Abstract
Limbal stem cells (LSC), which reside in the basal layer of the limbus, are thought to be responsible for corneal epithelial healing after injury. When the cornea is damaged, LSC start to proliferate, differentiate, and migrate to the site of injury. To characterize the signaling molecules ensuring communication between the cornea and LSC, we established a mouse model of mechanical corneal damage. The central cornea or limbal tissue was excised at different time intervals after injury, and the expression of genes in the explants was determined. It was observed that a number of genes for growth and differentiation factors were significantly upregulated in the cornea rapidly after injury. The ability of these factors to regulate the differentiation and proliferation of limbal cells was tested. It was found that the insulin-like growth factor-I (IGF-I), which is rapidly overexpressed after injury, enhances the expression of IGF receptor in limbal cells and induces the differentiation of LSC into cells expressing the corneal cell marker, cytokeratin K12, without any effect on limbal cell proliferation. In contrast, the epidermal growth factor (EGF) and fibroblast growth factor-β (FGF-β), which are also produced by the damaged corneal epithelium, supported limbal cell proliferation without any effect on their differentiation. Other factors did not affect limbal cell differentiation or proliferation. Thus, IGF-I was identified as the main factor stimulating the expression of IGF receptors in limbal cells and inducing the differentiation of LSC into cells expressing corneal epithelial cell markers. The proliferation of these cells was supported by EGF and FGF.
Introduction
The impaired or otherwise damaged cornea is thought to be healed by the cells that originate from the limbus. Although some degree of self-regenerative capacity of the corneal epithelium has been described in both the mouse [1] and in humans [2,3], the majority of cells migrating to the site of injury originate from limbal stem cells (LSC). It has been observed that in the case of LSC deficiency, the cornea cannot heal properly and its healing is associated with conjunctivilization, neovascularization, chronic inflammation, and persistent epithelial defects that may result in a loss of vision [4,5]. Conversely, such defects can be treated by the transplantation of limbal tissue or LSC [6,7]. It has been shown that after corneal damage, LSC start to proliferate and differentiate into transient amplifying cells, which migrate to the site of injury and give rise to cells expressing corneal epithelial cell markers [4,8].
The molecular basis of the interplay between the cornea and the limbus remains mostly unknown. Jia et al. [9] demonstrated that a large number of genes in corneal cells are upregulated after mechanical or chemical damage of the ocular surface. Among them, the genes coding for growth and differentiation factors represent a significant group. It was shown that the transforming growth factor-α (TGF-α), TGF-β, epidermal growth factor (EGF), hepatocyte growth factor (HGF), and fibroblast growth factor-β (FGF-β), which are produced by corneal epithelial cells, can modulate the proliferation and growth of cells on the ocular surface [10–12]. Another factor produced by the corneal epithelium, insulin-like growth factor-I (IGF-I), has been shown to support the proliferation of keratinocytes [13], enhance the production of the adherens-junction protein N-cadherin [14], stimulate the formation of the extracellular matrix [15], and increase the synthesis of collagen by keratinocytes [16]. The IGF signaling pathway has been shown to be involved in cell proliferation and differentiation, and it has an important role in growth regulation at both cellular and organism levels [17]. It suggests that IGF-I and other factors produced by the damaged corneal epithelium can be involved in the regulation of LSC differentiation, proliferation, and migration.
The identification of cornea-associated phenotypic markers, such as cytokeratins K3 and K12 or connexin 43, which are expressed by corneal cells, but are absent in the limbus [18,19], enables monitoring the differentiation of LSC into cells with the characteristics of corneal epithelial cells and the characterization of those factors responsible for the differentiation and proliferation of limbal cells. Previous studies have shown that supernatants from corneal cell cultures induce the differentiation of various types of stem cells, including hair follicule stem cells, mesenchymal stem cells (MSC), and embryonic stem cells, into cells expressing the corneal epithelial cell marker, K12 [20–23].
However, the key differentiation factor present in the corneal cell supernatants has not yet been identified. In the present study, a model of mechanically damaged cornea in the mouse has been used to characterize factors that are produced by the corneal epithelial cells after corneal injury, and which are responsible for differentiation and proliferation of LSC. Since these factors are produced by corneal cells after a superficial corneal epithelial damage and they induce the expression of the corneal cell marker, K12, in limbal cells, we suggest that these factors are involved in the regulation of corneal epithelial healing after the injury.
Materials and Methods
Animals
Mice of the inbred strain BALB/c of both sexes were used in the experiments at the age of 2–4 months. The animals were obtained from the breeding unit of the Institute of Molecular Genetics, Prague. The use of animals was approved by the local Animal Ethics Committee of the Institute of Molecular Genetics.
A model of corneal injury
The recipient BALB/c mice were deeply anesthetized by an intramuscular injection of a mixture of xylazine and ketamine (Rometar, Spofa, Prague, Czech Republic). The surface of the central cornea (only the epithelial layer) of the left eye was damaged by the epithelial debridement with a sharp needle (G23). The damage involved only the region in the central cornea (with a diameter 1 mm) and was made with a care to avoid injury of the corneal stroma or the limbus. The extent of damage can be observed under a microscope after a dissection of the cornea and mechanical separation of individual corneal layers. At different time intervals after the injury (3, 6, 9, 12, 24, or 48 h), the central cornea (the diameter 1 mm) and limbal tissue from control and damaged eyes were excised and tested for cell proliferation, factor production, or gene expression.
Preparation of a single-cell suspension from limbal tissue
A single-cell suspension from limbal tissues was obtained by enzyme digestion, as we have described [24]. In brief, limbal tissues (a narrow tissue between the cornea and the conjunctiva) from 10–12 BALB/c mice were cut out with scissors and subjected to 10 short (10 min each) trypsinization cycles. The released cells were harvested after each cycle, finally centrifuged (8 min at 250 g), and resuspended in the Dulbecco's modified Eagle's medium (DMEM; Sigma Corp., St. Louis, MO) containing 10% fetal calf serum (FCS; Sigma), antibiotics (100 U/mL of penicillin, 100 μg/mL of streptomycin), and 10 mM HEPES buffer (a complete DMEM).
Preparation of supernatants from cultures of corneal explants
Explants of the central cornea (1 mm in diameter, without any limbal tissue) from control and damaged eyes (24 h after the damage) were cultured in the complete DMEM (one cornea explant per 125 μL of medium) in 48-well tissue culture plates (Nunc, Roskilde, Denmark). After a 24-h incubation period, the supernatants were harvested and centrifuged at 400 g for 10 min, filtered through 0.22 μ filters, and stored at −80°C until used.
Determination of limbal cell proliferation
Limbal explants from individual eyes were incubated in a volume of 200 μL of complete DMEM in 96-well tissue culture plates (Nunc), and cell proliferation was determined by adding 3H-thymidine (1 μCi/well, Nuclear Research Institute, Rez, Czech Republic) for the last 6 h of the 48-h incubation period. To determine the effects of recombinant factors on limbal cell proliferation, limbal cells (25×103 cells/mL) from healthy eyes were cultured in a volume of 200 μL of complete DMEM in 96-well tissue culture plates in the absence or the presence of recombinant mouse (m)EGF, mFGF-β, human (h)TGF-β1, human keratinocyte growth factor (KGF), mHGF, or mIGF-1 (all factors were purchased from PeproTech, Rocky Hill, NJ, and were tested at concentrations ranging from 0.1 to 500 ng/mL). Cell proliferation was determined by measuring 3H-thymidine incorporation after a 48-h incubation period using a Tri-Carb 2900TR scintillation counter (Packard, Meridien, CT).
Detection of gene expression by real-time polymerase chain reaction
The expression of genes for EGF, FGF-β, TGF-β1, TGF-β2, KGF, HGF, IGF-I, IGF-II, IGF-IR, and IGF-IIR in corneal and limbal tissue or in cultured limbal cells was detected using quantitative real-time polymerase chain reaction (PCR). The central cornea or the limbus was excised using Vannas scissors at the indicated time intervals after the injury and transferred into Eppendorf tubes containing 500 μL of TRI Reagent (Molecular Research Center, Cincinnatti, OH). Total RNA was extracted using the TRI Reagent according to the manufacturer's instructions. One μg of total RNA was treated using deoxyribonuclease I (Sigma) and used for subsequent reverse transcription. The first-strand cDNA was synthesized using random primers (Promega, Madison, WI) in a total reaction volume of 25 μL using M-MLV Reverse Transcriptase (Promega). Quantitative real-time PCR was performed in an iCycler (BioRad, Hercules, CA), as previously described [25,26]. The primers used for amplification are shown in Table 1. The PCR parameters included denaturation at 95°C for 3 min, then 40 cycles at 95°C for 20 s, annealing at 60°C for 30 s, and elongation at 72°C for 30 s. Fluorescence data were collected at each cycle after an elongation step at 80°C for 5 s and were analyzed on the iCycler Detection system, Version 3.1. Each individual experiment was done in triplicate. A relative quantification model was applied to calculate the expression of the target gene in comparison to GAPDH used as an endogenous control.
Table 1.
Mouse Primer Sequences Used for Real-Time Polymerase Chain Reaction
| Gene | Sense primer | Antisense primer |
|---|---|---|
| GAPDH | AGAACATCATCCCTGCATCC | ACATTGGGGGTAGGAACAC |
| EGF | CATGCCCCACAGGATTTG | GGGCAGGAAACAAGTTCGT |
| FGF-β | TCTCATCAATCTCCAGTTCACAA | CTTGCGTTGATTGCTACTCCT |
| KGF | CGGCTCTACTGCAAGAACG | TGCTTGGAGTTGTAGTTTGACG |
| HGF | CACCCCTTGGGAGTATTGTG | GGGACATCAGTCTCATTCACAG |
| TGF-β1 | TGGAGCAACATGTGGAACTC | CAGCAGCCGGTTACCAAG |
| TGF-β2 | TGGAGTTCAGACACTCAACACA | AAGCTTCGGGATTTATGGTGT |
| K12 | GTGAGTCCGCTGGTGGTAAC | CATCAGCACAGCAGGAAGTG |
| IGF-I | TCGGCCTCATAGTACCCACT | ACGACATGATGTGTATCTTTATTGC |
| IGF-IR | GAGAATTTCCTTCACAATTCCATC | CACTTGCATGACGTCTCTCC |
| IGF-II | CGCTTCAGTTTGTCTGTTCG | GCAGCACTCTTCCACGATG |
| IGF-IIR | CCTTCTCTAGTGGATTGTCAAGTG | AGGGCGCTCAAGTCATACTC |
EGF, epidermal growth factor; FGF, fibroblast growth factor; KGF, keratinocyte growth factor; HGF, hepatocyte growth factor; TGF, transforming growth factor; IGF-I, insulin-like growth factor-I.
Immunostaining
Limbal cells or corneal epithelial cells (25×103 cells/mL) were cultured for 48 h in a volume of 1 mL of complete DMEM in 24-well tissue culture plates (Nunc) in the absence or the presence of IGF-I (100 ng/mL). Dead cells were marked using Hoechst 33258 dye (Invitrogen, Carlsbad, CA) and sorted out using a BD Influx Cell Sorter (BD Biosciences, Franklin Lakes, NJ). The remaining cells were fixed for 20 min with 4% paraformaldehyde and permeabilized for 10 min with 0.1% Triton X-100. The samples were incubated with goat polyclonal anti-K12 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature, then with a secondary donkey anti-goat IgG conjugated with Alexa Flour 594 (Invitrogen). The cells were rinsed with phosphate-buffered saline containing 0.05% TWEEN and fixed on glass slides with Mowiol 4-88 (Calbiochem, San Diego, CA) in the presence of the nuclear dye 4′,6′-diamidino-2-phenylindole (DAPI). Visualization of the fluorescent label was performed using a fluorescent microscope (Leica, Wetzlar, Germany). Control samples were run without the primary antibody.
The effect of neutralization antibody anti-IGF-I on the ability of corneal cell supernatants to induce differentiation of LSC
Supernatants from cultures of explants from damaged corneas were preincubated for 2 h in the absence or presence of 10 μg/mL of neutralization goat anti-murine anti-IGF-I antibody (Peprotech, Rocky Hill, NJ) and were added into cultures of freshly isolated limbal cells (25×103 cells/mL). After a 48-h cultivation period, the expression of K12 gene was determined by real-time PCR.
Statistical analysis
The statistical significance of differences between individual groups was calculated using the Student's t-test.
Results
Enhanced proliferative activity of limbal cells after corneal damage
The epithelium of the central cornea was mechanically damaged, and the limbal tissue was excised at the indicated time intervals after the injury. The limbal explants were cultured for 48 h, and the proliferation of cells was determined. As shown in Fig. 1A, limbal cells from the wounded eyes harvested 1 day after the injury proliferated significantly more strongly than did limbal cells from the control eyes. The proliferation of limbal cells on day 4 after the injury was, in contrast, rather decreased in comparison with limbal cells from control eyes and returned to a normal level 7 days after the injury.
FIG. 1.
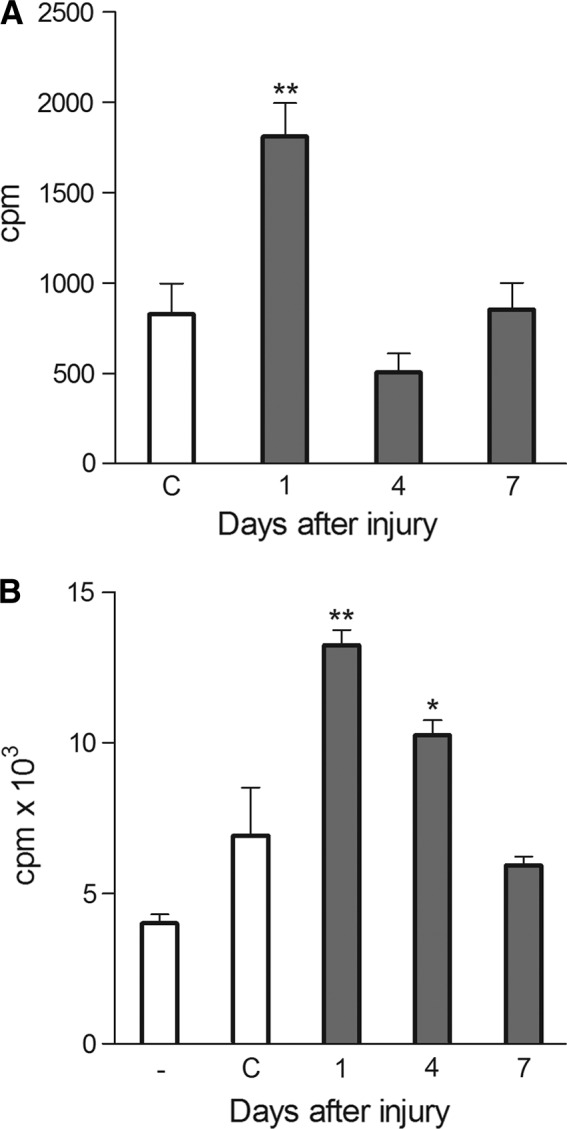
Proliferation of limbal cells after corneal damage. (A) The central cornea was mechanically damaged, the limbal tissue was excised at the indicated time intervals after the injury, and the proliferation of cells of the limbal explants was determined. (B) The corneas from control or damaged eyes were excised at the indicated time intervals after the injury, and the corneal explants were cultured for 24 h to prepare supernatants. Proliferation of limbal cells in cultures without supernatants (-) or in the presence of supernatants from control corneas (C) or corneas from damaged eyes was determined. Each bar represents mean±SD from 3–5 determinations. Values with asterisks are significantly (*P<0.01, **P<0.001) different from the controls (i.e., the proliferation of limbal explants from control eyes or limbal cell proliferation in cultures without supernatants).
To determine whether the enhanced proliferation of limbal cells observed after corneal damage was induced by a factor(s) produced by corneal cells, corneas from control or damaged eyes were cultured for 24 h. The supernatants from these cultures were added to cultures of limbal cells from control untreated mice. As shown in Fig. 1B, the supernatants from the cultures of corneas from damaged eyes induced a significantly stronger proliferation of limbal cells than supernatants from the cultures of corneal explants from control eyes.
Expression of genes in cells of the central cornea after injury
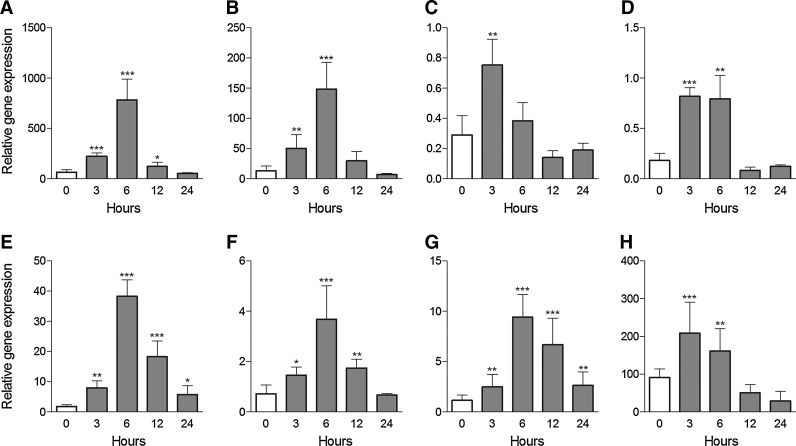
The epithelium of the central cornea was mechanically damaged and the corneas were excised at the indicated time intervals (0, 3, 6, 12, and 24 h) after the injury. The expression of genes for the tested factors was determined by real-time PCR. Figure 2 shows that the expression of genes for EGF, FGF-β, KGF, HGF, TGF-β1, IGF-I, and IGF-II was significantly upregulated already 3 or 6 h after the injury.
FIG. 2.
The expression of genes for growth and differentiation factors in corneal epithelial cells after corneal injury. Corneas were mechanically damaged and the central cornea was excised 0 (untreated eye), 3, 6, 12, or 24 h after the injury. The expression of genes for TGF-β1 (A), TGF-β2 (B), EGF (C), HGF (D), KGF (E), FGF-β (F), IGF-I (G), and IGF-II (H) was determined by real-time PCR. Each bar represents mean±SD from 3 determinations. Values with asterisks represent a statistically significant (*P<0.05, **P<0.01, ***P<0.001) increase in gene expression. EGF, epidermal growth factor; FGF, fibroblast growth factor; KGF, keratinocyte growth factor; HGF, hepatocyte growth factor; TGF, transforming growth factor; IGF-I, insulin-like growth factor-I; PCR, polymerase chain reaction.
The effects of recombinant factors on limbal cell proliferation
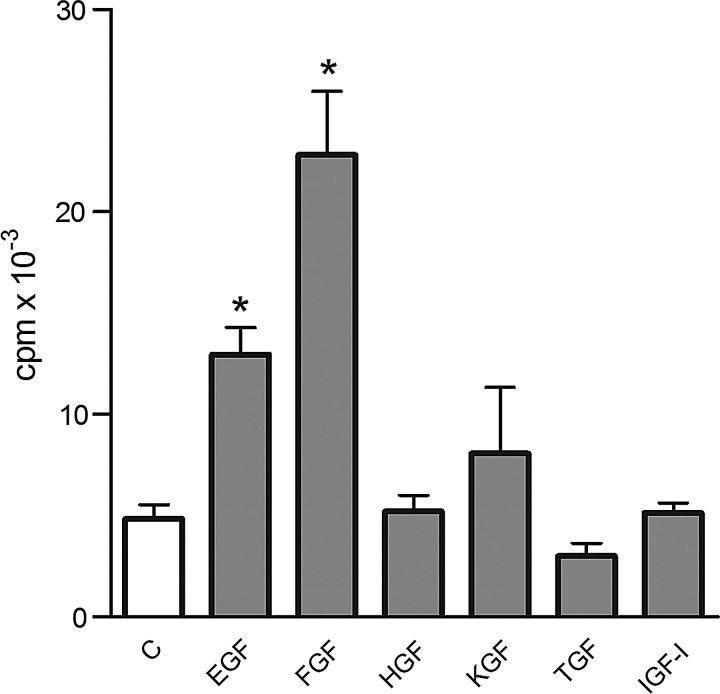
A single-cell suspension of limbal cells from control mice was incubated in the absence or in the presence of recombinant factors that had been identified as being produced by the damaged cornea. In preliminary experiments, all factors were tested over a wide range of concentrations (0.1–500 ng/mL); for the sake of brevity, the effects of only one concentration (10 ng/mL) are shown. As demonstrated in Fig. 3, EGF and FGF-β significantly stimulated the proliferation of limbal cells, KGF had less effect, and the other tested factors had only a marginal or no effect on limbal cell growth. Conversely, TGF-β, at higher concentrations, inhibited the proliferation of limbal cells (Fig. 3).
FIG. 3.
The effects of recombinant factors on limbal cell proliferation. A single-cell suspension was prepared by enzymatic digestion from the limbal tissue of control eyes, and the cells were cultured for 48 h without factors (C) or in the presence of 10 ng/mL of EGF, FGF-β, HGF, KGF, TGF-β, or IGF-I. Cell proliferation was determined according to 3H-thymidine incorporation. Each bar represents mean±SD from 4 determinations. Values with an asterisk are significantly (*P<0.001) different from the control cultures (cells cultured without added factors).
The effect of recombinant factors on limbal cell differentiation
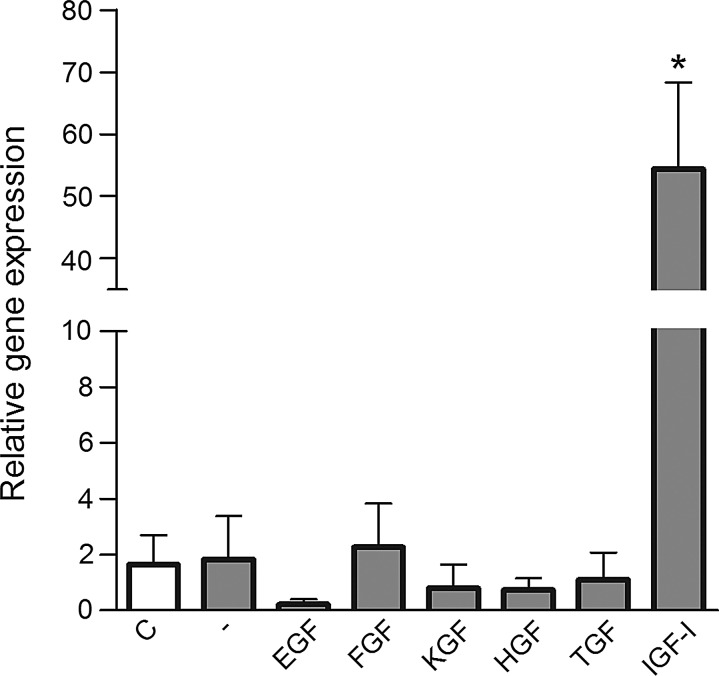
Since cytokeratin K12 is a molecule that can distinguish corneal epithelial and limbal cells (K12 is strongly expressed in the corneal epithelium, but is absent in the limbus), we cultured limbal cells in the presence of factors that are produced by the damaged cornea. After a 48-h incubation, the expression of the K12 gene was detected by real-time PCR. As shown in Fig. 4, only IGF-I induced the significant expression of K12 mRNA, while all other tested factors did not enhance expression of the K12 gene above the level seen in control cells (cultured in the absence of factors).
FIG. 4.
The effect of recombinant factors on K12 mRNA expression in cultured limbal cells. A single-cell suspension of limbal cells prepared from control eyes was cultured for 48 h without factors (-) or in the presence of the indicated recombinant factors, and the expression of the K12 gene was determined by real-time PCR. The level of K12 mRNA expression in fresh (nonstimulated) cells (C). Each bar represents mean±SD from 3 determinations. The value with an asterisk is significantly (*P<0.001) different from the control value (cells cultured without added factors).
The expression of K12 protein in limbal cells cultured in the presence of IGF-I
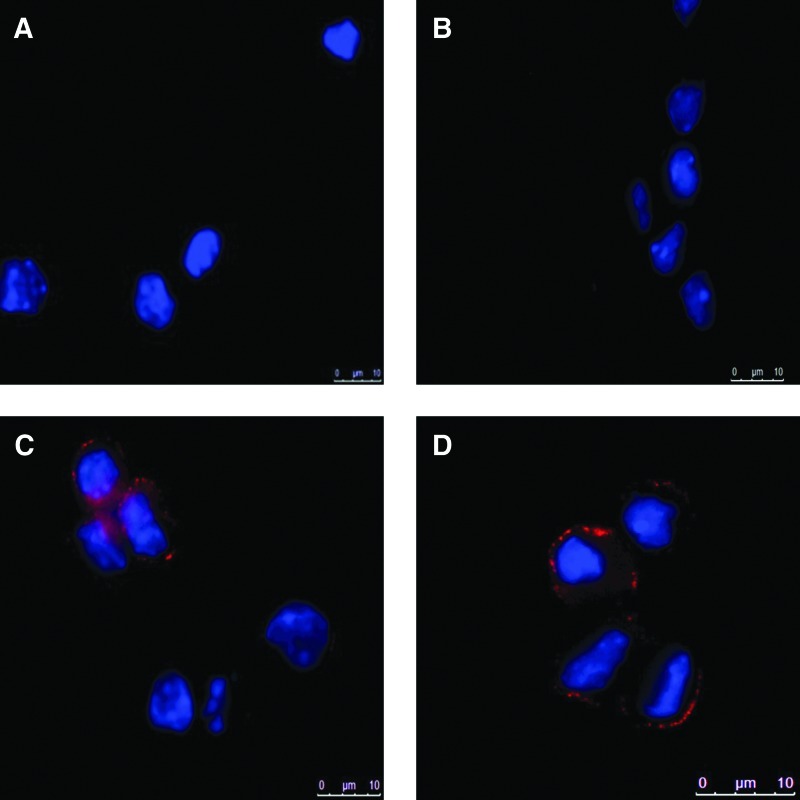
The differentiation potential of IGF-I was confirmed by immunostaining for the K12 protein. Limbal cells from control mice were cultured in the absence or in the presence of recombinant IGF-I, and the expression of K12 protein was detected by immunostaining using an anti-K12 antibody. As demonstrated in Fig. 5, clear positivity for K12 protein was detected in limbal cells incubated in the presence of IGF-I. Cultured corneal epithelial cells served as a positive control.
FIG. 5.
Immunostaining for K12 protein in limbal cells treated with IGF-I. A single-cell suspension prepared from the limbal tissue of control mice was incubated for 72 h with or without IGF-I (100 ng/mL), and the cells were stained with a goat antibody against mouse K12. The nuclei were stained with DAPI (blue). The limbal cells were cultured without IGF-I (A), with IGF-I, but with the primary antibody omitted during the staining procedure (B) or with IGF-I and with the K12 protein stained using anti-K12 antibody (C). As a positive control, cultured corneal epithelial cells were stained with the anti-K12 antibody (D). One representative experiment of 4 similar ones is shown. DAPI, 4′,6′-diamidino-2-phenylindole.
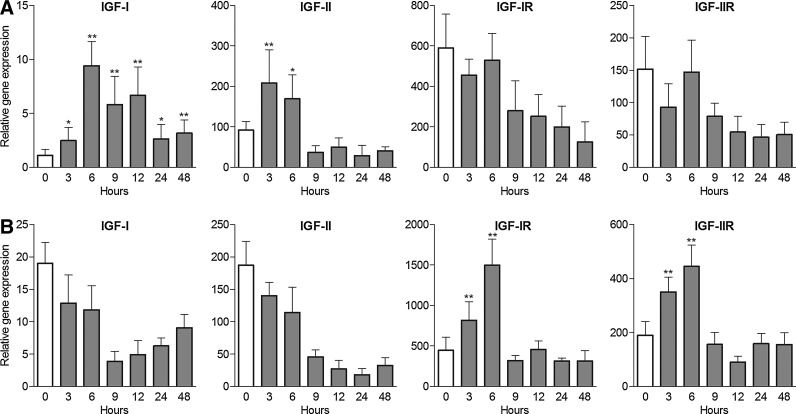
Kinetics of IGF-IR and IGF-IIR gene expression in the cornea and limbus after corneal injury
Although a number of genes in the corneal epithelium are rapidly upregulated after corneal injury (Fig. 2), the expression of genes for IGF-IR and IGF-IIR in the central cornea was simultaneously decreased (Fig. 6A). In contrast to the cornea, the expression of genes for IGF receptors in the limbus was significantly enhanced after corneal injury (Fig. 6B).
FIG. 6.
Kinetics of IGF-IR and IGF-IIR mRNA expression in the cornea and in the limbus after corneal injury. The cornea was mechanically damaged and the expression of IGF-IR and IGF-IIR mRNA in the explants of the central cornea (A) or the limbus (B) was determined by real-time PCR. Each bar represents mean±SD from 3 determinations. Values with asterisks represent a statistically significant (*P<0.01, **P<0.001) increase in gene expression.
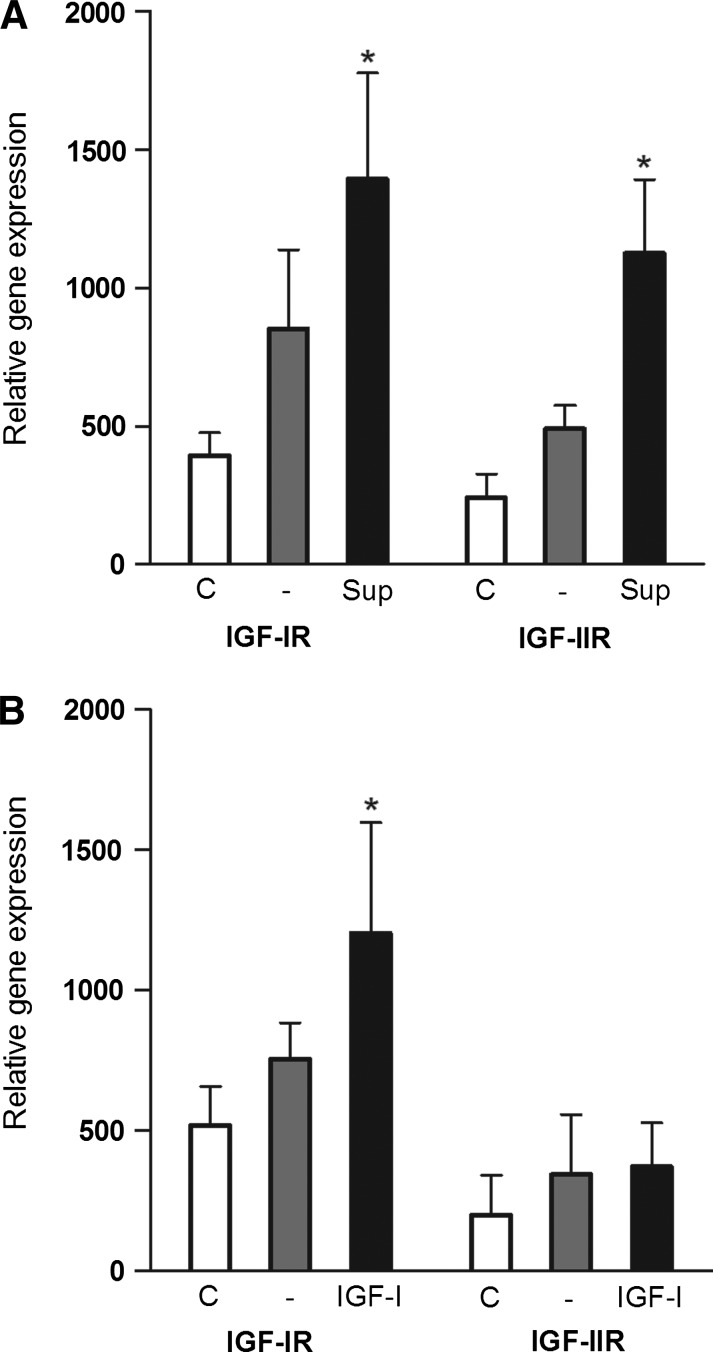
Regulation of IGF receptor expression in limbal cells by IGF-I
Next, we tested whether the enhanced expression of genes for IGF-IR and IGF-IIR that was observed in limbal cells after corneal injury was induced by a factor produced by corneal cells. Limbal cells from healthy eyes were incubated in supernatants from cultures of corneal explants, and the expression of genes for IGF-IR and IGF-IIR was determined. As demonstrated in Fig. 7A, the supernatants from cultures of corneal explants from damaged eyes significantly increased the expression of genes for IGF-IR and IGF-IIR. When limbal cells were cultured in the presence of recombinant IGF-I, a significant upregulation of IGF-IR gene expression was observed, without stimulation of IGF-IIR gene expression (Fig. 7B).
FIG. 7.
Regulation of IGF receptor expression by IGF-I. Limbal cells isolated from healthy eyes were cultured in the presence of supernatant from cultures of corneal explants (A) or with recombinant IGF-I (B), and the expression of IGF-IR and IGF-IIR mRNA was determined. The expression of IGF-IR or IGF-IIR mRNA was analyzed in freshly isolated limbal cells (C), in cells incubated for 6 h in culture medium without factors (-), in medium containing 20% of supernatant from cultures of corneal explants from damaged eyes (Sup) or in medium containing 250 ng/mL of IGF-I (IGF-I). Each bar represents mean±SD from 3 determinations. Values with an asterisk represent a statistically significant (*P<0.001) increase in gene expression (in comparison with cultures without supernatant or without IGF-I).
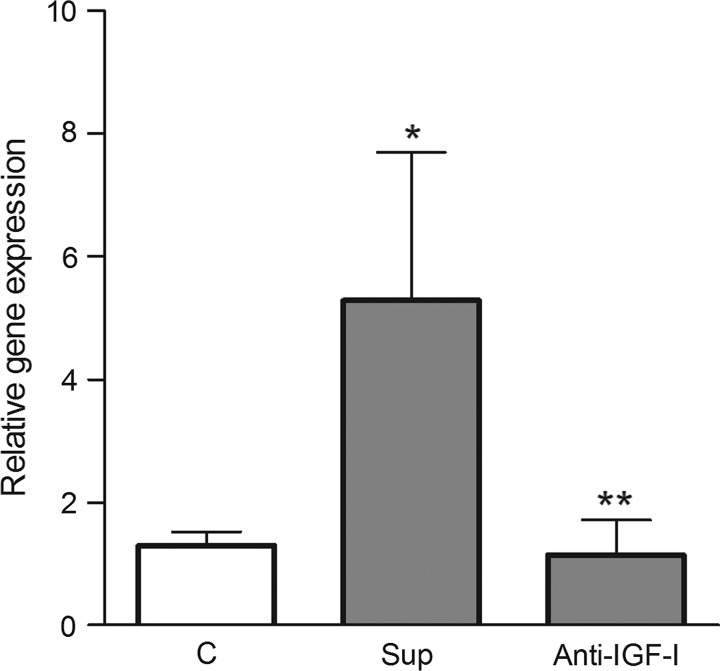
The effect of neutralization antibody anti-IGF-I on LSC differentiation
To prove that IGF-I present in the corneal cell supernatants is responsible for differentiation of limbal cells, the supernatants from cultures of explants from damaged corneas were preincubated with the neutralization antibody anti-IGF-I, and then added to the culture of limbal cells. Figure 8 shows that the expression of gene for K12 in limbal cells was significantly increased after incubation of cells with supernatants from damaged corneas and this increase was abrogated by preincubation of supernatants with the antibody, anti-IGF-I.
FIG. 8.
The effect of neutralization antibody anti-IGF-I on corneal cell supernatant-induced limbal stem cells differentiation. Limbal cells were cultured for 2 days in medium (C), in medium with supernatant from cultures of cells from damaged corneas (Sup) or in medium with supernatants, which were preincubated for 2 h with neutralization antibody anti-IGF-I (Anti-IGF-I). The expression of the gene for K12 was determined by real-time PCR. Each bar represents mean±SD from 3 determinations. The value with an asterisk is significantly (P<0.01) different from C, the value with 2 asterisks is significantly (P<0.05) different from a positive control (Sup).
Discussion
The impaired healing of the corneal epithelium in the case of LSC deficiency suggests that the corneal epithelium is regenerated from stem cells that reside in the limbus [27]. This suggestion is supported by the observation that corneal regeneration can be improved by the transplantation of limbal tissue or by a transfer of LSC [6,7]. These cells represent, in the healthy eye, a quiescent and slowly dividing cell population located in a specific niche that preserves their stemness [28]. LSC can be distinguished from corneal epithelial cells by phenotypic markers as well as by morphological and behavioral properties. Even though these stem cells are effectively protected in their niche, there must be a factor(s) produced by corneal cells after an injury that activates LSC and induces their proliferation, differentiation, and migration into the site of injury [8]. However, the molecular characteristics and the nature of those factors that overcome stemness and induce LSC differentiation remain unknown.
Previous studies that have described the inductive properties of limbal or the corneal cell-conditioned medium on the differentiation of stem cells into corneal epithelial lineages focused on embryonic stem cells [22,29], MSC [21,23], or adult tissue-specific stem cells [20]. These studies pointed out the role of the extracellular matrix of the stem cell niche and demonstrated the importance of factors present in the conditioned media. It was shown that it is possible to induce the expression of K12, a corneal cell-specific marker, in stem cells cultured in supernatants from corneal cells. However, the particular factors responsible for stem cell activation and differentiation have not been identified.
Here, we show that a number of genes for growth and differentiation factors are rapidly upregulated in cells of the corneal epithelium after an injury. These factors are able to reach the limbal region through the tear film, aqueous fluid, or the epithelial layer of the cornea. In the limbus, they induce the increased proliferative activity of LSC. We observed that limbal cells harvested from the eyes 1 day after a corneal injury have a significantly higher proliferative activity than limbal cells from the healthy eyes. To identify the molecules responsible for the activation of cell proliferation and differentiation, we cultured limbal cells in the presence of factors that had been found to be produced by cells of the damaged corneal epithelium. Among these factors, IGF-I was the only one that induced the expression of the gene for K12, a marker of terminally differentiated corneal epithelial cells. This marker is not expressed by limbal cells and has been used in previous studies to monitor the transdifferentiation of stem cells cultured in corneal cell supernatants into corneal epithelial cells [20–23]. Since IGF-I is produced by corneal epithelial cells rapidly after an injury, it may be the main candidate molecule responsible for the activation and differentiation of LSC into corneal epithelial cells. The production of IGF-I and IGF-II by corneal cells has been documented [14,30], and their multiple effects on ocular surface cells have been demonstrated [13–16,30]. Our study, thus extends the list of IGF functions, and we suggest that IGF-I is the main factor produced by the wounded cornea that induces the activation and differentiation of LSC into cells expressing corneal epithelial markers.
IGF-I is a member of the IGF signaling pathway, which regulates a number of cellular functions [17]. The system involves peptide hormones (IGF of type I and II), cell surface receptors (insulin receptor, IGF receptors of type I and II), and circulating binding proteins (IGF binding proteins of type 1–6). IGF-I is produced by the liver as a growth hormone and is synthesized also locally by many cell types under basal conditions and in response to inflammatory signals [31,32]. The effects of IGF-I and IGF-II are mediated through the IGF-I receptor, which initiates signaling cascades leading to regulation of a number of biological processes.
Our results also showed that the expression of IGF receptors in corneal cells is significantly downregulated after corneal injury. This mechanism can prevent the binding of locally synthesized IGF to corneal cells, and thus supports the penetration of IGF into the limbal region, where it stimulates the expression of IGF receptors and LSC differentiation. We demonstrated that supernatants from cultures of corneal cells stimulate IGF-IR and IGF-IIR expression in limbal cells, and IGF-I was identified as the factor that stimulates the expression of its own receptors in limbal cells. We also showed that IGF-I present in supernatants from corneal cell cultures is a molecule responsible for differentiation of limbal cells into cells expressing K12. The neutralization antibody anti-IGF-I completely abrogated the differential potential of corneal cell supernatants.
Our study also showed that IGF-I induces limbal cell differentiation, but it has no effect on limbal cell proliferation in the mouse. Previous studies have demonstrated that IGF-I and IGF-II can support the proliferation of keratinocytes [13] and, together with substance P, promote the growth of rabbit corneal epithelial cells [33,34]. A similar growth-promoting effect of KGF and HGF on limbal epithelial cells in the rabbit was described by Cheng et al. [10]. In our mouse model, IGF-I, which induced the differentiation of LSC, did not support the proliferation of limbal cells, even though it was tested over a wide range of concentrations. Similarly, KGF also had only a weak promoting effect on limbal cell proliferation. In contrast, 2 other factors produced by corneal epithelial cells after injury, EGF and FGF-β, stimulated the significant proliferation of limbal cells although they did not induce K12 expression. Thus, distinct factors regulate limbal cell proliferation and differentiation in the mouse.
It has been recently shown that IGF significantly improves the differentiation of MSC into hepatocyte-like cells [35], promotes the myogenic differentiation of mouse myoblasts [36,37], enhances the insulinogenic differentiation of human eyelid adipose stem cells [38], and supports the activation and differentiation of human cardiac stem cells [39,40]. Our results suggest that the differentiation-promoting activities of IGF are more general and also involve effects on stem cell differentiation in the eye.
The identification of differentiation and growth factors for LSC may have an impact on the targeted differentiation of stem cells for therapeutic purposes. Recently, the cultivation of LSC in vitro and their transfer onto the damaged ocular surface has become a prospective way to treat severe corneal injuries and LSC deficiencies [7,41]. The implementation of appropriate factors into cultures of LSC may increase the yield of the appropriate cell type for reparative and regenerative medicine.
Acknowledgments
This work was supported by grants P304/11/0653, P301/11/1568, and 310/08/H077 from the Grant Agency of the Czech Republic, grant KAN200520804 from the Grant Agency of the Academy of Sciences, projects MSM0021620858 and SVV 265211 from the Ministry of Education of the Czech Republic, and project RVO 68378050 from the Academy of Sciences of the Czech Republic.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Majo F. Rochat A. Nicolas M. Jaoude GA. Barrandon Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456:250–254. doi: 10.1038/nature07406. [DOI] [PubMed] [Google Scholar]
- 2.Chang C-Y. Green CR. McGhee CNJ. Sherwin T. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Invest Ophthalmol Vis Sci. 2008;2008;49:5279–5286. doi: 10.1167/iovs.07-1260. [DOI] [PubMed] [Google Scholar]
- 3.Dua HS. Miri A. Alomar T. Yeung AM. Said DG. The role of limbal stem cells in corneal epithelial maintenance: testing the dogma. Ophthalmology. 2009;116:856–863. doi: 10.1016/j.ophtha.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Tseng SC. Regulation and clinical implication of corneal epithelial stem cells. Mol Biol Rep. 1996;23:47–58. doi: 10.1007/BF00357072. [DOI] [PubMed] [Google Scholar]
- 5.Gomes JA. dos Santos MS. Cunha MC. Mascaro VL. Barros JN. de Sousa LB. Amniotic membrane transplantation for partial and total limbal stem cell deficiency secondary to chemical burn. Ophthalmology. 2003;110:466–473. doi: 10.1016/s0161-6420(02)01888-2. [DOI] [PubMed] [Google Scholar]
- 6.Du Y. Chen J. Funderburgh JL. Zhu X. Li L. Functional reconstruction of rabbit corneal epithelium by human limbal cells cultured on amniotic membrane. Mol Vis. 2003;9:635–643. [PMC free article] [PubMed] [Google Scholar]
- 7.Rama P. Matuska S. Paganoni G. Spinelli A. De Luca M. Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 8.Lehrer MS. Sun TT. Lavker RM. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J Cell Sci. 1998;111:2867–2875. doi: 10.1242/jcs.111.19.2867. [DOI] [PubMed] [Google Scholar]
- 9.Jia C. Zhu W. Ren S. Xi H. Li S. Wang Y. Comparison of genome-wide gene expression in suture- and alkali burn-induced murine corneal neovascularization. Mol Vis. 2011;17:2386–2399. [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng CC. Wang DY. Kao MH. Chen JK. The growth-promoting effect of KGF on limbal epithelial cells is mediated by upregulation of DeltaNp63alpha through the p38 pathway. J Cell Sci. 2009;122:4473–4480. doi: 10.1242/jcs.054791. [DOI] [PubMed] [Google Scholar]
- 11.Li DQ. Tseng SC. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995;163:64–79. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- 12.Wilson SE. He YG. Weng J. Zieske JD. Jester JV. Schultz GS. Effect of epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor, on proliferation, motility and differentiation of human corneal epithelial cells. Exp Eye Res. 1994;59:665–678. doi: 10.1006/exer.1994.1152. [DOI] [PubMed] [Google Scholar]
- 13.Jester JV. Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77:581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- 14.Ko JA. Yanai R. Nishida T. IGF-1 released by corneal epithelial cells induces up-regulation of N-cadherin in corneal fibroblasts. J Cell Physiol. 2009;221:254–261. doi: 10.1002/jcp.21850. [DOI] [PubMed] [Google Scholar]
- 15.Hassell JR. Birk DE. The molecular basis of corneal transparency. Exp Eye Res. 2010;91:326–335. doi: 10.1016/j.exer.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etheredge L. Kane BP. Hassell JR. The effect of growth factor signaling on keratocytes in vitro and its relationship to the phases of stromal wound repair. Invest Ophthalmol Vis Sci. 2009;50:3128–3136. doi: 10.1167/iovs.08-3077. [DOI] [PubMed] [Google Scholar]
- 17.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2009;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 18.Liu CY. Zhu G. Converse R. Kao CW. Nakanuta H. Tseng SC. Mui MM. Seyer J. Justice MJ. Stech ME, et al. Characterization and chromosomal localization of the cornea-specific murine keratin gene Krt1.12. J Biol Chem. 1994;269:24627–24636. [PubMed] [Google Scholar]
- 19.Matic M. Petrov IN. Chen S. Wang C. Dimitrijevich SD. Wolosin JM. Stem cells of the corneal epithelium lack connexins and metabolite transfer capacity. Differentiation. 1997;61:251–260. doi: 10.1046/j.1432-0436.1997.6140251.x. [DOI] [PubMed] [Google Scholar]
- 20.Blazejewska EA. Schlötzer-Schrehardt U. Zenkel M. Bachmann B. Chankiewitz E. Jacobi C. Kruse FE. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells. 2009;27:642–652. doi: 10.1634/stemcells.2008-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu S. Xing C. Han J. Tso MO. Hong J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol Vis. 2009;15:99–107. [PMC free article] [PubMed] [Google Scholar]
- 22.Homma R. Yoshikawa H. Takeno M. Kurokawa MS. Masuda C. Takada E. Tsubota K. Ueno S. Suzuki N. Induction of epithelial progenitors in vitro from mouse embryonic stem cells and application for reconstruction of damaged cornea in mice. Invest Ophthalmol Vis Sci. 2004;45:4320–4326. doi: 10.1167/iovs.04-0044. [DOI] [PubMed] [Google Scholar]
- 23.Jiang TS. Cai L. Ji WY. Hui YN. Wang YS. Hu D. Zhu J. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol Vis. 2010;16:1304–1316. [PMC free article] [PubMed] [Google Scholar]
- 24.Krulova M. Pokorna K. Lencova A. Zajicova A. Fric J. Filipec M. Forrester JV. Holan V. A rapid separation of two distinct populations of corneal epithelial cells with limbal stem cell characteristics in the mouse. Invest Ophthalmol Vis Sci. 2008;49:3903–3908. doi: 10.1167/iovs.08-1987. [DOI] [PubMed] [Google Scholar]
- 25.Holan V. Pokorna K. Prochazkova J. Krulova M. Zajicova A. Immunoregulatory properties of mouse limbal stem cells. J Immunol. 2010;184:2124–2129. doi: 10.4049/jimmunol.0903049. [DOI] [PubMed] [Google Scholar]
- 26.Svobodova E. Krulova M. Zajicova A. Prochazkova J. Trosan P. Holan V. The role of mouse mesenchymal stem cells in differentiation of naive T cells into anti-inflammatory regulatory T cell and proinflammatory helper T-cell 17 population. Stem Cells Dev. 2012;21:901–910. doi: 10.1089/scd.2011.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schermer A. Galvin S. Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W. Hayashida Y. Chen Y-T. Tseng SCG. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007;17:26–36. doi: 10.1038/sj.cr.7310137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad S. Stewart R. Yung S. Kolli S. Armstrong L. Stojkovic M. Figueiredo F. Lako M. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells. 2007;5:1145–1155. doi: 10.1634/stemcells.2006-0516. [DOI] [PubMed] [Google Scholar]
- 30.Izumi K. Kurosaka D. Iwata T. Oguchi Y. Tanaka Y. Mashima Y. Tsubota K. Involvement of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in corneal fibroblasts during corneal wound healing. Invest Ophthalmol Vis Sci. 2006;47:591–598. doi: 10.1167/iovs.05-0097. [DOI] [PubMed] [Google Scholar]
- 31.Maiter D. Underwood LE. Maes M. Ketelslegers JM. Acute downregulation of the somatogenic receptors in rat liver by a single injection of growth hormone. Endocrinology. 1988;122:1291–1296. doi: 10.1210/endo-122-4-1291. [DOI] [PubMed] [Google Scholar]
- 32.Smith TJ. Insuline-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacol Rev. 2010;62:199–236. doi: 10.1124/pr.109.002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura M. Chikama TI. Nishida T. Characterization of insulin-like growth factor-1 receptors in rabbit corneal epithelial cells. Exp Eye Res. 2000;70:199–204. doi: 10.1006/exer.1999.0775. [DOI] [PubMed] [Google Scholar]
- 34.Yamada N. Yanai R. Nakamura M. Inui M. Nishida T. Role of the C domain of IGFs in synergistic promotion, with a substance P-derived peptide, of rabbit corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2004;45:1125–1131. doi: 10.1167/iovs.03-0626. [DOI] [PubMed] [Google Scholar]
- 35.Ayatollahi M. Soleimani M. Geramizadeh B. Imanieh MH. Insulin-like growth factor 1 (IGF-I) improves hepatic differentiation of human bone marrow-derived mesenchymal stem cells. Cell Biol Int. 2011;35:1169–1176. doi: 10.1042/CBI20110016. [DOI] [PubMed] [Google Scholar]
- 36.Matheny RW., Jr. Nindl BC. Loss of IGF-IEa or IGF-IEb impairs myogenic differentiation. Endocrinology. 2011;152:1923–1934. doi: 10.1210/en.2010-1279. [DOI] [PubMed] [Google Scholar]
- 37.Shima A. Pham J. Blanco E. Barton ER. Sweeney HL. Matsuda R. IGF-I and vitamin C promote myogenic differentiation of mouse and human skeletal muscle cells at low temperatures. Exp Cell Res. 2011;317:356–366. doi: 10.1016/j.yexcr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Kang HM. Park S. Kim H. Insulin-like growth factor 2 enhances insulinogenic differentiation of human eyelid adipose stem cells via the insulin receptor. Cell Prolif. 2011;44:254–263. doi: 10.1111/j.1365-2184.2011.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D'Amario D. Cabral-Da-Silva MC. Zheng H. Fiorini C. Goichberg P. Steadman E. Ferreira-Martins J. Sanada F. Piccoli M, et al. Insulin-like growth factor-1 receptor identifies a pool of human cardiac stem cells with superior therapeutic potential for myocardial regeneration. Circ Res. 2011;108:1467–1481. doi: 10.1161/CIRCRESAHA.111.240648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Ellison GM. Torella D. Dellegrottaglie S. Perez-Martinez C. Perez de Prado A. Vicinanza C. Purushothaman S. Galuppo V. Iaconetti C, et al. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol. 2011;58:977–986. doi: 10.1016/j.jacc.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 41.Zajicova A. Pokorná K. Lencova A. Krulova M. Svobodova E. Kubinova S. Sykova E. Pradny M. Michalek J, et al. Treatment of ocular surface injuries by limbal and mesenchymal stem cells growing on nanofiber scaffolds. Cell Transplant. 2010;19:1281–1290. doi: 10.3727/096368910X509040. [DOI] [PubMed] [Google Scholar]