Summary
Store-operated calcium entry is an almost ubiquitous signaling pathway in eukaryotic cells. The plasma membrane store-operated channels are comprised of subunits of the recently discovered Orai proteins, the major one being Orai1.We have discovered that native Orai1, as well as expressed Orai1, exists in two forms in similar quantities: a longer form (Orai1α) of approximately 33 kDa, and a shorter form (Orai1β) of approximately 23 kDa. The Orai1β form arises from alternative translation initiation from a methionine at position 64, and possibly also 71, in the longer Orai1α form. In the sequence upstream of the initiation site of Orai1β, there is a poly-arginine sequence previously suggested to be involved in interaction of Orai1 with plasma membrane phosphatidylinositol-4,5-bisphosphate. The loss of this phospholipid binding domain would be expected to influence the mobility of Orai1 protein in the plasma membrane. Indeed, experiments utilizing fluorescence recovery after photobleaching (FRAP) revealed that the recovery half-time for Orai1β was significantly faster than for Orai1α. Since Orai1 must diffuse to sites of interaction with the Ca2+ sensor, STIM1, these two mobilities might provide for efficient recruitment of Orai1 subunits to sites of store-operated Ca2+ entry during agonist-induced Ca2+ signaling.
Key words: Store-operated calcium entry, Calcium channels, Orai channels, Alternative translation initiation
Introduction
The calcium ion (Ca2+) is an important second messenger, regulating almost all cell functions including muscle contraction, neurotransmitter release, proliferation, differentiation and even cell death. In the process of Ca2+ signaling, the Ca2+ concentration rises in the cytoplasm, where the free Ca2+ concentration is normally low, due to influx from the extracellular space and release from intracellular Ca2+ stores. A variety of surface receptors such as G-protein-coupled receptors and receptor tyrosine kinases are coupled to phospholipase C (PLC) activation. Upon receptor activation, PLC hydrolyses phosphatidylinositol-4,5-bisphosphate (PIP2) into inositol-1,4,5-trisphosphate (IP3) and diacylglycerol. IP3 activates IP3 receptors in the endoplasmic reticulum (ER) membrane resulting in Ca2+ release from the ER. This release of Ca2+ from the ER results in the depletion of stored Ca2+ which triggers Ca2+ influx across the plasma membrane through a process known as store-operated Ca2+ entry (SOCE) (Putney, 1986).
It was recently discovered that SOCE is composed of two essential membrane proteins, Orai1 and STIM1 (Feske et al., 2006; Liou et al., 2005; Roos et al., 2005; Vig et al., 2006; Zhang et al., 2006). Orai1 is a 4-pass transmembrane protein localized predominantly in the plasma membrane and forms a tetrameric functional SOC channel (Feske et al., 2006; Prakriya et al., 2006; Vig et al., 2006; Yeromin et al., 2006). Upon store depletion, STIM1, a Ca2+ sensor on the ER membrane, oligomerizes and migrates to special ER-PM junctions called puncta, and activates Orai1 apparently by direct interaction (Liou et al., 2005; Stathopulos et al., 2006; Wu et al., 2006; Zhang et al., 2005). Orai1 has two other homologues, Orai2 and Orai3 (Feske et al., 2006; Vig et al., 2006). All three Orai homologues can be activated by STIM1 upon store-depletion with some differences in magnitude of channel activity and pharmacology (DeHaven et al., 2008; Lis et al., 2007; Mercer et al., 2006).
Despite a growing accumulation of knowledge about the activation process of Orai1 by STIM1, the regulation of its expression is largely unknown. The Orai1 gene and protein are ubiquitously expressed (Gwack et al., 2007; McCarl et al., 2009). Orai1 is post-translationally glycosylated on an asparagine residue at position 223 (Gwack et al., 2007). However, the functional relevance of this glycosylation remains elusive. It has been reported that Orai1 is phosphorylated on serine residues at amino acids in positions 27 and 30 by protein kinase C, which negatively regulates Orai1 function (Kawasaki et al., 2010). Recently, it has been reported that the expression of Orai1 is suppressed during mitosis, contributing to suppression of SOCE (Smyth et al., 2009; Yu et al., 2009). Furthermore, Orai1 is internalized into endosomes during meiosis by caveolin-mediated endocytosis (Yu et al., 2010).
In this study, we report that the Orai1 gene is expressed in two different isoforms of Orai1 protein, Orai1α and Orai1β. The shorter isoform, Orai1β, is translated from a second or third methionine residue by alternative translation initiation. Both isoforms are ubiquitously expressed in human cell lines derived from different tissues and both localize similarly within cells as seen by confocal microscopy. In the sequence upstream of the initiation site of Orai1β, there is a poly-arginine sequence previously suggested to be involved in interaction of Orai1 with plasma membrane phosphatidylinositol-4,5-bisphosphate, as well as a sequence suggested to mediate interaction with caveolin. In the current study, we show that the shorter form of Orai1 has faster mobility in the plasma membrane. Since the roles of Orai channels in Ca2+ signaling pathways is expanding, it is possible that these distinct channel forms have specific roles in distinct modes of Ca2+ signaling.
Results
Orai1 is expressed as two isoforms in HEK293 cells
Since Orai1 has an N-glycosylation site on the asparagine residue 223, Western blotting showed several diffuse bands around 37 kDa (Fig. 1). The multiple bands disappeared following treatment with N-glycosidase F (PNGase F), an enzyme which hydrolyzes glycosylamine linkage of asparagine-linked oligosaccharides, leaving two bands instead (Fig. 1). Both bands were essentially eliminated by siRNA-mediated knockdown of Orai1, suggesting that both represent forms of Orai1 protein (Fig. 1). Both bands were recognized by two different antibodies raised against different epitopes (Fig. 1, supplementary material Fig. S2). The longer isoform, which we designate Orai1α, is about 33 kDa, which is similar to the predicted molecular mass (32.67 kDa) of full-length of Orai1. The shorter form, which we designate Orai1β, is about 23 kDa. The most likely mechanisms to produce multiple protein isoforms from a single gene are alternative splicing of the message, post-translational processing and alternative start sites of initiation. We found that the two forms of Orai1 were obtained either in native cells (as in Fig. 1) or when Orai1 was overexpressed from cDNA (Fig. 2A, first lane). This indicates that the two forms do not result from alternative splicing of message. We also analyzed the expression of Orai1 tagged with cyan fluorescent protein N- (ECFP-Orai1) or C-terminally (Orai1-ECFP) using an anti-GFP antibody. ECFP-Orai1 is expressed around 60 kDa, which is consistent with the estimated molecular mass. No second band was seen with ECFP-Orai1 (not shown), which could mean that the N-terminus is truncated by either alternative translation or post-translational modification (proteolysis). However, surprisingly, when Orai1-ECFP was overexpressed, again almost all of the expressed protein corresponded to the longer, Orai1α version (Fig. 2B, first lane). We noted that when the construct for Orai1-ECFP was made, as is often the case the Kozak sequence for the first methionine was improved (indeed, the Kozak sequence for the first methionine in the native 5′-untranslated region is rather weak). We considered that if the two forms of Orai1 arose due to alternative translation initiation, then strengthening the Kozak at the first initiation site might result in a majority of product formed from initiation at that site. To test this idea, we then made a construct of Orai1-ECFP but with the native 5′-untranslated region restored. When this construct was transfected into HEK293 cells, now two bands were seen, with the predicted sizes of ECFP-tagged Orai1α and Orai1β (Fig. 2B, second lane). This then suggests that the two Orai1 isoforms arise due to alternative translation initiation, and this possibility was investigated in more depth.
Fig. 1.
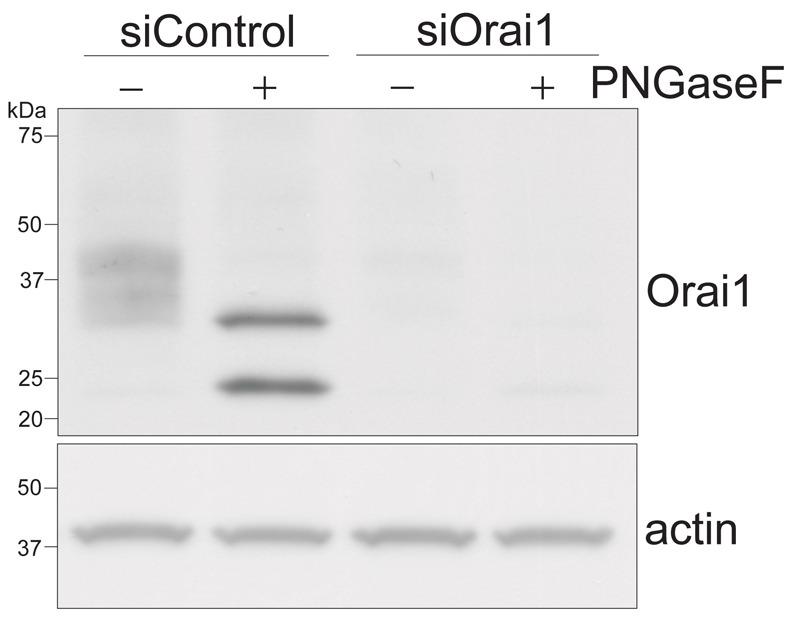
Endogenous Orai1 protein is expressed as two different isoforms in HEK293 cells. Western blot showing the expression of Orai1 protein in HEK293 cells transfected with negative control siRNA (siControl) or siRNA against human Orai1. Cells were lysed in RIPA buffer. After overnight treatment with PNGaseF, samples were dissolved by 4–20% gradient SDS-PAGE and analyzed by Western blotting using an anti-Orai1 antibody. Actin expression confirms that similar amounts of protein were loaded in each lane. Representative blot from three independent experiments is shown.
Fig. 2.
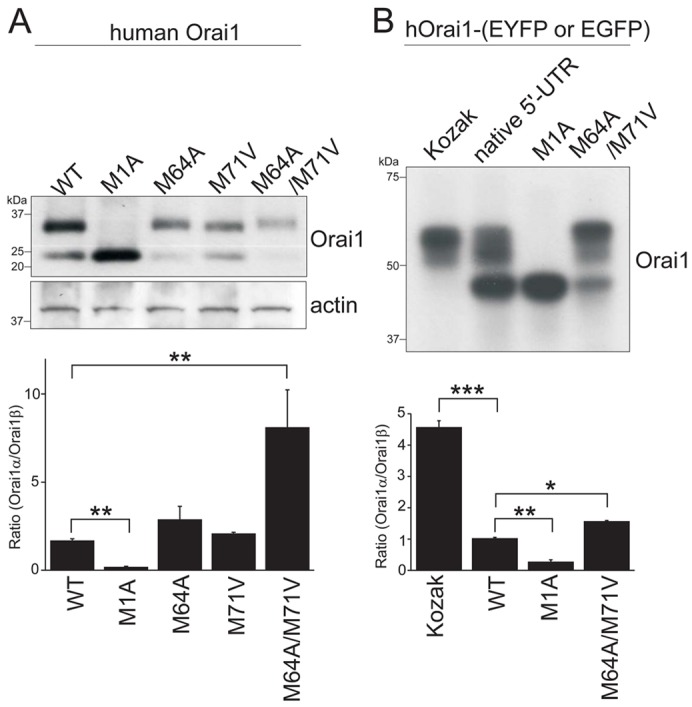
Two isoforms of Orai1 are translated by alternative translation initiation. (A) Western blot showing heterogeneously expressed Orai1 proteins in HEK293 cells transfected with the cDNAs of WT, first methionine (M1A) mutant, second methionine (M64A) mutant, third methionine (M71V) mutant and double mutant of second and third methionine (M64A/M71V) mutant of Orai1. (B) Western blot showing the expression of Orai1 proteins tagged with fluorescent protein in HEK293 cells transfected with the cDNAs of WT with Kozak sequence, WT with native 5′-UTR, M1A mutant and M64A/M71V double mutant of Orai1. Cell lysates were treated with PNGaseF overnight and resolved by 4–20% gradient (A) or 10% (B) SDS-PAGE. The blots were probed with anti-Orai1 antibody and anti-actin antibody for loading control. Representative data from at least three independent experiments are shown. Lower panels represent the ratio of Orai1α and Orai1β calculated by densitometric analysis. Data are means ± s.e.m.; *P<0.05, **P<0.01, ***P<0.001, one-way ANOVA followed by Tukey's test (n = 3).
Expression of two different isoforms of Orai1 is mediated by alternative translation initiation
To investigate the formation of alternative translation products of Orai1, we first mutated the first methionine codon (ATG) to that of alanine (GCG) to block the production of full-length Orai1 [Orai1(M1A)]. When expressed in HEK293 cells, Orai1(M1A) showed only one band after PNGase F treatment that has exactly same size as WT Orai1β (Fig. 2A). This result strongly suggested that the shorter isoform is translated from an alternative translation start site. Orai1 has two methionine residues downstream of the first in its N-terminus, amino acids 64 and 71. Next, we mutated these methionines to alanine or valine individually or in combination. These were expressed in HEK293 cells and their expression analyzed by Western blotting. While two single mutants showed the both longer and shorter isoforms of Orai1, the disruption of both methionines strongly suppressed the expression of the shorter form, Orai1β (Fig. 2A). Introduction of a methionine with Kozak properties upstream of position 64, at position 25 (S25M) resulted in a second band with mobility intermediate to Orai1α and Orai1β (supplementary material Fig. S2). These results suggest that both methionines at positions 64 and 71 may function as second translation initiation sites in Orai1. Similar results were obtained with the tagged, Orai1-EYFP (Fig. 2B). Orai1 mRNA has a relatively weak translation initiation site at the first methionine, which results in the production of protein translated from the second and third methionine codons. Note that in the M64A/M71A mutants, a small amount of Orai1β still appears to be formed; this is likely due to the very weak nature of the first initiation site, and the retention of some Kozak properties at the second site(s), even in the absence of a methionine (Kozak, 1989; Peabody, 1989). Note that expression of Orai1β from the M1A mutant construct is always very strong compared to WT and other mutants (Fig. 2). For example, in the Western analysis of the M1A mutant of Orai1-EGFP, the amount of loaded protein in the lane is reduced to half compared to other lanes because of the very strong expression of Orai1β from the M1A mutant.
N-terminal truncation in Orai1β does not affect subcellular localization
It is known that the N-terminal amino acid sequence is important for the membrane insertion process occurring immediate after translation, and for the later secretory pathway. Furthermore, recent findings have suggested a critical role of the Orai1 N-terminus in the regulation of its cell surface expression [(Yu et al., 2010), and see Discussion]. Therefore, we evaluated the localization of Orai1α and Orai1β in HEK293 cells and the ability of the two forms to form puncta in response to Ca2+ store depletion (Fig. 3). Equatorial confocal sections of resting HEK293 cells shown in Fig. 3A illustrate that EGFP- and EYFP-fused Orai1 proteins were almost exclusively localized in the plasma membrane, while mCherry-STIM1 fluorescence was distributed in cytoplasmic regions with a characteristic fibrillar pattern. Thapsigargin treatment (20 min) induced the accumulation of mCherry-STIM1 into puncta which locate in junctions between the ER and the plasma membrane. Although the change of Orai1 distribution was not as drastic as STIM1, both Orai1 forms also accumulated into the puncta and significantly co-localized with STIM1.
Fig. 3.
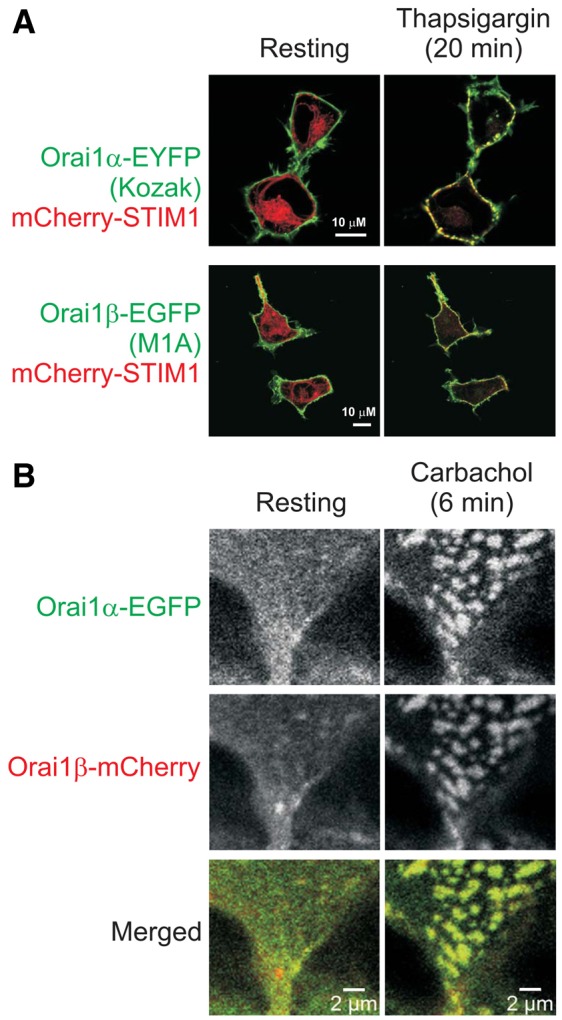
Both isoforms of Orai1 protein localize predominantly to the plasma membrane and translocate to puncta upon store-depletion. (A) Confocal images of HEK293 cells co-expressing Orai1-EYFP or Orai1-EGFP with mutations that result in expression of exclusively Orai1α or Orai1β forms, together with mCherry-STIM1. Confocal images were taken every 5 minutes. After collecting the first image, cells were treated with thapsigargin in the absence of extracellular Ca2+. The images taken just prior to, and 20 minutes after thapsigargin treatment are shown and are representative of at least three independent experiments. (B) Confocal images of HEK293 cells co-expressing Orai1α-EGFP and Orai1β-mCherry before and 6 minutes after store depletion by 0.5 mM carbachol. Results are representative of four independent experiments.
To better assess the localization of the two Orai1 forms during signaling, we imaged Orai1 puncta in cells simultaneously expressing STIM1, Orai1α-EGFP and Orai1β-mCherry following store depletion by the phospholipase C-activating agonist, carbachol. As shown in the cortical confocal sections of Fig. 3B, both forms appear to form puncta equally well and there was no apparent heterogeneity of the puncta with regard to their content of the two forms.
Both isoforms of Orai1 support store-operated Ca2+ entry and Icrac
We next analyzed thapsigargin (TG)-induced Ca2+ responses and Ca2+ release-activated Ca2+ current (Icrac) to determine whether there are functional differences between the two isoforms of Orai1. Both untagged Orai1 constructs were introduced into HEK293 cells with EYFP-STIM1. After thapsigargin treatment in nominally Ca2+-free solution, we added back extracellular Ca2+ by a step-wise increase in concentration. As we observed previously (Mercer et al., 2006), at the concentration of 0.1 mM Ca2+, there was little increase of SOCE in HEK293 cells co-expressing each Orai1 construct with EYFP-STIM1 compared to that in the cells expressing EYFP-STIM1 alone (Fig. 4A). At 0.3 mM and 1 mM extracellular Ca2+ concentration, cells over-expressing Orai1 and EYFP-STIM1 showed significantly larger Ca2+ increase than EYFP-STIM1-expressing control cells. However, there was no significant difference among Orai1 constructs (Fig. 4A). Next, we analyzed whether there were changes in the channel properties by recording Icrac. Although there were no significant differences in activation kinetics (half-times) or in the highly Ca2+-selective I-V relationship of Icrac among all Orai1 constructs, the average amplitude of Icrac recorded from cells co-expressing the Orai1 M64A/M71V mutant with EYFP-STIM1 was somewhat reduced compared to other two constructs (Fig. 4B). This result is consistent with the data of protein expression by Western blotting in which the M64A/M71V mutant of Orai1 gives less expression than other constructs (Fig. 2A).
Fig. 4.
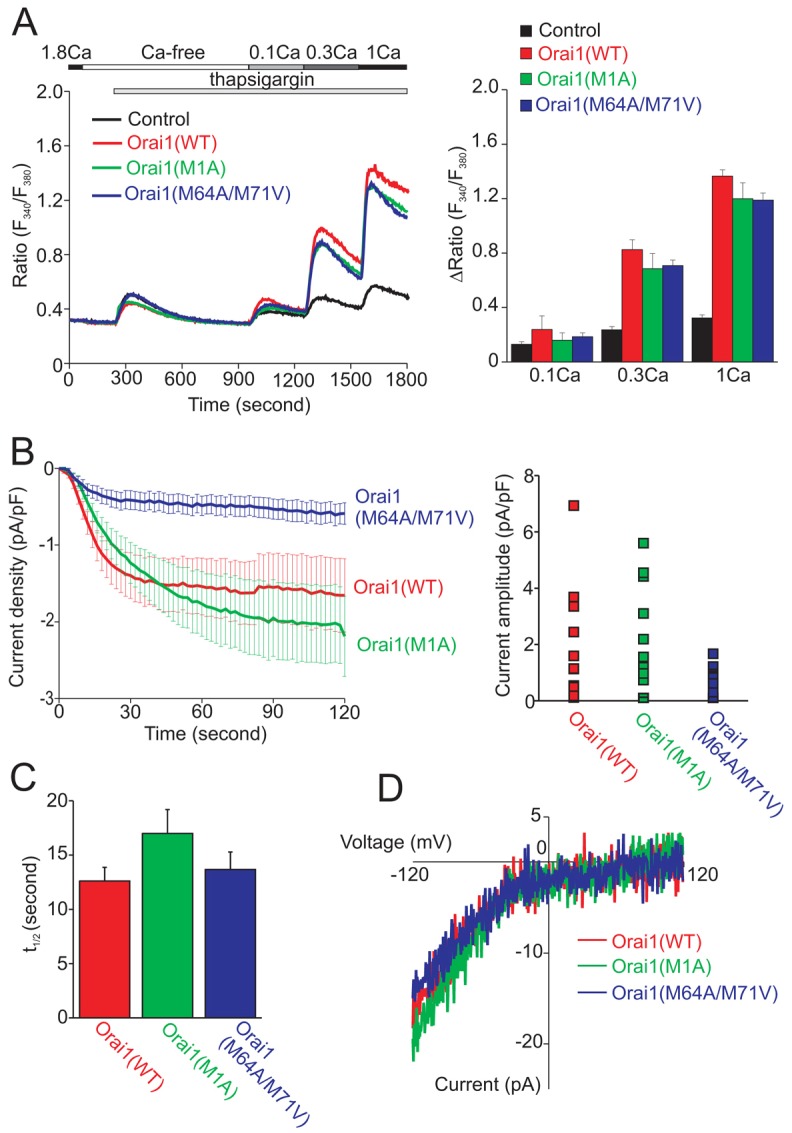
Long and short forms of Orai support SOCE and Icrac similarly. (A) Thapsigargin-induced Ca2+ responses in HEK293 cells co-expressing WT, M1A mutant or M64A/M71V mutant of Orai1 with EYFP-STIM1. Cells denoted control expressed EYFP-STIM1 alone. The left panel shows the time course of intracellular Ca2+ mobilization. The timing of extracellular solution exchange and addition of thapsigargin are indicated by horizontal bars above the traces. The right panel depicts the peak values (means ± s.e.m., n = 3) of Ca2+ responses after step-wise restoration of extracellular Ca2+. (B) Icrac recording from HEK293 cells co-expressing WT, M1A mutant or M64A/M71V mutant of Orai1 with EYFP-STIM1. The left panel demonstrates the time course of Icrac development after the establishment of the whole-cell configuration. The right panel shows the distribution of current amplitudes at the time point of 120 seconds for each cell. (C) Average half-times for development of Icrac. Data are means ± s.e.m. (n = 15 for WT, 13 for M1A mutant and 13 for M64A/M71V double mutant). (D) Representative I–V relationship from three cells, one from each condition that has a similar current amplitude.
We considered that downstream signaling due to store depletion and Icrac activation might differentially regulate the levels of the two Orai1 isoforms. However, no such difference was seen in cells activated with thapsigargin for 1 hour, whether in the presence or absence of extracellular Ca2+ (supplementary material Fig. S3).
Orai1α and Orai1β have distinct plasma membrane mobilities
Within the sequence upstream of the initiation site for Orai1β are sequences shown to interact with caveolin (Yu et al., 2010) and with PIP2 (Calloway et al., 2011). Loss of these domains might be expected to alter the mobility of Orai1 in the plasma membrane, since mobility is influenced by interaction with other membrane and subcellular components. To investigate this possibility, we assessed the mobilities of the two isoforms, expressed either singly or together by use of fluorescence recovery after photobleaching (FRAP), as described in the Materials and Methods. The recovery of both Orai forms occurred with two rate constants. The first with a half time of around 7 sec was similar for both forms and with both modes of expression. This rapid movement indicates molecules moving without impediment and without interaction with other cellular components. It may reflect excess quantities of the protein due to overexpression. For both forms there was a second, slower rate of recovery. For Orai1β this second, slower rate had a half time of 23.3±2.8 sec when expressed singly, and 39±6 sec when co-expressed with Orai1α. For the Orai1α isoform, the second component showed an even slower mobility with high standard deviation, predicting a heterogenic population. Fig. 5 shows a histogram of the half times for the two isoforms in cells transfected with either Orai1α-EGFP or Orai1β-EGFP alone (Fig. 5A) and in cells transfected with both isoforms, Orai1α-EGFP plus Orai1β-mCherry (Fig. 5B). In cells expressing the two isoforms singly or together with one another, the distribution of half-times for Orai1β appeared normal and uniformly distributed. Orai1α in both cases there appeared to be two populations. A smaller fraction of half times (24 out of 73 when expressed singly and 16 out of 54 when expressed together) had a similar half-time to that of Orai1β (25.6±2.0 sec and 32±7 sec, respectively). However, in both cases the larger proportion of measurements (49 out of 73 when expressed singly and 38 out of 54 when expressed together) revealed much longer half times of 59.0±12.0 sec and 62±12 sec, respectively. The basis for the minor proportion of faster diffusion rates is not known, as these occurred randomly, even with sequential measurements within a single cell.
Fig. 5.
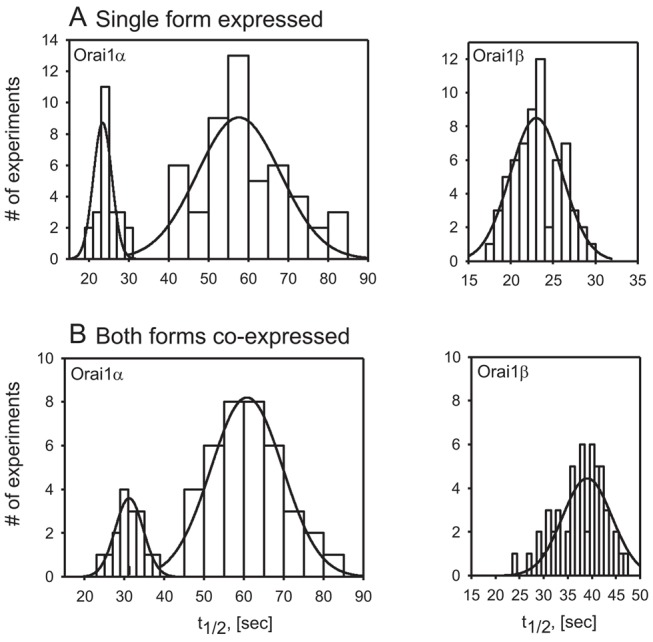
Distribution of FRAP recovery half-times for Orai1α and Orai1β. Half-times of recovery from FRAP were calculated as described in the Materials and Methods. (A) Singly expressed Orai1-GFP isoforms; Orai1α data distributed as two populations, a minor one averaging 26 seconds, and a major one averaging 59 seconds. Orai1β shows a homogenous distribution averaging 23 seconds. The data represent 73 FRAP recovery curves from 18 cells for Orai1α and 64 FRAP recovery curves from 13 cells for Orai1β, from three independent experiments. (B) Coexpressed Orai1α -GFP and Orai1β-mCherry isoforms; Orai1α data distributed as two populations, a minor one averaging 32 seconds, and a major one averaging 64 seconds. Orai1β shows a homogenous distribution averaging 39 seconds. The data represent 54 FRAP recovery curves from 20 cells, from three independent experiments. Scaling cluster sizes (or Δt1/2) were varied, Orai1β (Δt1/2 = 1 sec), Orai1α (Δt1/2 = 2 sec for the faster population and Δt1/2 = 5 sec for the slower population) for clearer presentation. The smooth curves are calculated normal distributions for the data.
Thus, when expressed either singly or together, the two forms exhibited clearly distinct mobilities as determined by rates of FRAP. This result strongly suggests that the two proteins do not combine to form hetero-tetrameric channels; if so, one would expect channels with a single, perhaps broad distribution of mobilities. However, it does seem that the mobilities of Orai1β, and perhaps of the minor population of Orai1α, are slowed somewhat when the two isoforms are co-expressed, while the slower mobility of the greater fraction of Orai1α was essentially unchanged. This many indicate some form of interaction between the two channel types, but this is not known at present.
Both Orai1α and Orai1β are expressed in other human cell lines
Finally, we analyzed protein expression of both the α and β isoforms of Orai1 in several human cell lines. We chose Jurkat T cells, HeLa cells, the keratinocyte cell line HaCaT, the squamous carcinoma cell line A431, a lung adenocarcinoma H441 and a colorectal adenocarcinoma T84 in addition to HEK293 cells. In all cell lines, both the longer and shorter isoforms of Orai1 were detected (Fig. 6). While the total expression levels of Orai1 were varied, the ratios of the isoforms were similar among the cell lines we examined. This result suggests that there may be no tissue-specific regulation of the alternative translation initiation of Orai1 protein at least in resting conditions.
Fig. 6.
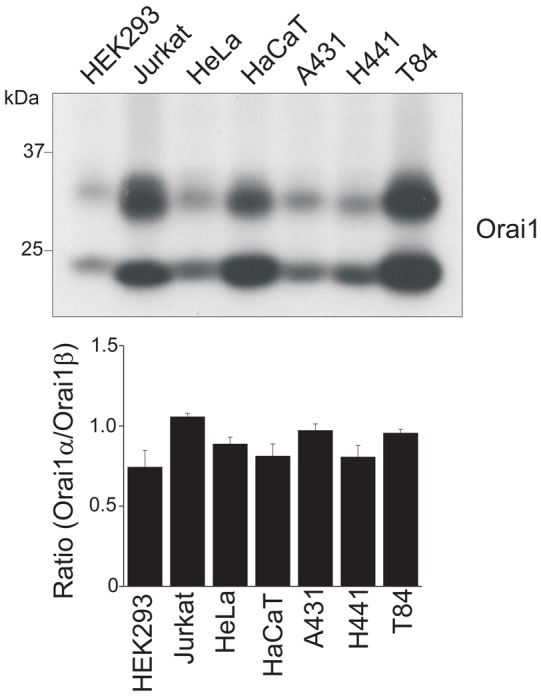
Expression of two isoforms of Orai1 in various human cell lines. Western blot showing the expression of endogenous Orai1 in HEK293 cells, Jurkat T cells, HeLa cells, the keratinocyte cell line HaCaT, squamous carcinoma A431 cells, lung adenocarcinoma H441 cells and colorectal adenocarcinoma T84 cells. Cell lysates from each cell type were treated with PNGaseF overnight and dissolved in 10% SDS-PAGE. 15 µg of total protein were loaded into each lane except HaCaT and T84. 7.5 µg of total proteins were loaded into the lanes of HaCaT and T84 due to higher expression of endogenous Orai1 protein these cell lines. The blot was probed with anti-Orai1 antibody. Representative data from three independent experiments are shown. Lower panel represents the ratio of Orai1α and Orai1β calculated by densitometric analysis. Data are means ± s.e.m.
Discussion
In this study, we demonstrate that Orai1 proteins are expressed in human cells as two different isoforms by alternative translation initiation. The longer form we designate Orai1α, and the shorter form, Orai1β. Site-directed mutagenesis revealed that there are two possible translation initiation sites giving rise to the short form at methionine residues 64 and 71. Since these two start sites are very close to one another, we have designated the short form as one entity, Orai1β, although it may be comprised of two forms differing by only 7 amino acids. We note that initiation at M71 would impair an established calmodulin-binding domain (residues 69–91) (Mullins et al., 2009). It is therefore conceivable, and perhaps likely that Orai1β initiates exclusively at M64, and only at M71 when M64 is mutated, but it is not possible to determine this with certainty. In most human cell lines, endogenous Orai1 is expressed as these two isoforms at least in resting conditions. Under the conditions of our assays, with the proteins overexpressed, we see no obvious difference in their activation or in the nature of the currents they produce.
Alternative translation initiation is one of the more important mechanisms for gene diversity (Kochetov, 2008). Based on the current model, the ribosome initiates translation upon scanning the first AUG from 5′ to 3′. However, the translation initiation site is also defined by the sequence around the first AUG. The canonical translation initiation site, which is known as the Kozak sequence, is RnnAUGG, in which R denotes a purine residue (Kochetov, 2008). Since the mRNA sequence of Orai1 around the first AUG is UCCAUGC, it would be too weak to efficiently stall the 40S ribosome at that site for translation initiation.
Despite many reports of distinct forms of ion channels due to alternative splicing of message (for example, Gross et al., 2007; Oberwinkler et al., 2005; Standley et al., 2003; Zhang and Saffen, 2001), we are aware of only one instance of multiple channel isoforms due to alternative translation initiation (Simkin et al., 2008; Thomas et al., 2008). To date, there have been no reports describing the expression of two different isoforms of Orai1. Since Orai1 is a highly glycosylated protein, complete removal of the sugar chain by PNGaseF treatment is required to detect the two bands clearly in Western analysis (Fig. 1). It is possible that many cDNA clones of Orai1 used in expression studies have been modified with strong Kozak sequences for optimal expression in mammalian cells, which would thus obscure the expression of the shorter isoform of Orai1β. This prediction was confirmed by our observation that an Orai1-EYFP construct modified with a conventional Kozak sequence (GACGGCACCATG) expressed almost exclusively the longer Orai1α protein fused to EYFP (Fig. 2B). The Orai1 cDNA clone (Origene; Cat# SC316298) used in the current study retains the original 5′ untranslated region. Thus, we could observe clear expression of the two different isoforms, as seen in untransfected cells. In HEK293 cells, the WT Orai1 cDNA clone expressed slightly more of the longer Orai1α form than the shorter Orai1β form. Densitometry analysis revealed about 50% more expression of the longer isoform (Orai1α/Orai1β = 1.547±0.11; mean ± s.e.m.; n = 4).
In addition to the interaction with STIM1, there are reports of other mechanisms of regulating Orai1 that involve interactions with residues found only in the longer Orai1α. Kawasaki et al. demonstrated that serine residues at the position of 27 and 30 are phosphorylated by protein kinase Cβ, which is important for negative regulation of SOCE (Kawasaki et al., 2010). We thus might presume that the relative amounts of the two isoforms would dictate sensitivity to regulation by protein kinase C. However, we were unable to test this idea as we have consistently failed to see any effect of phorbol ester on thapsigargin-activated store-operated Ca2+ entry in HEK293 cells, in either the untransfected cells or in cells overexpressing STIM1 and Orai1.
Yu et al. recently reported caveolin (Cav)-mediated internalization of Orai1 during meiosis (Yu et al., 2010). Although deletion of the first 70 amino acids had no effect on plasma membrane expression in oocytes, deletion of the N-terminus prevented internalization of Orai1 during meiosis. Amino acids 52–60 of Orai1 were identified as a Cav binding motif. This observation is quite interesting because one of the more common and important functions of alternative translation initiation is to alter localization of the gene product. We did not observe any difference in localization between the long and short forms of Orai1 when expressed in HEK293 cells, in resting cells or after store-depletion (Fig. 4). However, since HEK293 cells do not express a lot of Cav proteins, this system may not be suitable for the analysis of the contribution of Cav protein in the regulation of SOCE. There may be certain conditions (for example during meiosis) and/or specific cells or tissues in which only full-length Orai1 is regulated in a Cav-dependent manner. Recently, a N-terminal polyarginine sequence (amino acids 28–33) was suggested to be a binding site for phosphatidylinositol-4,5-bisphosphate (PIP2) and to regulate lateral distribution of Orai1 in the plasma membrane (Calloway et al., 2011). The observations of Cav and PIP2 binding strongly suggest the full-length Orai1 will be under strict spatio-temporal regulation in the plasma membrane. Consistent with this idea, we found that in most measurements, Orai1α exhibited a substantially slower rate of recovery from FRAP, indicating that its mobility in the plasma membrane may be restricted by interaction with other components.
Despite this difference in mobility, we did not observe any striking functional difference between the full-length and truncated shorter isoforms in experiments utilizing overexpression of each of the two proteins. It has been previously reported that the deletion of N-terminal amino acids 1–73 reduced STIM1-mediated Orai1 activation slightly (Li et al., 2007; Yuan et al., 2009). However, the isolated SOAR domain, a region in STIM1 critical for the activation of Orai1, can fully activate an Orai1 N-terminal truncation mutant (Yuan et al., 2009). Yuan et al. proposed that four proline residues at positions 3, 5, 39 and 40 are critical for the interaction with polybasic domain of STIM1, which is important for the SOAR domain to be released from auto-inhibition. In our study, we did not see significant reduction of Icrac or store-operated Ca2+ entry in HEK293 cells overexpressing Orai1 (M1A) mutant and STIM1. However, it is quite possible that subtle regulatory effects are lost due to overexpression of both Orai1 and STIM1. It is well documented that the polybasic domain of STIM1 is not necessary for Orai1 activation under conditions of overexpression (for example, Smyth et al., 2009).
In summary, we have demonstrated that in humans, Orai1 exists in two isoforms due to alternative translation initiation. The two isoforms are found in similar quantities in cell lines derived from a variety of different tissues. The longer form, Orai1α, retains sequences predicted to mediate interaction with other cellular components, and in FRAP recovery experiments, Orai1α was shown to have slower plasma membrane mobility than the shorter Orai1β. Many possibilities exist with regard to the physiological roles of these distinct channel forms. For example, Orai1 has an additional role in regulating TRPC1 trafficking (Cheng et al., 2011), and in forming arachidonic acid gating channels when combined with Orai3 (Shuttleworth, 2009). There is evidence that distinct store-operated channels may regulate specific signaling pathways , and this may depend upon their localization and interaction with other molecules. These possibilities will need to be the focus of continued research on this centrally important signaling molecule.
Materials and Methods
Cell culture
HEK293 cells were obtained from ATCC and cultured in DMEM (Invitrogen) supplemented with 10% fetal bovine serum. Cells were maintained at 37°C in a humidifier incubator set at 5% CO2.
cDNA constructs, siRNA and transfection
Human Orai1 cDNA encoding full 5′ and 3′ untranslated regions from the Orai1 message was obtained from Origene. Site-direct mutagenesis of methionine 1, 64 and 71 to alanine or valine was carried out by using Quikchange II XL (Stratagene) according to the manufacturer's instructions. These mutant Orai1 cDNAs were amplified by PCR and subcloned into pEGFP-N1 vector (Clontech) to fuse EGFP on their C-termini. HEK293 cells were transfected with plasmid DNAs by using Amaxa nucleofection (Lonza) according to the manufacturer's instructions. To knock down endogenous Orai1 in HEK293 cells, the cells were transfected with siRNA against human Orai1 as described previously (Mercer et al., 2006). HEK293 cells were transfected with siRNAs by using Hiperfect (QIAGEN) according to the manufacturer's instructions and used for experiments 48 hours after transfection.
Cell lysis and Western blotting
HEK293 cells were lysed in RIPA buffer [137 mM NaCl, 20 mM Tris-HCl, pH 7.4, 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 0.5% sodium deoxycholate, 2 mM EDTA, 5 mM sodium orthovanadate and protease inhibitor cocktail (Roche)]. To remove N-glycosylation, lysates were treated with N glycosidase F (Roche or Sigma) at 37°C overnight. Cell lysates were resolved by a 4–20% gradient or 10% fixed SDS-PAGE and subjected to immunoblot analysis with anti-human Orai1 (Sigma; 1:5000 or Thermo; 1:3000), anti-actin (Sigma; 1:3000) or anti-GFP antibody (Abcam; 1:1000). The bands were scanned and the density of each band was determined using ImageJ software.
Confocal imaging
Fluorescence images were acquired with a confocal laser-scanning microscope (LSM710; Carl Zeiss) using the 488 nm line of an argon laser for excitation and a 493 nm to 552 nm band-pass filter for emission for EGFP, the 514 nm line of an argon laser for excitation and 517 nm to 575 nm for emission for EYFP, or the 561 nm line of a HeNe laser for excitation and 578 nm to 694 nm for emission for mCherry. The specimens were viewed at a zoom of 2.4× using plan oil objectives (Plan-Apochromat 63×, numerical aperture 1.4, Oil DIC M27). Images were taken every 30 seconds for 20 minutes.
Fluorescence recovery after photobleaching (FRAP)
FRAP measurements were carried out on a LSM710 confocal microscope (Carl Zeiss, Jena, Germany) using an C-Apochromat 40×/1.2 W objective at a zoom of 2.6×. EGFP was excited with the 488 nm line of 25 mW Argon laser at 1–4% power to minimalize the bleaching of the sample during monitoring. Fluorescence emission was detected between 492–602 nm. The pinhole was set at 1.5 Airy Unit (1.5 µm resolution) and no line averaging was used. The detection gain 640V was chosen such that the Orai1-EGFP pool at the plasma membrane was almost saturating the dynamic level of the detectors. Bleaching area (ROI) was 2.6 µm wide square, and not more than 5% of the whole plasma membrane of the targeted cell. 10 bleach iterations, 180 msec of bleaching time, at 100% transmission of a 25 mW 488 nm laser were sufficient to bleach 80–90% of EGFP. Three pre-bleach images were taken to assess noise. Image size, 81.6 µm×81.6 µm, was chosen to be able to monitor two neighboring cells to calculate the overall photo-bleaching and evaluate focal plane drifts during post bleaching data acquisition. Generally, three to six FRAP curves from a particular cell were collected by repeated bleaching at the same ROI. The monitored fluorescence recovery plateau was between 100–150 sec at 23°C with mobile fraction >85%. Sample rate was 120–280 images at a rate of 2.1 image/s. 13 cells (64 FRAP curves) and 18 cells (73 FRAP curves) of Orai1β and Orai1α were obtained from three independent experiments. FRAP curves were collected from Orai1α and Orai1β coexpressed cells by using two parallel laser lines, 488 nm (70%) and 561 nm (45% of a 15mW laser) for bleaching. Sample rate was 1 image/s, recording two channels simultaneously using line scanning. Again, three pre-bleach images were taken to assess noise. Twenty coexpressed cells (54 FRAP curves) were acquired from three independent experiments.
In order to compare recovery curves for different cells, we normalized the data to correct for variations in protein expression levels, background fluorescence, and loss of fluorescence during bleaching. The background photobleaching correction was calculated from a neighboring cell. To calculate the percentage of mobile fractions of the two Orai1 forms the pre-bleach intensity was set as 1 for normalization.
To obtain the rate of mobility in the two Orai1 isoforms, we set as 1 the difference between the fluorescence intensities at the asymptote and that immediately following the bleach. To reduce the noise sufficiently to determine the optimal number of exponentials to fit the recovery kinetics, 64 normalized FRAP curves from 13 cells (Orai1β) were averaged using a house written macro of SigmaPlot (Systat Software, CA). Two exponential fitting gave the best parameter dependencies and standard deviations (SD). Then each normalized recovery curve was fitted with two exponential recovery curves. Statistical analysis, t-test of the half times and their distribution was calculated by SigmaPlot software. An illustration of the method and an example of raw FRAP data obtained in this way is given in supplementary material Fig. S1.
Ca2+ imaging
Measurement of changes in [Ca2+]i was performed as we previously described (DeHaven et al., 2008). Cells were loaded with the fluorescent Ca2+ indicator Fura-5F. The fluorescence ratio images were recorded from cells incubated in HBSS: 145 mM NaCl, 3 mM KCl, 1.8 mM CaCl2, 1.2 mM MgCl2, 10 mM HEPES, 10 mM glucose (pH 7.4 adjusted with NaOH). CaCl2 was omitted in Ca2+-free solution.
Electrophysiology
Whole-cell currents were measured as described previously (DeHaven et al., 2008). The standard extracellular HBSS contained: 145 mM NaCl, 3 mM KCl, 10 mM CsCl, 1.2 mM MgCl2, 2 mM CaCl2, 10 mM glucose and 10 mM HEPES (adjusted to pH 7.4 with NaOH). The intracellular pipette solution contained: 145 mM Cs-methanesulfonate, 10 mM BAPTA, 10 mM HEPES and 8 mM MgCl2 (adjusted to pH 7.2 with CsOH) together with the addition of 25 µM IP3 (hexasodium salt). Currents were acquired with pCLAMP-10 (Axon Instruments) and analyzed with Clampfit (Axon Instruments).
Supplementary Material
Acknowledgments
We thank Drs Stephen Shears and Robert Oakley for helpful comments.
Footnotes
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.104919/-/DC1
References
- Calloway N., Owens T., Corwith K., Rodgers W., Holowka D., Baird B. (2011). Stimulated association of STIM1 and Orai1 is regulated by the balance of PtdIns(4,5)P2 between distinct membrane pools. J. Cell Sci. 124, 2602–2610 10.1242/jcs.084178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. T., Liu X., Ong H. L., Swaim W., Ambudkar I. S. (2011). Local Ca2+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca2+ signals required for specific cell functions. PLoS Biol. 9, e1001025 10.1371/journal.pbio.1001025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaven W. I., Smyth J. T., Boyles R. R., Bird G. S., Putney J. W., Jr (2008). Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. J. Biol. Chem. 283, 19265–19273 10.1074/jbc.M801535200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S. H., Tanasa B., Hogan P. G., Lewis R. S., Daly M., Rao A. (2006). A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185 10.1038/nature04702 [DOI] [PubMed] [Google Scholar]
- Gross S. A., Wissenbach U., Philipp S. E., Freichel M., Cavalié A., Flockerzi V. (2007). Murine ORAI2 splice variants form functional Ca2+ release-activated Ca2+ (CRAC) channels. J. Biol. Chem. 282, 19375–19384 10.1074/jbc.M701962200 [DOI] [PubMed] [Google Scholar]
- Gwack Y., Srikanth S., Feske S., Cruz–Guilloty F., Oh–hora M., Neems D. S., Hogan P. G., Rao A. (2007). Biochemical and functional characterization of Orai proteins. J. Biol. Chem. 282, 16232–16243 10.1074/jbc.M609630200 [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Ueyama T., Lange I., Feske S., Saito N. (2010). Protein kinase C-induced phosphorylation of Orai1 regulates the intracellular Ca2+ level via the store-operated Ca2+ channel. J. Biol. Chem. 285, 25720–25730 10.1074/jbc.M109.022996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochetov A. V. (2008). Alternative translation start sites and hidden coding potential of eukaryotic mRNAs. Bioessays 30, 683–691 10.1002/bies.20771 [DOI] [PubMed] [Google Scholar]
- Kozak M. (1989). Context effects and inefficient initiation at non-AUG codons in eucaryotic cell-free translation systems. Mol. Cell. Biol. 9, 5073–5080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Lu J., Xu P., Xie X., Chen L., Xu T. (2007). Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J. Biol. Chem. 282, 29448–29456 10.1074/jbc.M703573200 [DOI] [PubMed] [Google Scholar]
- Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E., Jr and Meyer T. (2005). STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 15, 1235–1241 10.1016/j.cub.2005.05.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis A., Peinelt C., Beck A., Parvez S., Monteilh–Zoller M., Fleig A., Penner R. (2007). CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr. Biol. 17, 794–800 10.1016/j.cub.2007.03.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarl C. A., Picard C., Khalil S., Kawasaki T., Röther J., Papolos A., Kutok J., Hivroz C., Ledeist F., Plogmann K., et al. (2009). ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J. Allergy Clin. Immunol. 124, 1311–1318.e7 10.1016/j.jaci.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. C., Dehaven W. I., Smyth J. T., Wedel B., Boyles R. R., Bird G. S., Putney J. W., Jr (2006). Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J. Biol. Chem. 281, 24979–24990 10.1074/jbc.M604589200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins F. M., Park C. Y., Dolmetsch R. E., Lewis R. S. (2009). STIM1 and calmodulin interact with Orai1 to induce Ca2+-dependent inactivation of CRAC channels. Proc. Natl. Acad. Sci. USA 106, 15495–15500 10.1073/pnas.0906781106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberwinkler J., Lis A., Giehl K. M., Flockerzi V., Philipp S. E. (2005). Alternative splicing switches the divalent cation selectivity of TRPM3 channels. J. Biol. Chem. 280, 22540–22548 10.1074/jbc.M503092200 [DOI] [PubMed] [Google Scholar]
- Peabody D. S. (1989). Translation initiation at non-AUG triplets in mammalian cells. J. Biol. Chem. 264, 5031–5035 [PubMed] [Google Scholar]
- Prakriya M., Feske S., Gwack Y., Srikanth S., Rao A., Hogan P. G. (2006). Orai1 is an essential pore subunit of the CRAC channel. Nature 443, 230–233 10.1038/nature05122 [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr (1986). A model for receptor-regulated calcium entry. Cell Calcium 7, 1–12 10.1016/0143-4160(86)90026-6 [DOI] [PubMed] [Google Scholar]
- Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., et al. (2005). STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 169, 435–445 10.1083/jcb.200502019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth T. J. (2009). Arachidonic acid, ARC channels, and Orai proteins. Cell Calcium 45, 602–610 10.1016/j.ceca.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin D., Cavanaugh E. J., Kim D. (2008). Control of the single channel conductance of K2P10.1 (TREK-2) by the amino-terminus: role of alternative translation initiation. J. Physiol. 586, 5651–5663 10.1113/jphysiol.2008.161927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth J. T., Petranka J. G., Boyles R. R., DeHaven W. I., Fukushima M., Johnson K. L., Williams J. G., Putney J. W., Jr (2009). Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat. Cell Biol. 11, 1465–1472 10.1038/ncb1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley S., Roche K. W., McCallum J., Sans N., Wenthold R. J. (2003). PDZ domain suppression of an ER retention signal in NMDA receptor NR1 splice variants. Neuron 28, 887–898 10.1016/S0896-6273(00)00161-6 [DOI] [PubMed] [Google Scholar]
- Stathopulos P. B., Li G. Y., Plevin M. J., Ames J. B., Ikura M. (2006). Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J. Biol. Chem. 281, 35855–35862 10.1074/jbc.M608247200 [DOI] [PubMed] [Google Scholar]
- Thomas D., Plant L. D., Wilkens C. M., McCrossan Z. A., Goldstein S. A. N. (2008). Alternative translation initiation in rat brain yields K2P2.1 potassium channels permeable to sodium. Neuron 58, 859–870 10.1016/j.neuron.2008.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig M., Peinelt C., Beck A., Koomoa D. L., Rabah D., Koblan–Huberson M., Kraft S., Turner H., Fleig A., Penner R., et al. (2006). CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223 10.1126/science.1127883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. M., Buchanan J., Luik R. M., Lewis R. S. (2006). Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 174, 803–813 10.1083/jcb.200604014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromin A. V., Zhang S. L., Jiang W., Yu Y., Safrina O., Cahalan M. D. (2006). Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 443, 226–229 10.1038/nature05108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Sun L., Machaca K. (2009). Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proc. Natl. Acad. Sci. USA 106, 17401–17406 10.1073/pnas.0904651106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Sun L., Machaca K. (2010). Constitutive recycling of the store-operated Ca2+ channel Orai1 and its internalization during meiosis. J. Cell Biol. 191, 523–535 10.1083/jcb.201006022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J. P., Zeng W., Dorwart M. R., Choi Y. J., Worley P. F., Muallem S. (2009). SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat. Cell Biol. 11, 337–343 10.1038/ncb1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Saffen D. (2001). Muscarinic acetylcholine receptor regulation of TRP6 Ca2+ channel isoforms. Molecular structures and functional characterization. J. Biol. Chem. 276, 13331–13339 10.1074/jbc.M008914200 [DOI] [PubMed] [Google Scholar]
- Zhang S. L., Yu Y., Roos J., Kozak J. A., Deerinck T. J., Ellisman M. H., Stauderman K. A., Cahalan M. D. (2005). STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905 10.1038/nature04147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. L., Yeromin A. V., Zhang X. H., Yu Y., Safrina O., Penna A., Roos J., Stauderman K. A., Cahalan M. D. (2006). Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc. Natl. Acad. Sci. USA 103, 9357–9362 10.1073/pnas.0603161103 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


