anti-GFP antibody (Abcam) diluted 1/100 in a washing buffer. Next day, the endogenous peroxidase was blocked with 3% H2O2 for 10 min in the dark. Sections were subsequently washed with PBS and incubated with a biotinylated horseradish peroxidase–avidin complex (ImmunoPure® ABC Peroxidase Staining Kit; Pierce). Finally, sections were stained with the peroxidase substrate diaminobenzidine tetrahydrochloride (DAB; Pierce) and counterstained with hematoxylin. Moreover, each specimen was routinely stained with hematoxylin/eosin to evaluate tissue morphology and the presence of inflammatory cell infiltrates.
Enzyme-linked immunosorbent assay
Rabbit sera were obtained at 8 and 16 weeks of age. Blood was taken from the lateral saphenous vein and left coagulating for ∼30 min. Samples were centrifuged twice at 3000 g for 10 min and kept at −80°C until use.
The generation of a rabbit humoral immune response against the EGFP reporter protein was assessed by enzyme-linked immunosorbent assay (ELISA). Maxisorb ELISA 96-well plates (Nunc) were coated overnight at 4°C with 0.1 μg/well of recombinant EGFP (BioVision) diluted in 50 μL of 50 mM carbonate buffer (pH 9.6). After several washes with PBS-T (PBS plus 0.05% Tween 20), plates were blocked with 300 μL of 3% fish gelatin (Sigma-Aldrich) diluted in PBS for 2 h at room temperature. Serum samples diluted 1: 500 with a dilution buffer (PBS-T + 3% BSA) were incubated in triplicate wells for 2 h at room temperature. The plates were then washed again with PBS-T, and 100 μL of secondary antibody (biotin-conjugated goat anti-rabbit IgG, diluted 1:200,000) (Sigma-Aldrich) was further incubated for 2 h at 37°C. Subsequently, plates were washed with PBS, incubated for 30 min with biotinylated horseradish peroxidase–avidin complex (ImmunoPure ABC Peroxidase Staining Kit; Pierce), and developed using 1-Step Slow TMB (Pierce) for 30 min. Finally, the reaction was stopped with 2 M H2SO4, and the corresponding absorbances were measured at 450 nm on an ELISA plate reader. A standard curve was prepared by serial dilutions of a polyclonal anti-GFP antibody (MBL) in rabbit serum (prediluted 1: 500 with dilution buffer) to establish the range of sensitivity.
Mixed lymphocyte culture
After mechanical disaggregation on a culture dish, rabbit spleens were homogenized by gently flushing with a 21G needle. The cellular splenocyte suspension was filtered through a 70-μm nylon filter to remove cellular debris, incubated for 30 min with a lysis buffer (0.15 M NH4Cl, 10 mM KHCO3, 0.1 mM EDTA) at 4°C, and centrifuged at 300 g for 5 min.
Splenocytes were washed twice with PBS and finally cultured in a complete DMEM at 37°C and 5% CO2 atmosphere. Cell viability was assessed by trypan blue exclusion. To study the effect of ASCs on lymphocyte reactivity, a 2-way mixed lymphocyte culture (MLC) was performed. Lymphoid cells were seeded in triplicate at a concentration of 2×105 cells/well in 96-well, U-bottom microtiter cell plates (Corning), in the presence or absence of 1×105 ASCs (passage 2) preplated overnight before the coculture experiment. After a 5-day incubation period, cell proliferation was determined using the Amersham cell proliferation Biotrack ELISA system (GE Healthcare Amersham Biosciences).
Results
We intra-amniotically administered a unique dose of 2×105 EGFP+-ASCs (100 μl/fetus) into fetal rabbits at day E17 (control fetuses were injected with an identical volume of PBS) [14]. At E31 (14 days after intervention), pregnant rabbits were sacrificed (samples of gonads and uterus from the does and placenta from the pups were frozen for DNA extraction), and experimental pups were retrieved by visual identification after caesarean section, reanimated, and weighted. Regarding survival, 22 fetuses from 4 pregnant does were intervened (18 were injected with EGFP+-ASCs, and 4 were injected with PBS), and 3 abortions were documented: 2 abortions among the ASC-treated fetuses and 1 abortion among the PBS-treated fetuses. Thus, the overall survival rate at birth was 86.4%. Furthermore, no anatomical defects were detected in any of the treated fetuses. They maintained a correct weight at the termination of pregnancy according to a normal development pattern. No significant weight differences were found between EGFP+-ASC- and PBS-treated young rabbits (mean weight of EGFP+-ASC-treated fetuses: 56.49±8.24 g, n=16, vs. control PBS-treated fetuses: 57.43±12.59 g, n=3) (P=0.8670, Student's t-test). Therefore, neither the intrinsic features of the EGFP+-ASCs nor the delivery procedure generated toxicity to the in utero-treated fetuses beyond the standard risk from the surgical intervention.
After reanimation, 19 treated pups were placed to the care of a foster mother and 17 survived to adulthood (14 EGFP+-ASC-treated fetuses and 3 PBS-treated fetuses). Analogously to that recently reported for in utero EGFP+-fl-MSC transplantation in the fetal rabbits [14], 2 casualties were documented among the EGFP+-ASC-treated pups due to nurturer rejection. The corresponding necropsies did not reveal any pathological sign in the deceased animals.
To assess engraftment of transplanted EGFP+-ASCs, young rabbits were sacrificed at 8 weeks (5 EGFP+-ASC-treated and 2 PBS-treated) and 16 weeks (9 EGFP+-ASC-treated and 1 PBS-treated) after in utero intervention, and tissues were collected. Genomic DNA isolated from the harvested tissues was employed to detect integrated proviral sequences (Fig. 1B and Table 1). PCR analysis of the EGFP sequence at 8 weeks after transplantation showed the presence of donor cells in several tissues from 3 of 5 recipients (60%), similar to the frequency observed at 16 weeks [6 of 9 recipients (66.7%)]. Each recipient exhibited a different distribution pattern of donor cells, but their presence in the lung and trachea was most frequent, accordingly to the intra-amniotic route of administration. To note, we also detected the presence of donor cells in some placentas (frequency: 2/14, 14.3%). In contrast, gonadal tissue was PCR negative. Moreover, all PBS-treated control animals were completely negative for the presence of EGFP+-ASC proviral sequences (data not shown).
Table 1.
Efficacy of Prenatal Xenogeneic EGFP+-ASC Transplantation in the Rabbit
| |
|
|
|
EGFP+-ASC Biodistributionb |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gestational Period | Time of analysis after in utero intervention (weeks) | Delivery route (N)a | Provirus-positive animals | Heart | Liver | Kidney | Lung | Trachea | Esophagus | Gonads | Placenta |
| E17 | 8 | IA (5) | 3/5 (60%) | 0/5 (0%) | 1/5 (20%) | 1/5 (20%) | 0/5 (0%) | 2/5 (40%) | 1/5 (20%) | 0/5 (0%) | 0/5 (0%) |
| 16 | IA (9) | 6/9 (66.7%) | 0/9 (0%) | 0/9 (0%) | 1/9 (11.1%) | 5/9 (55.6%) | 1/9 (11.1%) | 0/9 (0%) | 0/9 (0%) | 2/9 (22.2%) | |
IA, intraamniotic. (N), number of EGFP+-ASC-treated fetuses.
The presence of proviral DNA in the different tissues from the in utero-treated fetuses was analyzed after genomic DNA extraction and polymerase chain reaction amplification using EGFP-specific primers. For each tissue, frequencies are displayed as number of provirus-positive animals/total number of EGFP+-ASC-treated animals analyzed.
ASC, adipose tissue-derived mesenchymal stem cell; EGFP, enhanced green fluorescent protein.
We next wanted to confirm fetal homing and engraftment of EGFP+-ASCs into the different rabbit tissues. Therefore, we screened transgenic EGFP expression of tissue sections positive for the presence of pWPT-EGFP provirus sequences from EGFP+-ASC-treated rabbits, as previously described [14]. However, we were not able to substantiate engraftment of xenogeneic EGFP+-ASCs into any postnatal in utero-treated rabbit tissue by immunohistochemistry. We were also unsuccessful determining EGFP expression at the transcriptional level by real-time quantitative-PCR (RT-qPCR) (data not shown). Additional examination of the different tissue sections did not reveal the presence of cellular infiltrates in any of the analyzed samples, suggesting absence of immune–inflammatory activity.
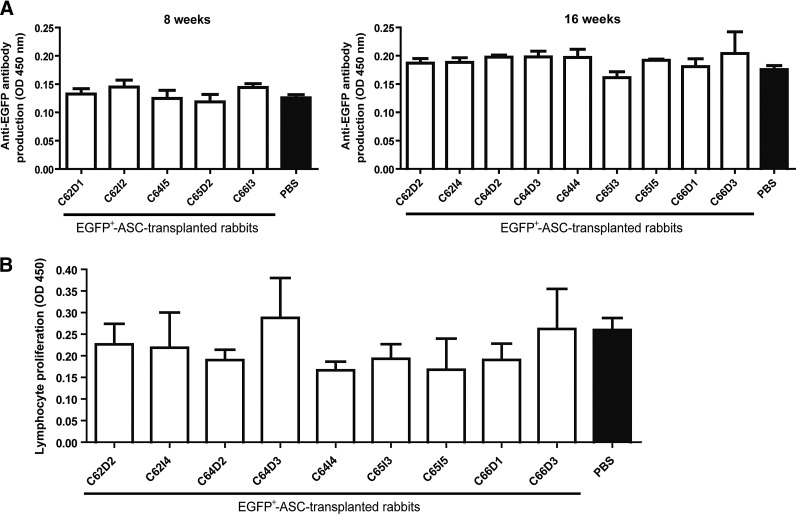
Subsequently, we wished to determine the immunogenicity of human ASCs after prenatal transplantation. For this purpose, we first analyzed whether young rabbits that underwent prenatal transplantation of EGFP+-ASCs overexpressing the reporter EGFP protein were able to develop a humoral immune response against the ASC-derived EGFP transgene product. A specific ELISA assay detecting the presence of antibodies against EGFP [14] was performed on sera harvested from young rabbits after in utero EGFP+-ASC delivery (Fig. 2A). There was no evidence of anti-EGFP antibodies in any of the analyzed animals at both 8 and 16 weeks after in utero transplantation.
FIG. 2.
Lack of immunogenicity of EGFP+-ASCs in the rabbit host. (A) Specific enzyme-linked immunosorbent assay (ELISA) to detect the presence of antibodies against EGFP. Serum samples from the in utero EGFP+-ASC-transplanted young rabbits were collected at sacrifice, and the presence of antibodies against the transgenic EGFP protein was analyzed by ELISA. (B) Xenogeneic lymphocyte proliferation assessed by mixed lymphocyte culture (MLC). About 2×105 splenocytes from the in utero EGFP+-ASC-transplanted young rabbits were cocultured with 1×105 xenogeneic ASCs during 5 days in triplicate wells, and cell proliferation was assessed by ELISA. White column labels refer to the rabbit identification, analyzed 16 weeks after transplantation. Individual values represent the mean±SD from technical replicates (n=3). Phosphate-buffered saline (PBS) (black columns) refer to the mean±SD values obtained from control, in utero PBS-treated young rabbits. Statistically significant differences among EGFP+-ASC-transplanted and control PBS-treated rabbits in both the ELISA, and the MLC assays were estimated using the nonparametric Mann-Whitney U test.
Finally, through an MLC assay [14], we analyzed whether young rabbits that underwent prenatal transplantation of EGFP+-ASCs were able to induce a cellular immune response against these xenogeneic cells at 16 weeks after transplantation. Mononuclear cells isolated from the spleen of in utero EGFP+-ASC-transplanted young rabbits were unable to proliferate when exposed to xenogeneic human ASCs (Fig. 2B), analogously to that observed from mononuclear cells isolated from an in utero PBS-treated rabbit spleen. This suggests absence of an immune reaction against the transplanted human ASCs.
Discussion
Although the most common source of human MSCs is the adult bone marrow (aBM-MSCs), the invasive methods used for their obtention and the low number of MSCs present in the bone marrow (<0.01% of the bone marrow mononuclear cell population [18]) stress the need to identify alternative MSC sources. Over the last decade, ASCs have arisen as a highly attractive alternative source of MSCs. They are easily obtained from adipose tissue lipoaspirates using a straightforward collagenase-based isolation protocol. Furthermore, the abundance of ASCs in the original tissue and their in vitro expansion capacity outperform that from BM-MSCs [19].
Here, we have presented evidence of the overall safety, biodistribution, and limited engraftment capacity of genetically engineered EGFP-expressing adult human ASCs after xenogeneic fetal transplantation into the pregnant rabbit model. We have demonstrated that xenogeneic in utero transplantation of lentiviral vector-transduced human ASCs is safe both for the recipient dams and for the intervened offspring. Concerning the overall engraftment frequency of xenogeneic EGFP+-ASCs into the transplanted fetuses, 60% and 66.7% of intra-amniotically administered E17 animals had detectable pWPT-EGFP provirus at 8 and 16 weeks after EGFP+-ASC delivery, respectively. These frequencies are analogous to those observed in our previous study using rabbit fetal liver MSCs transduced with a retrovirus vector expressing the EGFP reporter (EGFP+-flMSCs) through the same route of administration [14]. Moreover, in both studies, the MSC biodistribution pattern appeared essentially restricted to the primary target organs according to the route of administration (lung and trachea for intra-amniotic administration). Both the frequency and depth of fetal breathing movements where the amniotic fluid is taken in would facilitate direct contact and further homing and engraftment of EGFP+-ASCs into respiratory tissues, as we and others have proven using viral vectors [16,20].
The limited engraftment of EGFP+-ASCs in our immunocompetent fetal rabbits indicates the lack of competitive advantage of these cells in prenatal recipient niches, similarly to that reported for in utero hematopoietic cell transplantation [21]. However, the rather stable levels of microchimerism achieved by the EGFP+-ASCs in the transplanted young rabbit tissues at both 8 and 16 weeks suggest that the engrafted cells were able to overcome the fetal immune barrier to transplantation. In that sense, it has been recently shown that in addition of being nonimmunogenic human ASCs display an even lower susceptibility to natural killer (NK) cell-mediated lysis than bone marrow-derived MSCs [22]. Indeed, it has been shown that in addition to durable donor-specific T-cell tolerance [23], the presence of microchimerism induces NK cell hyporesponsiveness [24], both necessary to overcome rejection.
Intriguingly, although human ASCs could engraft into the fetal tissues, being detectable by whole-tissue analysis of the proviral sequence through PCR, we were unable to detect EGFP transgene expression in any of the PCR-positive tissue samples, analyzed both by immunohistochemistry and by RT-qPCR. Because only a very small fraction of the ASCs administered in utero could undergo sustained engraftment, their detection at the cellular level would be complicated. However, with a comparable low-level engraftment, in our previously study using rabbit EGFP+-fl-MSCs, we could identify a few patchy areas expressing EGFP through immunohistochemistry. More likely, the lack of transgene expression detection might reflect the outcome of epigenetic events acting over the integrated EGFP-containing provirus. We have recently shown that the EGFP+-ASCs gradually downregulated EGFP transgene expression from integrated gamma-retroviral vectors. Transgene extinction occurred both in undifferentiated ASCs and in ASCs differentiated into 2 different cell lineages (adipocytes and osteocytes), and correlated with increases in H3 histone deacetylation and CpG dinucleotide methylation within the proviral region [15]. Furthermore, epigenetic regulation has also been able to silence lentiviral vector-mediated transgene expression during stem cell differentiation in vivo, in transgenic mice [25,26]. This could limit the efficacy of genetically engineered ASCs for tissue repair/regeneration applications. In that case, the optimization of integrative vectors introducing insulators or other cis-regulatory elements allowing the maintenance of high-level transgene expression [27] should be prioritary.
In summary, the results from this report support the potential of intrauterine allogeneic transplantation of ASCs, either unmodified or genetically engineered using optimized integrative vectors, leading to limited while sustained engraftment after prenatal application. Therefore, due to their intrinsic immunoregulatory features, ASCs might become an alternative to fetal MSCs, the latter more susceptible to ethical considerations, attaining microchimerism and leading to prenatal tolerance induction. This would selectively enhance donor cell competition, which may become an essential step to boost successful postnatal ASC transplantation [28,29] for the early treatment/prevention of many devastating hereditary disorders.
Acknowledgments
This work was supported by the grants 01/1417 from the Fondo de Investigaciones Sanitarias (FIS-ISCIII) to E.G., from 2009SGR1490 (Generalitat de Catalunya) and from Fundación Sira Carrasco para ayuda a la fibrosis quística to J.M.A., and by a fellowship from Associació Catalana de Fibrosi Quística to I.M. J.M.A. is sponsored by the Researchers Stabilization Program from the SNS-Dpt., Salut Generalitat de Catalunya (Exp. CES06/012). We wish to thank Didier Trono (Geneve, Switzerland) for both the pWPT-EGFP vector and the pMD2.G vector encoding the pantropic VSV-G envelope glycoprotein.
Author Disclosure Statement
The authors declare no potential conflicts of interest.
References
- 1.Mehta V. Abi Nader K. Waddington S. David AL. Organ targeted prenatal gene therapy—how far are we? Prenat Diagn. 2011;31:720–734. doi: 10.1002/pd.2787. [DOI] [PubMed] [Google Scholar]
- 2.Gonzaga S. Henriques-Coelho T. Davey M. Zoltick PW. Leite-Moreira AF. Correia-Pinto J. Flake AW. Cystic adenomatoid malformations are induced by localized FGF10 overexpression in fetal rat lung. Am J Respir Cell Mol Biol. 2008;39:346–355. doi: 10.1165/rcmb.2007-0290OC. [DOI] [PubMed] [Google Scholar]
- 3.Themis M. Waddington SN. Schmidt M. von Kalle C. Wang Y. Al-Allaf F. Gregory LG. Nivsarkar M. Themis M, et al. Oncogenesis following delivery of a nonprimate lentiviral gene therapy vector to fetal and neonatal mice. Mol Ther. 2005;12:763–771. doi: 10.1016/j.ymthe.2005.07.358. [DOI] [PubMed] [Google Scholar]
- 4.Park PJ. Colletti E. Ozturk F. Wood JA. Tellez J. Almeida-Porada G. Porada C. Factors determining the risk of inadvertent retroviral transduction of male germ cells after in utero gene transfer in sheep. Hum Gene Ther. 2009;20:201–215. doi: 10.1089/hum.2007.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cumano A. Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 6.Roybal JL. Santore MT. Flake AW. Stem cell and genetic therapies for the fetus. Semin Fetal Neonatal Med. 2010;15:46–51. doi: 10.1016/j.siny.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Almeida-Porada G. Porada CD. Tran N. Zanjani ED. Cotransplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood. 2000;95:3620–3627. [PubMed] [Google Scholar]
- 8.Mosna F. Sensebé L. Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user's guide. Stem Cells Dev. 2010;19:1449–1470. doi: 10.1089/scd.2010.0140. [DOI] [PubMed] [Google Scholar]
- 9.Ghannam S. Bouffi C. Djouad F. Jorgensen C. Noël D. Immunosuppression by mesenchymal stem cells: mechanisms and clinical applications. Stem Cell Res Ther. 2010;1:2. doi: 10.1186/scrt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernardo ME. Locatelli F. Fibbe WE. Mesenchymal stromal cells. Ann New York Acad Sci. 2009;1176:101–117. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen CP. Liu SH. Huang JP. Aplin JD. Wu YH. Chen PC. Hu CS. Ko CC. Lee MY. Chen CY. Engraftment potential of human placenta-derived mesenchymal stem cells after in utero transplantation in rats. Hum Reprod. 2009;24:154–165. doi: 10.1093/humrep/den356. [DOI] [PubMed] [Google Scholar]
- 12.Ersek A. Pixley JS. Goodrich AD. Porada CD. Almeida-Porada G. Thain DS. Zanjani ED. Persistent circulating human insulin in sheep transplanted in utero with human mesenchymal stem cells. Exp Hematol. 2010;38:311–320. doi: 10.1016/j.exphem.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacKenzie TC. Flake AW. Multilineage differentiation of human MSC after in utero transplantation. Cytotherapy. 2001;3:403–405. doi: 10.1080/146532401753277571. [DOI] [PubMed] [Google Scholar]
- 14.Moreno R. Martínez-González I. Rosal M. Nadal M. Petriz J. Gratacós E. Aran JM. Fetal liver-derived mesenchymal stem cell engraftment after allogeneic in utero transplantation into rabbits. Stem Cells Dev. 2012;21:284–295. doi: 10.1089/scd.2010.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreno R. Martínez I. Petriz J. Nadal M. Tintoré X. Gonzalez JR. Gratacós E. Aran JM. The β-interferon scaffold attachment region confers high-level transgene expression and avoids extinction by epigenetic modifications of integrated provirus in adipose tissue-derived human mesenchymal stem cells. Tissue Eng Part C. 2011;17:275–287. doi: 10.1089/ten.TEC.2010.0383. [DOI] [PubMed] [Google Scholar]
- 16.Moreno R. Rosal M. Martínez I. Vilardell F. Gonzalez JR. Petriz J. Hernandez-Andrade E. Gratacós E. Aran JM. Restricted transgene persistence after lentiviral vector-mediated fetal gene transfer in the pregnant rabbit model. J Gene Med. 2008;10:951–964. doi: 10.1002/jgm.1227. [DOI] [PubMed] [Google Scholar]
- 17.Moreno R. Rosal M. Cabrero L. Gratacós E. Aran JM. Feasibility of retroviral vector-mediated in utero gene transfer to the fetal rabbit. Fetal Diagn Ther. 2005;20:485–493. doi: 10.1159/000088036. [DOI] [PubMed] [Google Scholar]
- 18.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 19.Bunnell BA. Flaat M. Gagliardi C. Patel B. Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckley SMK. Waddington SN. Jezzard S. Lawrence L. Schneider H. Holder MV. Themis M. Coutelle C. Factors influencing adenovirus-mediated airway transduction in fetal mice. Mol Ther. 2005;12:484–492. doi: 10.1016/j.ymthe.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Westgren M. In utero stem cell transplantation. Semin Reprod Med. 2006;24:348–357. doi: 10.1055/s-2006-952156. [DOI] [PubMed] [Google Scholar]
- 22.DelaRosa O. Sánchez-Correa B. Morgado S. Ramírez C. Del Río B. Menta R. Lombardo E. Tarazona R. Casado JG. Human adipose-derived stem cells impair natural killer cell function and exhibit low susceptibility to natural killer-mediated lysis. Stem Cells Dev. 2011;21:1333–1343. doi: 10.1089/scd.2011.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HB. Shaaban AF. Yang EY. Liechty KW. Flake AW. Microchimerism and tolerance after in utero bone marrow transplantation in mice. J Surg Res. 1998;77:1–5. doi: 10.1006/jsre.1997.5255. [DOI] [PubMed] [Google Scholar]
- 24.Durkin ET. Jones KA. Rajesh D. Shaaban AF. Early chimerism threshold predicts sustained engraftment and NK-cell tolerance in prenatal allogeneic chimeras. Blood. 2008;112:5245–5253. doi: 10.1182/blood-2007-12-128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofmann A. Kessler B. Ewerling S. Kabermann A. Brem G. Wolf E. Pfeifer A. Epigenetic regulation of lentiviral transgene vectors in a large animal model. Mol Ther. 2006;13:59–66. doi: 10.1016/j.ymthe.2005.07.685. [DOI] [PubMed] [Google Scholar]
- 26.Herbst F. Ball CR. Tuorto F. Nowrouzi A. Wang W. Zavidij O. Dieter SM. Fessler S. van der Hoeven F, et al. Extensive methylation of promoter sequences silences lentiviral transgene expression during stem cell differentiation in vivo. Mol Ther. 2012;20:1014–1021. doi: 10.1038/mt.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giles KE. Gowher H. Ghirlando R. Jin C. Felsenfeld G. Chromatin boundaries, insulators, and long-range interactions in the nucleus. Cold Spring Harb Symp Quant Biol. 2010;75:79–85. doi: 10.1101/sqb.2010.75.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrier E. Lee TH. Busch MP. Cowan MJ. Induction of tolerance in nondefective mice after in utero transplantation of major histocompatibility complex-mismatched fetal hematopoietic stem cells. Blood. 1995;86:4681–4690. [PubMed] [Google Scholar]
- 29.Hajdu K. Tanigawara S. McLean LK. Cowan MJ. Golbus MS. In utero allogeneic hematopoietic stem cell transplantation to induce tolerance. Fetal Diagn Ther. 1996;11:241–248. doi: 10.1159/000264309. [DOI] [PubMed] [Google Scholar]