Abstract
One of the strategies to improve the outcome of anti-erbB2-mediated immunotherapy is to combine anti-erbB2 antibodies with T-cell-based adoptive immunotherapy, which can be achieved by expressing anti-erbB2 mAb on the surface of T cells. A single-chain variable fragment (scFv) from an anti-erbB2 mAb has been expressed on T cell surface to bind to erbB2-positive cells, and CD3ζ has been expressed as a fusion partner at C terminus of this scFv to transduce signals. T cells grafted with this chimeric scFv/CD3ζ were able to specifically attack target tumor cells with no MHC/Ag restriction. To test the effects of CD28 signal on cellular activation and antitumor effectiveness of chimeric scFv/CD3ζ-modified T cells, we constructed a recombinant anti-erbB2 scFv/Fc/CD28/CD3ζ gene in a retroviral vector. T cells expressing anti-erbB2 scFv/Fc/CD28/CD3ζ specifically lyzed erbB2-positive target tumor cells and secreted not only interferon-γ (IFN-γ) but also IL-2 after binding to their target cells. Our data indicate that CD3 and CD28 signaling can be delivered in one molecule, which is sufficient for complete T cell activation without exogenous B7/CD28 co-stimulation.
Key words: erbB2, gene therapy, immunotherapy, recombinant gene, scFv, T cell, tumor
Introduction
HER2/c-erbB-2 is an oncogene encoding a growth factor receptor (HER2/c-erbB-2 receptor).1 Studies showed that erbB2 overexpression was present in about 30% of breast cancer patients.2 These patients were insensitive to chemotherapy and endocrine therapy and tended to relapse earlier, thus with a short survival time.3,4 In addition to breast cancer, erbB2 was overexpressed in ovarian cancer, prostate cancer, nonsmall-cell lung cancer, nasopharyngeal cancer, colorectal cancer, gastric cancer, bladder cancer, renal cell carcinoma, and other primary malignant tumors.2,5,6 erbB2 has been shown to play a key role in tumor progression and is one of the important targets for the biological therapy of cancers.7,8
Anti-erbB2 monoclonal antibody Herceptin has been generated and applied in clinical cancer therapy,9 but its efficiency for erbB2-overexpressing breast cancer patients was only 12%–34%.10,11 In search for alternative solutions, current attempts have been made to produce scFv (single-chain variable fragment) by genetic engineering for gene therapy.12,13 Compared with monoclonal antibody therapy, scFv gene therapy, with equivalent antigen-binding ability, has several advantages, such as stronger penetrability in solid tumors, longer stable expression, and much lower cost of production.14
CD3ζ is an important subunit of major signal transduction molecule CD3 inside T cell membrane, transmitting outside antigen stimulation signal, and is essential in activating T cells to lyze tumor cells. Studies showed that CD3ζ was low expressed in T cells of cancer patients, leading to T cell anergy for antitumor.15,16 Several groups have reported that chimeric receptors consisting of an extracellular scFv and an intracellular CD3ζ domain are capable of redirecting T cells specific to tumor cells expressing target epitopes.17,18
According to the dual signal model of T cell activation, a co-stimulatory signal in addition to signaling through the TCR/CD3 complex is required for efficient activation of T cells, which results in proliferation, cytokine secretion, and targeted lysis. Hombach et al. reported in their anti-CEA scFv-mediated T cell adoptive immunotherapy study that cellular proliferation and antigen-induced IL-2 secretion of grafted T cells required both CD3ζ and CD28 signals, and these two signals could be delivered together in a combined immunoreceptor molecule.19
This study was intended to improve the effectiveness of scFv gene therapy by constructing the fusion gene anti-erbB2 scFv-Fc-CD28-CD3ζ via genetic engineering. In this fusion gene, anti-erbB2 scFv is expected to make fusion protein specifically target to erbB2-overexpressed tumor cells, meanwhile CD28 and CD3ζ were expected to co-stimulate T cells when modified T cells bind to target cells.
Materials and Methods
Cell lines and culture
The erbB2-positive/negative-expressing human breast cancer cell line SK-BR-3/MCF7 and human lymphoma T cell line Jurkat were obtained, respectively, from Cell Bank of Shanghai Institutes for Biological Sciences and China Center for Type Culture Collection. They were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) (Gibco), with 5% CO2, at 37°C.
Plasmids
The plasmid pSecTag2B containing signal peptide of IgGκ was purchased from Invitrogen. The recombinant plasmid pPIC9K/scA21 containing anti-erbB2 scFv was kindly provided by Prof. Jing Liu.20 The recombinant plasmid pBULLET containing anti-CEA scFv/Fc/CD28/CD3ζ was kindly provided by Dr. Hinrich Abken.19
Construction of recombinant expression vector
DNA fragments IgGκ, anti-erbB2-scFv, and Fc-CD28-CD3ζ were amplified, respectively, from plasmid pSecTag2B, recombinant plasmid pPIC9K/scA21, and recombinant plasmid pBULLET by polymerase chain reaction (PCR) (Pyrobest Taq; TaKaRa). These DNA fragments were then connected together via splicing by overlap extension–PCR (SOE-PCR) (Pyrobest Taq, Ex Taq; TaKaRa), forming the fusion gene anti-erbB2-scFv-Fc-CD28-CD3ζ with an IgGκ signal peptide at the N-terminus. Primers for regular PCR and SOE-PCR (5′-3′) are as follows (see Table 1). After the fusion gene was cloned through TA cloning and confirmed by the restriction enzyme digestion analysis, PCR, and sequencing, it was inserted into the retrovirus expression vector pLNCX at HindIII and ClaI (TaKaRa) sites, forming a recombinant eukaryotic expression vector pLNCX/anti-erbB2 scFv-Fc-CD28-CD3ζ, which was then confirmed by restriction enzyme digestion analysis, PCR, and sequencing.
Table 1.
Primers for Regular Polymerase Chain Reaction and Splicing by Overlap Extension–Polymerase Chain Reaction (5′-3′)
| Fragments | Primers |
|---|---|
| IgGκ | 1F: GCT GGA AGC TTA GCA TGG AGA CAG ACA CAC |
| 1R: GTC AGC ACA ATG TCG TCA CCA GTG GAA CCT | |
| Anti-erbB2 scFv | 2F: CTG GTG ACG ACA TTG TGC TGA CCC AAA CT |
| 2R: TCG GCT GAC GAG ACG GTG ACT GAG GTT | |
| Fc-CD28-CD3ζ | 3F: CCG TCT CGT CAG CCG AGC CCA AAT CTC CT |
| 3R: AAC CAT CGA TCA GCA TCT CTC CAG TAT TAG CG |
The underlined sequences are the sites for restriction digest, the gray-highlighted sequences are complimentary tailer sequences, and the dotted sequences are initiation or termination codons, respectively. 1F and 2R were used as primers for amplifying the IgGκ/anti-erbB2 scFv fragment in SOE-PCR. 1F and 3R were used as primers for amplifying the IgGκ/anti-erbB2 scFv/Fc/CD28/CD3ζ fragment in SOE-PCR.
scFv, single-chain variable fragment; SOE-PCR, splicing by overlap extension–polymerase chain reaction.
Preparation of human peripheral T lymphocytes (PTLs)
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized peripheral blood of normal donors by using a lymphocyte separation medium (Ficoll-Hypaque) via density-gradient centrifugation. Lymphocytes were isolated from PBMCs by a glass adhesion assay. Further isolation and purification of T lymphocytes were accomplished by a cotton column. Purified PTLs were cultured in a medium supplemented with 1 μg/mL PHA-L (Roche) and 200 U/mL recombinant human IL-2 (rhIL-2). Starting from day 3, keeping rhIL-2, whereas PHA-L was replaced by 50 ng/mL anti-CD3 mAb OKT3. At day 7, stimulated PTLs were harvested. Aliquot of cells were prepared for electroporation and for flow cytometry analysis (FITC anti-human CD3 and FITC mouse IgG2aκ; eBioscience).
Generation of recombinant gene-modified T cells
Jurkat cells and PTLs were transfected with pLNCX/anti-erbB2 scFv-Fc-CD28-CD3ζ, by electroporation (Bio-Rad Gene Pulser MXcell), and then were selected with around 800 μg/mL G418 for about 2 weeks. Cultures of PTLs were still supplemented with 200 U/mL IL-2 and 50 ng/mL OKT3. The stably transfected Jurkat cells and PTLs were further expanded for studies.
Detection of expression of fusion gene
The fusion gene in stably transfected Jurkat cells and PTLs was amplified by regular PCR, using forward primer 5′ atccacgctgttttgacctc 3′ and reverse primer 5′ caggtggggtctttcattcc 3′. For detecting fusion protein expression, stably transfected cells were analyzed by flow cytometry (FITC mouse anti-human IgG Fc and FITC mouse IgG1κ; BD Pharmingen™). Cell lysates of stably transfected Jurkat cells and PTLs were also examined by Western blot (mouse anti-human CD3ζ mAb conjugated to HRP; Santa Cruz).
Binding of transfected cells to target cells
Transfected PTLs were cocultured with erbB2-positive SKBR3 cells for 48 hours. The binding of transfected PTLs to SKBR3 cells and the status of SKBR3 were observed by a microscope.
Analysis of cytokine production
Transfected PTLs were cocultured with erbB2-positive SKBR3 cells for 72 hours. ELISA kits (R&D) for detecting IL-2 and interferon-γ (IFN-γ) were used to analyze supernatants of cells following the manufacturer's instructions. Untransfected PTLs and erbB2-negative MCF-7 cells were taken as two negative controls.
Nonradioactive cytotoxicity assay
Transfected PTLs were cocultured with erbB2-positive SKBR3 cells for 72 hours in a 96-well plate. Untransfected PTLs and erbB2-negative MCF-7 cells were taken as two negative controls. By using cytotoxicity LDH detection kit (Genmed), following the manufacturer's instructions, specific LDH release from target cells in a cell-free supernatant was detected. The amount of LDH release was used to assess the lysis of target cells, which can be translated into the effectiveness of effector cells. Percent cytotoxicity was calculated as follows, according to O.D. values: cytotoxicity%=(experimental−effector spontaneous−target spontaneous)/(target maximum−target spontaneous)×100%.
Statistical analysis
Probability (p) values were calculated by using SPSS 14.0 software. The means of groups were compared via one-way analysis of variance (ANOVA). First, homogeneity of variance was tested, if equal variances assumed, and then p values were calculated by LSD (according to SPSS/Help Topics/Base System/Analyzing Data/One-Way ANOVA/One-Way ANOVA Post Hoc Tests/Equal Variances Assumed/LSD). Differences were considered statistically significant when p<0.05.
Results
Construction of recombinant expression vector
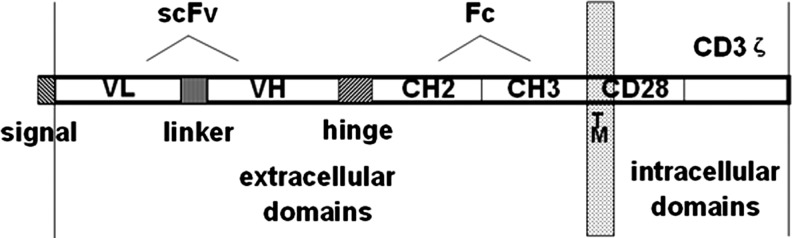
The DNA fragments coding for signal peptides, anti-erbB2 scFv, and Fc-CD28-CD3ζ were amplified by PCR from their source plasmids. All three fragments were linked together by SOE-PCR. The correct sequence of fusion gene signal peptide-anti-erbB2-scFv-Fc-CD28-CD3ζ (Fig. 1) was finally confirmed by sequencing when it was inserted in pMD19-T simple vector and in the pLNCX vector. The recombinant eukaryotic expression vector pLNCX/signal peptide-anti-erbB2-scFv-Fc-CD28-CD3ζ was constructed successfully.
FIG. 1.
The schematic diagram of fusion gene signal peptide-anti-erbB2-scFv-Fc-CD28-CD3ζ. Signal, signal peptide for human IgGκ light chain; linker, (G4S)4; hinge, human IgG1 hinge; TM, transmembrane domain of CD28. scFv, single-chain variable fragment.
Preparation of human PTLs
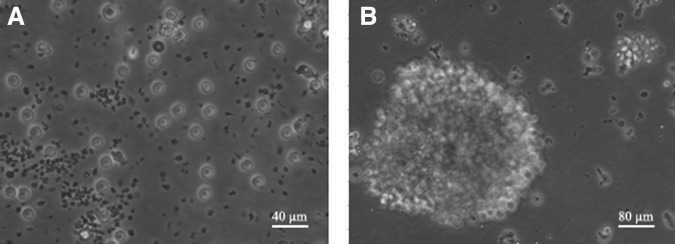
For the preparation of following transfection, PTLs were isolated, purified, and stimulated as detailed in the Materials and Methods section. Compared with the unstimulated control, which did not proliferate well and died gradually (Fig. 2A), the prepared human PTLs grew in good status and proliferated abundantly to cluster together (Fig. 2B). Compared with the isotype control, the CD3-positive rate of purified and stimulated PTLs detected by flow cytometry was 98.42% (Fig. 3).
FIG. 2.
The isolated, purified human PTLs observed by microscopy. PTLs were isolated, purified, and then stimulated by PHA-L, OKT3, and IL-2 for 7 days (B), or as a control, PTLs were not stimulated (A). The PTLs were observed by a 200× (B) or 400× (A) microscope. PTL, peripheral T lymphocytes.
FIG. 3.
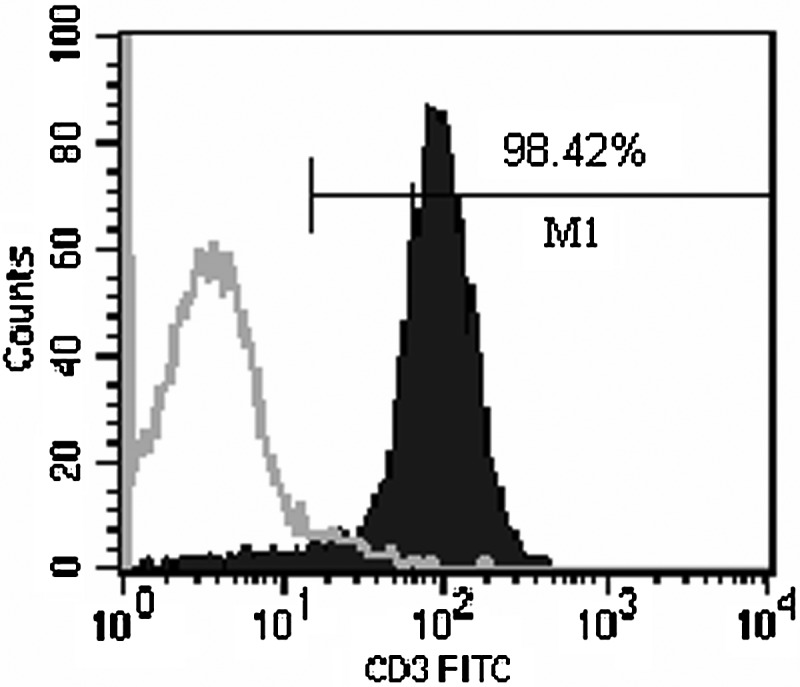
Analysis of a CD3-positive cell population in stimulated PTLs by flow cytometry. Stimulated PTLs were incubated with an FITC-labeled mouse anti-human CD3 mAb, and analyzed by flow cytometry (right curve). The incubation of PTLs with an FITC-labeled mouse IgG 2aκ was used as an isotype control (left curve).
Expression of fusion protein in transfected Jurkat cells and PTLs
Jurkat cells and PTLs were transfected with the fusion gene by electroporation. The existence of fusion gene in stably transfected Jurkat cells and PTLs was detected by PCR (Fig. 4). The expression of fusion gene was checked by flow cytometry (Fig. 5) and Western blot (Fig. 6). Compared with the isotype control, the fusion protein was expressed in 76.77% stably transfected Jurkat cells, as shown by flow cytometry (Fig. 5), and by using FITC mouse anti-human IgG (Fc), the Fc fragment in the fusion gene was confirmed to be expressed on the cell surface. Both transfected Jurkat cells and PTLs expressed not only endogenous CD3ζ (∼21 kD) but also the expected exogenous (fusion protein) CD3ζ (67 kD), as shown by Western blot (Fig. 6).
FIG. 4.
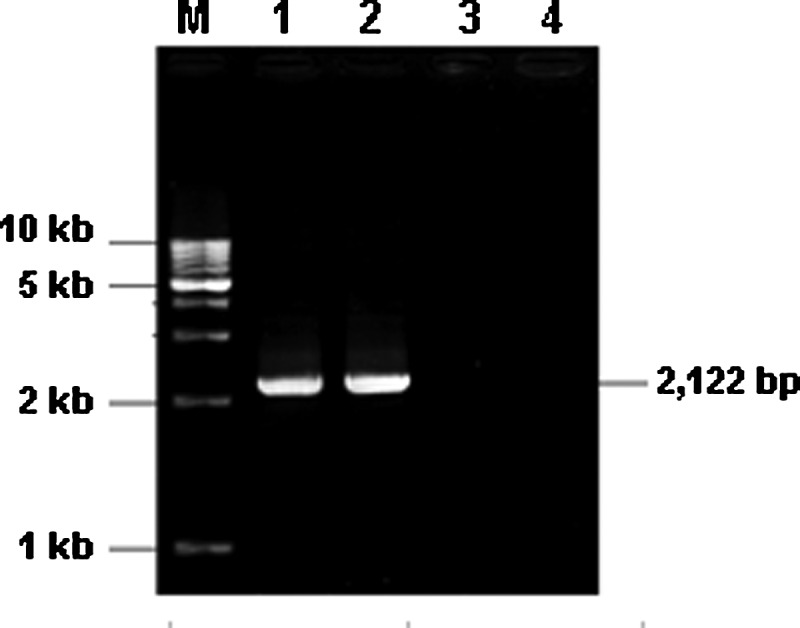
Analysis of the fusion gene in stably transfected Jurkat cells and PTLs by PCR. PCR products of transfected Jurkat cells (lane 1), transfected PTLs (lane 2), untransfected Jurkat cells (lane 3), and untransfected PTLs (lane 4) were analyzed by agarose gel electrophoresis. The size of the positive band was 2122 bp.
FIG. 5.
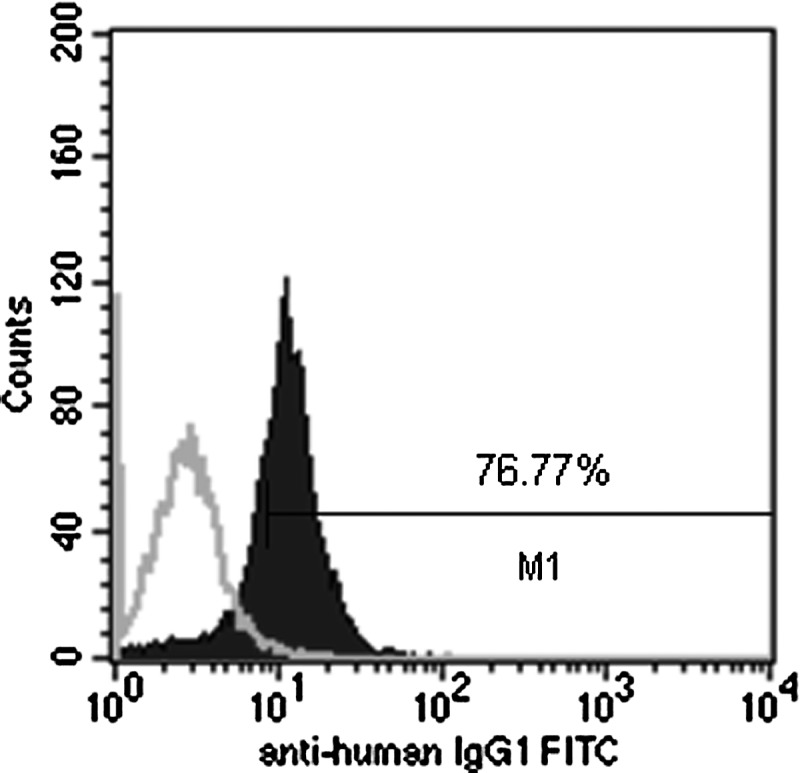
Analysis of a fusion gene-positive population in stably transfected Jurkat cells by flow cytometry. Transfected Jurkat cells were incubated with an FITC-labeled mouse anti-human IgG (Fc) and analyzed by flow cytometry (right curve), or with an FITC-labeled mouse IgG1κ as an isotype control (left curve).
FIG. 6.

Analysis of fusion gene expression in stably transfected Jurkat cells and PTLs by Western blot. Whole-cell lysates of transfected Jurkat cells (lane 1), transfected PTLs (lane 2), untransfected Jurkat cells (lane 3), and untransfected PTLs (lane 4) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to a membrane, and probed with a mouse anti-human CD3ζ mAb conjugated to HRP. The size of endogenous CD3ζ was 21 kD, while the size of exogenous (fusion protein) CD3ζ was 67 kD.
The binding activity of transfected PTLs to erbB2-positive tumor cells
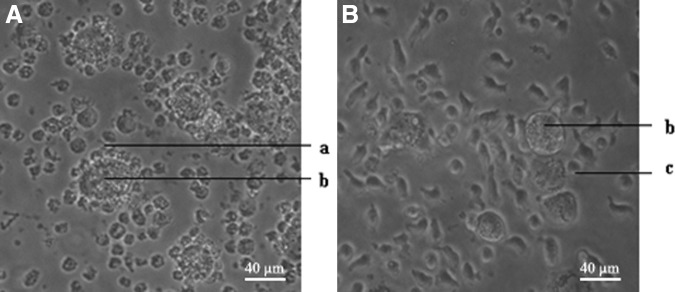
To examine the binding activity of anti-erbB2 scFv expressed on PTL surface, transfected or untransfected PTLs were cocultured with erbB2-positive SKBR3 cells for 48 hours. Transfected PTLs encircled SKBR3 cells, as arranged in a garland (Fig. 7A), and the number of viable SKBR3 cells reduced obviously, SKBR3 cells became lyzed, whereas untransfected PTLs did not bind around SKBR3 (Fig. 7B). The data showed that transfected PTLs could make use of not only cellular immunity but also humoral immunity to eliminate erbB2-positive tumor cells.
FIG. 7.
The binding activity of transfected PTLs to target cells. The transfected (A) or untransfected (B) PTLs were cocultured with erbB2-positive SKBR3 cells for 48 hours and observed by microscopy (400×). (a) Transfected PTLs, (b) SKBR3, and (c) untransfected PTLs.
The activation of transfected PTLs
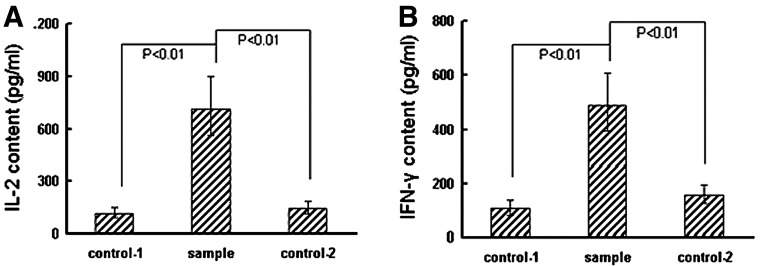
To analyze the effects of the binding of transfected PTLs to target cells on transfected PTLs, that is, the effects of the transfected CD3ζ fragment, transfected PTLs were cocultured with SKBR3 cells for 72 hours. The contents of both IL-2 and IFN-γ in the supernatant increased significantly than those of negative control-1 (p<0.01) and negative control-2 (p<0.01) respectively (Fig. 8).
FIG. 8.
Detection of IL-2 and IFN-γ by ELISA. Transfected PTLs were cocultured with erbB2-positive SKBR3 cells for 72 hours. Supernatant from the culture was, respectively, detected by ELISA for IL-2 (A) or IFN-γ (B). Supernatants from transfected PTLs cocultured with erbB2-negative MCF7 cells (control 1) and from untransfected PTLs cocultured with erbB2-positive SKBR3 cells (control 2) were used as negative controls. ELISA, enzyme-linked immunosorbent assay.
The effectiveness of transfected PTLs to lyze erbB2-positive tumor cells
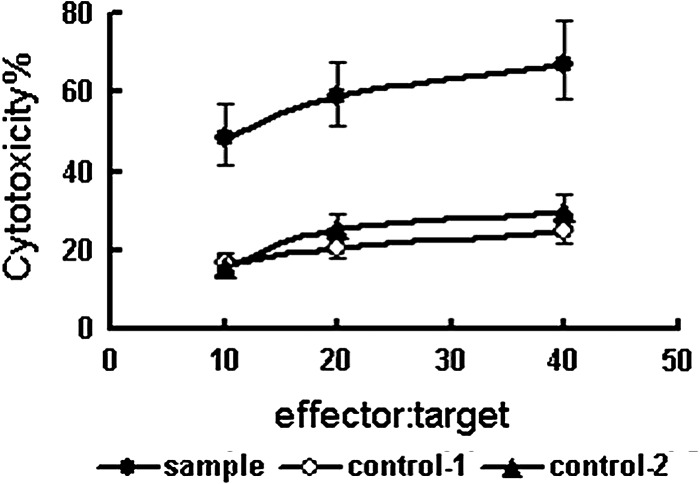
To analyze the effects of the binding of transfected PTLs to target cells on target cells, transfected PTLs (effector) were cocultured with SKBR3 cells (target) for 72 hours. As determined at effector: target ratios of 10, 20, and 40, the effectiveness of transfected PTLs to lyze SKBR3 cells was significantly higher than negative control-1 (p<0.01) and negative control-2 (p<0.01) respectively (Fig. 9).
FIG. 9.
Analysis of the cytotoxicity of effector cells by detecting specific LDH release from target cells. Transfected PTLs were cocultured with erbB2-positive SKBR3 cells for 72 hours. Supernatant from the culture was detected by using cytotoxicity LDH detection kit for LDH released from lyzed target cells. Supernatants from transfected PTLs cocultured with erbB2-negative MCF7 cells (control 1) and from untransfected PTLs cocultured with erbB2-positive SKBR3 cells (control 2) were used as negative controls.
Discussion
To construct the fusion gene, we used SOE-PCR21 in a two-step process, to connect three DNA fragments (IgGκ, anti-erb2 scFv, and Fc-CD28-CD3ζ), each of which was amplified, respectively, by PCR. The key for SOE-PCR method relies on the design of overlap extension primers. In addition to following the general rules of primer design, we also considered the characteristic and length of overlap extension region,22,23 to ensure the correct connection of DNA fragments. The correct sequence of the fusion gene has been dually confirmed by sequencing it in a recombinant clone vector and expression vector.
Lymphoma T cell line (Jurkat) cells can be subcultured unlimitedly. We first employed it to transfect the fusion gene, so that the related techniques and methods can be optimized for the expected level of fusion protein expression. If the fusion gene could be expressed in T cell line cells, it would be expected that it could also be expressed in PTLs. While PTLs are primary cells, they can only be subcultured very limitedly. The experiments to detect binding ability, T cell activation, and targeted lysis are all bound to adopting PTLs.24 Furthermore, to obtain transfected PTLs is the prerequisite for any subsequent antitumor experiments in vivo. So, we finally transfected PTLs.
Electroporation has been extensively applied,25 available for not only suspending cells but also large-size DNA (>65 kb). It is user-friendly, and its transfection efficiency is high.26 We once tried a lipofectine 2000 kit for transfection, but the efficiency was quite limited. As a possible explanation, our recombinant expression vector was too large to transfect, and our receptor cells were suspended.27 We optimized the electroporation condition according to the reported27–31: (1) purified plasmid (no endotoxin, low content of RNA and protein), (2) took high concentration of plasmid (≥1 μg/μL), (3) added carrier DNA (e.g., salmon sperm DNA), (4) linearized plasmid (digested by PvuI), and (5) kept in ice-bath for 5 minutes before electroporation.
Since the cells are very fragile after electroporation, we improved cell survival by adding trehalose in the recovery medium at a final concentration of 50 mmol/L, for stabilizing DNA, protein, and cell membrane.32 Given to the fact that apoptosis is the main death of cells after electroporation, we can add a caspase inhibitor in the recovery medium.33 For PTLs are primary cells with a short life span, during G418 selection, IL-2 and OKT3 were added every other day,34 and FBS concentration was increased to 15%–20%. According to the reported, during the generation of PTLs, it could be a better choice to add irradiated allogeneic PBMC feeder cells or irradiated allogeneic EBV-transformed lymphoblastoid cell line cells.35
Alvarez et al. succeeded in applying recombinant adenoviral anti-erbB2 scFv for phase-I clinical gene therapy of ovarian carcinoma.36 Still there are some concerns remained. For instance, anti-erbB2 scFv had a lower affinity and specificity than monoclonal antibody, no antibody-dependent cellular cytotoxicity for lack of Fc,37 and less-efficient and stable expression of secretory scFv than anchored scFv.38 Some strategies have been attempted for improvements. Barker et al.39 improved the specificity and efficacy of adenoviral anti-erbB2 scFv gene delivery to ovarian carcinoma. In one study,40 scFv was replaced with the Herceptin whole-antibody gene. In some studies, a dual antitumor function fusion protein was made by fusing anti-erbB2 scFv with IL-2,41 SEC-2,7 TNF-α,1 caspase-3,42 RNase,43 or CD16.44 For recombinant anti-erbB2 scFv/ETA constructed by Von Minckwitz et al., potent antitumor activity was demonstrated in vitro and in animal models, and was applied in a phase-I clinical trial of erbB2-overexpressing tumors.45 In other studies, the fusion gene anti-erbB2 scFv/B7.246 or anti-erbB2 scFv/CD8647 was constructed for activating T cells.
Anti-erbB2 monoclonal antibodies have been used in the treatment of erbB2-positive malignant tumors. Using the anti-erbB2 antibody to mobilize T-cell- based immunity is one of the strategies to enhance the efficacy of anti-erbB2-based immunotherapy. T or NK cells can be grafted with immunoreceptors containing chimeric extracellular scFv and intracellular CD3ζ. ScFv against CEA,19 CD19,48 CD2017,49–51 CA72-4, CD44, PSMA,52 HMW-MAA,53 CD33,18 TAG72,54 NCAM, EGFRvIII,55 and EGP-256 has been made in this chimeric structure. ScFv was expressed on the T or NK cell surface to bind to its specific antigen target, whereas CD3ζ was expressed as a fusion partner to transduce signals. ScFv/CD3ζ gene modified-T or NK cells can specifically bind to its antigen just as TCR binds to Ag-MHC. However, the former does not need to recognize MHC. Signals induced by scFv binding to its antigen are able to activate T cells grafted with the immunoreceptors. Jensen et al.49 have reported that recombinant anti-CD20 scFv/CD3ζ gene-modified T cells display redirected MHC-unrestricted CD20-specific lymphoma cell cytolysis and are activated to produce Tc1 cytokines (e.g., IFN-γ). Chimeric scFv/CD3ζ is the basic part of the immunoreceptors. CD28 was added to the immunoreceptors as a costimulation signal by Hombach et al.19 They constructed anti-CEA scFv/CD28/CD3ζ gene-modified T cells. Comparing with anti-CEA scFv/CD3ζ gene-modified T cells, they found that when binding to CEA-positive tumor cells, both kinds of T cells could lyze tumor cells with the similar efficiency and secrete a high level of IFN-γ. T cells modified with the anti-CEA scFv/CD28/CD3ζ gene, but not with the anti-CEA scFv/CD3ζ gene, also secrete a high level of IL-2, which means the immunoreceptors containing CD28 can completely activate the modified T cells after binding to their target cells.
Secretion of IL-2 by T cells grafted with immunoreceptors containing both CD3ζ and CD28 intracellular signal elements is important for the therapeutical efficacy. IL-2 plays a key role for T cell proliferation and Th1-based cellular immunity.57 Targeting of tumor cells by receptor-grafted T cells without additional CD28 signaling is expected to end in a limited immune response despite a high IFN-γ secretion level. Particularly, the acquisition of additional effector cells at the tumor site, for example, NK cells, will depend on the presence of IL-2. CD28 costimulation, in addition to IL-2 secretion, synergistically prevents activation-induced T cells from death by upregulation of the antiapoptotic proteins bcl-x and bcl-2.58 Secretion of an amount of IL-2 and sustained proliferation of grafted T cells determine a long-lasting antitumor response of modified T cells.
We demonstrate in this study that the MHC-independent Ag recognition enables receptor-grafted T cells to exert efficient cytolysis of erbB2-positive target cells and produce not only IFN-γ but also IL-2. This means CD3ζ signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3ζ signaling receptor molecule, as previously reported by Hombach et al.19
In this study, the recombinant eukaryotic expression vector pLNCX/signal peptide-anti-erbB2-scFv-Fc-CD28-CD3ζ was constructed successfully with the correct sequence. Upon transfection, the fusion gene could be stably expressed on the surface of human Jurkat cells and PTLs at a high rate and level. Grafted PTLs could bind to erbB2-positive tumor cells specifically, be activated significantly, and lyze target cells efficiently in vitro. Grafted PTLs constructed by our study were equipped with both cellular and humoral antitumor immune function, allowing a way to eradicate erbB2-positive tumor cells. Our study would lay an experimental foundation for antitumor gene therapy by targeting erbB2 receptors and activating PTLs. Grafted PTLs constructed by our study could be applied to any other erbB2-positive tumors besides breast cancer. PTLs harboring antibody gene can target to any tumor-associated antigens on the cell surface; namely, the strategy of designing the fusion gene in our study could be applied to any cell surface tumor-associated antigen.
Acknowledgments
This material is based upon work funded by the Zhejiang Provincial Natural Science Foundation of China under Grant No. Y205171, the Joint Research Foundation for Overseas Chinese of Wenzhou Science and Technology Bureau of China under Grant No. H20080059, and the Science and Technology Project for Overseas Chinese. We thank Dr. Hinrich Abken and Prof. Jing Liu for sharing the recombinant plasmids utilized in this study. We thank Prof. Jianxin Lu, Prof. Yingxia Tan, Dr. Yongxian Hu, and Dr. Lijing Bu for their help.
Disclosure Statement
No conflict of interest exists for any of the authors.
References
- 1.Huang TH. Morrison SL. A trimeric anti-HER2/neu ScFv and tumor necrosis factor-alpha fusion protein induces HER2/neu signaling and facilitates repair of injured epithelia. J Pharmacol Exp Therapeut. 2006;316:983. doi: 10.1124/jpet.105.095513. [DOI] [PubMed] [Google Scholar]
- 2.Yu D. Hung MC. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene. 2000;19:6115. doi: 10.1038/sj.onc.1203972. [DOI] [PubMed] [Google Scholar]
- 3.Revillion F. Bonneterre J. Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer. 1998;34:791. doi: 10.1016/s0959-8049(97)10157-5. [DOI] [PubMed] [Google Scholar]
- 4.Houston SJ. Plunkett TA. Barnes DM, et al. Overexpression of c-erbB2 is an independent marker of resistance to endocrine therapy in advanced breast cancer. Br J Cancer. 1999;79:1220. doi: 10.1038/sj.bjc.6690196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kershaw MH. Westwood JA. Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gravalos C. Jimeno A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523. doi: 10.1093/annonc/mdn169. [DOI] [PubMed] [Google Scholar]
- 7.Ming-Kai X. Cheng-Gang Z. Gene expression and function study of fusion immunotoxin anti-Her-2-scFv-SEC2 in Escherichia coli. Appl Microbiol Biotechnol. 2006;70:78. doi: 10.1007/s00253-005-0049-z. [DOI] [PubMed] [Google Scholar]
- 8.Cai Z. Zhang H. Liu J, et al. Targeting erbB receptors. Semin Cell Dev Biol. 2010;21:961. doi: 10.1016/j.semcdb.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeon CH. Pegram MD. Anti-erbB-2 antibody trastuzumab in the treatment of HER2-amplified breast cancer. Investig New Drugs. 2005;23:391. doi: 10.1007/s10637-005-2899-8. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Angulo AM. Hortobagyi GN. Esteva FJ. Adjuvant therapy with trastuzumab for HER-2/neu-positive breast cancer. Oncologist. 2006;11:857. doi: 10.1634/theoncologist.11-8-857. [DOI] [PubMed] [Google Scholar]
- 11.Osako T. Ito Y. Takahashi S, et al. Efficacy and safety of trastuzumab plus capecitabine in heavily pretreated patients with HER2-positive metastatic breast cancer. Cancer Chemother Pharmacol. 2008;62:159. doi: 10.1007/s00280-007-0586-5. [DOI] [PubMed] [Google Scholar]
- 12.Wright MJ. Deonarain MP. Phage display of chelating recombinant antibody libraries. Mol Immunol. 2007;44:2860. doi: 10.1016/j.molimm.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Berry LJ. Moeller M. Darcy PK. Adoptive immunotherapy for cancer: The next generation of gene-engineered immune cells. Tissue Antigens. 2009;74:277. doi: 10.1111/j.1399-0039.2009.01336.x. [DOI] [PubMed] [Google Scholar]
- 14.Beckman RA. Weiner LM. Davis HM. Antibody constructs in cancer therapy: Protein engineering strategies to improve exposure in solid tumors. Cancer. 2007;109:170. doi: 10.1002/cncr.22402. [DOI] [PubMed] [Google Scholar]
- 15.Schule J. Bergkvist L. Hakansson L, et al. Down-regulation of the CD3-zeta chain in sentinel node biopsies from breast cancer patients. Breast Cancer Res Treat. 2002;74:33. doi: 10.1023/a:1016009913699. [DOI] [PubMed] [Google Scholar]
- 16.Shibuya TY. Nugyen N. McLaren CE, et al. Clinical significance of poor CD3 response in head and neck cancer. Clin Cancer Res. 2002;8:745. [PubMed] [Google Scholar]
- 17.Wang J. Press OW. Lindgren CG, et al. Cellular immunotherapy for follicular lymphoma using genetically modified CD20-specific CD8+ cytotoxic T lymphocytes. Mol Ther. 2004;9:577. doi: 10.1016/j.ymthe.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Schirrmann T. Pecher G. Specific targeting of CD33(+) leukemia cells by a natural killer cell line modified with a chimeric receptor. Leuk Res. 2005;29:301. doi: 10.1016/j.leukres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Hombach A. Wieczarkowiecz A. Marquardt T, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001;167:6123. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 20.Hu S. Li L. Qiao J, et al. Codon optimization, expression, and characterization of an internalizing anti-ErbB2 single-chain antibody in Pichia pastoris. Protein Expr Purif. 2006;47:249. doi: 10.1016/j.pep.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Warrens AN. Jones MD. Lechler RI. Splicing by overlap extension by PCR using asymmetric amplification: An improved technique for the generation of hybrid proteins of immunological interest. Gene. 1997;186:2. doi: 10.1016/s0378-1119(96)00674-9. [DOI] [PubMed] [Google Scholar]
- 22.Jones ML. Barnard RT. Chimerization of multiple antibody classes using splice overlap extension PCR. Biotechniques. 2005;38:181. doi: 10.2144/05382BM01. [DOI] [PubMed] [Google Scholar]
- 23.Heckman KL. Pease LR. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc. 2007;2:924. doi: 10.1038/nprot.2007.132. [DOI] [PubMed] [Google Scholar]
- 24.Cox MA. Zajac AJ. Shaping successful and unsuccessful CD8 T cell responses following infection. J Biomed Biotechnol. 2010;2010:159152. doi: 10.1155/2010/159152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faurie C. Rebersek M. Golzio M, et al. Electro-mediated gene transfer and expression are controlled by the life-time of DNA/membrane complex formation. J Gene Med. 2010;12:117. doi: 10.1002/jgm.1414. [DOI] [PubMed] [Google Scholar]
- 26.Maurisse R. De Semir D. Emamekhoo H, et al. Comparative transfection of DNA into primary and transformed mammalian cells from different lineages. BMC Biotechnol. 2010;10:9. doi: 10.1186/1472-6750-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooper LJ. Ausubel L. Gutierrez M, et al. Manufacturing of gene-modified cytotoxic T lymphocytes for autologous cellular therapy for lymphoma. Cytotherapy. 2006;8:105. doi: 10.1080/14653240600620176. [DOI] [PubMed] [Google Scholar]
- 28.Wang J. Jensen M. Lin Y, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Human Gene Ther. 2007;18:712. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- 29.Escoffre JM. Portet T. Wasungu L, et al. What is (still not) known of the mechanism by which electroporation mediates gene transfer and expression in cells and tissues. Mol Biotechnol. 2009;41:286. doi: 10.1007/s12033-008-9121-0. [DOI] [PubMed] [Google Scholar]
- 30.Escoffre JM. Bellard E. Golzio M, et al. Transgene expression of transfected supercoiled plasmid DNA concatemers in mammalian cells. J Gene Med. 2009;11:1071. doi: 10.1002/jgm.1384. [DOI] [PubMed] [Google Scholar]
- 31.Maucksch C. Bohla A. Hoffmann F, et al. Transgene expression of transfected supercoiled plasmid DNA concatemers in mammalian cells. J Gene Med. 2009;11:444. doi: 10.1002/jgm.1310. [DOI] [PubMed] [Google Scholar]
- 32.Jain NK. Roy I. Effect of trehalose on protein structure. Protein Sci. 2009;18:24. doi: 10.1002/pro.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuki N. Takeda M. Ishikawa T, et al. Activation of caspases and apoptosis in response to low-voltage electric pulses. Oncol Rep. 2010;23:1425. doi: 10.3892/or_00000780. [DOI] [PubMed] [Google Scholar]
- 34.Meehan KR. Wu J. Webber SM, et al. Development of a clinical model for ex vivo expansion of multiple populations of effector cells for adoptive cellular therapy. Cytotherapy. 2008;10:30. doi: 10.1080/14653240701762398. [DOI] [PubMed] [Google Scholar]
- 35.Jensen MC. Clarke P. Tan G, et al. Human T lymphocyte genetic modification with naked DNA. Mol Ther. 2000;1:49. doi: 10.1006/mthe.1999.0012. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez RD. Barnes MN. Gomez-Navarro J, et al. A cancer gene therapy approach utilizing an anti-erbB-2 single-chain antibody-encoding adenovirus (AD21): A phase I trial. Clin Cancer Res. 2000;6:3081. [PubMed] [Google Scholar]
- 37.Clemenceau B. Congy-Jolivet N. Gallot G, et al. Antibody-dependent cellular cytotoxicity (ADCC) is mediated by genetically modified antigen-specific human T lymphocytes. Blood. 2006;107:4669. doi: 10.1182/blood-2005-09-3775. [DOI] [PubMed] [Google Scholar]
- 38.Arafat WO. Gomez-Navarro J. Buchsbaum DJ, et al. Effective single chain antibody (scFv) concentrations in vivo via adenoviral vector mediated expression of secretory scFv. Gene Ther. 2002;9:256. doi: 10.1038/sj.gt.3301639. [DOI] [PubMed] [Google Scholar]
- 39.Barker SD. Dmitriev IP. Nettelbeck DM, et al. Combined transcriptional and transductional targeting improves the specificity and efficacy of adenoviral gene delivery to ovarian carcinoma. Gene Ther. 2003;10:1198. doi: 10.1038/sj.gt.3301974. [DOI] [PubMed] [Google Scholar]
- 40.Guo MG. Jiang MH. Yang Q, et al. Gene therapy for ovarian cancers by adenovirus-mediated complete antibody gene. Zhonghua Yi Xue Za Zhi. 2004;84:1147. [PubMed] [Google Scholar]
- 41.Shen YC. Wang XH. Wang XM, et al. High efficient mammalian expression and secretion of a functional humanized single-chain Fv/human interleukin-2 molecules. World J Gastroenterol. 2006;12:3859. doi: 10.3748/wjg.v12.i24.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agadjanian H. Weaver JJ. Mahammed A, et al. Specific delivery of corroles to cells via noncovalent conjugates with viral proteins. Pharmaceut Res. 2006;23:367. doi: 10.1007/s11095-005-9225-1. [DOI] [PubMed] [Google Scholar]
- 43.Edelweiss E. Balandin TG. Ivanova JL, et al. Barnase as a new therapeutic agent triggering apoptosis in human cancer cells. PloS One. 2008;3:e2434. doi: 10.1371/journal.pone.0002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shahied LS. Tang Y. Alpaugh RK, et al. Bispecific minibodies targeting HER2/neu and CD16 exhibit improved tumor lysis when placed in a divalent tumor antigen binding format. J Biol Chem. 2004;279:53907. doi: 10.1074/jbc.M407888200. [DOI] [PubMed] [Google Scholar]
- 45.von Minckwitz G. Harder S. Hovelmann S, et al. Phase I clinical study of the recombinant antibody toxin scFv(FRP5)-ETA specific for the ErbB2/HER2 receptor in patients with advanced solid malignomas. Breast Cancer Res. 2005;7:R617. doi: 10.1186/bcr1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall KW. Marks JD. Engineering and characterization of a novel fusion protein incorporating B7.2 and an anti-ErbB-2 single-chain antibody fragment for the activation of Jurkat T cells. J Immunother. 2001;24:27. doi: 10.1097/00002371-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Biburger M. Weth R. Wels WS. A novel bispecific tetravalent antibody fusion protein to target costimulatory activity for T-cell activation to tumor cells overexpressing ErbB2/HER2. J Mol Biol. 2005;346:1299. doi: 10.1016/j.jmb.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 48.Cooper LJ. Topp MS. Serrano LM, et al. T-cell clones can be rendered specific for CD19: Toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 49.Jensen MC. Cooper LJ. Wu AM, et al. Engineered CD20-specific primary human cytotoxic T lymphocytes for targeting B-cell malignancy. Cytotherapy. 2003;5:131. doi: 10.1080/14653240310001028. [DOI] [PubMed] [Google Scholar]
- 50.Yu K. Hu Y. Tan Y, et al. Immunotherapy of lymphomas with T cells modified by anti-CD20 scFv/CD28/CD3zeta recombinant gene. Leuk Lymphoma. 2008;49:1368. doi: 10.1080/10428190802064958. [DOI] [PubMed] [Google Scholar]
- 51.Till BG. Jensen MC. Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maher J. Brentjens RJ. Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol. 2002;20:70. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 53.Losch FO. Muller R. Mutschler B, et al. Activation of T cells via tumor antigen specific chimeric receptors: The role of the intracellular signaling domain. Int J Cancer. 2003;103:399. doi: 10.1002/ijc.10826. [DOI] [PubMed] [Google Scholar]
- 54.Patel SD. Ge Y. Moskalenko M, et al. Anti-tumor CC49-zeta CD4 T cells possess both cytolytic and helper functions. J Immunother. 2000;23:661. doi: 10.1097/00002371-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Bullain SS. Sahin A. Szentirmai O, et al. Genetically engineered T cells to target EGFRvIII expressing glioblastoma. J Neurooncol. 2009;94:373. doi: 10.1007/s11060-009-9889-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ren-Heidenreich L. Mordini R. Hayman GT, et al. Comparison of the TCR zeta-chain with the FcR gamma-chain in chimeric TCR constructs for T cell activation and apoptosis. Cancer Immunol Immunother. 2002;51:417. doi: 10.1007/s00262-002-0301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morel PA. Oriss TB. Crossregulation between Th1 and Th2 cells. Crit Rev Immunol. 1998;18:275. doi: 10.1615/critrevimmunol.v18.i4.10. [DOI] [PubMed] [Google Scholar]
- 58.Mueller DL. Seiffert S. Fang W, et al. Differential regulation of bcl-2 and bcl-x by CD3, CD28, and the IL-2 receptor in cloned CD4+ helper T cells. A model for the long-term survival of memory cells. J Immunol. 1996;156:1764. [PubMed] [Google Scholar]