Abstract
Spermatogenesis is a complex biochemical event, involving the participation of the hypothalamus and the pituitary gland via the secretion of the hypothalamus hormone GnRH, and two pituitary hormones FSH and LH. Thus, the hypothalamus-pituitary-testicular axis is a crucial regulatory axis for testicular function. Recent studies have shown that in the microenvironment of the seminiferous epithelium, wherein each Sertoli cell is supporting ~30-50 germ cells at different stages of their development, locally produced autocrine and paracrine factors are also involved in spermatogenesis, in particular at the level of cell junctions. These cell junctions at the Sertoli-Sertoli and Sertoli-germ cell interface are crucial to coordinate different events of spermatogenesis by sending signals back-and-forth between Sertoli and germ cells, in order to precisely regulate spermatogonial cells renewal by mitosis, cell cycle progression, meiosis, spermiogenesis, germ cell movement across the epithelium, spermiation and germ cell apoptosis. In this minireview, we provide an update on these latest findings for an emerging new concept regarding the presence of a local “apical ectoplasmic specialization – blood-testis barrier – hemidesmosome/basement membrane” functional axis that regulates the events of spermiation and blood-testis barrier (BTB) restructuring via paracrine/autocrine factors and polarity proteins produced locally in the seminiferous epithelium. These findings provide a new window of research for investigators in the field to tackle the functional regulation of spermatogenesis.
Keywords: testis, spermatogenesis, seminiferous epithelial cycle, adherens junction, tight junction, ectoplasmic specialization, blood-testis barrier, hemidesmosome, basement membrane, laminin chains, collagen chains, cytokines
1. Introduction
Seminiferous tubule is the functional unit in the testis that produces spermatozoa (haploid, 1n) from spermatogonia (diploid, 2n) during spermatogenesis. Spermatogenesis takes place in the seminiferous epithelium of the seminiferous tubule, which is composed of four distinctive phases: mitosis, meiosis, spermiogenesis, and spermiation that occur during the 14 stages of seminiferous epithelial cycle of spermatogenesis in rats, 12 stages in mice, and 6 stages in men. The seminiferous epithelium is composed of the Sertoli cell and germ cells lying adjacent to the tunica propria with physical contact with the basement membrane (see Fig. 1). Spermatogenesis is supported by the pituitary hormone follicle stimulating hormone (FSH) and the male sex hormone testosterone produced by Leydig cells in the interstitium with the FSH receptor and androgen receptor restricted to Sertoli cells in the seminiferous epithelium (for reviews, see [1-4]). In short, the Sertoli cell in the seminiferous epithelium is the target for both FSH and testosterone, illustrating its crucial role in spermatogenesis in supporting germ cell maturation [2,4]. Besides, estrogens also play an important role in germ cell development, such as apoptosis [5]. Recent studies have demonstrated that cytochrome P450 aromatase (P450arom) which transforms androgens into estrogens irreversible, and estrogen receptors (ERα and ERβ) that mediate the action of estrogens are found in germ cells including spermatocytes, round spermatids, and elongated spermatids, besides Leydig and Sertoli cells (for a review, see [6]). These thus illustrate that cells in the seminiferous epithelium are capable of producing estrogens from testosterone to regulate spermatogenesis (for reviews, see [5-7]).
Figure 1. The schematic drawing illustrating the local “apical ES-BTB-basement membrane/hemidesmosome” regulatory axis, coordinating the events of spermiation and BTB restructuring that occur simultaneously at stage VIII of the seminiferous epithelial cycle of spermatogenesis in adult rat tests.

At spermiation, the α6β1-integrin/laminin-333 adhesion complex at the apical ES is disrupted, likely via the action of MMP-2 at the site, with the generation of the biologically active laminin fragments. These fragments were shown to induce BTB disruption directly, such as by reducing the steady-state levels of integral membrane proteins at the BTB. Additionally, cytokines (e.g., TNFα, TGF-β2, and TGF-β3) and testosterone were also shown to induce protein endocytosis at the BTB. The combination of these thus ‘de-stabilizes’ the BTB, facilitating the transit of primary preleptotene spermatocytes at the BTB. Furthermore, these laminin fragments can also disrupt the BTB indirectly via effects on the hemidesmosome, such as by reducing the β1-integrin level in the hemidesmosome, this, in turn, further ‘de-stabilizes’ the BTB integrity. Additionally, biologically active collagen fragments, such as the NC1 domain, can also ‘de-stabilizes’ the BTB, working in concert with the laminin fragment, perhaps via FAK and c-Src at the site. More important, testosterone, which is known to promote BTB integrity, can enhance de novo synthesis of TJ-proteins (e.g., occludin), as well as promoting transcytosis of endocytosed proteins from the ‘old’ TJ-fibrils above the primary spermatocyte in transit at the BTB to the ‘new’ TJ-fibrils beneath the cell. Furthermore, as discussed in the text, TNFα, was also shown to induce androgen receptor expression (see text for detail). Thus, TNFα can promote the assembly of new BTB behind a primary spermatocyte in transit while it perturbs the ‘old’ TJ-fibril above the migrating germ cell. In short, this novel functional axis in the seminiferous epithelium coordinates the events of spermiation and BTB restructuring which take place concurrently at stage VIII of the epithelial cycle. It is anticipated that this model will be updated in the years to come based on additional findings in the field. This model, however, serves as a reference for investigators in the field.
The seminiferous epithelium in the rat testis is segregated into the basal and the apical (adluminal) compartment by the blood-testis barrier (BTB) which is created by adjacent Sertoli cells via co-existing tight junction (TJ), basal ectoplasmic specialization [a testis-specific atypical adherens junction (AJ) type], and desmosome-like junction (for a review, see [8]) (see Fig. 1). Mitosis takes place in the basal compartment in which type A spermatogonia undergo mitosis to produce additional germ cells, some of which differentiate into type B spermatogonia and primary preleptotene spermatocytes [1-2]. Preleptotene spermatocytes are the germ cells in transit at the BTB at stages VIII-IX of the seminiferous epithelial cycle in rats while differentiating into leptotene and zygotene spermatocytes. Once the primary spermatocytes enter the apical compartment, they differentiate into diplotene spermatocytes and undergo diakinesis at stages X-XIII. These spermatocytes then enter metaphase I to undergo meiosis I, to be followed immediately by meiosis II to form haploid spermatids at stage XIV of the epithelial cycle in rat testes [9]. Thus, there are extensive restructuring of cell junctions at the Sertoli-Sertoli cell and Sertoli-spermatocyte interface to facilitate the transit of primary spermatocytes at stages VIII-IX of the epithelial cycle. Once spermatids are formed, they undergo an extensive phase of developmental changes known as spermiogenesis, which is typified by the condensation of the genetic material to form the nucleus in the spermatid head, the formation of the acrosome above the spermatid head, elongation of the tail, and the packaging of the mitochondria into the mid-piece. Spermatids can be classified into steps 1 to 19 in rats depending on the phases of their development [1,9]. At stage VIII of the epithelial cycle, fully developed spermatids (i.e., spermatozoa) undergo spermiation, thus, spermatozoa detach from the seminiferous epithelium, entering the tubular lumen for their further maturation in the epididymis. This thus involves disruption of the cell adhesion complex at the elongated spermatid-Sertoli cell interface.
While these morphological changes during spermatogenesis are known for almost six decades [10-12], the molecular and biochemical mechanism(s) that regulate and/or coordinate these junction restructuring events that take place at the opposite ends of the Sertoli cell epithelium, namely BTB restructuring and spermiation, remain largely unexplored and unknown until recently. In this short review, we attempt to provide a critical discussion based on recent findings in the field regarding the regulation of these events in the microenvironment of the seminiferous epithelium. For instance, biologically active laminin peptides generated locally at the apical ectoplasmic specialization (apical ES) during spermiation can regulate BTB restructuring [13], and coordinate these two seemingly unrelated events in the seminiferous epithelium that occur simultaneously at stage VIII of the epithelial cycle (Fig. 1). We also discuss recent findings that components of the polarity protein complexes (e.g., Par3, Par6, and 14-3-3 also known as Par5) at the BTB [14-15] and proteins (e.g., β1-integrin) at the hemidesmosome [13] also play a crucial role to maintain the homeostasis of the BTB during spermatogenesis (Fig. 1). Taken collectively, these data illustrate the presence of an autocrine/paracrine apical ES-BTB-hemidesmosome functional axis to regulate the local cellular events in the microenvironment of the seminiferous epithelium during spermatogenesis (Fig. 1). These findings also illustrate several potential targets that can be manipulated to induce infertility and to understand the mechanism(s) by which environmental toxicants induce reproductive dysfunction in males.
2. Ectoplasmic specialization (ES)
ES is an atypical adherens junction (AJ) type uniquely found in the testis, which appears at the interface between Sertoli cells and step 8 spermatids during spermiogenesis known as apical ES (for recent reviews, see [16-18]). Once apical ES appears, it is the only anchoring device present between these cells which persists through step 19 spermatids in rat testes until spermiation (for a review, see [16]). However, ES is also found between Sertoli cells at the BTB, coexisting with TJ, gap junction and desmosome-like junction to constitute the BTB [18]. Ultrastructurally, ES is typified by the presence of actin filament bundles sandwiched between cisternae of endoplasmic reticulum and the plasma membrane of the Sertoli cell with this feature limited only to the Sertoli cell side at the apical ES, but they are present on both sides of the two adjacent Sertoli cells at the basal ES at the BTB. Some of the known adhesion protein complexes and their peripheral adaptors at the apical ES and basal ES are cadherin/catenin, and nectin/afadin [19]. The best studied cell adhesion complex at the apical ES, however, is the laminin 333-α6β1-integrin in the rat testes wherein laminin α3β3γ3 chains are restricted to the elongating/elongated spermatids [20] with the α6β1-integrin residing on Sertoli cells [21-22]. Studies by immunohistochemistry and dual-labeled immunofluorescence analysis have demonstrated laminins α3 and γ3 chains co-localized to the same site at the apical ES, most predominantly expressed at stages VII-VIII tubules prior to spermiation [20]. This pattern of stage-specific localization in the seminiferous epithelium of adult rat testes coincided with MMP-2 as earlier reported [23], implicating MMP-2 can proteolytically cleave the laminins at the apical ES at spermiation in stage VIII of the seminiferous epithelial cycle (Fig. 1).
3. The blood-testis barrier (BTB)
The BTB is an important ultrastructure in the seminiferous epithelium of mammalian testes. In rats, the BTB forms at ~ages 15-17 post-partum created by adjacent Sertoli cells near the basement membrane [19]. Under electron microscopy, the BTB is composed of TJ, basal ES, gap junction and desmosome-like junction that forms one of the tightest blood-tissue barriers in mammals [24-26]. Some of the known adhesion protein complexes that constitute the BTB are shown in Figs. 1 and 2. Recent studies have shown that androgens (e.g., testosterone) are crucial to maintain the integrity of the BTB [27-30]. For instance, testosterone was shown to facilitate the reassembly of the BTB following exposure of the Sertoli cell BTB to cadmium [31], an environmental known to induce testicular injury via its rapid and disruptive effects on the BTB [32] by causing disruption of the TJ-fibrils at the site [33] (for a review, see [34]). Recent studies have also demonstrated that the occludin/ZO-1/FAK is a crucial regulatory complex at the BTB [35], and a loss of function of FAK in this complex by RNAi using FAK-specific siRNA duplexes in Sertoli cells cultured in vitro with an established TJ-barrier can render the BTB to become insusceptible to cadmium-induced BTB disruption [31]. Additionally, a disruption of the gap junction function by silencing connexin 43 and plakophilin-2 simultaneously, but not connexin 43 alone, a novel protein complex at the BTB, by RNAi can also perturb protein distribution at the Sertoli-Sertoli cell interface, such as the occludin-ZO-1 protein complex, moving from the cell surface to cytosol, transiently disrupt the Sertoli cell TJ-permeability barrier when assessed by transepithelial electrical resistance (TER) across the Sertoli cell epithelium [36]. This recent finding illustrates not only the significance of the connexin 43/plakophilin-2 protein complex at the BTB, it demonstrates the physiological significance for the co-existing junction types at the BTB. For instance, it is likely that the gap junction and desmosome-like junction provide the necessary cross-talk to coordinate the function of multiple junctions at the BTB to effectively safe-guard the immunological barrier function during the transit of primary spermatocytes at the BTB during the seminiferous epithelial cycle, such as at stages VIII-IX.
Figure 2. A local regulatory mechanism to facilitate the transit of primary preleptotene spermatocytes at the BTB while maintaining its immunological barrier function.
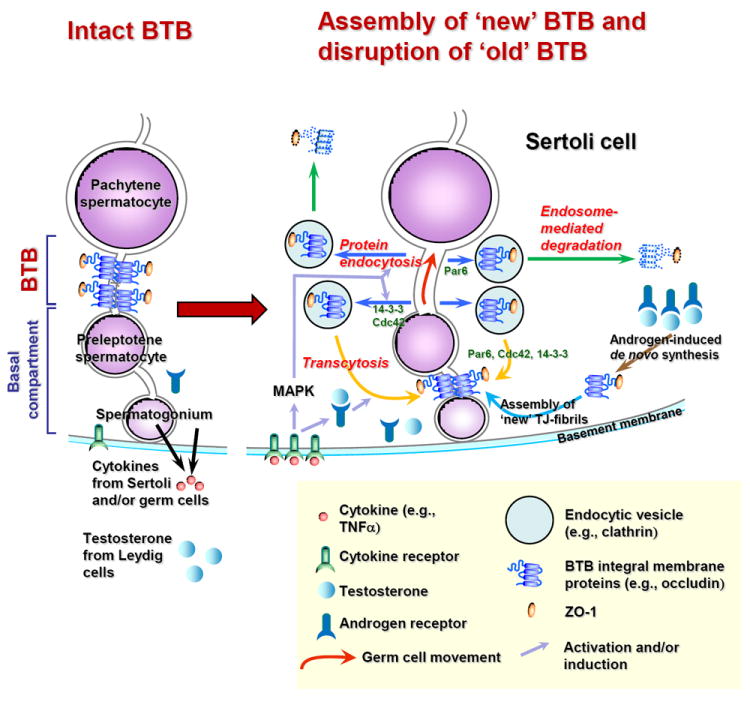
As discussed in detail in the text, this model illustrates the coordinated effects of cytokines (e.g., TNFα, TGF-β2, TGF-β3) and testosterone, involving polarity proteins (e.g., Par3, Par6, 14-3-3, Cdc42) on junction dynamics in the testis. This model also illustrates the intricate interactions of cytokines, testosterone and polarity proteins on protein endocytosis, endosome-mediated degradation and transcytosis (recycling) and de novo synthesis to facilitate the transit of primary preleptotene spermatocytes at the BTB while the immunological barrier function can be maintained.
4. Basement membrane
In mammalian testes, the basement membrane is a modified form of extracellular matrix, also called basal lamina, that is known to regulate Sertoli cell function (for a review, see [37]). The basement membrane is composed of two major building blocks: type IV collagen and laminins (for a review, see [38]). Recent studies have shown that the inclusion of an anti-collagen antibody in Sertoli cells cultured in vitro with an establish TJ-barrier could reversible perturb the TER across the cell epithelium [39], illustrating a blockade of the collagen function (such as scaffold) can perturb the Sertoli cell BTB function. This finding also illustrates the presence of functional cross-talk between the basement membrane and the Sertoli cell in the seminiferous epithelium. While the basement membrane also maintains pools of proteases, protease inhibitors, cytokines and growth factors, many of which are known regulators of Sertoli cell functions, such as BTB integrity and cell cycle progression, very few studies are found in the literature to define the role(s) of basement membrane in spermatogenesis. In one of these few studies, TNFα was shown to induce Sertoli cell MMP-9 production and promote the activation pro-MMP-9 [39]. As collagen α3(IV) chain is a putative substrate of MMP-9, and fragments of collagen chain (e.g., its non-collagenous domain 1, NC1 domain) are biologically active peptides that are known to regulate multiple biological events including cell movement at the cell-matrix focal adhesion complex (also known as focal contact) (for a review, see [38]). Thus, we have speculated that TNFα in the basement membrane can regulate Sertoli cell BTB function via its effects on the MMP-9, which can generate biologically active fragments (e.g., NC1 domain) from collagen a3 (IV) chain to regulate Sertoli cell TJ-permeability barrier (Fig. 1). This is an area of research that should be functionally addressed in future studies.
5. Hemidesmosome
While focal contact (a cell-matrix actin-based anchoring junction type, also known as focal adhesion complex) is not found in the testis, hemidesmosome (a cell-matrix intermediate filament-based anchoring junction) is readily detected in the testis by electron microscopy [3,38]. However, it biochemical composition remains unexplored. Recent study has shown that laminin α2 chain and β1-integrin are two putative components of the hemidesmosome in the rat testis [13]. Moreover, a disruption of the β1-integrin function by RNAi at the hemidesmosome in Sertoli cells cultured in vitro appears to perturb the Sertoli cell TJ-permeability barrier [13], illustrating a blockade of hemidesmosome function can perturb cross-talk between the two ultrastructures namely BTB and hemidesmosome in the testis. In fact, studies in cancer biology have supported a functional axis between hemidesmosome and the blood-tissue barrier. For instance, during TJ disruption and a loss of cell polarity at the time of oncogenesis, an increase in interactions between TJ-associated proteins (e.g., ErbB, an epidermal growth factor receptor variant) and α6β4-integrin in the hemidesmosome was detected, this ‘newly’ formed protein complex, in turn, served as the docking platform for signal transduction to promote cell proliferation [40]. Much research is needed to explore the functional significance of hemidesmosome in spermatogenesis, in particular its cross-talk with BTB and perhaps apical ES during the seminiferous epithelial cycle.
6. The apical ES-BTB-hemidesmosome functional axis
As discussed in section 2, biologically active laminin fragments are likely generated at spermiation via the action of MMP-2 on the laminin chains at the apical ES [13,23] (Fig. 1). Indeed, recombinant protein fragments of both laminin β3 (e.g., domain I) and γ3 chains (e.g., domain IV) were capable of perturbing the Sertoli cell TJ-permeability barrier and/or the steady-state levels of BTB-associated proteins occludin and JAM-A, and hemidesmosome-associated protein β1-integrin [13]. Additionally, these findings were validated by overexpression of laminin γ3 domain IV or laminin β3 domain I in primary Sertoli cells cultured in vitro with an established TJ-barrier that mimics the BTB in vivo [13]. These findings illustrate the presence of a functional axis between the apical ES and BTB via the production of an autocrine-like laminin peptide fragment at the apical ES at spermiation near the luminal edge of the seminiferous epithelium, which can directly modulate the BTB function near the basement membrane (Fig. 1). Since the expression of β1-integrin, hemidesmosome-associated protein, in these primary Sertoli cell cultures with a functional TJ-barrier was shown to be inhibited by transient overexpression of laminin fragments or following the inclusion of the recombinant proteins in the media, these findings suggest the likely presence of a functional loop between the apical ES/BTB and the hemidesmosome that the laminin peptide fragment can also indirectly modulate the BTB function. Indeed, a knock-down of β1-integrin by using specific siRNA duplexes versus non-targeting control siRNA duplexes has shown that a disruption of the hemidesmosome function leads to a transient disruption of the Sertoli cell barrier function [13]. We thus propose the presence of a functional apical ES-BTB-hemidesmosome axis in the seminiferous epithelium that can coordinate and regulate the two cellular events namely apical ES disruption at spermiation and BTB restructuring to facilitate the transit of primary preleptotene spermatocytes that occur at the opposite ends of the seminiferous epithelium at stage VIII of the epithelial cycle (Fig. 1).
7. Cytokines and testosterone on junction restructuring in the testis
Cytokines, such as TNFα, TGF-β2, and TGF-β3, and testosterone are important regulators of testicular functions including junction dynamics in the testis (for reviews, see [3,38,41-42]). We herein provide brief background information for these molecules and summarize recent findings in the field pertinent to their role in regulating spermatogenesis in particular junction dynamics in the testis.
7.1. TNFα (tumor necrosis factor-α)
TNFα was initially identified as a cytokine produced by endotoxin-stimulated macrophages that induced necrosis of transplanted tumors [43]. TNFα (~50 kDa) is a homotrimer consisting of three identical subunits of ~17 kDa each. In the mammalian testis, such as rodents, TNFα is a product of interstitial macrophages, round and elongating spermatids, pachytene spermatocytes [44] and Sertoli cells [39]. TNFα binds to either one of its two receptors: TNFR1(p55, CD120a) and TNFR2 (p75, CD120b), which are mostly restricted to Sertoli and Leydig cells in the testis (but apoptotic germ cells express TNRF1). TNFα exerts its biological effects by regulating inflammatory responses, such as stimulating IL-1 and IL-6 production [45]. It is noted that the expression of TNFα receptor by Sertoli cells is induced by FSH [46]. TNFα is also known to induce cell death (e.g., germ cell apoptosis [47]) via its binding to TNFR1, through the subsequent interaction with the TNFR-associated death domain protein (TRADD) or the Fas-associated death domain protein (FADD), to be followed by an activation of the caspase-dependent apoptotic pathway (e.g., procaspase 8) [48] or the mitochondrial pathway such as Bid and Bax [49]. Indeed, TNFR1-positive germ cells are apoptotic, TNFα can also trigger germ cell apoptosis by acting together with the local cell death regulatory systems, such as the Fas-Fas ligand system [50]. The binding of TNFα to TNFR1 can induce proinflammatory response (e.g., cell proliferation) by activating NF-κB and possibly p38 MAPK in multiple cell epithelia [42,51]. TNFα is an inhibitor of Leydig cell steroidogenesis via the NFκB signaling pathway [52] by blocking the expression and/or protein production of Leydig cell steroidogenic enzymes and/or proteins, such as P450scc, P450c17, 3β-hydroxysteroid dehydrogenase [53-55] and steroidogenic acute regulatory protein (StAR) [56]. However, TNFα also stimulates Sertoli cell androgen receptor expression by up-regulating NFκB [57]. Additionally, TNFα enhances the expression of plasminogen activator inhibitor-1 (PAI1) by peritubular myoid cells in the testis [58], illustrating its involvement in the homeostasis of proteolytic events (e.g., phagocytic reabsorption and proteolytic degradation of residual bodies by Sertoli cells at spermiation) in the seminiferous epithelium during spermatogenesis. Recent studies have also demonstrated that TNFα perturbs blood-retinal barrier, blood-intestine barrier, and Sertoli cell BTB (for a review, see [38]). TNFα was shown to disrupt the Sertoli cell BTB integrity by first activating matrix metalloprotease-9 (MMP-9) in the basement membrane, which, in turn, cleaved the collagen α3(V) chain (a major component of the basement membrane), perturbing the scaffolding function of the basal lamina, leading to BTB disruption [39]. Administration of TNFα to adult rat testes in vivo was shown to induce reversible BTB disruption [59], confirming it reversible disruptive effects on the Sertoli cell TJ-permeability barrier in vitro [39]. In short, TNFα has multiple biological effects on any somatic cells (e.g., Sertoli cells) and/or germ cells in the testis and other organs, thus, its stimulatory, inhibitory, pro-apoptotic, pro-inflammatory, or destructive effects in cell epithelia depends on the receptor subtype engaged, and the expression and/or presence of specific adaptors or target proteins within the cell.
7.2. Transforming growth factor-β2 and −β3 (TGF-β2 and −β3)
TGF-β1, -β2 and −β3 are homodimers of ~25 kDa consisting of two identical subunits of ~12 kDa, are their receptors (type I, TβRI, 55 kDa; and type II, TβRII, 85 kDa) are found in the testes and they are crucial to spermatogenesis (for reviews, see [60-62]. In adult testes, TGF-βs are expressed in early spermatids, pachytene spermatocytes, Sertoli cells, peritubular myoid cells and Leydig cells [60,62]. However, TGF-β2 and −β3, but not TGF-β1, were shown to have disruptive but reversible effects on the Sertoli cell TJ-permeability barrier function, apparently mediated via the p38 MAPK signaling pathway [63-64]. Subsequent in vivo studies have confirmed the earlier in vitro results illustrating the reversible disruptive effects of TGF-β3 on the BTB integrity is mediated via the p38 MAPK signaling pathway [65-67]. Moreover, TGF-β2 or −β3 induces its disruptive effects on the BTB integrity via enhancing the kinetics of clathrin-mediated internalization of occludin, JAM-A and/or N-cadherin at the BTB using techniques of biotinylation and endocytosis assay [67-68]. More important, TGF-β3 targets the endocytosed proteins to the endosome-mediated intracellular degradation pathway, such that the endocytosed proteins (e.g., occludin) associated more extensively with Rab 9, a late endosome marker, reducing the steady-state level of integral membrane proteins at the BTB, thereby compromising the TJ-barrier integrity [68].
7.3. Testosterone
The role of testosterone in regulating and maintaining spermatogenesis is known for decades [2,69]. For instance, the intratesticular testosterone levels found in rete testis fluid and seminiferous tubule fluid in both men and rodents are 50-100 fold higher than those found in the systemic circulation [70-71], which apparently is needed to maintain germ cell development and normal Sertoli cell physiology in the seminiferous epithelium. Even though androgen receptor is mostly restricted to Sertoli cells in males [72] to mediate androgen action, germ cells are intimately associated with Sertoli cells in the seminiferous epithelium via specialized cell junctions (e.g., gap junctions) and cytoplasmic bridges [73] through which, chemical signals, biological factors, and/or biomolecules induced by androgen can be transported back-and-forth between these cells. Besides genomic action, recent studies have shown that androgen can also exert its effects via non-genomic action without involving the androgen receptor by activating c-Src/ERK1/2 in the EGFR (epidermal growth factor receptor) signaling pathway (for a recent review, see [4]). It is known that testosterone is crucial to maintain elongating/elongated spermatid adhesion in the testis, in particular the apical ES in adult testes [74-76], possibly involving phosphorylated (activated)-FAK [77-78]. Also, testosterone is important to maintain the BTB integrity in rodents [27-28]. Recent studies have shown that testosterone, similar to cytokines (e.g., TGF-β2, TGF-β3 and TNFα), also enhance the endocytosis of integral membrane proteins at the BTB (e.g., occludin, N-cadherin), however, the endocytosed proteins are rapidly recycled back to the Sertoli cell surface, perhaps to a different cellular location via transcytosis [68]. Yet, cytokine-induced endocytosed proteins are targeted to endosome-mediated degradation as illustrated by their increased association with the late endosome marker Rab9 [68].
7.4. The coordinated effects of cytokines and testosterone to facilitate the transit of primary preleptotene spermatocytes at the BTB while maintaining the immunological barrier function
As discussed above, cytokines and testosterone have opposing effects on the integrity of the junctional complexes in the testes with cytokines (e.g., TNFα, TGF-β3) promote BTB disruption [39,59,65,67,79] and testosterone promotes BTB assembly and/or maintenance [27-28], respectively. The findings recently reported [67-68] and summarized above regarding the differential effects of cytokines (e.g., TGF-β2, TGF-β3, TNFα) and testosterone on protein endocytosis, recycling and endosome-mediated degradation in Sertoli cell BTB are significant. Collectively, these findings illustrate that cytokines are likely working in concert with testosterone to assemble “new” TJ-fibrils behind a primary preleptotene spermatocyte in transit at the BTB via the action of testosterone or TNFα-induced androgen receptor production to: (i) augment androgen action by inducing de novo synthesis of BTB integral membrane proteins [30] and/or via (ii) promote transcytosis [68] by relocating integral membrane proteins from the “old” to the “new” BTB site; whereas cytokines (e.g., TGF-β2, TGF-β3, TNFα) induce the dissolution of the “old” TJ-fibrils via enhanced endocytosis and endosome-mediated degradation above the spermatocyte in transit (see Fig. 2). Using such an efficient mechanism, the immunological barrier can be maintained during the transit of preleptotene spermatocyte at the BTB at stage VIII of the epithelial cycle (Fig. 2). It is likely that polarity proteins, such as Par3, Par6, and 14-3-3 are involved in these events since these proteins were recently shown to be involved in protein endocytosis at the Sertoli cell BTB [14-15] (Fig. 2). It is likely that testosterone- and/or cytokine-induced endocytosis and the subsequent recycling or endosome-mediated degradation is facilitated by these polarity proteins. For instance, it was shown that the transient knock-down of Par3 or Par6 in Sertoli cells cultured in vitro with an established TJ-permeability barrier that mimics the BTB in vivo caused a mislocalization of JAM-A and/or N-cadherin, the corresponding integral membrane protein at the TJ and basal ES at the BTB in rat testes, from the Sertoli cell surface to cytosol, inducing transient TJ-barrier disruption [14]. Subsequent studies have shown that this mislocalization is likely the result of an enhanced protein endocytosis induced by 14-3-3 [15]. These findings also illustrate that polarity proteins are working in concert with protein complexes at the BTB to modulate BTB restructuring during the seminiferous epithelial cycle of spermatogenesis.
8. Future perspectives
We have herein reported the presence of a novel functional axis in the seminiferous epithelium of adult rat testes known as the apical ES-BTB-hemidesmosome axis or the apical ES-BTB-basement membrane axis (Fig. 1), illustrating cross-talk between the different cellular ultrastructures in the seminiferous epithelium to coordinate different cellular events during the epithelial cycle. Obviously, there are many open questions remain to be addressed in future studies. For instance, what is the precise biochemical composition of the hemidesmosome in adult testes besides laminin α2 and β1-integrin? What is the downstream signaling event of β1-integrin at the hemidesmosome following its activation by the biologically active laminin fragments? Is there any cross-talk between β1-integrin found at the apical ES and hemidesmosome in the testis? What is the target protein(s) at the BTB that is being activated by the biologically active laminin fragments; is that the FAK in the recently identified occludin/ZO-1/FAK protein complex [31,35]? What is the physiological and/or functional relationship between cytokines and testosterone that modulate BTB restructuring? Can cytokines modulate androgen receptor expression by Sertoli cells? What is the biological mechanism(s) that generates biologically active laminin fragments and/or collagen fragments to regulate BTB function directly and/or indirectly via hemidesmosome? The two models depicted herein (see Figs. 1 and 2) will also serve as the basis for many future functional studies, and many of the above open questions can now be tackled. It is obvious that recent advances in cell and molecular biology and biochemistry have yielded unprecedented opportunities for investigators in the field for the years to come.
Footnotes
The writing of this review was supported by grants from the National Institutes of Health (NICHD, R01 HD056034; U54 HD029990 Project 5) to CYC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Kretser D, Kerr J. The cytology of the testis. In: Knobil E, et al., editors. The Physiology of Reproduction. Raven Press; New York: 1988. pp. 837–932. [Google Scholar]
- 2.Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neil JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 1363–1434. [Google Scholar]
- 3.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 4.Walker WH. Molecular mechanisms of testosterone action in spermatogenesis. Steroids. 2009;74:602–607. doi: 10.1016/j.steroids.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Shaha C. Estrogens and spermatogenesis. In: Cheng CY, Austin TX, editors. Molecular Mechanisms in Spermatogenesis. Landes Bioscience and Springer Science+Business Media; 2008. pp. 42–64. [Google Scholar]
- 6.Carreau S. Estrogen: Roles in spermatogenesis. Immun Endocr Metab Agents Med Chem. 2008;8:59–65. [Google Scholar]
- 7.Hess RA. Estrogen in the adult male reproductive tract: A review. Reprod Biol Endocrinol. 2003;1(52):1–14. doi: 10.1186/1477-7827-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 9.Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3:404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- 10.LeBlond C, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann NY Acad Sci. 1952;55:548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- 11.Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–235. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- 12.Clermont Y, Oko R, Hermo L. Cell biology of mammalian spermatogenesis. In: Desjardins C, Ewing L, editors. Cell and Molecular Biology of the Testis. Oxford University Press; New York: 1993. pp. 332–376. [Google Scholar]
- 13.Yan HHY, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong EWP, Sun S, Li MWM, Lee WM, Cheng CY. 14-3-3 protein is a cell adhesion regulator in the seminiferous epithelium of rat testes. Endocrinology. 2009 doi: 10.1210/en.2009-0427. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan HHY, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis? BioEssays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan HHY, Mruk DD, Cheng CY. Junction restructuring and spermatogenesis: The biology, regulation, and implication in male contraceptive development. Curr Top Dev Biol. 2008;80:57–92. doi: 10.1016/S0070-2153(07)80002-0. [DOI] [PubMed] [Google Scholar]
- 19.Mruk DD, Cheng CY. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan HHY, Cheng CY. Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- 21.Palombi F, Salanova M, Tarone G, Farini D, Stefanini M. Distribution of β1 integrin subunit in rat seminiferous epithelium. Biol Reprod. 1992;47:1173–1182. doi: 10.1095/biolreprod47.6.1173. [DOI] [PubMed] [Google Scholar]
- 22.Salanova M, Ricci G, Boitani C, Stefanini M, De Grossi S, Palombi F. Junctional contacts between Sertoli cells in normal and aspermatogenic rat seminiferous epithelium contain α6β1 integrins, and their formation is controlled by follicle-stimulating hormone. Biol Reprod. 1998;58:371–378. doi: 10.1095/biolreprod58.2.371. [DOI] [PubMed] [Google Scholar]
- 23.Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70:945–964. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- 24.Setchell BP. Blood-testis barrier, junctional and transport proteins and spermatogenesis. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience/Springer Science; 2008. pp. 212–233. [DOI] [PubMed] [Google Scholar]
- 25.Vogl A, Vaid K, Guttman J. The Sertoli cell cytoskeleton. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience/Springer Science; 2008. pp. 186–211. [Google Scholar]
- 26.Yan HHN, Mruk DD, Lee WM, Cheng CY. Cross-talk between tight and anchoring junctions - lesson from the testis. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience and Springer Science+Business Media; 2008. pp. 234–254. [Google Scholar]
- 27.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci USA. 2005;102:16696–16670. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, Wu CC, di Sant’Agnese PA, deMesy-Bentley KL, Tzeng CR, Chang C. Androgen receptor in Sertoli cell is essential for germ cell nursery and junction complex formation in mouse testes. Endocrinology. 2006;147:5624–5633. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- 29.Janecki A, Jakubowiak A, Steinberger A. Regulation of transepithelial electrical resistance in two-compartment Sertoli cell cultures: in vitro model of the blood-testis barrier. Endocrinology. 1991;129:1489–1496. doi: 10.1210/endo-129-3-1489. [DOI] [PubMed] [Google Scholar]
- 30.Chung NPY, Cheng CY. Is cadmium chloride-induced inter-Sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142:1878–1888. doi: 10.1210/endo.142.5.8145. [DOI] [PubMed] [Google Scholar]
- 31.Siu ER, Wong EWP, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood-testis barrier regulator. Proc Natl Acad Sci USA. 2009;106:9298–9303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the “blood-testis barrier” after injection of cadmium chloride in the rat. J Endocrinol. 1970;47:81–86. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- 33.Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol Reprod. 1993;49:840–849. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- 34.Siu ER, Mruk DD, Porto CS, Cheng CY. Cadmium-induced testicular injury. Toxicol Appl Pharmacol. 2009;238:240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siu ER, Wong EWP, Mruk DD, Sze KL, Porto CS, Cheng CY. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology. 2009;150:3336–3344. doi: 10.1210/en.2008-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a signaling complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci USA. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dym M. Basement membrane regulation of Sertoli cells. Endocr Rev. 1994;15:102–115. doi: 10.1210/edrv-15-1-102. [DOI] [PubMed] [Google Scholar]
- 38.Siu MKY, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. BioEssays. 2004;26:978–992. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- 39.Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-α gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloprotease-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 40.Carraway C, KL C. Sequestration segregation of receptor kinases in epithelial cells: Implications for ErbB2 oncogenesis. SciSTEK. 2007;381:re3. doi: 10.1126/stke.3812007re3. [DOI] [PubMed] [Google Scholar]
- 41.Skinner M. Secretion of growth factors and other regulatory factors. In: Russell L, Griswold M, editors. The Sertoli Cell. Cache River Press; Clearwater: 1993. pp. 237–247. [Google Scholar]
- 42.O’Bryan MK, Hedger MP. Inflammatory networks in the control of spermatogenesis. Chronic inflammation in an immunologically privileged tissue? In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin, TX: Landes Bioscience and Springer Science+Business Media; 2008. pp. 92–114. [DOI] [PubMed] [Google Scholar]
- 43.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De S, Chen H, Pace J, Hunt J, Terranova P, Enders G. Expression of tumor necrosis factor-α in mouse spermatogenic cells. Endocrinology. 1993;133:389–396. doi: 10.1210/endo.133.1.8319585. [DOI] [PubMed] [Google Scholar]
- 45.Cerami A. Inflammatory cytokines. Clin Immunol Immunopath. 1992;62:S3–S10. doi: 10.1016/0090-1229(92)90035-m. [DOI] [PubMed] [Google Scholar]
- 46.Mauduit C, Besset V, Caussanel V, Benahmed M. Tumor necrosis factor α receptor p55 is under hormonal (follicle-stimulating hormone) control in testicular Sertoli cells. Biochem Biophys Res Commun. 1996;224:631–637. doi: 10.1006/bbrc.1996.1077. [DOI] [PubMed] [Google Scholar]
- 47.Theas MS, Rival C, Jarazo-Dietrich S, Jacobo P, G VA, Lustig L. Tumor necrosis factor-α released by testicular macrophages induces apoptosis of germ cells in autoimmune orchitis. Human Reprod. 2008;23:1865–1872. doi: 10.1093/humrep/den240. [DOI] [PubMed] [Google Scholar]
- 48.Hsu H, Shu HB, Pan MG, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathway. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 49.Pei Y, Xing D, Gao X, L L, Chen T. Real-time monitoring full length bid interacting with Bax during TNF-α-induced apoptosis. Apoptosis. 2007;12:1681–1690. doi: 10.1007/s10495-007-0091-7. [DOI] [PubMed] [Google Scholar]
- 50.Riccioli A, Starace D, D’Alessio A, Starace G, Padula F, De Cesaris P, Filippini A, Ziapro E. TNF-α and IFN-γ regulate expression and function of the Fas system in the seminiferous epithelium. J Immunol. 2000;165:743–749. doi: 10.4049/jimmunol.165.2.743. [DOI] [PubMed] [Google Scholar]
- 51.Idriss HT, Naismith JH. TNFα and the TNF receptor superfamily: structure-funciton relationship(s) Microsc Res Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 52.Hong CY, Park JH, Ahn RS, Im SY, Choi HS, Soh J, Mellon SH, Lee K. Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor nectrosis factor α. Mol Cell Biol. 2004;24:2593–2604. doi: 10.1128/MCB.24.7.2593-2604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X, Younblood GL, Payne AH, Hales DB. Tumor necrosis factor-α inhibition of 17α-hydroxylase/C17-20 lyase gene (Cyp17) expression. Endocrinology. 1995;136:3519–3526. doi: 10.1210/endo.136.8.7628389. [DOI] [PubMed] [Google Scholar]
- 54.Xiong Y, Hales D. The role of tumor necrosis factor-α in the regulation of mouse Leydig cell steroidogenesis. Endocrinology. 1993;132:2438–2444. doi: 10.1210/endo.132.6.8504748. [DOI] [PubMed] [Google Scholar]
- 55.Xiong Y, Hales DB. Immune-endocrine interactions in the mouse testis: Cytokine-mediated inhibition of Leydig cell steroidogenesis. Endocrine. 1994;2:223–228. [Google Scholar]
- 56.Mauduit C, Gasnier F, Rey C, Chauvin MA, Stocco DM, Louisot P, Benahmed M. Tumor necrosis factor-α inhibits Leydig cell steroidogenesis through a decrease in steroidogenic acute regulatory protein expression. Endocrinology. 1998;139:2863–2868. doi: 10.1210/endo.139.6.6077. [DOI] [PubMed] [Google Scholar]
- 57.Delfino FJ, Boustead JN, Fix C, Walker WH. NF-κB and TNFα stimulate androgen receptor expression in Sertoli cells. Mol Cell Endocrinol. 2003;201:1–12. doi: 10.1016/s0303-7207(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 58.Le Magueresse-Battistoni B, Pernod G, Kolodie L, Morera A, Benahmed M. Tumor necrosis factor-α regulates plasminogen activator inhibitor-1 in rat testicular peritubular cells. Endocrinology. 1997;138:1097–1105. doi: 10.1210/endo.138.3.4963. [DOI] [PubMed] [Google Scholar]
- 59.Li MWM, Xia W, Mruk DD, Wang CQF, Yan HHY, Siu MKY, Lui WY, Lee WM, Cheng CY. TNFα reversibly disrupts the blood-testis barrier and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- 60.Lui W, Lee W, Cheng C. TGF-βs: their role in testicular function and Sertoli cell tight junction dynamics. Int J Androl. 2003;26:147–160. doi: 10.1046/j.1365-2605.2003.00410.x. [DOI] [PubMed] [Google Scholar]
- 61.Loveland KL, Dias V, Meachem S, Rajpert-De Meyts E. The transforming growth factor-β superfamily in early spermatogenesis: Potential relevance to testicular dysgenesis. Int J Androl. 2007;30:377–384. doi: 10.1111/j.1365-2605.2007.00785.x. [DOI] [PubMed] [Google Scholar]
- 62.Guazzone VA, Jacobo P, Theas M, Lustig L. Cytokines and chemokines in testicular inflammation: A brief review. Microsc Res Techn. 2009 doi: 10.1002/jemt.20704. in press. [DOI] [PubMed] [Google Scholar]
- 63.Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–1877. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- 64.Lui WY, Lee WM, Cheng CY. Transforming growth factor-β3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod. 2003;68:1597–1612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- 65.Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-β3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- 66.Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- 67.Xia W, Wong EWP, Mruk DD, Cheng CY. TGF-β3 and TNFα perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: A new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan HHY, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zirkin BR. Spermatogenesis: its regulation by testosterone and FSH. Semin Cell Dev Biol. 1998;9:417–421. doi: 10.1006/scdb.1998.0253. [DOI] [PubMed] [Google Scholar]
- 70.Turner T, Jones C, Howards S, Ewing L, Zegeye B, Gunsalus G. On the androgen microenvironment of maturing spermatozoa. Endocrinology. 1984;115:1925–1932. doi: 10.1210/endo-115-5-1925. [DOI] [PubMed] [Google Scholar]
- 71.Jarow JP, Zirkin BR. The androgen microenvironment of teh human testis and hormonal control of spermatogenesis. Ann NY Acad Sci. 2005;1061 doi: 10.1196/annals.1336.023. [DOI] [PubMed] [Google Scholar]
- 72.Griswold MD, Heckert L, Linder C. The molecular biology of the FSH receptor. J Steroid Biochem Mol Biol. 1995;53:215–218. doi: 10.1016/0960-0760(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 73.Fawcett DW. Intercellular bridges. Exp Cell Res. 1961;8:174–187. doi: 10.1016/0014-4827(61)90347-0. [DOI] [PubMed] [Google Scholar]
- 74.Beardsley A, O’Donnell L. Characterization of normal spermiation and spermiation failure induced by hormone suppression in adult rats. Biol Reprod. 2003;68:1299–1307. doi: 10.1095/biolreprod.102.009811. [DOI] [PubMed] [Google Scholar]
- 75.Zhang J, Wong CH, Xia W, Mruk DD, Lee NPY, Lee WM, Cheng CY. Regulation of Sertoli-germ cell adherens junction dynamics via changes in protein-protein interactions of the N-cadherin-β-catenin protein complex which are possibly mediated by c-Src and myotubularin-related protein 2: An in vivo study using an androgen suppression model. Endocrinology. 2005;146:1268–1284. doi: 10.1210/en.2004-1194. [DOI] [PubMed] [Google Scholar]
- 76.Xia W, Wong CH, Lee NPY, Lee WM, Cheng CY. Disruption of Sertoli-germ cell adhesion function in the seminiferous epithelium of the rat testis can be limited to adherens junctions without affecting the blood-testis barrier integrity: An in vivo study using an androgen suppression model. J Cell Physiol. 2005;205:141–157. doi: 10.1002/jcp.20377. [DOI] [PubMed] [Google Scholar]
- 77.Beardsley A, Robertson DM, O’Donnell L. A complex containing α6β1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J Endocr. 2006;190:759–770. doi: 10.1677/joe.1.06867. [DOI] [PubMed] [Google Scholar]
- 78.Siu MKY, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of β1-integrin and focal adhesion complex (FAC)-associated proteins. Endocrinology. 2003;144:2141–2163. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- 79.Xia W, Mruk DD, Lee WM, Cheng CY. Differential interactions between transforming growth factor-β3/TβR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem. 2006;281:16799–16813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]


