Abstract
Lysyl oxidase (LOX), a copper-dependent amine oxidase known to function both intracellularly and extracellularly, is implicated in promoting tumor progression and hypoxic metastasis in certain malignancies. Nonsmall cell lung cancer (NSCLC) is a highly aggressive cancer with poor prognosis worldwide. However, the role and molecular mechanism by which LOX involving in hypoxic NSCLC invasion and migration are poorly understood. This study explores the effect of LOX on invasion and migration of NSCLC cells under hypoxic conditions. Small interfering RNA (siRNA) targeting LOX was used to silence LOX expression of hypoxic NSCLC cells, SPCA1 and A549. Cellular invasive and migratory potentials were determined by matrigel invasion and migration assays. Expression of LOX, Src, Src activation (Tyr418 phosphorylation of Src), and Snail were evaluated by real-time PCR and western blot, respectively. The results showed that LOX mRNA and protein expression were upregulated under hypoxic conditions in NSCLC cells. Knockdown of LOX led to inhibition of hypoxia-induced invasion and migration. Phosphorylated Src (Tyr418) and Snail proteins were decreased along with LOX downregulation. Our data provide molecular evidences that LOX is mechanistically linked to increased invasion and migration of hypoxic NSCLC cells, and may serve as an antimetastasis target of human NSCLC.
Key words: hypoxia, invasion, lysyl oxidase, migration, nonsmall cell lung cancer, Snail, Src signaling pathway
Introduction
Non-small cell lung cancer (NSCLC), the most common type of lung cancer, is nowadays the leading cause of cancer-related deaths worldwide. Metastasis is the principal cause of treatment failure and the most lethal consequence of tumor progression. Although the introduction of adjuvant chemotherapeutic regimens and radiotherapy has improved the outcome of patients with NSCLC, overall survival remains poor in advanced lung cancer patients.1,2 Therefore, identification of novel molecular targets to block the metastasis is urgently required.
Hypoxia, the most important and prevailing character of microenvironments in solid tumors, is closely associated with metastasis and poor prognosis.3 Emerging lines of evidences suggest that hypoxia facilitates tumor metastasis via upregulation of many genes such as LAMP3, CCR7, β-Catenin, integrin, fibronectin, and so on.4–7 However, the precise molecular mechanism underlying this process remains unknown.
Lysyl oxidase (LOX), an extracellular copper-dependent amine oxidase, catalyzes the exchange of an amine to an aldehyde group on a peptidyl lysine, producing hydrogen peroxide (H2O2) and ammonia as by-products of catalytic activity. In extracellular matrix (ECM), LOX acts to initiate the covalent cross-linking of collagen and elastin molecules, and thereby it increases insoluble matrix deposition and tensile strength.8 Additionally, several reports have demonstrated that LOX may have intracellular functions, such as the regulation of motility/migration, gene transcription, and cell differentiation.8–10 LOX is associated with metastasis and poor survival in patients with breast cancer or head and neck cancer, and is also a hypoxia inducible gene.3,11,12 In breast cancer cells, LOX has been shown to facilitate tumor cell invasion and migration, and LOX inhibition abrogates metastasis in an orthotopic model of breast cancer.3,13,14 The molecular mechanism by which LOX facilitates a migratory phenotype in breast cancer cell lines is ascribed to H2O2-mediated phosphorylation and activation of the FAK/Src signaling complex and p130Cas/Crk/DOCK180 signaling pathway, a downstream target of the FAK/Src signaling complex.15,16 In mouse models of breast cancer, Erler and coworkers had shown that tumor-secreted LOX protein promotes metastasis by mediating the recruitment of bone marrow-derived cells to “premetastatic niches.”17 In addition, emerging work has demonstrated that LOX proteins can stabilize the Snail protein, a critical regulator of epithelial–mesenchymal transition.18–20
In human NSCLC cancer, variations in tissue LOX expression may correlate with invasive and metastatic potential.21 Most recently, Wilgus and partners demonstrated that LOX is an independent predictor of poor prognosis in patients with early stage lung adenocarcinoma.22 However, the role and molecular mechanism by which LOX involve in invasion and migration induced by hypoxia in human NSCLC cells have not yet been elucidated. Our previous studies showed that hypoxia promotes invasion and adhesion of NSCLC cells.23 In the present study, we demonstrate that LOX is involved in hypoxic invasion and migration in human lung adenocarcinoma cells, SPCA1 and A549. Moreover, the underlying molecular mechanism was explored.
Materials and methods
Cells and culture conditions
Human lung adenocarcinoma cell lines, SPCA1 (China Center for Type Culture Collection, CCTCC) and A549 (ATCC), were both cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen), supplemented with 10% heat-inactivated fetal bovine serum (FBS; GIBCO), 100 U/mL penicillin, 100 U/mL streptomycin, and 2 mM l-glutamine at 37°C in a 5% CO2 incubator. Cells were passaged every 2–3 days to maintain exponential growth. Hypoxic exposure was carried out under 0.5% oxygen, 5% CO2, and 94.5% nitrogen (Galaxy R CO2 incubator; RS Biotech) at 37°C for 24 hours. At the same time, normoxic cells were placed at 37°C in a 19% O2 and 5% CO2 incubator (BBD6220; Heraeus) until harvest. The study was approved by the Review Board of Shandong Cancer Hospital and Institute.
LOX small interfering RNA transfection
For LOX knockdown, cells were transfected with 100 nM of LOX small interfering RNA (siRNA) using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. LOX siRNA targeting nucleotides 1108–1128 of human LOX mRNA sequence (accession S78694), and nonsense siRNA used as control for nonspecific effects were synthesized by Genepharma. The sequences for the LOX siRNA and control siRNA were as follows: LOX sense: 5′-CAUUACCACAGUAUGGAUGUU-3′; antisense: 5′-CAUCCAUACUGUGGUAAUGUU-3′. control sense: 5′-UUCUCCGAACGUGUCACGUTT-3′; antisense: 5′-ACGUGACACGUUCGGAGAATT-3′. Cells were incubated for 24 hours under normoxia after transfection, then exposed to hypoxia (0.5% O2) for 24 hours prior to assays.
Quantitative real-time PCR
Total RNA was isolated from cells with TRIzol reagent (Invitrogen) following the manufacturer's protocols. Reverse transcription was performed by Super-Script II reverse transcriptase (TaKaRa RT kit; Dalian). Quantitative real-time PCR was set up in triplicate for each sample using TaKaRa SYBR® Premix Ex Taq™ quantitative PCR kit. Relative levels of LOX and Snail mRNA were normalized to β-actin mRNA. Primers for LOX (forward 5′–TGATGCCAACACCCAGAGGA-3′; reverse 5′-CGAATGTCACAGCGCACAAC-3′), Snail (forward 5′-GACCACTATGCCGCGCTCTT-3′; reverse 5′- TCGCTGTAGTTAGGCTTCCGATT-3′), and β-actin (forward 5′-TTAGTTGCGTTACACCCTTTC-3′; reverse 5′-GCTGTCACCTTCACCGTTC-3′) were synthesized by Sangon. Using the ΔCt method, the relative gene expression in a particular sample was expressed with 2−ΔΔCt value.24
Western blot analysis
Cells of each group were quickly washed once with precold PBS and homogenized in extraction buffer containing 0.15 M NaCl, 50 mM Tris-Cl (pH 7.4), 2 mM EDTA (pH 8.0), 2 mM EGTA (pH 8.0), 0.5% Triton-100, 5 mM DTT, 0. 2 mM PMSF, 1 mM NaF, 2 mM Na3VO4, and 2 μg/mL aprotinin. The lysates were incubated on ice for 4 hours, then centrifuged at 10,000 g for 15 minutes at 4°C. Proteins were quantified by Bradford method. 50 μg of protein underwent SDS-PAGE and was transferred to PVDF membrane using an electronic Bio-Rad transfer apparatus. The membranes were blocked for 2 hours at room temperature in 1×TBS-0.05% Tween 20 (TBS-T) containing 5% dry milk powder. The primary antibodies, rabbit antihuman Src antibody (Abcam), rabbit antihuman Src (pTyr418; Invitrogen; antibody), rabbit antihuman Snail antibody (Abcam), rabbit antihuman LOX antibody (Sigma-Aldrich), and mouse antihuman β-actin antibody (Santa Cruz Biotechnology) were added at a ratio of 1:1000, 1:500, 1:1000, 1:500, and 1:2000, respectively, and incubated overnight at 4°C. The membrane was washed with TBST thrice for a total of 30 minutes. Then, the secondary antibody, horseradish peroxidase-labeled rabbit antimouse or goat antirabbit immunoglobulin G secondary antibody (1:2000; Santa Cruz Biotechnology), was added and incubated at room temperature for 2 hours. The membrane was washed thrice and reacted with ECL plus reagent (Amersham Biosciences) for 5 minutes and exposed onto Kodak Imaging film. Radiographs were scanned and analyzed by Multi-image light cabinet and software (Alpha Innotech Corporation). The amount of each protein sample was controlled by β-actin.
Invasion and migration assays
To evaluate the effect of LOX siRNA transfections on the invasion of hypoxic cells, we used a previously described assay that employs reconstituted basement membrane matrigel (BD Biosciences) as the substrate for invasion. Briefly, SPCA1 and A549 cells were transfected with LOX siRNA and control siRNA 24 hours under normoxic condition, then transferred onto the top of matrigel-coated invasion chambers (24-well insert, 8 μm pore size; BD Biosciences) in a serum-free DMEM. DMEM containing 10% fetal calf serum was added to the lower chamber as a chemoattractant. Following 24 hours incubation under hypoxic conditions, cells on the surface of the matrigel-coated polycarbonate membrane (noninvading cells) were removed by scraping with a cotton swab. Cells that invaded the matrigel and the pores of the underlying membrane were stained with 0.1% crystal violet. Cells were counted under fluorescence microscopy (100×). Cell migration was performed using a similar approach without matrigel coating.
Statistical analysis
SPSS 13.0 software was used to perform statistical analysis. Results are expressed as mean of three independent experiments (±SD). Differences between groups were analyzed by the two-tailed Student's t-test. p-values <0.05 were considered statistically significant.
Results
LOX expression in NSCLC cells were induced by hypoxia
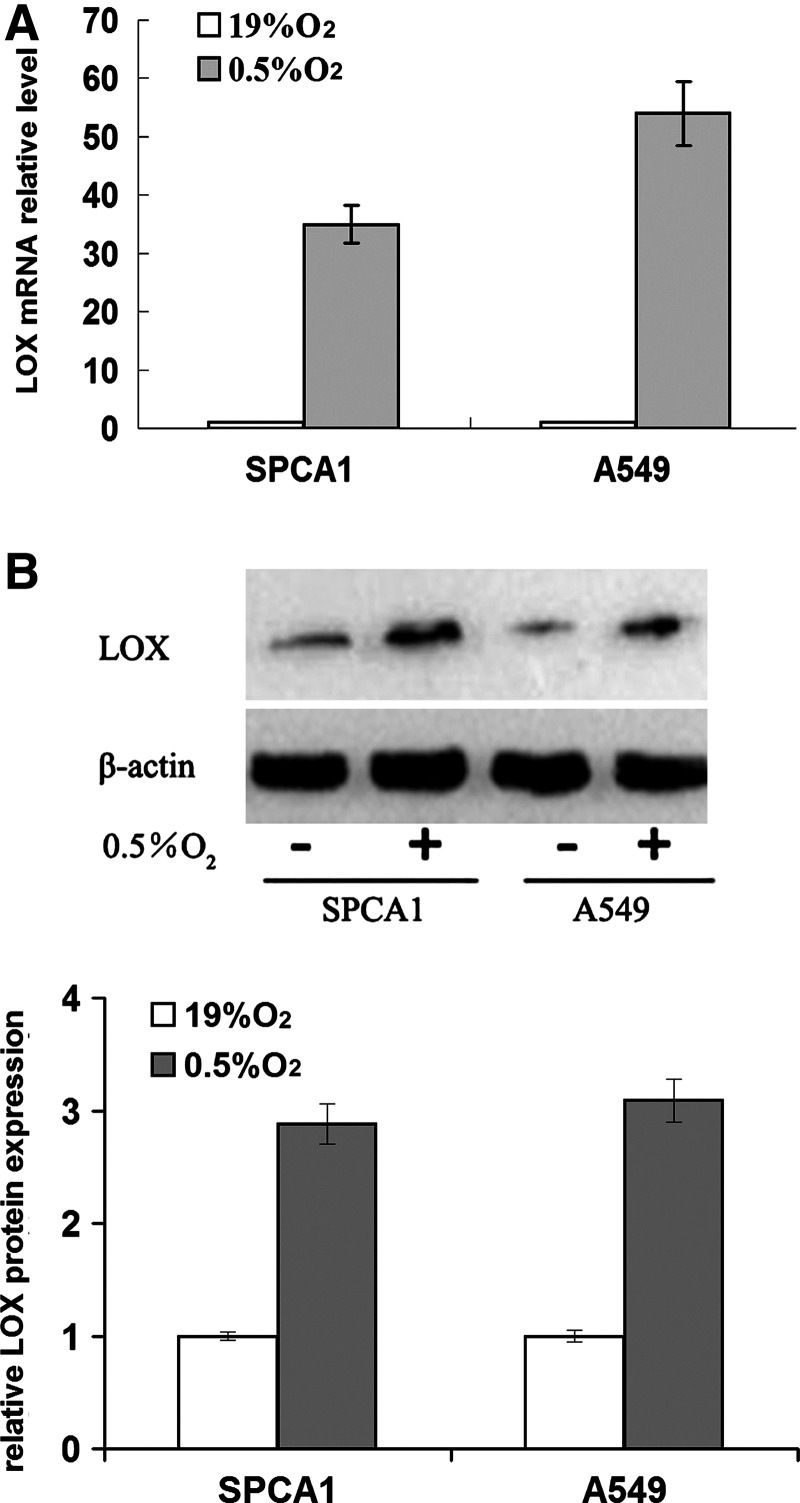
To assess the effect of hypoxia on LOX expression in NSCLC cells, SPCA1 and A549 cells were exposed to normoxia (19% O2) or hypoxia (0.5%O2, 5% CO2, and 94.5% N2) for 24 hours and harvested for the preparation of total RNA and cellular lysates. The levels of LOX mRNA and protein were determined by SYBR real-time PCR and western blot assays, respectively. Compared with normoxia, LOX mRNA (Fig. 1A) and protein (Fig. 1B) in NSCLC cells under hypoxic condition were both greatly upregulated, suggesting that hypoxia promotes LOX expression on transcriptional and post-transcriptional levels.
FIG. 1.
Effect of hypoxia on lysyl oxidase (LOX) expression in nonsmall cell lung cancer (NSCLC) Cells. Cells were exposed to either normoxia (19% O2) or hypoxia(0.5% O2) for 24 hours. LOX mRNA and protein expression were determined by quantitative real-time PCR and western blot, respectively. (A) LOX mRNA expression in SPCA1 and A549 cells. Relative fold LOX expression was determined by comparison to cells cultured under 19% O2 and arbitrarily set at 1.0. (B) Western blot was performed to detect LOX protein expression in SPCA1 and A549 cells. Quantification of relative LOX protein expression in SPCA1 and A549 cells following hypoxia was performed.
Knockdown of LOX inhibits hypoxia-induced invasion and migration of NSCLC cells
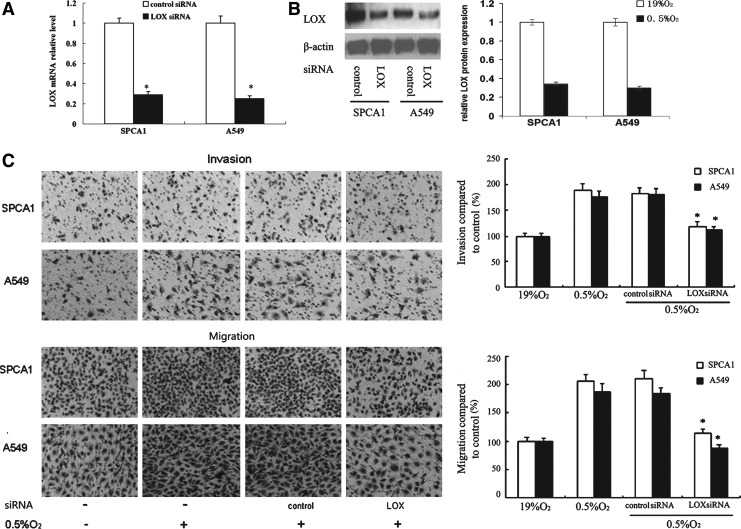
To knockdown LOX, a 21-base pair siRNA construct, LOX siRNA, was applied. SPCA1 and A549 cells transfected with LOX siRNA demonstrated LOX knockdown at both the transcription and translation levels (Fig. 2A, B).At the same time, another LOX siRNA targeting nucleotides 783–803 of human LOX mRNA sequence also demonstrated similar RNAi efficacy (data not shown). The sequences for this LOX siRNA were as follows: sense: 5′-CUACUACGAUACUUAUGAAUU-3′; antisense: 5′-UUCAUAAGUAUCGUAGUAGUU-3′.
FIG. 2.
Knockdown of LOX by specific small interfering RNA (siRNA) attenuated LOX expression, invasion and migration of NSCLC cells under hypoxia. At 24 hours post-transfection, cells were exposed to hypoxia (0.5% O2) for 24 hours and harvested for LOX mRNA and protein analysis, and in vitro invasion and migration potential evaluation. (A) LOX mRNA expression was analyzed by quantitative real-time PCR and normalized to β-actin expression. (B) LOX protein level was analyzed by western blot. β-actin was used as the internal standard. Relative LOX protein expression was determined by comparison to control siRNA transfected cells and arbitrarily set at 1.0. (C) Cells in transwell chamber were incubated under normoxic or hypoxic conditions for 24 hours following transfection of cells with control or LOX siRNA. The data are standardized against control in normoxia and presented as relative cell invasion and migration. *p<0.05
Compared with normoxic (19% O2) cells, invasion and migration of hypoxic cells (0.5% O2) were markedly enhanced. LOX knockdown significantly reduces cell invasion by 35% in SPCA1 and 38% in A549 compared with control siRNA group (Fig. 2C), respectively. In cell migration, the reductions were 46% in SPCA1 and 52% in A549, respectively (Fig. 2C). The enhanced invasive and migrative abilities by hypoxia were dramatically repressed by knockdown of LOX expression.
LOX knockdown attenuates Src activation and Snail protein expression induced by hypoxia in NSCLC cells
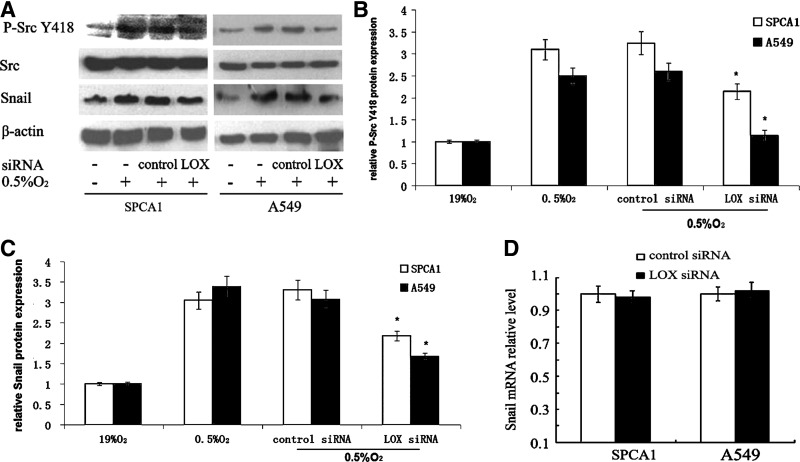
Src, a nonreceptor protein tyrosine kinase, participates in multiple physiological processes such as cell adhesion, movement, proliferation, and survival. To evaluate the role of Src activation in LOX-induced invasion of hypoxic NSCLC cells, both Src and phosphorylated Src (Tyr418) in cells transfected with LOX siRNA were determined. Src was not affected either by hypoxia or LOX interference. However, phosphorylated Src (Tyr418) dramatically increased in response to hypoxia, and it reduced by LOX interference (Fig. 3A, B). The present findings demonstrated that Src activation contributes to hypoxia-induced invasion and migration, and mediated by LOX.
FIG. 3.
LOX silencing attenuates increased Src activation and Snail expression in NSCLC cells under hypoxia. At 24 hours after transfection under normoxia, NSCLC cells were exposed to hypoxia (0.5% O2) for another 24 hours, then harvested for Src, activated Src Y418, Snail protein, and Snail mRNA level analysis. (A) Src, activated Src Y418 and Snail protein expression in NSCLC cells. (B) Relative activated Src Y418 protein expression was determined by comparison to cells cultured under 19% O2 and arbitrarily set at 1.0. *p<0.05. (C) Relative Snail protein expression was determined by comparison to cells cultured under 19% O2 and arbitrarily set at 1.0. *p<0.05. (D) Snail mRNA level in cells transfected with control or LOX siRNA under hypoxia. Each experiment was performed thrice and representative data are shown.
Another critical molecule, Snail, which was acknowledged to involve in cancer metastasis, was also investigated in this study. We found that silencing of LOX weakened the elevated Snail protein expression level induced by hypoxia (Fig. 3A, C). However, cells transfected with control siRNA displayed similar Snail protein expression as the untransfected cells (Fig. 3A, C). Interestingly, LOX silencing did not result in significant change at Snail mRNA level (Fig. 3D).These results indicated that LOX influences Snail expression at post-transcriptional level rather than transcription level.
Discussion
LOX, a hypoxia-inducible ECM protein, has attracted more attention in recent years with its importance in tumor progression.3,12–19,25,26 LOX possesses a canonical and functional hypoxia response element in its promoter region. Induction of LOX by hypoxia is mediated by hypoxia-inducible factor (HIF). The significant correlation between hypoxia and increased LOX expression was displayed in many hypoxic human tumor cells and tissues, such as breast and head and neck.3,11 Interestingly, compelling evidence most recently reveals that LOX and HIF-1 act in synergy to foster colon carcinoma formation, and HIF-1/LOX mutual regulation is a pivotal mechanism in the adaptation of tumor cells to hypoxia.27 In the present study, we first showed that LOX mRNA expressions were greatly upregulated in SPCA1 and A549 cells when exposed to 0.5% O2 for 24 hours (Fig. 1A), so were LOX proteins (Fig. 1B), supporting the notion that LOX is a hypoxia-responsive gene. In addition, LOX inhibition can effectively impair the increased invasive and migratory potentials induced by hypoxia (Fig. 2), indicating that metastatic ability of hypoxic NSCLC cells were linked to LOX.
The molecular mechanism of LOX-mediated promotion of metastasis remains unclear. Src, the first discovered oncogene, has been reported to be regulated by LOX and Src overexpression and/or overactivity appear to have a role in tumor development and metastases of NSCLC.15,28 Activated Src phosphorylates FAK, leading to the induction of various signaling pathways that regulate cell adhesion and migration. In the present study, it is revealed that knockdown of LOX lead to a reduction of Src activation (Fig. 3A, B), indicating that Src activity is associated with LOX under hypoxic conditions. For the time being, the specific mechanism by which LOX regulates the phosphorylation and activation of Src remains largely unknown. Payne and partners showed that LOX regulates Src activation through the production of H2O2.15 Similar to the notion, Pez et al. demonstrated in colon cancer cells that the H2O2 generation by LOX activity contributes to the regulation of HIF-1α expression in hypoxia, through the PI3K/Akt pathway.27 In addition, LOX may regulate Src through other mechanisms, such as an interaction with integrins and the stimulation of outside-in signaling cascades.15 The detailed molecular mechanism by which LOX affects Src activation of hypoxic NSCLC cells will be further explored in future work.
A large pool of evidences has suggested that the transcription factor Snail plays a key role in cancer metastasis and malignant progression.29–32 Overexpression of Snail is widespread in numerous human cancers, including lung cancer.29,32 Hung and coworkers reported that overexpression of Snail was shown in 55.2% of primary NSCLC patients and associated with a shorter overall survival.32 The molecular mechanism controlling the expression and function of Snail has only recently been studied. Particularly, post-translational modifications influencing Snail protein stability and/or nuclear translocation have attracted more attention. Peinado and colleagues reported that the catalytic domain of LOX, LOXL, LOXL2, and LOXL3 interacted with Snail in the repressor SNAG domain and that Snail Lys98 and Lys137 were necessary for LOX-Snail binding.18,19 To address the relationship between LOX and Snail in hypoxic NSCLC cells, Snail protein and mRNA expression levels were analyzed. Interestingly, we found that upregulation of Snail protein expression induced by hypoxia could be reduced by LOX silencing (Fig. 3A, C). However, LOX inhibition did not alter Snail mRNA expression level (Fig. 3D). Similar to our results, Sahlgren and partners demonstrated that in hypoxic SKOV-3 cells, LOX inhibitor β-aminoproprionitrile reduced Snail protein levels rather than Snail mRNA expression, which were augmented by hypoxia, indicating that LOX exerts an effect on Snail protein stability.20
Taken together, the present data suggest that LOX affects hypoxic lung cancer invasion and migration through Src signaling pathway and Snail protein activity. Our findings provide evidence that LOX may serve as a potential therapeutic target for lung cancer treatment. Clearly, for the development of such a therapeutic strategy for clinical use, a suitable vector system is necessary. Concurrently, the current results are still preliminary, and further exploration is needed to address detailed molecular mechanism for LOX involving in hypoxic NSCLC cells metastasis. Moreover, in vivo experiments would be necessary to validate the correlation for LOX and lung cancer metastasis. The relevant work is currently under investigation.
Acknowledgments
This work was supported by grants from Shandong Province Science Foundation (ZR2010HZ002, ZR2009CM141) and Shandong Health Department (2009HZ087).
Disclosure Statement
No competing financial interests exist.
References
- 1.Herbst RS. Heymach JV. Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soon YY. Stockler MR. Askie LM, et al. Duration of chemotherapy for advanced non-small-cell lung cancer: A systematic review and meta-analysis of randomized trials. J Clin Oncol. 2009;27:3277. doi: 10.1200/JCO.2008.19.4522. [DOI] [PubMed] [Google Scholar]
- 3.Erler JT. Bennewith KL. Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 4.Mujcic H. Rzymski T. Rouschop KM, et al. Hypoxic activation of the unfolded protein response (UPR) induces expression of the metastasis-associated gene LAMP3. Radiother Oncol. 2009;92:450. doi: 10.1016/j.radonc.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Li Y. Qiu X. Zhang S, et al. Hypoxia induced CCR7 expression via HIF-1 alpha and HIF-2 alpha correlates with migration and invasion in lung cancer cells. Cancer Biol Ther. 2009;8:322. doi: 10.4161/cbt.8.4.7332. [DOI] [PubMed] [Google Scholar]
- 6.Zhao JH. Luo Y. Jiang YG, et al. Knockdown of β-Catenin through shRNA cause a reversal of EMT and metastatic phenotypes induced by HIF-1α. Cancer Invest. 2011;29:377. doi: 10.3109/07357907.2010.512595. [DOI] [PubMed] [Google Scholar]
- 7.Ryu MH. Park HM. Chung J, et al. Hypoxia-inducible factor-1alpha mediates oral squamous cell carcinoma invasion via upregulation of alpha5 integrin and fibronectin. Biochem Biophys Res Commun. 2010;393:11. doi: 10.1016/j.bbrc.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 8.Kagan HM. Li W. Lysyl oxidase: Properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 9.Li W. Liu G. Chou IN, et al. Hydrogen peroxide-mediated, lysyl oxidase-dependent chemotaxis of vascular smooth muscle cells. J Cell Biochem. 2000;78:550. [PubMed] [Google Scholar]
- 10.Giampuzzi M. Oleggini R. Di Donato A. Demonstration of in vitro interaction between tumor suppressor lysyl oxidase and histones H1 and H2: Definition of the regions involved. Biochim Biophys Acta. 2003;1647:245. doi: 10.1016/s1570-9639(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 11.Denko NC. Fontana LA. Hudson KM, et al. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene. 2003;22:5907. doi: 10.1038/sj.onc.1206703. [DOI] [PubMed] [Google Scholar]
- 12.Postovit LM. Abbott DE. Payne SL, et al. Hypoxia/reoxygenation: A dynamic regulator of lysyl oxidase-facilitated breast cancer migration. J Cell Biochem. 2008;103:1369. doi: 10.1002/jcb.21517. [DOI] [PubMed] [Google Scholar]
- 13.Polgar N. Fogelgren B. Shipley JM, et al. Lysyl oxidase interacts with hormone placental lactogen and synergistically promotes breast epithelial cell proliferation and migration. J Biol Chem. 2007;282:3262. doi: 10.1074/jbc.M609407200. [DOI] [PubMed] [Google Scholar]
- 14.Kirschmann DA. Seftor EA. Fong SF, et al. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002;62:4478. [PubMed] [Google Scholar]
- 15.Payne SL. Fogelgren B. Hess AR, et al. Lysyl oxidase regulates breast cancer cell migration and adhesion through a hydrogen peroxide-mediated mechanism. Cancer Res. 2005;65:11429. doi: 10.1158/0008-5472.CAN-05-1274. [DOI] [PubMed] [Google Scholar]
- 16.Payne SL. Hendrix MJ. Kirschmann DA. Lysyl oxidase regulates actin filament formation through the p130(Cas)/Crk/DOCK180 signaling complex. J Cell Biochem. 2006;98:827. doi: 10.1002/jcb.20792. [DOI] [PubMed] [Google Scholar]
- 17.Erler JT. Bennewith KL. Cox TR, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peinado H. Portillo F. Cano A. Switching on-off Snail: LOXL2 versus GSK3β. Cell Cycle. 2005;4:1749. doi: 10.4161/cc.4.12.2224. [DOI] [PubMed] [Google Scholar]
- 19.Peinado H. Del Carmen Iglesias-de la Cruz M. Olmeda D, et al. A molecular role for lysyl oxidase-like 2 enzyme in Snail regulation and tumor progression. EMBO J. 2005;24:3446. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahlgren C. Gustafsson MV. Jin S, et al. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci U S A. 2008;105:6392. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woznick AR. Braddock AL. Dulai M, et al. Lysyl oxidase expression in bronchogenic carcinoma. Am J Surg. 2005;189:297. doi: 10.1016/j.amjsurg.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 22.Wilgus ML. Borczuk AC. Stoopler M, et al. Lysyl oxidase: A lung adenocarcinoma biomarker of invasion and survival. Cancer. 2011;117:2186. doi: 10.1002/cncr.25768. [DOI] [PubMed] [Google Scholar]
- 23.Liu YL. Yu JM. Song XR, et al. Regulation of the chemokine receptor CXCR4 and metastasis by hypoxia-inducible factor in non small cell lung cancer cell lines. Cancer Biol Ther. 2006;5:1320. doi: 10.4161/cbt.5.10.3162. [DOI] [PubMed] [Google Scholar]
- 24.Liu H. Song X. Liu C, et al. Knockdown of astrocyte elevated gene-1 inhibits proliferation and enhancing chemo-sensitivity to cisplatin or doxorubicin in neuroblastoma cells. J Exp Clin Cancer Res. 2009;28:19. doi: 10.1186/1756-9966-28-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santhanam AN. Baker AR. Hegamyer G, et al. Pdcd4 repression of lysyl oxidase inhibits hypoxia-induced breast cancer cell invasion. Oncogene. 2010;29:3921. doi: 10.1038/onc.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bondareva A. Downey CM. Ayres F, et al. The lysyl oxidase inhibitor, beta-aminopropionitrile, diminishes the metastatic colonization potential of circulating breast cancer cells. PLoS One. 2009;4:e5620. doi: 10.1371/journal.pone.0005620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pez F. Dayan F. Durivault J, et al. The HIF-1-inducible lysyl oxidase activates HIF-1 via the Akt pathway in a positive regulation loop and synergizes with HIF-1 in promoting tumor cell growth. Cancer Res. 2011;71:1647. doi: 10.1158/0008-5472.CAN-10-1516. [DOI] [PubMed] [Google Scholar]
- 28.Giaccone G. Zucali PA. Src as a potential therapeutic target in non-small-cell lung cancer. Ann Oncol. 2008;19:1219. doi: 10.1093/annonc/mdn048. [DOI] [PubMed] [Google Scholar]
- 29.Yanagawa J. Walser TC. Zhu LX, et al. Snail promotes CXCR2 ligand-dependent tumor progression in non-small cell lung carcinoma. Clin Cancer Res. 2009;15:6820. doi: 10.1158/1078-0432.CCR-09-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundgren K. Nordenskjöld B. Landberg G. Hypoxia, Snail and incomplete epithelial-mesenchymal transition in breast cancer. Br J Cancer. 2009;101:1769. doi: 10.1038/sj.bjc.6605369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang MH. Chen CL. Chau GY, et al. Comprehensive analysis of the independent effect of twist and Snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50:1464. doi: 10.1002/hep.23221. [DOI] [PubMed] [Google Scholar]
- 32.Hung JJ. Yang MH. Hsu HS, et al. Prognostic significance of hypoxia-inducible factor-1alpha, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax. 2009;64:1082. doi: 10.1136/thx.2009.115691. [DOI] [PubMed] [Google Scholar]