Abstract
Wnt/β-catenin signaling can influence the proliferation and differentiation of progenitor populations in the hippocampus and subventricular zone, known germinal centers in the adult mouse brain. It is not known whether β-catenin signaling occurs in quiescent glial progenitors in cortex or spinal cord, nor is it known whether β-catenin is involved in the activation of glial progenitor populations after injury. Using a β-catenin reporter mouse (BATGAL mouse), we show that β-catenin signaling occurs in NG2 chondroitin sulfate proteoglycan+ (NG2) progenitors in the cortex, in subcallosal zone (SCZ) progenitors, and in subependymal cells surrounding the central canal. Interestingly, cells with β-catenin signaling increased in the cortex and SCZ following traumatic brain injury (TBI) but did not following spinal cord injury. Initially after TBI, β-catenin signaling was predominantly increased in a subset of NG2+ progenitors in the cortex. One week following injury, the majority of β-catenin signaling appeared in reactive astrocytes but not oligodendrocytes. Bromodeoxyuridine (BrdU) paradigms and Ki-67 staining showed that the increase in β-catenin signaling occurred in newly born cells and was sustained after cell division. Dividing cells with β-catenin signaling were initially NG2+; however, by four days after a single injection of BrdU, they were predominantly astrocytes. Infusing animals with the mitotic inhibitor cytosine arabinoside prevented the increase of β-catenin signaling in the cortex, confirming that the majority of β-catenin signaling after TBI occurs in newly born cells. These data argue for manipulating the Wnt/β-catenin pathway after TBI as a way to modify post-traumatic gliogenesis.
Keywords: Wnt, Neural stem cell, Gliosis, Brain injury, Spinal cord injury
Introduction
Wnt/β-catenin signaling influences the proliferation and/or differentiation of various stem cell and progenitor populations, such as skin [1], hematopoietic [2], intestinal [3], and embryonic stem cells [4, 5]. During nervous system development, β-catenin signaling drives the proliferation of neuronal stem cells leading to the correct number of neurons in the developing embryo [6]. β-catenin signaling has also been shown to be important in progenitor populations in the adult brain, specifically for neurogenesis in the subgranular zone (SGZ) and subventricular zone (SVZ) [7, 8]. It is unknown, however, if β-catenin signaling occurs in glial progenitor populations in the adult brain, specifically NG2 chondroitin sulfate proteoglycan+ (NG2) progenitors and those residing in the subcallosal zone (SCZ).
Injury to the brain and spinal cord results in gliosis due to intense astrocyte hypertrophy and an increase in the number of reactive astrocytes following injury [9, 10]. The origin of reactive astrocytes following injury has been under intense debate with different labs proposing NG2+ cells [11, 12], SVZ cells [13], and dedifferentiated astrocytes [14] as the source of these newly born cells. In addition to their unknown origin, the signaling pathways that instruct the response of reactive astrocytes are unclear. Recent research has begun to implicate the transcriptional activator signal transducers and activators of transcription three (STAT3) [15] and the extracellular signal-regulated kinase (ERK) mitogen-activated protein kinase (MAPK) signaling pathway [16] in this process. Understanding the signaling pathways that contribute to reactive gliosis is crucial for developing therapies that promote wound healing and regeneration.
In part due to its role in stem cell populations, β-catenin signaling has been shown to be important in the response to injury of various tissues. Recently, we and others have shown that β-catenin signaling is required for fin regeneration in zebrafish [17] and enhances liver regeneration in mice after hepatectomy [18]. Other work has shown that β-catenin signaling is necessary for hair follicle regeneration [19] and retinal regeneration [20]. The aim of the present study was to determine if β-catenin signaling occurs in glial progenitors in the adult brain and if it participates in the glial response to injury in the nervous system.
Methods
Animals
Three-month-old male and 4-month-old female BATGAL mice [21] were used for traumatic brain injury (TBI) studies and spinal cord injury (SCI) experiments, respectively. All animal-related procedures were approved by the Institutional Animal Care and Use Committee of the University of Washington and were conducted in accordance with the guidelines of the NIH.
Traumatic Brain Injury
Littermates from nine litters (n = 43) were divided across four groups: control, 3 days post-injury (dpi), 7 dpi, and 28 dpi. Mice (n = 32) were anesthetized with intraperitoneal (i.p.) injections of avertin (12.6% tribromoethanol in 0.6% tert-amyl alcohol), a midline scalp incision was made, soft tissues were reflected, and a 3.5 mm by 3.5 mm craniotomy was performed, centered 2 mm left of midline, halfway between bregma and lambda. A 2-mm metal probe attached to the Ohio State University contusion device (Ling Dynamic Systems, http://www.lds-group.com) was gently lowered onto the dura until contact was visualized by the dampening of oscillations on an oscilloscope. Then brain deformation (mild TBI) of 1.4 mm at a velocity of 4.3 m/second was performed. After injury, the craniotomy was filled with Gelfoam (Upjohn, Kalamazoo, MI, http://www.pfizer.com); the skin was closed with a combination of sutures and staples. Animals were kept on a heating pad (38°C) until fully awake. Postoperatively, lactated Ringer’s solution and analgesic (buprenorphine, Reckitt Benckiser Healthcare, Hull, U.K., http://www.rb.com) were administered as needed. Control mice (n = 11) were anesthetized only.
Spinal Cord Injury
Mice from two litters were divided into control (n = 4) and injured (n = 5) groups. Injured mice, anesthetized as above, underwent a midthoracic (T9) laminectomy; iridectomy scissors were used to make a hemisection lesion by cutting the dorsal spinal cord tissue until the central canal (~0.3 mm deep). After injury, muscle and skin were closed in layers and postoperative care was as above. Control mice were anesthetized only.
Intercerebroventricular Infusion of Cytosine Arabinoside
For the cytosine arabinoside (AraC) experiments, mice (n = 11) received a TBI as above. After recovery of hemostasis, a surgical drill was used to make a small hole at −0.34 mm from bregma, 1.00 mm right of the central sulcus, contralateral to the TBI. The outlet of an Alzet osmotic minipump (model 1007D, Durect Corporation, Cupertino, CA, http://www.durect.com) was secured to the skull with superglue, and the body of the pump was placed subcutaneously behind the shoulder blades of the mouse. This pump delivered either cytosine arabinoside (AraC) (40 ug/day) in artificial cerebral spinal fluid (aCSF = 150 mM Na, 3 mM K, 1.4 mM Ca, 0.8 mM Mg, 1 mM P, 155 mM Cl, 1 mg/ml of bovine serum albumin (BSA) (Sigma, St. Louis, MO, http://www.sigmaaldrich.com) or aCSF only at a rate of 0.5 μl/day for 7 days until tissue was collected as below. A researcher blind to the TBI decided which animals received AraC (n = 6) or aCSF (n = 5). Postoperative care was as above.
Bromodeoxyuridine Injections
There were two bromodeoxyuridine (BrdU) injection paradigms used in these experiments. For analyzing proliferation in the uninjured SCZ (Fig. 1) (n = 4), i.p. injections of BrdU (50 mg/kg; seven injections total) were given once every 2 hours [22] and the mice were processed for immunohistochemistry 2 hours after the last injection. For analyzing proliferation after TBI (n = 32), a single i.p. injection of BrdU (200 mg/kg) was given on the third day postinjury. Control mice (n = 11) were given a single i.p. injection of BrdU (200 mg/kg) three days after being anesthetized in the exact same manner as the other experimental groups. Animals were processed for immunohistochemistry 2 hours (control and day 3 time point), 4 days (day 7 time point), or 25 days (day 28 time point) after the BrdU injection.
Figure 1.
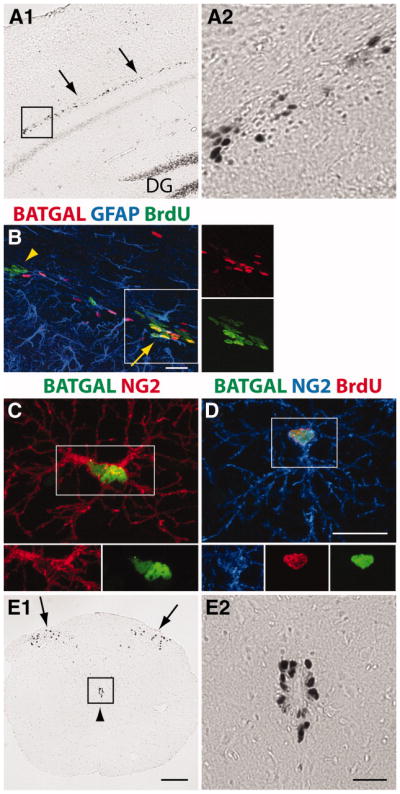
β-catenin signaling is a characteristic of progenitors in the nervous system. (A1–A2): X-gal reaction in a coronal brain section showing expression of a β-catenin signaling (BATGAL) reporter in cells of the subcallosal zone (SCZ) (arrows). (A2): Higher magnification of inset. (B): Immunohistochemistry showing colocalization of the BATGAL reporter (antibody against β-galactosidase shown in red) in a portion of BrdU+ (green) cells in the SCZ of an adult mouse (arrow). Arrowhead designates a cluster of BrdU+ cells that are BATGAL−. Some BATGAL+ cells are immunopositive for GFAP (blue). (C): Colocalization of two NG2+ (red) cells in the cortex with β-catenin signaling (green). (D): A small subpopulation of BrdU+ (red) NG2+ (blue) cells in the cortex of adult mice has β-catenin signaling (green). (E1–E2): X-gal reaction showing expression of BATGAL reporter in cells in the substantia gelatinosa (arrows) and in cells surrounding the central canal (arrowhead). (E2): Higher magnification of inset. Scale bars: (A1, E1) 200 μm, (A2, E2) 50 μm, (B–D) 20 μm. Abbreviations: DG, dentate gyrus; BrdU, bromodeoxyuridine; GFAP, glial fibrillary acidic protein.
Tissue Collection
Animals were deeply anesthetized and perfused transcardially with 10 ml of 0.9% saline followed by 50 ml of 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB, pH 7.4). Brains and spinal cords were removed and post-fixed in PFA for 10 hours at 4°C, rinsed in PB, and transferred to 30% sucrose in PB for 36–48 hours of cryoprotection. Spinal cords were cut into 1 mm sections surrounding the lesion; three of these sections were embedded in OCT medium and flash frozen for cryosectioning. Serial 20 μm coronal spinal cord sections were mounted on Permafrost plus slides (Thermo Fisher Scientific, Waltham, MA, http://www.thermo.com) in a one-in-six series and stored at −80°C. Brains were mounted on a sliding microtome and cut into 50 μm coronal sections. These free-floating sections were collected in a one-in-six series, placed in cryoprotectant (30% glycerol, 30% ethylene glycol in 0.1 M PB), and stored at −20°C.
X-Gal Reaction
β-Galactosidase was visualized using the staining kit and protocol from Specialty Media (Millipore, Billerica, MA, http://www.millipore.com).
Immunohistochemistry
Free floating sections were rinsed 3× in phosphate buffered saline (PBS) and put in a block solution consisting of 5% goat serum (Sigma, St. Louis, MO, http://www.sigmaaldrich.com) and 0.6% Triton X-100 (Amresco, Solon, OH, http://www.amresco-inc.com) in PBS for 1 hour. Sections processed for anti-BrdU staining were first incubated with 1.5N HCl for 25 minutes at 37°C. These sections were rinsed 8× in PBS over 30 minutes before being placed in block. Primary antibody cocktails were made in block using combinations of the following antibodies: rabbit anti-β-galactosidase (1:2000, Cappel Antibodies, ICN Biomedical, Aurora, CA, http://www.valeant.com/), mouse anti-β-galactosidase (1:200, Promega, Madison, WI, http://www.promega.com), rat anti-BrdU (bromodeoxyuridine, 1:300, Novus Biologicals, Littleton, CO, http://www.novusbio.com), guinea pig anti-GFAP (glial fibrillary acidic protein; 1:2000, Advanced ImmunoChemical Inc. Long Beach, CA, http://www.advimmuno.com), rabbit anti-NG2 (chondroitin sulfate proteoglycan; 1:500, a generous gift from W. Stallcup, Burnham Institute, http://www.burnham.org), rabbit anti-GSTπ (glutathione s-transferase π; 1:1250, Millipore, Billerica, MA, http://www.millipore.com), rabbit anti-Ki-67 (1:750, AbD Serotec, Raleigh, NC, http://www.abdserotec.com), and goat anti-nestin (1:750, Santa Cruz Biotechnology, Santa Cruz, CA, http://www.scbt.com). After incubating with primary antibodies for 24–48 hours at 4°C, sections were rinsed 8× over 12 hours with 0.1% Triton X-100 (PBST, pH 7.4), incubated for 2 hours at room temperature with the appropriate secondary antibodies: anti-rat 488, -mouse 488, -rabbit 647, 594, or -guinea pig 647 (1:400, all from Invitrogen, Carlsbad, CA, http://www.invitrogen.com), and rinsed 8× over 6 hours with PBST. The last rinse was in PB and included the nuclear marker, 4′,6-diamidino-2-phenylindole (1:1000). Sections were mounted on Permafrost plus slides and coverslipped with gelvatol. Slides with spinal cord sections were treated as above except with shorter washing periods.
Cell and Molecular Phenotype Quantification
Serial coronal sections of brain, each spaced 250 μm apart, were mounted on slides. For BATGAL+ cell quantification, every BATGAL+ cell was counted in one 50 μm section at Bregma −1.58 ± 0.125 mm (n = 8–11 animals per group). Two contours were used in our analysis: (1) A cortex contour was drawn around the cortex with the dorsal edge of the corpus callosum as the ventral boundary medially. A horizontal line extending from the dorsal edge of the lateral ventricle provided the ventral boundary of the cortex contour laterally (supporting information Fig. 1). (2) A SCZ contour stretched from the dorsal edge of the corpus callosum to the dorsal edge of the CA1 pyramidal cell layer of the hippocampus, with the lateral ventricle and midline as lateral and medial borders, respectively (supporting information Fig. 1). We refer to this region as the SCZ contour, although it includes the corpus callosum, SCZ, and the dorsal aspect of the hippocampus. The SCZ contour does not include the SVZ because of its posterior location. Cells within these contours were counted using Stereo Investigator (MicroBrightField, Williston, VT, http://www.mbfbioscience.com). Cell density was calculated using the following equation: . For BATGAL+ cell phenotyping, multiple label immunofluorescent confocal z-stack images were collected and analyzed with a confocal microscope (Nikon Eclipse TE200 or Nikon A1R confocal microscope, Nikon Instruments Inc., Melville, NY, http://www.nikon.com). A minimum of 50 cells (mean of 100) were counted across 1–4 sections with four animals per group. For BATGAL+ BrdU+ cell phenotyping, a minimum of 25 cells (mean of 50) were counted across 2–4 sections with 4–9 animals per group. Cell density of phenotyped BATGAL+ cells was estimated using the following equation: .
Statistical Analysis
Differences among experimental groups were evaluated by a one-way ANOVA followed by Tukey’s Multiple Comparison Test. An unpaired Student’s t-test evaluated differences in cell phenotypes and differences between AraC and aCSF infusions. For all statistical analyses, significance was accepted at a p-value of 0.05. Error bars in graphs are SEM.
Results
β-Catenin Signaling Occurs in Progenitor Populations of the Uninjured Nervous System
Areas of high β-catenin signaling in the uninjured adult brain were found using the BATGAL reporter mouse [21]. Upon transduction of a Wnt signal, β-catenin is stabilized in the cytoplasm, translocates to the nucleus, and binds to TCF/LEF transcription factors converting them to activators of β-catenin dependent transcription [23]. With LacZ driven by a siamois promoter and seven T-cell factor/lymphoid enhancer factor (TCF/LEF) binding sites, the BATGAL mouse allows the visualization of cells with transcriptionally active β-catenin signaling through the expression of nuclear localized β-galactosidase [8, 24, 25]. BATGAL+ cells are defined as cells with intense nuclear β-galactosidase staining, indicating transcriptionally active β-catenin.
Confirming previous studies, BATGAL+ cells were found in the SGZ of the hippocampus and the SVZ, two known germinal centers in the adult brain [7, 8] (data not shown). Interestingly, BATGAL+ cells were also found in a band just below the corpus callosum (Fig. 1A1–1A2, arrows). This band of cells appears to be in a similar location as progenitors of the SCZ, a third germinal center in the adult brain [22]. Injecting BrdU, we find BrdU+ nuclei colocalized with BATGAL+ nuclei, indicating that a portion of the newly dividing cells had active β-catenin signaling (Fig. 1B, arrow). Some clusters of BrdU+ cells had a high degree of BATGAL+ colocalization and some clusters were devoid of any staining for the β-catenin signaling reporter (arrowhead). We quantified that 20% of BATGAL+ cells in the SCZ were BrdU+. Many of the nondividing BATGAL+ cells were glial fibrillary acidic protein (GFAP) labeled astrocytes.
A few BATGAL+ cells with high β-galactosidase expression were also found scattered throughout the cortex (Fig. 1C). These BATGAL+ cells colocalized with antibodies against the NG2 proteoglycan. We estimate ~2% of NG2+ cells in the cortex colocalized with the BATGAL+ signal, and a few of these NG2+BATGAL+ cells were also positive for BrdU (Fig. 1D). These data indicate that β-catenin signaling occurs in a subset of NG2+ cells in the cortex and is sometimes coincident with cell division.
Examination of the spinal cord of BATGAL reporter mice revealed two major areas of β-catenin signaling, the substantia gelatinosa and the area surrounding the central canal (Fig. 1E1–1E2). In contrast to the findings in the cortex, active β-catenin signaling was not observed in NG2+ cells in the spinal cord.
These data expand the known progenitor populations in the adult nervous system that exhibit active β-catenin signaling. Given that these progenitor populations are known to become activated and proliferate after injury [11, 26], the response of cells with β-catenin signaling after injury was examined next.
β-Catenin Signaling Increases After Traumatic Brain Injury but Not After Spinal Cord Injury
To determine the distribution and frequency of cells with active β-catenin signaling following injury, BATGAL reporter mice were subjected to a mild traumatic brain injury (TBI). Littermates comprised four groups: uninjured controls and animals harvested at 3, 7, and 28 days post-injury (dpi). Coronal histological sections were divided into two areas for analysis: (1) the cortex and (2) an area including the corpus collosum, SCZ, and dorsal hippocampus above the CA1 pyramidal layer (supporting information Fig. 1). The corpus callosum and the dorsal hippocampus were included in the analysis of the SCZ because previous studies have proposed that these progenitors may migrate either toward the cortex or toward the hippocampal CA1 pyramidal layer after injury [13, 27].
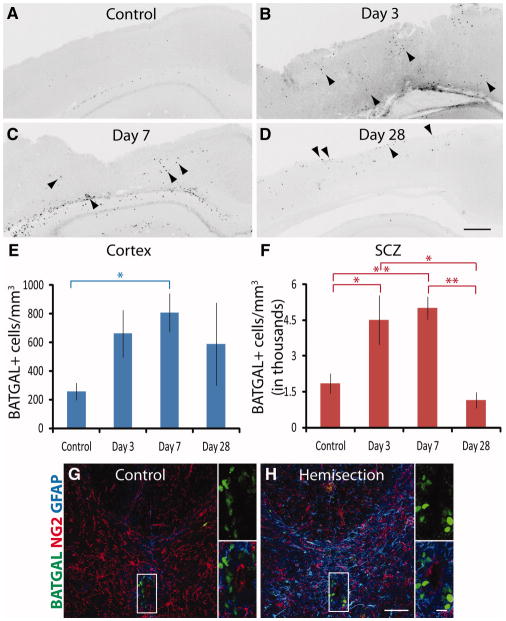
BATGAL+ cells increased in the cortex and in the SCZ following TBI (Fig. 2A–2F). Three days post-injury, BATGAL+ cells were increased throughout the injured cortex (Fig. 2B, arrowheads). By day 7, the number of BATGAL+ cells in the cortex and SCZ had increased and cells were often clustered together (Fig. 2C, arrowheads). The majority of β-catenin signaling was near the dorsal surface of the cortex by day 28 (Fig. 2D, arrowheads). The increase in BATGAL+ cells in the cortex became statistically significant at day 7 and remained elevated at day 28 in some animals (Fig. 2E). BATGAL+ cells surrounding the SCZ increased significantly 3 and 7 dpi and fell at or below control levels at 28 dpi. Using mean values for control and day 7 animals, BATGAL+ cells increased 3.3-fold in the cortex and 2.7-fold in the SCZ.
Figure 2.
β-catenin signaling increases in cortex and subcallosal zone after traumatic brain injury but not after spinal cord injury. (A–D): Coronal sections from mouse brain immunolabeled with an antibody against the β-catenin reporter in control animals and at 3, 7, and 28 days post-injury. Arrowheads indicate examples of cells positive for the reporter. (E): Quantification of cells in the cortex shows a significant increase of β-catenin signaling at day 7 (*, p < .05). (F): Quantification of cells in the subcallosal zone shows significant increases of β-catenin signaling at day 3 and day 7 (*, p < .05; **, p < .01). For (E) and (F), an ANOVA followed by Tukey’s Multiple Comparison Test was performed for all time points in the cortex and subcallosal zone (n = 8–12 mice per group). (G–H): Coronal sections of spinal cord from control and hemisection animals immunolabeled with antibodies against the BATGAL reporter (green), NG2 (red), and GFAP (blue) (n = 4–8 mice). Smaller panels are higher magnification views of the insets. Scale bars: (A–D) 300 μm, (G–H) 50 μm, insets 10 μm.
Abbreviations: GFAP, glial fibrillary acidic protein; SCZ, subcallosal zone.
To determine if β-catenin signaling increases in other parts of the nervous system after injury, BATGAL reporter mice received a dorsal spinal hemisection injury (SCI) (Fig. 2G–2H). Although BATGAL+ cells increased after TBI, BATGAL+ cells did not increase in the spinal cord or subependymal zone (inset) after SCI.
β-Catenin Signaling Occurs in NG2+ and GFAP+ Cells in the Cortex After TBI
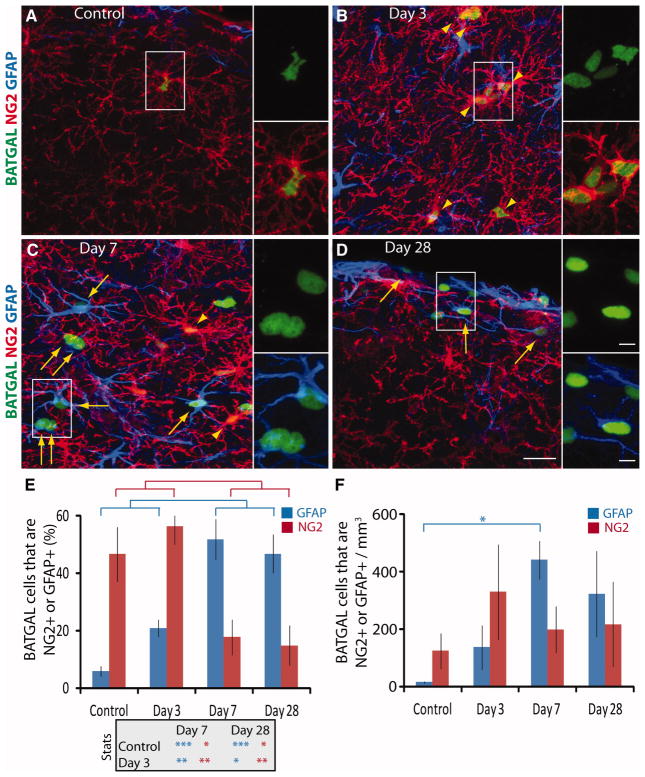
Immunofluorescence showed that the majority of BATGAL+ cells were NG2+ (arrowheads) or GFAP+ (arrows) after TBI (Fig. 3A–3D). The intensity of NG2 staining and the density of NG2 cells increased between control and day 3, concomitant with an increase in BATGAL+ NG2+ cells (Fig. 3A–3B, arrowheads). These cells were located either directly in the injury core (cortex under the injury site that is surrounded by hypertrophied GFAP+ astrocytes) or they were located among hypertrophied GFAP+ astrocytes surrounding the core. NG2+ BATGAL+ cells in the injury core often appeared to be wrapped around the vasculature and were considered pericytes, similar to observations by others [28, 29]. NG2+ BATGAL+ cells in the parenchyma surrounding the core were polydendritic with multiple branched processes (arrowheads). By 7 dpi, the majority of BATGAL+ cells colabeled with antibodies against GFAP. These GFAP+ BATGAL+ astrocytes often appeared as couplets surrounding the injury core (Fig. 3C, arrows). GFAP+ BATGAL+ astrocytes were also found in day 28 animals, often near the dorsal edge of the injured cortex (Fig. 3D, arrows).
Figure 3.
The majority of β-catenin reporter cells in the cortex shift from being NG2+ in control and day 3 animals to being GFAP+ in day 7 and 28 animals. (A–D): Representative images of triple immunofluorescence for NG2+ (red), GFAP+ (blue), and the BATGAL reporter (green), with arrowheads pointing to NG2+ cells and arrows pointing to GFAP+ cells that are BATGAL+. Smaller panels are higher magnification views of the insets. (E): Quantification shows significant differences in the percentage of BATGAL+ cells that are either NG2+ or GFAP+ over time (*, p < .05; **, p < .01; ***, p < .001), with a transition from a greater percentage of NG2+ BATGAL+ cells at day 3 to a greater percentage of GFAP+ BATGAL+ cells at day 7. (F): Calculating the total number of BATGAL+ cells that are either NG2+ or GFAP+ using information from (E) and stereological counts from Figure 2E shows a significant increase in GFAP+ cells with β-catenin signaling at day 7 (*, p < .05). An ANOVA followed by Tukey’s Multiple Comparison Test was performed for all time points (n = 4). Scale bars: 20 μm, 5 μm insets. Abbreviations: GFAP, glial fibrillary acidic protein.
Quantification of the phenotype of BATGAL+ cells showed the majority of BATGAL+ cells were NG2+ in control and day 3 animals; however, the phenotype of the majority of BATGAL+ cells shifted to GFAP+ astrocytes in day 7 and day 28 animals (Fig. 3E). Specifically, the percentage of BATGAL+ cells that were NG2+ decreased from 56 ± 6% to 18 ± 6%, whereas the percentage of BATGAL+ cells that were GFAP+ increased from 21 ± 3% to 52 ± 7% between day 3 and day 7. Interestingly, the actual number of BATGAL+ NG2+ cells stayed constant during this time despite a decrease in the relative percentage of BATGAL+ cells that were NG2+ (Fig. 3F). On the other hand, the number of GFAP+ BATGAL+ cells increased >5-fold between days 3 and 7. This indicates that the decrease in the percentage of NG2+ BATGAL+ cells is due to an increase in total BATGAL+ cells, the majority of which are GFAP+. Although β-catenin signaling occurred in NG2+ cells and Olig2+ cells (supporting information Fig. 2A–2C), it did not occur in mature oligodendrocytes as analyzed with antibodies against glutathione s-transferase π (GSTπ) (supporting information Fig. 2D–2E).
β-Catenin Signaling Occurs in Newly Born Cells After TBI
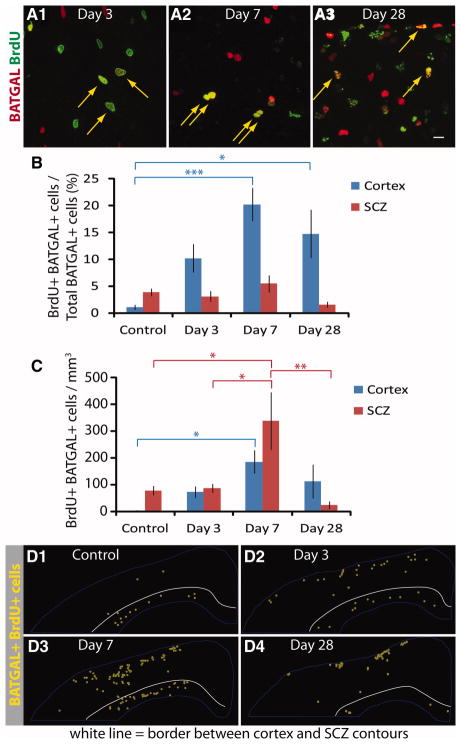
The increase in β-catenin signaling after TBI could occur in post-mitotic cells, or it could be due to newly born cells signaling through β-catenin. To examine this directly, BrdU was injected once at 3 dpi to label proliferating cells; animals were fixed 2 hours, 4 days, or 25 days after the injection. BrdU+ BATGAL+ colocalized cells were found at all time points after injury (Fig. 4A1–4A3, arrows). BrdU+ BATGAL+ cells were often found in couplets at day 7 (Fig. 4A2) and near the dorsal surface of the cortex at day 28 (Fig. 4A3). Quantifying this response, 10 ± 3% of BATGAL+ cells were immunoreactive for BrdU at day 3 and this increased to 20 ± 3% of BATGAL+ cells at day 7 (Fig. 4B), suggesting that β-catenin signaling predominantly occurs in a dividing cell population in the cortex. Although the majority of BATGAL+ cells are from a proliferating cell population, BATGAL+ BrdU+ cells represent only 1–2% of the total BrdU labeled cells (supporting information Table 1). The percentage of BATGAL+ cells that are BrdU+ did not change in the SCZ; however, the actual number of BATGAL+ BrdU+ cells in the SCZ increased significantly at day 7 (Fig. 4B–4C).
Figure 4.
β-catenin reporter cells divide after injury. (A1–A3): Representative images showing immunofluorescence of BrdU+ (green) and BATGAL+ cells (red) after injury. Arrows point to colocalization of BrdU and BATGAL reporter in cells. (B): Quantification of BrdU+ BATGAL+ cells shows that 20% of β-catenin reporter cells at day 7 incorporated BrdU on day 3 (*, p < .05; ***, p < .001). (C): Calculating the total number of BATGAL+ cells that are BrdU+ using information from (B) and stereological counts from Figure 2E shows a significant increase in BrdU+ BATGAL+ cells at day 7 (*, p < .05; **, p < .01). An ANOVA followed by Tukey’s Multiple Comparison Test was performed for all time points (n = 8–12). (D1–D4): Spatial distribution of BrdU+ β-catenin reporter cells after injury. The stereological location of BrdU+ BATGAL+ cells was determined for one section in each of four different animals per time point. These images were overlaid to show the composite results of these four animals. The white line indicates the border between the cortex and SCZ contours. Scale bars: 20 μm. Abbreviations: BrdU, bromodeoxyuridine; SCZ, subcallosal zone.
Stacks of images from four animals were aligned and merged to show a spatial representation of the BATGAL+ BrdU+ cells at the various time points (Fig. 4D1–4D4). At 3 dpi, dividing BATGAL+ cells were located throughout the cortex and a few cells were in the SCZ (Fig. 4D2). The numbers of dividing BATGAL+ cells increased in the cortex and the SCZ at day 7 (Fig. 4D3). By 28 dpi, the BATGAL+ BrdU+ population was primarily near the dorsal surface of the cortex (Fig. 4D4). These data point to two important findings: (1) dividing BATGAL+ cells are located throughout the cortex at day 3 and not sequestered to a specific anatomical location or germinal center and (2) the location of dividing BATGAL+ cells changes over time and is associated with known areas of gliosis. Although it is tempting to conclude that the BATGAL+ BrdU+ cells at day 3 contributed to the increase of BATGAL+ BrdU+ at day 7, the data presented here neither confirm nor refute this hypothesis. The BATGAL+ BrdU+ cells at 3 dpi could have lost their expression of β-galactosidase by 7 dpi, becoming BATGAL BrdU+ cells. On the other hand, BATGAL BrdU+ cells at day 3 might not have expressed β-galactosidase until day 7.
β-Catenin Signaling Is Sustained in Cells That Have Proliferated
To investigate whether β-catenin signaling occurs only when cells are proliferating or whether it is sustained in cells that have proliferated a few days earlier, staining with antibodies against the proliferation marker Ki-67 was performed (Fig. 5A–5B). Only 4 ± 1% of BATGAL+ cells are Ki-67+ in the cortex at day 7, whereas 20 ± 3% of BATGAL+ cells are BrdU+ (Fig. 5C). This illustrates that β-catenin signaling is not exclusively tied to proliferation in the cortex, but it is sustained in cells that have previously proliferated. Conversely, in the SCZ, the percentage of BATGAL+ cells that are Ki-67+ or BrdU+ is similar (Fig. 5D).
Figure 5.
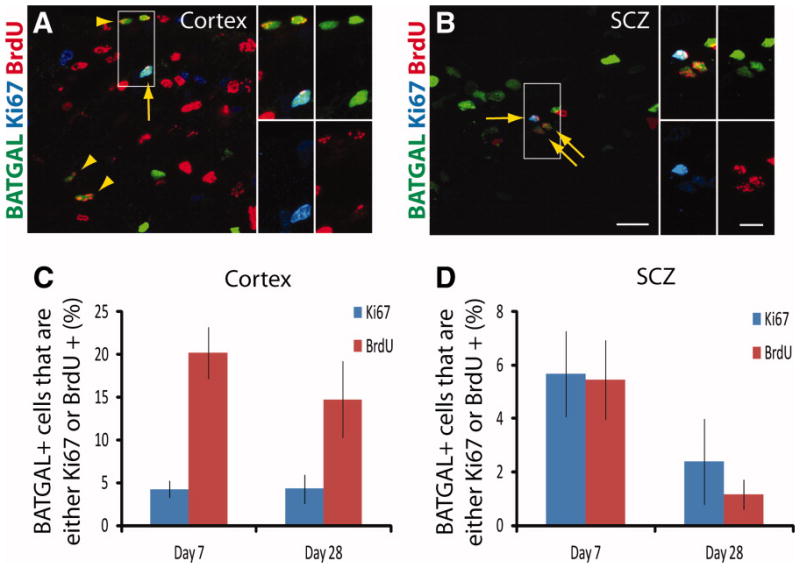
β-catenin signaling is sustained in cells of the cortex that have proliferated. (A–B): Representative images of triple immunofluorescence for BrdU (red), Ki-67 (blue), and the BATGAL reporter (green) in a day 7 animal. Arrowheads indicate BATGAL+ cells that are BrdU+ but not Ki-67+. Arrows point to BATGAL+ cells that are both Ki-67 and BrdU+. Smaller panels illustrate higher magnification views of the insets. (C–D): Graphing the proliferation data illustrates differences in the percentage of BATGAL+ cells that are either Ki-67+ or BrdU+ in the cortex but not in the SCZ (n = 7). Scale bars: 20 μm, 10 μm insets. Abbreviations: BrdU, bromodeoxyuridine; SCZ, subcallosal zone.
The Majority of Newly Born Cells in the Cortex with β-Catenin Signaling Are NG2+ and GFAP+
To characterize the phenotype of the dividing BATGAL+ cell population, staining with antibodies against the BATGAL reporter, BrdU, NG2, and GFAP was performed. Dividing BATGAL+ cells expressing either NG2 or GFAP were found at 3 and 7 dpi (Fig. 6A–6D). At 3 dpi, GFAP+ BATGAL+ BrdU+ cells as well as GFAP+ BATGAL BrdU+ were present, suggesting that β-catenin signaling is not necessary for astrocytes to divide, or it is only necessary at a specific step in cell division (Fig. 6C). Another plausible explanation is that β-catenin signaling occurs in a subpopulation of astrocytes. At 7 dpi, many GFAP+ BATGAL+ BrdU+ cells were found in couplets (Fig. 6D), suggesting a common ancestor that was in S-phase at day 3.
Figure 6.
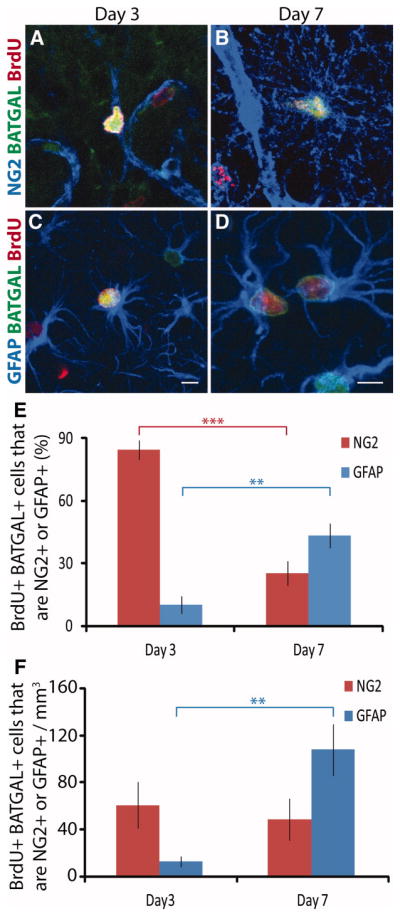
The phenotype of the majority of BrdU+ β-catenin reporter cells in the cortex shifts from being NG2+ at day 3 to GFAP+ at day 7. (A–B): Representative images from cortex of immunofluorescence for BrdU+ (red), NG2+ (blue), and BATGAL+ cells (green) at day 3 and day 7. (C–D): Representative images from cortex of immunofluorescence for BrdU+ (red), GFAP+ (blue), and BATGAL+ cells (green) at day 3 and day 7. (E): The percentage of BrdU+ BATGAL+ cells in the cortex that are either NG2+ or GFAP+ changes over time (**, p < .01; ***, p < .001). (F): Calculating the total number of BrdU+ β-catenin reporter cells that are either NG2+ or GFAP+ using information from (E) and stereological counts from Figures 2E and 4C shows a significant increase in the number of BrdU+ BATGAL+ GFAP+ cells at 7 days post-injury. (**, p < .01). An unpaired t-test was performed (n = 4–9). Scale bars: 5 μm. Abbreviations: BrdU, bromodeoxyuridine; GFAP, glial fibrillary acidic protein.
Quantification of the phenotype of BrdU+ BATGAL+ cells indicated that 85 ± 5% of the dividing BATGAL+ population expressed NG2 at 3 dpi, and this shifted to 25 ± 6% four days later (Fig. 6E). Reciprocally, only 10 ± 4% of the dividing BATGAL+ population expressed GFAP at 3 dpi, and this increased to 43 ± 6% by 7 dpi (Fig. 6E). Although the percentage of dividing BATGAL+ cells that were NG2+ decreased significantly between day 3 and day 7, the actual number of dividing BATGAL+ cells that were NG2+ stayed the same (Fig. 6F). The number of dividing BATGAL+ cells that were GFAP+ increased. This indicates that the decreased percentage of BATGAL+ cells that express NG2 is not due to loss of BATGAL+ NG2+ cells but rather is due to the increase in the number of BATGAL+ GFAP+ cells.
Cell Division Is Required for the Increase in β-Catenin Signaling in the Cortex but Not SCZ After TBI
To tease apart the contribution of newly born cells versus post-mitotic cells to the increase in BATGAL+ cells after TBI, cytosine arabinoside (AraC) was administered from the time of injury until the tissue was harvested 7 days later to kill mitotically active cells. Control animals (infused with aCSF) had an increase in BATGAL+ cells in the cortex and SCZ similar to previous uninfused experiments (Fig. 7A). Animals infused with AraC did not have an increase in BATGAL+ cells in the cortex (Fig. 7B); however, AraC did not reduce the number of BATGAL+ cells in the SCZ following TBI. These data confirm the BrdU studies and indicate that the majority of β-catenin signaling occurs in newly born cells in the cortex. A large number of post-mitotic cells of the SCZ have active β-catenin signaling in spite of a mitotic kill after injury. Taken together, these data suggest a difference in signaling pathways between GFAP-labeled astrocytes in the cortex and GFAP-labeled astrocytes in the SCZ.
Figure 7.
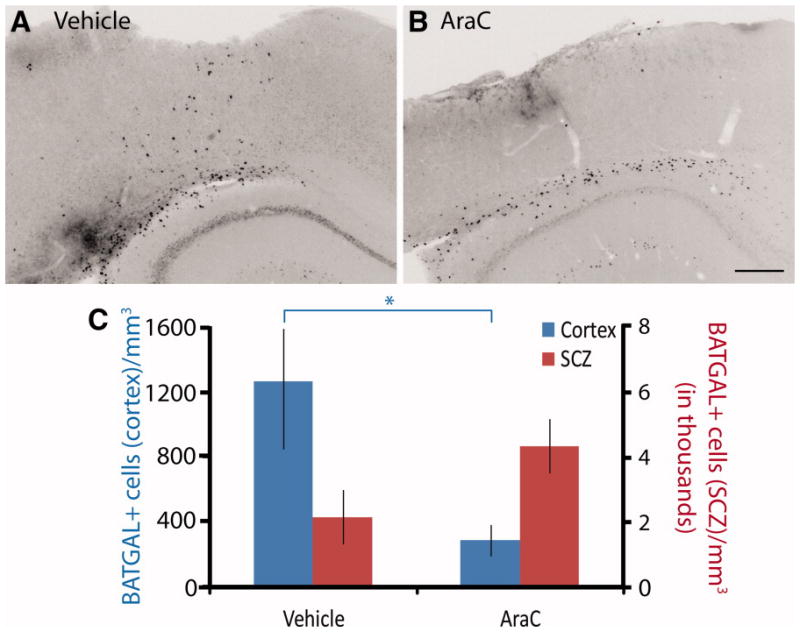
The increase in β-catenin reporter cells after traumatic brain injury is dependent on cell division. (A-B): Immunohistochemistry in coronal brain sections showing the response of β-catenin reporter cells to TBI in vehicle (aCSF) and AraC infused animals. (C): The increase in β-catenin reporter cells in cortex of vehicle treated mice is attenuated in cortex with AraC infusion; however, the change in β-catenin reporter cells in the subcallosal zone due to AraC is not significant (*, p < .05). An unpaired t-test was performed (n = 5–6). Scale bars: 300 μm. Abbreviations: AraC, cytosine arabinoside; SCZ, subcallosal zone.
Discussion
The current study demonstrates that β-catenin signaling occurs in glial progenitor populations in the adult brain and is increased after TBI, coincident with injury-induced gliosis. We also show that amplification of glia with active β-catenin signaling in the injured cortex relies on proliferation. These data argue that β-catenin signaling plays a role in the control of astrogliogenesis following cortical injury.
β-Catenin Signaling in Progenitor Populations in the Nervous System
Previous work has shown that β-catenin signaling is important for neurogenesis in the developing nervous system [6, 30, 31] as well as in adult mice [7, 8]. The current study presents the first evidence showing that β-catenin signaling also occurs in adult glial progenitor populations, specifically NG2+ progenitors and SCZ progenitors in the brain and in an area where subependymal progenitors are located in the spinal cord.
It is interesting that β-catenin signaling was found only in a small subset of NG2+ progenitors. NG2+ cells are not a homogeneous population of cells but rather differ in their functionality and multipotency [32–36]. Recently, Rivers et al. found separate dividing and nondividing populations of NG2+ cells in the adult corpus callosum [37]. Lytle et al. found two populations of NG2+ cells that differed in their lineage, transcription factor expression, and response to SCI [38]. It may be the case that signaling pathways such as β-catenin are the molecular determinants of phenotypic differences among various NG2+ cells in the adult brain. Using a Cre-Lox system to stably express β-catenin in Olig-1 expressing cells, Ye et al. completely abolished oligodendrocyte development in embryonic and postnatal time points [39]. If β-catenin’s role is similar in adult NG2+ cells as in development, perhaps β-catenin signaling inhibits oligodendrocyte formation in a small subset of adult NG2+ cells and keeps them in an immature, progenitor state.
An additional novel finding is that β-catenin signaling only occurred in NG2+ progenitors in the brain but not in the spinal cord. This could be due to differences in NG2+ cells, differences in ligand expression, or differences in BATGAL transgene expression in these two regions. Although found throughout the nervous system, the cell fate decisions of NG2+ cells differ depending on where they reside [36, 37, 40]. The finding of β-catenin signaling in a subset of NG2+ cells in the brain but not the spinal cord reveals another level of heterogeneity in this progenitor population.
Similar to its appearance in a specific subset of NG2+ cells, β-catenin signaling was found only in a subset of SCZ progenitors. This may be explained by considering that progenitors in the SCZ are at various stages of self renewal and differentiation and that only a specific step involves the transcriptional activity of β-catenin. Furthermore, lineage tracing studies have shown that SCZ progenitors become oligodendrocytes and astrocytes [22]. Taking the recent findings that β-catenin signaling inhibits oligodendrocyte development [39, 41], perhaps β-catenin signaling keeps the progenitors in an undifferentiated state or instructs them to become astrocytes.
β-Catenin Signaling After Nervous System Injury
A third novel finding of the current study is that β-catenin signaling increased after TBI and that this increase occurred in newly born NG2+ cells and GFAP+ reactive astrocytes in the injured cortex. A previous report showed an increase in β-catenin protein in hippocampal extracts after TBI [42]. Our work extends that analysis in multiple ways by assaying transcriptionally active β-catenin, examining other areas of the brain, and determining the phenotype of these cells.
Surprisingly, an increase in β-catenin signaling was not observed in NG2+ cells or GFAP+ cells after SCI. Previous research has shown that the glial response to injury is different in the brain and in the spinal cord [43]. Perhaps differences in β-catenin signaling in NG2+ cells and reactive astrocytes between the two areas of the central nervous system (CNS) underlie the difference in gliosis and scar formation between the brain and the spinal cord. Recently, Fancy et al. have shown that ~15% of oligodendrocyte precursors increase β-catenin signaling after a demyelination lesion [44]. Our analysis of BATGAL+ staining after SCI did not corroborate these findings, possibly due to the different type of spinal cord injury performed and the fact that our analysis was concentrated on the injury epicenter and surrounding glial scar where remyelination is likely limited.
Origin of Reactive Astrocytes in Gliosis Following Injury
Currently there are three competing hypotheses regarding the origin of reactive astrocytes following injury. The source of newly born astrocytes could be from (1) GFAP+ astrocytes that dedifferentiate then divide, (2) NG2+ progenitors found throughout the parenchyma, (3) progenitors migrating from the SVZ or SCZ, or from a combination of these three sources [11, 13, 14]. The data presented in this paper do not specifically support one of these hypotheses but implicate β-catenin in post-traumatic gliogenesis regardless of the origin of newly born astrocytes.
Buffo et al. recently proposed that reactive astrocytes following injury were derived from dedifferentiated GFAP+ cells [14]. Our results show β-catenin signaling in a subset of dividing astrocytes 3 dpi, a time coincident with the proposed dedifferentiation. Often these GFAP+ BATGAL+ cells were nestin+ as well (supporting information Fig. 3). GFAP+ BATGAL+ BrdU+ cells were also found as couplets at 7 dpi, suggesting that they came from a common ancestor. In addition, Wnt/β-catenin signaling has already been shown to be required for the dedifferention of epithelial cells to form hair follicles [19] and for the dedifferentiation of Mueller glia to form retinal cells [20] after injury. Collectively, this is compelling evidence indicating that the Wnt/β-catenin pathway may be part of the dedifferentiation or subsequent differentiation process of reactive astrocytes.
Several investigators have proposed that reactive astrocytes are derived in part from NG2+ progenitors after injury. Recent research using BrdU paradigms [11, 45] and genetic fate mapping [12, 46] has suggested that NG2+ progenitors could generate a subpopulation of astrocytes and were a source of proliferative gliosis after injury. Given that ~80% of dividing BATGAL+ cells were NG2+ at 3 dpi and NG2+ BATGAL+ cells were found in areas of BrdU+ GFAP+ astrocytes, our results suggest that β-catenin signaling is important in the proliferation or differentiation of this NG2+ population following injury.
It has also been suggested that reactive astrocytes following TBI are derived from cells originating in the SVZ and SCZ [13, 47]. Cells with active β-catenin signaling are found in the SCZ, and BATGAL+ BrdU+ cells are found in the SCZ at 3 and 7 dpi, but not at day 28. These results are consistent with BATGAL+ cells migrating toward the lesion; however, it is equally plausible that they died or divided so many times as to dilute their BrdU below immunofluorescence detection.
Molecular Mechanisms That Instruct Reactive Glosis Following Injury
As researchers have begun to propose the cellular origin of the glial scar, they have also initiated studies into the signaling pathways that instruct astrogliosis following injury. The transcription factor STAT3 has been found to be a critical regulator of reactive astrocytes, and its absence disrupted scar formation following SCI [15]. Others have shown that the Ras-MEK-ERK signaling cascade is increased in reactive astrocytes after injury [16]. Interestingly, the Wnt/β-catenin pathway has been shown to upregulate the expression of STAT3 in various contexts such as zebrafish gastrulation [48], embryonic stem cell self-renewal [49], and human esophageal squamous cell carcinoma [50]. In addition, novel ways in which mitogen-activated protein kinase pathways cross-talk with the Wnt/β-catenin pathway are being discovered [51]. Our research shows that β-catenin signaling increases in post-traumatic gliogenesis and provides a new example of potential cross-talk between various signaling pathways. The findings presented in this study implicate β-catenin signaling as an important target for the development of interventions to modify gliogenesis and improve regeneration after brain injury.
Supplementary Material
Supplemental Figure 1. Example of cortex and subcallosal zone (SCZ) contours used in quantification. (A) X-gal reaction in a coronal brain section showing expression of a β-catenin signaling (BATGAL) reporter. (B) An example of the contours drawn for the cortex (blue) and SCZ (red). An arrow points to a band of BATGAL+ cells that were not included in the quantification because it is similar in intensity and location at all time points regardless of injury.
Supplemental Figure 2. β-catenin reporter cells in the cortex colocalize with Olig2+ progenitors but not mature oligodendrocytes. (A–B) Representative images of double immunofluorescence for Olig2 (red) and the BATGAL reporter (green) in day 3 and day 7 animals. Arrows point to BATGAL+ cells that co-label with antibodies against Olig2. (C) Quantification of the percentage of BATGAL+ cells that are Olig2+. (D) Representative images of double immunofluorescence for GSTπ (red) and the BATGAL reporter (green) illustrating no overlap between these two cell populations. An ANOVA was performed for all time points in (C) (n = 3–5). Scale bars: 20 μm. Abbreviations: GSTπ, glutathione s-transferase π.
Supplemental Figure 3. The majority of GFAP+ β-catenin reporter cells co-label for nestin at day 3 and day 7, but not day 28. (A–D) Representative images of triple immunofluorescence for nestin (red), GFAP+ (blue), and the BATGAL reporter (green). Arrowheads indicate BATGAL+ cells that are nestin+ but predominantly GFAP−. Arrows point to BATGAL+ cells that are GFAP+ but predominantly nestin−. Smaller panels illustrate separate channel views of the insets. (n = 3–4) Scale bars: 20 μm. Abbreviations: GFAP, glial fibrillary acidic protein.
Supplemental Table 1. A table showing the number of proliferating BATGAL+ cells as a percentage of total BrdU+ cells
Acknowledgments
We wish to thank Denise Inman, Kathy Davidson, Andy Chien, Richard James, and Nathan Camp for helpful comments on the manuscript; Ramona Hicks and Andy Fink for initial discussions and assistance with TBI procedure; William Stallcup for the gift of anti-NG2 antibody. This work was supported by NIH RO1 GM073887 (R.T.M.), NIH NSO46724 (P.J.H.), and NIH NIG5 T32GM07108 (B.D.W). R.T.M is an Investigator of the HHMI.
Footnotes
Disclosure of Potential Conflicts of Interest
This work and B.D.W. were supported by NIH RO1 GM073887 (R.T.M.), NIH NSO46724 (P.J.H.), and NIH NIG5 T32GM07108 (B.D.W.).
Additional supporting information available online.
Contributor Information
Randall T. Moon, Email: rtmoon@u.washington.edu.
Philip J. Horner, Email: phorner@u.washington.edu.
References
- 1.Merrill BJ, Gat U, DasGupta R, et al. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 3.Pinto D, Gregorieff A, Begthel H, et al. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dravid G, Ye Z, Hammond H, et al. Defining the role of Wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells. 2005;23:1489–1501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- 5.Sato N, Meijer L, Skaltsounis L, et al. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 6.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 7.Adachi K, Mirzadeh Z, Sakaguchi M, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 8.Lie DC, Colamarino SA, Song HJ, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 9.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 10.Norton WT, Aquino DA, Hozumi I, et al. Quantitative aspects of reactive gliosis: A review. Neurochem Res. 1992;17:877–885. doi: 10.1007/BF00993263. [DOI] [PubMed] [Google Scholar]
- 11.Alonso G. NG2 proteoglycan-expressing cells of the adult rat brain: Possible involvement in the formation of glial scar astrocytes following stab wound. Glia. 2005;49:318–338. doi: 10.1002/glia.20121. [DOI] [PubMed] [Google Scholar]
- 12.Sellers DL, Maris DO, Horner PJ. Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury. J Neurosci. 2009;29:6722–6733. doi: 10.1523/JNEUROSCI.4538-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salman H, Ghosh P, Kernie SG. Subventricular zone neural stem cells remodel the brain following traumatic injury in adult mice. J Neurotrauma. 2004;21:283–292. doi: 10.1089/089771504322972077. [DOI] [PubMed] [Google Scholar]
- 14.Buffo A, Rite I, Tripathi P, et al. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann JE, Imura T, Song B, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito M, Natsume A, Takeuchi H, et al. Type I Interferon Inhibits Astrocytic Gliosis and Promotes Functional Recovery after Spinal Cord Injury by Deactivation of the MEK/ERK Pathway. J Neurotrauma. 2009;26:41–53. doi: 10.1089/neu.2008.0646. [DOI] [PubMed] [Google Scholar]
- 17.Stoick-Cooper CL, Weidinger G, Riehle KJ, et al. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134:479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- 18.Goessling W, North TE, Lord AM, et al. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev Biol. 2008;320:161–174. doi: 10.1016/j.ydbio.2008.05.526. [DOI] [PubMed] [Google Scholar]
- 19.Ito M, Yang Z, Andl T, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 20.Osakada F, Ooto S, Akagi T, et al. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27:4210–4219. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maretto S, Cordenonsi M, Dupont S, et al. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seri B, Herrera DG, Gritti A, et al. Composition and organization of the SCZ: A large germinal layer containing neural stem cells in the adult mammalian brain. Cereb Cortex. 2006;16 (Suppl 1):i103–111. doi: 10.1093/cercor/bhk027. [DOI] [PubMed] [Google Scholar]
- 23.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 24.Kalani MY, Cheshier SH, Cord BJ, et al. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci U S A. 2008;105:16970–16975. doi: 10.1073/pnas.0808616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liebner S, Corada M, Bangsow T, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mothe AJ, Tator CH. Proliferation, migration, and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131:177–187. doi: 10.1016/j.neuroscience.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Nakatomi H, Kuriu T, Okabe S, et al. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 28.Hampton DW, Rhodes KE, Zhao C, et al. The responses of oligodendrocyte precursor cells, astrocytes and microglia to a cortical stab injury, in the brain. Neuroscience. 2004;127:813–820. doi: 10.1016/j.neuroscience.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 29.Ozerdem U, Grako KA, Dahlin-Huppe K, et al. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- 30.Gulacsi AA, Anderson SA. Beta-catenin-mediated Wnt signaling regulates neurogenesis in the ventral telencephalon. Nature Neurosci. 2008;11:1383–1391. doi: 10.1038/nn.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zechner D, Fujita Y, Hulsken J, et al. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 32.Belachew S, Chittajallu R, Aguirre AA, et al. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butt AM, Kiff J, Hubbard P, et al. Synantocytes: New functions for novel NG2 expressing glia. J Neurocytol. 2002;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- 34.Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561:109–122. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horner PJ, Thallmair M, Gage FH. Defining the NG2-expressing cell of the adult CNS. J Neurocytol. 2002;31:469–480. doi: 10.1023/a:1025739630398. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–157. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 37.Rivers LE, Young KM, Rizzi M, et al. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nature Neurosci. 2008;11:1392–1401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lytle JM, Chittajallu R, Wrathall JR, et al. NG2 cell response in the CNP-EGFP mouse after contusive spinal cord injury. Glia. 2009;57:270–285. doi: 10.1002/glia.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye F, Chen Y, Hoang T, et al. HDAC1 and HDAC2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-TCF interaction. Nature Neurosci. 2009 doi: 10.1038/nn.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu X, Hill RA, Nishiyama A. NG2 cells generate oligodendrocytes and gray matter astrocytes in the spinal cord. Neuron Glia Biol. 2008;4:19–26. doi: 10.1017/S1740925X09000015. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu T, Kagawa T, Wada T, et al. Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. Developmental Biology. 2005;282:397–410. doi: 10.1016/j.ydbio.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 42.Shapira M, Licht A, Milman A, et al. Role of glycogen synthase kinase-3beta in early depressive behavior induced by mild traumatic brain injury. Mol Cell Neurosci. 2007;34:571–577. doi: 10.1016/j.mcn.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Schnell L, Fearn S, Klassen H, et al. Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur J Neurosci. 1999;11:3648–3658. doi: 10.1046/j.1460-9568.1999.00792.x. [DOI] [PubMed] [Google Scholar]
- 44.Fancy SP, Baranzini SE, Zhao C, et al. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 2009;23:1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao JW, Raha-Chowdhury R, Fawcett JW, et al. Astrocytes and oligodendrocytes can be generated from NG2+ progenitors after acute brain injury: Intracellular localization of oligodendrocyte transcription factor 2 is associated with their fate choice. Eur J Neurosci. 2009;29:1853–1869. doi: 10.1111/j.1460-9568.2009.06736.x. [DOI] [PubMed] [Google Scholar]
- 46.Burns KA, Murphy B, Danzer SC, et al. Developmental and post-injury cortical gliogenesis: A genetic fate-mapping study with Nestin-CreER mice. Glia. 2009;57:1115–1129. doi: 10.1002/glia.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramaswamy S, Goings GE, Soderstrom KE, et al. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005;1053:38–53. doi: 10.1016/j.brainres.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 48.Yamashita S, Miyagi C, Carmany-Rampey A, et al. Stat3 Controls Cell Movements during Zebrafish Gastrulation. Dev Cell. 2002;2:363–375. doi: 10.1016/s1534-5807(02)00126-0. [DOI] [PubMed] [Google Scholar]
- 49.Hao J, Li TG, Qi X, et al. WNT/beta-catenin pathway up-regulates Stat3 and converges on LIF to prevent differentiation of mouse embryonic stem cells. Dev Biol. 2006;290:81–91. doi: 10.1016/j.ydbio.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Yan S, Zhou C, Zhang W, et al. beta-Catenin/TCF pathway upregulates STAT3 expression in human esophageal squamous cell carcinoma. Cancer Lett. 2008;271:85–97. doi: 10.1016/j.canlet.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 51.Bikkavilli RK, Malbon CC. Mitogen-activated protein kinases and Wnt/beta-catenin signaling: Molecular conversations among signaling pathways. Commun Integr Biol. 2009;2:46–49. doi: 10.4161/cib.2.1.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Example of cortex and subcallosal zone (SCZ) contours used in quantification. (A) X-gal reaction in a coronal brain section showing expression of a β-catenin signaling (BATGAL) reporter. (B) An example of the contours drawn for the cortex (blue) and SCZ (red). An arrow points to a band of BATGAL+ cells that were not included in the quantification because it is similar in intensity and location at all time points regardless of injury.
Supplemental Figure 2. β-catenin reporter cells in the cortex colocalize with Olig2+ progenitors but not mature oligodendrocytes. (A–B) Representative images of double immunofluorescence for Olig2 (red) and the BATGAL reporter (green) in day 3 and day 7 animals. Arrows point to BATGAL+ cells that co-label with antibodies against Olig2. (C) Quantification of the percentage of BATGAL+ cells that are Olig2+. (D) Representative images of double immunofluorescence for GSTπ (red) and the BATGAL reporter (green) illustrating no overlap between these two cell populations. An ANOVA was performed for all time points in (C) (n = 3–5). Scale bars: 20 μm. Abbreviations: GSTπ, glutathione s-transferase π.
Supplemental Figure 3. The majority of GFAP+ β-catenin reporter cells co-label for nestin at day 3 and day 7, but not day 28. (A–D) Representative images of triple immunofluorescence for nestin (red), GFAP+ (blue), and the BATGAL reporter (green). Arrowheads indicate BATGAL+ cells that are nestin+ but predominantly GFAP−. Arrows point to BATGAL+ cells that are GFAP+ but predominantly nestin−. Smaller panels illustrate separate channel views of the insets. (n = 3–4) Scale bars: 20 μm. Abbreviations: GFAP, glial fibrillary acidic protein.
Supplemental Table 1. A table showing the number of proliferating BATGAL+ cells as a percentage of total BrdU+ cells