Abstract
Bisphenol A, an estrogenic environmental toxicant, has been implicated to have hazardous effects to reproductive health in humans and rodents. However, there are conflicting reports in the literature regarding its effects to male reproductive function. In this study, it was shown that in adult rats treated with acute doses of bisphenol A, a small but statistically insignificant percentage of seminiferous tubules in the testes displayed signs of germ cell loss, consistent with some earlier reports. It also failed to disrupt the blood-testis barrier in vivo. This is possibly due to the low bioavailability of free bisphenol A in the systemic circulation. However, bisphenol A disrupted the blood-testis barrier when administered to immature 20-day-old rats, consistent with earlier reports concerning the higher susceptibility of immature rats towards bisphenol A. This observation was confirmed using primary Sertoli cells cultured in vitro with established tight junction-permeability barrier that mimicked the blood-testis barrier in vivo. The reversible disruption of Sertoli cell tight junction barrier by bisphenol A was associated with an activation of ERK, and a decline in the levels of selected proteins at the tight junction, basal ectoplasmic specialization, and gap junction at the blood-testis barrier. Studies by dual-labeled immunofluorescence analysis and biotinylation techniques also illustrated declining levels of occludin, connexin 43, and N-cadherin at the cell-cell interface following bisphenol A treatment. In summary, bisphenol A reversibly perturbs the integrity of the blood-testis barrier in Sertoli cells, which can also serve as a suitable model for studying the dynamics of the blood-testis barrier.
Keywords: Testis, spermatogenesis, seminiferous epithelial cycle, tight junction, anchoring junction, Sertoli cells, bisphenol A
INTRODUCTION
The presence of the blood-testis barrier (BTB) in adult mammalian testes, such as rodents was described a century ago (Setchell, 2008). Its biological significance began to be deciphered in the 1950 s when the seminiferous epithelial cycle was reported (Clermont, 1972; LeBlond and Clermont, 1952; Parvinen, 1982). The BTB is essential to (i) confer cell polarity, (ii) maintain an immunological barrier to segregate the events of post-meiotic germ cell development from the systemic circulation, and (iii) provide a ‘fence’ function to restrict the paracellular diffusion of molecules (e.g., water, drugs, electrolytes), nutrients, hormones and biomolecules between adjacent Sertoli cells (Dym and Fawcett, 1970; Pelletier and Byers, 1992; Setchell, 1980). However, it must be restructured at stage VIII-IX of the seminiferous epithelial cycle (Russell, 1977) to facilitate the transit of primary preleptotene spermatocytes at the BTB, while differentiating into leptotene and zygotene spermatocytes (Hess and de Franca, 2008). Without this timely movement of developing primary spermatocytes across the BTB at stages VIII-IX of the epithelial cycle, spermatogenesis is disrupted, leading to infertility.
The mechanism(s) that maintains the BTB integrity during spermatogenesis and regulates its timely restructuring at stages VIII-IX of the epithelial cycle while maintaining the immunological barrier, remain largely unexplored. This is largely due to the lack of a reversible model to examine the BTB dynamics. In fact, there are some in vivo models that are available in the field to study the biology and regulation of the BTB, including the use of cadmium (Hew et al., 1993; Lui et al., 2003; Wong et al., 2004), glycerol (Wiebe et al., 2000), or cytokines (e.g. TGF-β3 and TNFα) (Li et al., 2006; Xia et al., 2006; Xia et al., 2009). However, either these methods were prohibitively expensive (e.g. the use of recombinant cytokines) or the disruptive effects of these models were irreversible (e.g. the use of toxicants cadmium and glycerol). In the latter ones, it is difficult to discern if changes in target genes during the BTB disruption is indeed part of the physiological BTB restructuring process during spermatogenesis or a result of general toxicity. Thus, a better and more economical model in which BTB can be reversibly disrupted would be well received by investigators in the field.
It was reported that the Sertoli cell junction at the BTB acts as an early target of environmental toxicants, including bisphenol A (BPA) (Fiorini et al., 2004). BPA, an estrogenic environmental toxicant, is used for the manufacturing of polycarbonate- and epoxy resin-based plastics. The general public is widely exposed to it but it is rapidly metabolized in the body (National Toxicology Program, 2008; Vandenberg et al., 2009). This renders them less toxic than other toxicants, such as cadmium chloride, where both were shown to decrease the junction protein level in a Sertoli cell line (Fiorini et al., 2004). Some in vivo studies have shown that BPA however appears to primarily disrupt the spermiogenesis via the apical ectoplasmic specialization (ES) (an atypical testis-specific adherens junction, AJ) when administered to adult rats and mice, without severe effects on the status of spermatogenesis (Takahashi and Oishi, 2001; Toyama et al., 2004). We sought to examine if this relatively inexpensive and less disruptive environmental toxicant could be used to develop a model for studying the assembly and disassembly of the BTB. We report herein some unexpected but interesting findings regarding the use of BPA both in vivo and in vitro for studying the BTB function.
MATERIALS AND METHODS
Animals
Sprague-Dawley rats and Wistar rats were obtained from Charles River Labs (Kingston, NY). The use of animals for the experiments reported herein was approved by the Animal Use and Care Committee of the Rockefeller University with the Protocol Number 06018.
BPA treatment to immature and adult rats
BPA, which was dissolved in absolute ethanol and diluted in corn oil (Sigma-Aldrich), was fed to Wistar rats by gavage since oral feeding was reported to be the primary route of exposure to BPA in humans (National Toxicology Program, 2008). Rats were treated at corresponding doses daily for 5 or 6 consecutive days in different regimens (see Fig. 1). Rats were treated with multiple doses of BPA due to its short biological half-life in rodents and primates including humans and monkeys (National Toxicology Program, 2008). In this study, the BPA treatment covered a range of 0.02 to 50 mg/kg b.w. per day due to its non-monotonic response observed for some endpoints (Vandenberg et al., 2009). The first day of the drug administration was designated as day 1. Rats were terminated at specified time points in each regimen (see Fig. 1) with n = 4-7. In humans, the daily intake of BPA was estimated to be ~2.5-4 μg/person/day (Lakind and Naiman, 2008). Thus, the regimens that were used in our study mimicked acute short-term or accidental exposure to BPA. Since rats fed with BPA following 5 daily doses did not show any signs of tubular damage in pilot experiments, a vehicle control group was not included in subsequent experiments as reported in Fig. 1. Only an untreated control group (see Fig. 1) was used to avoid the unnecessary slaughter of animals. However, a vehicle control group was included in the study to examine the effects of BPA on the BTB integrity in immature rats when a statistically significant effect was detected based on pilot experiments.
Fig. 1. A study to assess the effects of BPA on the body weight, testis weight, and status of spermatogenesis in adult rats.
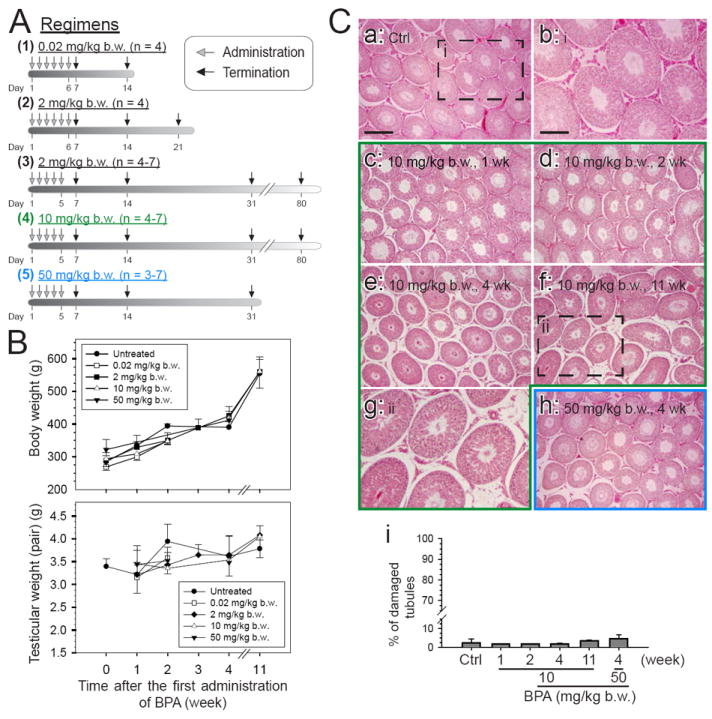
The five regimens used in this study and the number of rats per regimen are shown in (A). Adult Wistar rats, from 275 to 350 g b.w., were treated with BPA in corn oil by gavage at the desired concentration in a volume of ~600 μl per rat. Normal untreated rats serve as the control. (B) The body weight and testis weight were recorded and analyzed. There was no statistically significant change in both parameters among the control and treatment groups. (C) Morphological studies were performed by staining paraffin sections of testes with eosin and hematoxylin from selected time points in treatment groups and normal rats and were shown in (C: a – h). The boxed areas in C: a and C: f were magnified and shown in C: b and C: g, respectively. The percentage of damaged tubule was assessed by the presence of germ cells, including elongating spermatids, round spermatids and spermatocytes, in the tubule lumen, excluding stage VIII at which spermiation occurs (C: i). Seminiferous tubules on an entire cross-section from each testis at each time point were assessed. Scale bar in C: a = 300 μm, which applies to C: c – f, and h; scale bar in C: b = 150 μm, which applies to C: g.
Primary Sertoli cell cultures
Sertoli cells were isolated from testes of 20-day-old Sprague-Dawley rats as earlier described (Cheng et al., 1986). Freshly isolated Sertoli cells were plated on Matrigel (BD Biosciences)-coated bicameral units or 6 to 24-well dishes at 0.5 – 1.0 × 106 cells/cm2 in serum-free F12/DMEM media (Sigma-Aldrich) containing different growth factors and antibiotics as described (Li et al., 2009). About 36 hr thereafter, cultures were subjected to a hypotonic treatment with 20 mM Tris (pH 7.4) for 2.5 min as described (Galdieri et al., 1981) to lyze residual germ cells. As such, the Sertoli cell purity in these cultures was greater than 98% with negligible contaminations of either germ cells (e.g., elongating and elongated spermatids) or Leydig cells as earlier characterized with the use of RT-PCR, immunoblotting and electron microscopy (Lee et al., 2003). In this culture system, functional TJ-permeability barrier, which mimicked the BTB in vivo morphologically and functionally, was established on day 3 – 4. This was assessed with the TER across the cell epithelium (Lui et al., 2001) and monitored by electron microscopy, in which ultrastructures of TJ, ES and desmosome-like junction were observed (Siu et al., 2005). Apical ES was absent in the primary Sertoli cell cultures since elongating and elongated spermatids were removed in the hypotonic treatment step. Cells were treated with either vehicle control (0.1 % ethanol), BPA at 40 μM or 200 μM on day 3 to 5 after the functional TJ-barrier was formed in cultured Sertoli cells. These concentrations of BPA were selected for in vitro experiments since BPA at 40 μM was reported to induce junction disruption in the SerW3 Sertoli cell line (Fiorini et al., 2004), and 200 μM of BPA is equivalent to ~45 mg/kg b.w. in the in vivo animal study assuming rats with the body weight at 300 g with a volume of ~0.3 L.
Immunoblot analysis
Protein lysates were prepared as described in the NP-40 lysis buffer [50 mM Tris, pH 8.0 containing 0.15 M NaCl, 10% glycerol (v/v), 1% NP-40 (v/v), 2 mM EGTA], supplemented with protease inhibitor cocktail (~10 μl/ml) and phosphatase inhibitor cocktails 1 and 2 (~20 μl/ml) (Sigma Aldrich). The concentration of protein lysate was estimated using the DC protein assay kit (Bio-Rad Laboratories). Proteins were resolved by SDS-PAGE and the target proteins were probed with corresponding antibodies by immunoblot analysis. The source of antibodies and their working dilutions using for various experiments were shown in Table 1. Immunoblot analysis was performed as earlier described (Yan et al., 2008). Cytoskeletal proteins, namely actin and vimentin, serve as protein loading controls.
Table 1.
Antibodies obtained from different vendors and their usage for different experiments in this report
| Target | Vendor | Catalog no. | Lot no. | Host species* | Usage (Dilution) |
|---|---|---|---|---|---|
| Actin | SCBT | sc-1616 | H0808 | Goat | IB (1:300) |
| α-Caten | SCBT | sc-7894 | A2705 | Rabbit | IB (1:200) |
| β-Catenin | SCBT | sc-7199 | L0507 | Rabbit | IB (1:200) |
| γ-Catenin | SCBT | sc-7900 | K2204 | Rabbit | IB (1:200) |
| Connexin 43 | Cell Signaling Technology | 3512 | 11/2006 | Rabbit | IB (1:200), IF (1:100) |
| Connexin 43 | BD Biosciences | 612400 | 612400 | Mouse | IF (1:100) |
| c-Src | SCBT | sc-19 | C0708 | Rabbit | IB (1:200) |
| E-cadherin | SCBT | sc-7870 | E2605 | Rabbit | IB (1:200) |
| ERK1/2 | Cell Signaling Technology | 9102 | 10 | Rabbit | IB (1:200) |
| p-ERK1/2 (Thr202/Tyr 204) | Cell Signaling Technology | 9101S | 13 | Rabbit | IB (1:200) |
| β1-integrin | BD Biosciences | 610468 | 35714 | Mouse | IB (1:200) |
| JAM-A | Invitrogen | 36-1700 | 370923A | Rabbit | IB (1:250) |
| N-Cadherin | SCBT | sc-7939 | N0907 | Rabbit | IB (1:200), IF (1:100) |
| Nectin-3 | SCBT | sc-14806 | K261 | Goat | IB (1:200) |
| Occludin | Invitrogen | 71-1500 | 366532A | Rabbit | IB (1:250), IF (1:100) |
| Vimentin | SCBT | sc-6260 | D1805 | Mouse | IB (1:200) |
| ZO-1 | Invitrogen | 61-7300 | 450085A | Rabbit | IB (1:250), IF (1:100) |
Abbreviations used: IB – immunoblot analysis, IF – immunofluorescence microscopy, SCBT – Santa Cruz Biotechnology.
Remarks: Antibodies raised in rabbit and goat are polyclonal antibodies while those raised in mouse are monoclonal antibodies. All antibodies, as indicated by the manufacturers, cross-reacted with the corresponding target proteins in rats.
Fluorescence microscopy and dual-labeled immunofluorescence analysis
Dual-labeled immunofluorescence analysis was performed using different combinations of primary antibodies and secondary antibodies (see Table 1). ProLong gold antifade reagent with DAPI (Invitrogen) was used as the mounting medium. Fluorescence and bright field microscopy were performed using the Olympus BX61 Fluorescent Microscope and images were captured using the Olympus DP71 digital camera. All image files were acquired and saved in TIFF format using the MicroSuite Five imaging software package (Version 1224, Olympus Soft Imaging Solutions Corp, Lakewood, CO). Fluorescence images were analyzed and merged using the Adobe PhotoShop CS3 extended (Version 10.0). For immunostaining experiments of cultured Sertoli cells, ~500 cells were examined from 10 randomly selected fields in each group. For studies using cross sections of testes, ~80 seminiferous tubules were examined from 10-20 randomly selected fields on each section to assess tubule damage.
In vivo BTB integrity assay
The BTB integrity assay in vivo was performed as described (Li et al., 2006) using inulin-FITC (Mr 4,500) (Sigma-Aldrich) as the tracer. This assay is based on the ability of the BTB to block the diffusion of a molecular probe (such as inulin-FITC) in the systemic circulation in the interstitium from traversing the BTB. The BTB would be considered leaky when the tracer could traverse the TJ-barrier from the basal to the apical compartment of the seminiferous epithelium (Li et al., 2006). Inulin-FITC was dissolved in PBS and ~200 μl (containing ~1 mg of the fluorescence probe) was administered via a 28-gauge needle into the jugular vein of each rat, which was injected i.m. with Ketamine HCl/Xylazine (60/10 mg/kg b.w.) as the anesthetic and analgesic. Rats were euthanized about 30 min after the tracer administration by CO2 asphyxiation. Testes were collected and frozen in liquid nitrogen. Frozen sections of testes (about 10 μm thick) were obtained in a cryostat at -20°C. The diffusion of the tracer in the seminiferous epithelium was monitored using fluorescence microscopy as described above. Rats treated with a single dose of cadmium chloride (5 mg/kg b.w. via i.p.) for ~3 days, which is known to disrupt the BTB (Wong et al., 2004), served as the positive control, and rats without any drug treatment served as the negative controls.
Assessment of the Sertoli cell BTB integrity in vitro
Sertoli cells cultured in vitro on Matrigel-coated bicameral units and/or dishes is known to assemble the TJ-permeability barrier that mimics the BTB in vivo physiologically and ultrastructurally (Grima et al., 1992; Janecki et al., 1992), which has been used extensively by investigators in the field to study BTB dynamics. To assess the establishment of the Sertoli cell TJ-barrier, TER across the Sertoli cell epithelium in the presence or absence of BPA was monitored daily following Sertoli cell plating as described (Grima et al., 1992; Janecki et al., 1992; Lui et al., 2003). To assess the specificity and reversibility of BPA-induced TJ-barrier disruption, BPA (or CdCl2) was removed by rinsing the Sertoli cells with fresh F12/DMEM (2 times, 5 min each).
Electron microscopy
Electron microscopy was performed at the Rockefeller University Bio-Imaging Resource Center to assess the effects of BPA on the BTB integrity. Sertoli cells isolated from 20-day-old rat testes were plated on Matrigel-coated 60-mm dishes at 0.5 × 106 cells/cm2, and ~36-hr thereafter, cells were hypotonically treatment as describe above to lyze residual germ cells (Galdieri et al., 1981). On day 5, cells were fixed in 2.5% (v/v) glutaraldehyde/2.5% (w/v) paraformaldehyde in 0.1 M cacodylate buffer (pH 7.5 at 22°C) for 1 hr. Cells were post-fixed in 1% OsO4 (v/v) in 0.1 M cacodylate buffer and stained with 2% (w/v) uranyl acetate. After dehydration in ascending graded ethanol, cells were detached from the 60-mm dishes with propylene oxide treatment and embedded in EPON (Electron Microscopy Sciences, Fort Washington, PA) blocks as described (Brown and Farquhar, 1989). Silver sections were obtained in a Reichert Ultracult II ultramicrotome (Reichert Inc., Depew, NY) and electron micrographs were obtained with a JEOL 100CXII electron microscope (JEOL USA Inc., Peabody, MA) at 80 kV.
Cytotoxicity assay
Sertoli cells were cultured at 0.5 × 106 cm2 on Matrigel-coated 96-well plate. Cells were treated with the toxicant at the corresponding concentrations for 24 hours. The cytotoxicity of BPA was monitored using the XTT (sodium 3’-[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hyrate) assay kit (Cell Proliferation Kit II) obtained from Roche Diagnostics. This assay quantified the ability of mitochondrial dehyrogenases in viable cells to cleave the yellowish tetrazolium salt XTT to an orange formazan dye at 35 °C. The XTT labeling reagent mix was added into each well except for the control wells to obtain the background absorbance caused by the phenol red containing F12/DMEM. The plate was then returned to the 35 °C incubator and the absorbance at 450 nm and the reference wavelength 655 nm was measured using the BioRad 680 Microplate Reader for a number of times from 1 to 24 hours before the absorbance was saturated.
Biotinylation of cell surface proteins to assess the turnover rate of cell surface proteins
Proteins on the cell surface of Sertoli cells cultured alone for 3-4 days with an intact cell epithelium were biotinylated using sulfo-NHS-SS-biotin (Pierce, Thermo Fisher Scientific) at 4 °C for 30 min. Non-bound biotins were quenched with 50 mM ammonium chloride solution (Wachtel et al., 1999). Cells were then incubated in 200 μM BPA or 0.1% ethanol at 35 °C for 1, 2 or 4 hr to allow the endocytosis and intracellular degradation of biotinylated cell surface proteins. Cell lysates were collected in NP40 lysis buffer. Biotinylated proteins were isolated from cell lysates using UltraLink Immobilized NeutrAvidin Protein Plus and resolved in SDS-PAGE for immunoblotting using the corresponding specific antibodies to assess the level of cellular biotinylated proteins. Using this approach, the fate of the biotinylated Sertoli cell surface proteins, namely occludin and N-cadherin, at the BTB in the absence (vehicle control) or presence of BPA for specified time was quantified and it represents the net result of protein endocytosis, endosome-mediated degradation, recycling and transcytosis.
Statistical analyses
GB-STAT Statistical Analysis software package (Version 7.0, Dynamic Microsystems) was used to perform statistical analyses of data. Each experiment was repeated at least 4 times, excluding pilot experiments for assessing the optimal termination schedule. One-way ANOVA, which was followed by Tukey/Kramer procedure or two-tailed Dunnett’s procedure, and Student’s t test were performed.
RESULTS
BPA did not significantly disrupt the spermatogenesis in adult rats
Adult Wistar rats (~300 gm b.w.) were treated daily by gavage with acute doses of BPA (0.02, 2, 10, or 50 mg/kg b.w.) for 5-6 days in a total of 5 different regimens and rats were terminated at specified time points (Fig. 1A). No significant differences were detected at 1- to 11-weeks between treatment and control groups regarding the body weight and testis weight (Fig. 1B). The status of spermatogenesis was assessed by histological analysis using hematoxylin and eosin staining of paraffin sections (Fig. 1C). There was a small but statistically insignificant percentage of seminiferous tubules that displayed signs of germ cell loss in the 10 and 50 mg/kg treatment groups versus controls (Fig. 1C), illustrating the reproductive functions of these rats were not affected. The steady-state levels of different TJ, ES, gap junction (GJ) and peripheral adaptor proteins were assessed by immunoblot analysis. No significant difference was detected for most of these proteins, except for a mild but significant decline of occludin and nectin-3 in the high acute dose (10 or 50 mg/kg b.w.) groups versus controls (Fig. 2A, B). These findings illustrate that the spermatogenesis in adult rat testes remains unaffected by the acute administration of BPA in the five different treatment regimens.
Fig. 2. Immunoblot analysis to assess any alteration in the steady-state levels of proteins found at different junction types in the seminiferous epithelium following BPA treatment.
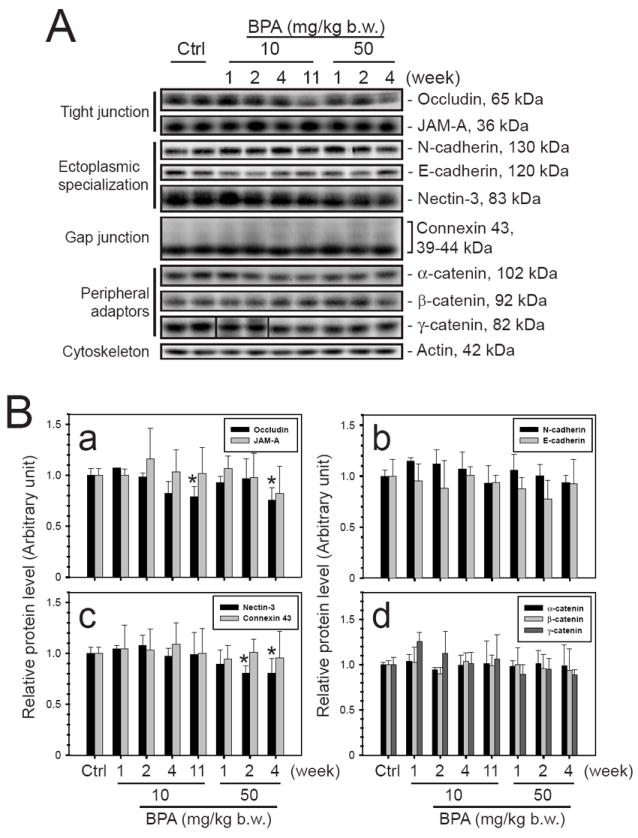
The steady-state levels of various junction proteins in testis samples from two BPA treatment groups, 10 and 50 mg/kg b.w., were assessed by immunoblotting. (A) The levels of integral membrane proteins of the tight junction, ectoplasmic specialization, and gap junction as well as peripheral adaptor proteins at these junctions were shown. (B) Their protein levels in testes from 3 different rats were analyzed. A mild, but statistically significant, decline in the protein level of TJ protein occludin and apical ES protein nectin-3 was detected. Statistical analysis was performed using one way ANOVA test and Dunnett’s procedure to compare the treatment groups against the control group. All treatment groups are not significantly different from the control group unless otherwise specified. *, P < 0.05.
BPA did not disrupt the BTB integrity in adult rats
Possible effects of acute dose of BPA to the BTB integrity were next examined using samples obtained from Regimen 5 (50 mg/kg b.w., 5 daily doses) (Fig. 1A) by 4 weeks post-treatment (Fig. 3A, B). Dual-labeled immunofluorescent analysis (Fig. 3A) showed that the basal ES integral membrane protein N-cadherin (green) and TJ-integral membrane protein occludin (red) were co-localized to the same site in the seminiferous epithelium near the basal compartment. These observations were consistent with their localization at the BTB (Lee et al., 2003; Wong et al., 2004) (Fig. 3A e-h and m-p). Occludin, but not N-cadherin, showed a mild loss from the BTB site (Fig. 3A n vs. f), consistent with the immunoblot results (Fig. 2). The relative localization and/or distribution of two other BTB proteins, namely basal ES adaptor protein β-catenin (green) and TJ-integral membrane protein JAM-A (red), were not altered at the BTB (Fig. 3B). The distribution of F-actin, which was stained with phalloidin-FITC, in the seminiferous epithelium from selected time points in Regimens 4 and 5 (see Fig. 1) were not observably different from the controls (Fig. 4A). These findings suggest that the BTB integrity was not compromised following the BPA treatment.
Fig. 3. An immunofluorescent microscopy study to investigate the distribution of junction proteins at the BTB in testes following BPA treatment.
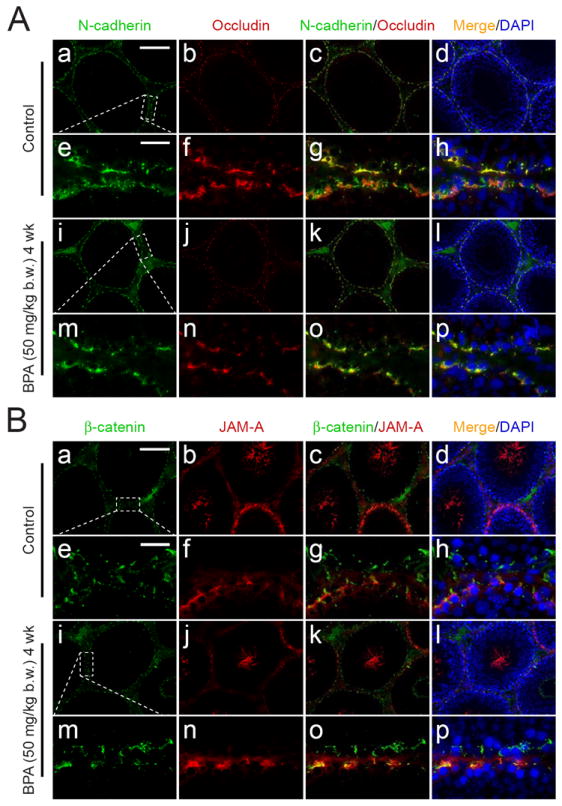
Localizations of junction proteins present at the BTB were studied using immunofluorescent microscopy. The fluorescence staining in cross-sections of testes from rats sacrificed 4 weeks after their first treatment with BPA (50 mg/kg b.w.) was compared against untreated controls. (A) N-Cadherin (FITC) and occludin (CY3) were detected and co-localized at the BTB in virtually all stages of the seminiferous epithelial cycle in the control (A: a – h). No observable difference can be detected in the BPA treatment group (A: i – p). The occludin staining appears as a ring at the BTB surrounding the entire seminiferous tubule (A: b). However, its intensity became diminished and appeared to be disrupted slightly in the BPA treatment group (A: n versus f). (B) β-Catenin (FITC) and JAM-A (CY3) in both treatment and control groups co-localized partially at the BTB (B: a – h versus i – p). While β-catenin was detected at the BTB in virtually all stages (B: a, d), JAM-A was abundantly expressed at the BTB from late stage VIII to about stage XIV (B: b, d). JAM-A was also found to associate with departing elongated spermatids (i.e. spermatozoa) (see B: b, d to note the JAM-A fluorescent staining in the tubule lumen). The micrographs shown here are results of a representative experiment. This experiment was repeated three times using 3 different rats in each group. Scale bars in A: a and B: a = 120 μm, which apply to A: b – d, i – l and B: b – d, i – l; scale bars in A: e and B: e = 25 μm, which apply to A: e – h, m – p and B: e – h, m – p.
Fig. 4. A study to examine the BTB integrity in vivo following BPA treatment.
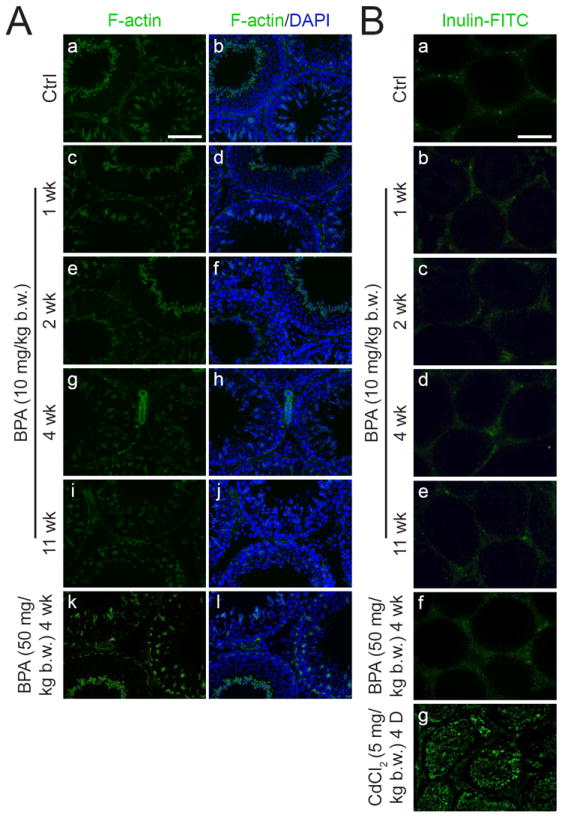
The integrity of the BTB was assessed by (A) the localization of filamentous actin (F-actin) in the seminiferous epithelium and (B) the ability of the BTB to block the passage of a fluorescent tracer into the adluminal compartment. (A) The localization of F-actin at the BTB in the epithelium in normal rats (A: a, b) versus rats treated with BPA (A: c – l) with no observable differences were noted. (B) The BTB integrity was assessed by its ability to block the diffusion of inulin-FITC (~4.5 kDa) from the systemic circulation to the apical compartment and restricted it to the BTB region (B: a). When the BTB became damaged after CdCl2 treatment (5 mg/kg b.w., i.p., for 4 days), the fluorescent tracer was detected in almost the entire epithelium (B: g) (positive control). In the BPA treatment groups (10 and 50 mg/kg b.w.) (B: b – f), the fluorescent tracer was also restricted by the BTB from entering into the apical compartment of the epithelium, similar to the control (B: a). The micrographs shown here are the representative results of two experiments where n = 3 rats for each treatment group. For the BPA (10 mg/kg b.w.) treatment group, the data shown were the results using one rat for each time point. Similar observations were obtained in the treatment group using 2 mg/kg b.w. (see Fig. 1) with n = 2 rats per time point. Scale bar in A: a = 100 μm, which applies to (A: b – l); scale bar in B: a = 200 μm, which applies to B: b - g.
To validate these findings, an in vivo BTB integrity assay was performed as described (Li et al., 2006). The integrity of the BTB is assessed by its ability to limit the diffusion of a small fluorescent tracer, inulin-FITC, from the basal to the apical compartment of the seminiferous epithelium. As shown in Fig. 4B, rats receiving acute doses of BPA in regimens 4 and 5 (Fig. 1) displayed normal BTB function. The diffusion of inulin-FITC across the TJ-barrier was limited (see Fig. 4Bb-f vs. a). This is drastically different from rats treated for 4 days with CdCl2 (5 mg/kg b.w., i.p.) (Fig. 4Bg), a known disruptor of the BTB in adult rats (Wong et al., 2004).
BPA disrupted the BTB integrity in immature rats
It is known that only free BPA in the systemic circulation is biologically active. When adult mammals, including rats and humans, ingested bisphenol A, it is rapidly metabolized in the liver to its non-toxic BPA-glucuronide form, which is then excreted by the kidney. The ability of immature rats to metabolize BPA is nonetheless less efficient (Chapin et al., 2008; National Toxicology Program, 2008). The effect of BPA on the BTB in immature rats was then examined. In the treatment groups, 20-day-old rats were treated by gavage daily with BPA (50 mg/kg b.w.) or 17β-estradiol (E2, 0.2 mg/kg b.w.) for 6 days. In the control groups rats were treated with corn oil for 5 days (negative control) or CdCl2 (5 mg/kg b.w.) at 25 days of age (positive control). The BTB integrity was assessed when rats were at 28 days of age. It is noted that BPA treated pups displayed a disruption of the BTB integrity (Fig. 5c vs. a). At acute dose, E2 was also shown to be disruptive to the BTB integrity (Fig. 5d vs. a). Thus, when administered to immature rats, BPA was able to compromise the BTB integrity.
Fig. 5. Assessment of the effect of BPA on the BTB integrity in immature rats.
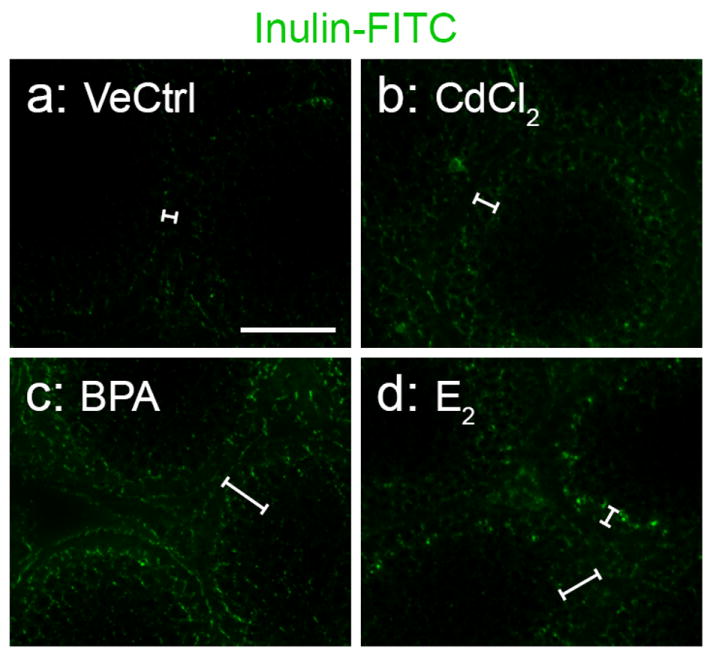
Rats were treated by gavage daily from 20- to 25-day old, with BPA (50 mg/kg b.w.), 17β-estradiol (E2, 0.2 mg/kg b.w.), or ethanol in corn oil (2.5%) (vehicle control) for 6 consecutive doses. For the positive control, rats were treated with a single dose of CdCl2 (5 mg/kg b.w., i.p.) when they were 25-day-old which is known in induce BTB disruption by day 3 thereafter (Wong et al., 2004). The integrity of the BTB was assessed, using inulin-FITC as the tracer, when rats were 28 days of age. White brackets were used to indicate the distance of the tracer diffused into the seminiferous epithelium from the basement membrane. A diffusion of inulin-FTIC into the adluminal compartment of the seminiferous epithelium was observed in the BPA (c), CdCl2 (b) and E2 (d) treatment groups, when compared to the vehicle control group (a). This indicates that the BTB in pups is more vulnerable towards exposure to BPA. Scale bar in a = 75 μm, which applies to b – d.
BPA perturbed the Sertoli cell TJ-permeability barrier function via the redistribution of proteins at the cell-cell interface
When Sertoli cells were cultured in vitro, a functional TJ-permeability barrier was established by ~day 3. This was manifested by the ability of the cell epithelium to resist the passage of a short pulse of electrical current as quantified by the transepithelial electrical resistance (TER) (Fig. 6A). This in vitro system has been widely used by investigators in the field to study the BTB dynamics (Byers et al., 1986; Grima et al., 1992; Janecki et al., 1992; Okanlawon and Dym, 1996). While BPA at 40 μM had no apparent effect on the Sertoli cell TJ-barrier function, BPA at 200 μM was shown to reversibly perturb the TJ-barrier. A removal of BPA by day 4 allowed the disrupted TJ-barrier to ‘reseal’ by day 5 (Fig. 6A). This is in contrast to CdCl2, which disrupted the Sertoli cell TJ-barrier irreversibly (Fig. 6A), similar to its disruptive effects in vivo (Wong et al., 2004).
Fig. 6. A study to assess the effect of BPA on the tight junction permeability barrier function and localization of junction proteins in Sertoli cells in vitro.
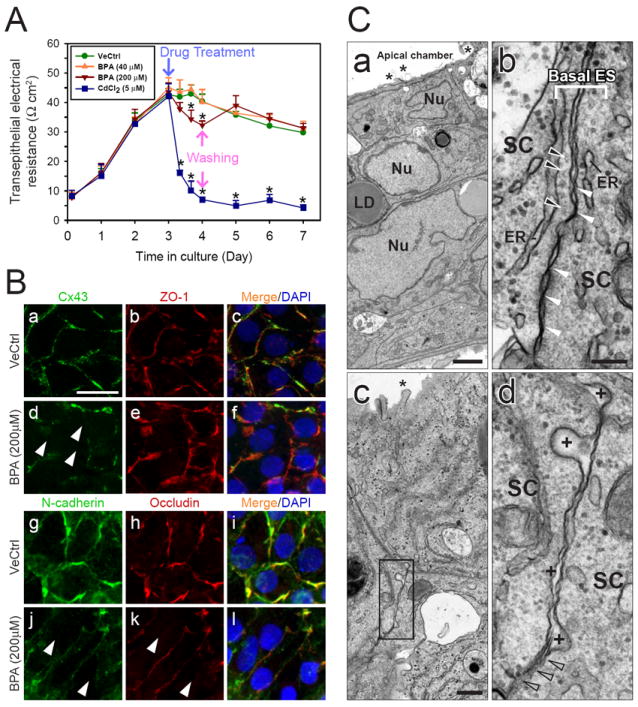
(A) Sertoli cells were plated at 1.0 × 106 cells/cm2 on Matrigel-coated bicameral units and the TJ-barrier assembly was monitored by TER measurement. On day 3, cells were treated with 0.1% ethanol (vehicle control, VeCtrl), BPA or CdCl2. For the BPA (200 μM) and CdCl2 treatment groups, cells were washed 24 h thereafter and replenished with F12/DMEM without the toxicant. Cells treated with CdCl2 showed a significant and irreversible TJ-barrier disruption. BPA caused a mild reversible damage to the TJ-barrier at 200 μM, but not 40 μM. *, P < 0.01. (B) Immunofluorescent microscopy was performed using Sertoli cells cultured at 0.1 − 0.2 × 106 cells/cm2. On day 3, cells were treated with 200 μM BPA or vehicle control for 24 h. The localization of connexin 43 (Cx43, FITC) and ZO-1 (CY3) (B: a – f) and N-cadherin (FITC) and occludin (CY3) (B: g –l) were shown. White arrowheads indicate the disappearance of corresponding junction proteins at the cell-cell interface. A decline in the level of junction proteins, namely connexin 43, ZO-1, N-cadherin and occludin, at the cell-cell interface was noted following BPA treatment (B: a – c, and g – i vs. d – f and j –l). Results shown in (A) and (B) are the representative results from three experiments. (C) Electron microscopy was used to assess the ultrastructures at the cell-cell interface in cells treated on day 5 with 0.1% ethanol (C: a and b) or 200 μM BPA (C: c and d) for 24 hours. (C: a) Cell-cell junctions and an intact cell epithelium that mimicked the seminiferous epithelium in vivo were established. Microvilli (see asterisk) are typical cellular structures found in the apical side of cultured Sertoli cells. Nu, Sertoli cell nucleus; LD, lipid droplet. (C: b) This is a magnified view of the BTB found between cultured Sertoli cells. Tight junctions appeared as ‘kisses’ between two adjacent Sertoli cells (SC) (see white arrowheads). Basal ES is typified by the presence of actin filament bundles (see black arrowheads) sandwiched between endoplasmic reticulum (ER) and the SC plasma membrane. (C: c) shows the typical ultrastructure of the cell-cell interface between Sertoli cells following BPA treatment. The boxed area in (C: c) was magnified and shown in (C: d). It was noted that following treatment of Sertoli cells with BPA, the intercellular space between SC widened (see “+”) and the typical TJ ‘kisses’ was absent. Instead, there were signs of disruption of cell junctions (see gray arrowheads). Scale bar in B: a = 20 μm, which applies to B: b – l; scale bar in C: a = 2 μm; scale bar in C: b = 0.2 μm, which applies to C: d; scale bar in C: c = 0.5 μm.
The effect of BPA on the distribution of junction proteins at the Sertoli cell interface was then examined by dual-immunofluorescence studies (Fig. 6B). Sertoli cells cultured for 3 days were treated with BPA at 200 μM for 24 hr. The cellular distribution of Cx43 (green), ZO-1 (red) (Fig. 6Ba-f), N-cadherin (green) and occludin (red) (Fig. 6B g-l) were examined. It was shown that the BPA treatment led to a decline of Cx43, N-cadherin and occludin, but not ZO-1, at the Sertoli cell interface (see white arrowheads in d, j and k in Fig. 6B). The mislocalization of these proteins from the TJ-fibrils at the site would affect the cell adhesion, thereby compromising the TJ-barrier function as shown in Fig. 6A.
These observations were further validated using the electron microscopy (Fig. 6C). It was noted that Sertoli cells cultured in vitro displayed the characteristic microvilli (see ‘*’ in a), and ultrastructures of the BTB (Fig. 6Cb). These include the presence of TJ (see the ‘kisses’ in b) and the basal ES, which is typified by the presence of actin filaments (see black arrowheads in b) sandwiched between the cisternae of endoplasmic reticulum (ER, in b) and the Sertoli cell plasma membrane. Following the BPA treatment, the typical TJ and basal ES ultrastructures were not observed in some areas (Fig. 6Cc,d). Instead, there are numerous widened intercellular space between two adjacent Sertoli cells (see ‘+’ in Fig. 6Cd). These findings thus illustrate that BPA was capable of disrupting the Sertoli cell BTB reversibly in vitro.
BPA affected BTB-associated proteins in Sertoli cells cultured in vitro at a non-cytotoxic dose
When BPA was applied at a wide range of concentrations, from 0.01 to 800 μM, to Sertoli cells cultured in vitro with an established TJ-barrier, the dosings at 0.01 to 500 μM were not cytotoxic to Sertoli cells (Fig. 7A). This illustrates that findings shown in Fig. 4 were not the result of cytotoxicity. Immunoblot analyses showed that BPA caused a dose-dependent reduction in several junction proteins. These include TJ proteins, namely occludin, JAM-A, and ZO-1, basal ES proteins, namely N-cadherin, α-catenin, and β-catenin, GJ protein Cx43, hemidesmosomal protein β1-integrin and signaling protein kinase c-Src (Fig. 7B, C). In addition, BPA activated the pERK1/2, without changing the level of total ERK1/2 (Fig. 7B, Cd).
Fig. 7. A study to assess the effects of BPA on junction proteins using cultured primary Sertoli cells.
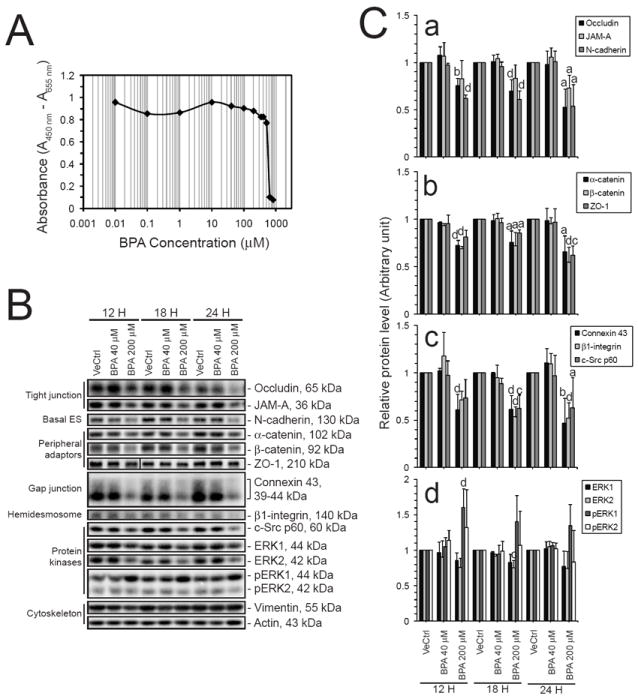
(A) The cytotoxicity of BPA on Sertoli cells cultured at 0.5 × 106 cells/cm2 was assessed. BPA was added to cell cultures on day 5 at the desired concentrations. The XTT cytotoxicity assay was performed as described in Materials and Methods. This study indicated that BPA was non-cytotoxic at 40 μM and 200 μM. (B) Immunoblot analysis of selected target proteins found at the BTB in rats was performed to validate the dose-dependent effects of BPA on the TJ-barrier function in primary Sertoli cell cultures. The immunoblotting results were analyzed and plotted in (C). Protein levels of integral membrane proteins from different junction types and their associated adaptors were investigated. Significant decline could be detected in all the markers studied (C: a – c). Protein levels of selected signaling kinases were also studied. There was a significant increase in the level of phosphorylated ERK1 (C: d). The results in (A) and (B) are the representative data from three independent experiments, with n = 3 for (C). Statistical analysis was performed using the one-way ANOVA test and Tukey/Kramer procedure. All treatment groups were not significantly different unless otherwise specified. a, P < 0.05 versus vehicle control and 40 μM BPA; b, P < 0.05 versus vehicle control and P < 0.01 versus 40 μM BPA; c, P < 0.01 versus vehicle control and P < 0.05 versus 40 μM BPA; d, P < 0.01 versus vehicle control and 40 μM BPA.
BPA disrupted the BTB through enhancing the turnover rate of transmembrane junction proteins
The mechanism by which BPA disrupted the Sertoli cell TJ-permeability barrier function in vitro was then examined. The turnover rate of junction proteins at the cell surface following the BPA treatment was examined with the biotinylation technique. Cell surface proteins were first biotinylated at 4 °C, at which endocytosis would be inhibited. Then the cultured cells were treated with either vehicle control or BPA at 200 μM, and incubated at 35 °C to resume endocytosis and degradation. Cell lysates were collected after specified period of time. Biotinylated proteins were separated and resolved in SDS-PAGE. Levels of biotinylated target proteins in the cell were assessed with corresponding antibodies. Thus, the turnover rate of cell surface proteins biotinylated at time 0 was assessed, regardless of their cellular localization in Sertoli cells. The turnover rates of occludin and N-cadherin were shown to increase following the BPA treatment (Fig. 8). These findings are consistent with results in the immunofluorescence microscopy study shown in Fig. 6B. BPA could perturb the BTB integrity through a redistribution and degradation of junction proteins at the cell surface.
Fig. 8. A study to examine the turnover rate of junction proteins at the cell surface following the BPA treatment.
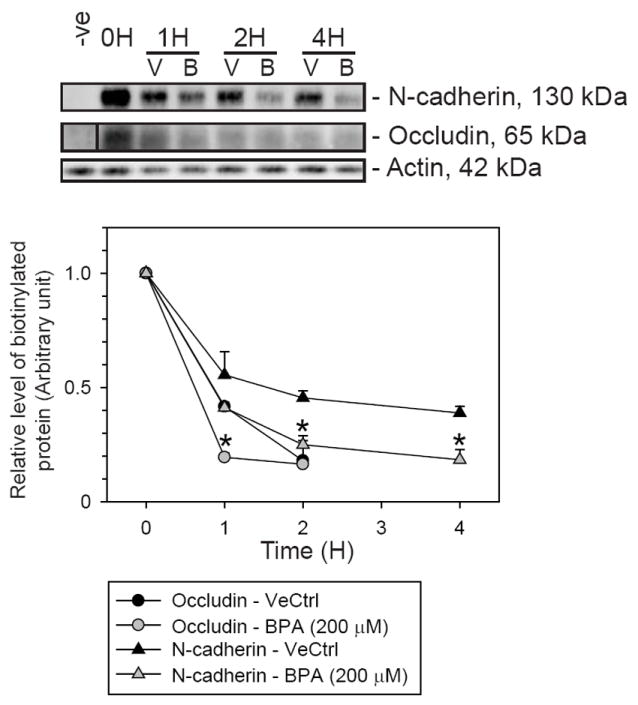
(A) The study was performed with Sertoli cells cultured at 0.5 × 106 cells/cm2 on Matrigel-coated dishes on day 5. Proteins on the Sertoli cell surface were biotinylated using sulfo-NHS-SS-biotin at 4 °C. The non-bound biotin was quenched. Cells were then incubated in 200 μM BPA or 0.1% ethanol (vehicle control, VeCtrl) at 35 °C to allow the endocytosis and degradation of biotinylated cell surface proteins to occur for specified period of time. Cell lysates were obtained. Biotinylated proteins were isolated from cell lysates using UltraLink Immobilized NeutrAvidin Protein Plus and resolved in SDS-PAGE. The amount of biotinylated proteins remaining in the cell was examined with immunoblotting. Following the treatment of cells with BPA, there was a decrease in the level of biotinylated occludin and N-cadherin versus vehicle control. This suggested that BPA could enhance the turnover rate of occludin and N-cadherin to disrupt the cell adhesion.
DISCUSSION
Differential responses in the BTB of adult and immature rats, and Sertoli cells in vitro upon exposure to BPA
BPA, an estrogenic environmental contaminant, leaches from polycarbonate- and epoxy resin-based food containers and water bottles, and enters the food chain. Once absorbed, BPA is rapidly metabolized in the liver to its non-toxic BPA-glucuronide form and excreted in the urine. Only free BPA in the systemic circulation acts as an endocrine disruptor (EFSA, 2006; National Toxicology Program, 2008).
There are a few reports in the literature exploring the reproductive toxicity of BPA in adult rodents [for a review, see (Chapin et al., 2008)]. These findings include a decline in daily sperm production in mice (Al-Hiyasat and Darmani, 2006; Al-Hiyasat et al., 2002), some structural deformation of spermatids (Takahashi and Oishi, 2001; Toyama et al., 2004), and the disruption of the apical ES, but not the BTB, in selected tubules examined by electron microscopy in adult rats and mice (Toyama et al., 2004). Our findings are in agreement with these earlier findings that the gross spermatogenesis status in adult rats following BPA treatment remained relatively intact. There are also reports demonstrating statistically insignificant changes in the testis weight in adult rats following BPA treatment (Ashby et al., 2003; Sakaue et al., 2001; Yamasaki et al., 2002) while some reported an increase (when BPA was used at 600-1000 mg/kg b.w.) (Yamasaki et al., 2002) or a decrease (Akingbemi et al., 2004; Chitra et al., 2003; Kawai et al., 2003). These reports used different regimens, including the route of administration, duration of exposure, dosage and age of the treated animals, and may be housed under different conditions, such as the material of the cage and water bottle and the chow used. All these would affect the experimental outcomes, at least to some extent. As such, we found it difficult to compare our findings with some previous reports.
Nonetheless, these earlier findings and the results reported herein support the notion that prenatal or neonatal rats appear to be more sensitive to BPA exposure versus adult animals. In this context, it is of interest to note that several recent reviews have summarized findings in the field that BPA probably causes irreversible developmental defects in rodents and humans (Chapin et al., 2008; Vandenberg et al., 2009). Infants and children are thus more susceptible to BPA exposure. Another reason is that BPA can be metabolized faster into its biologically inactive form in adult than in children, reducing the duration that the reproductive organs are exposed to BPA. This thus explains the susceptibility of the BTB to BPA-induced disruption in immature rats versus adult rats as reported here. The lack of any changes in the BTB integrity observed in adult rats is likely due to the lower concentration of biologically active BPA in circulation instead of differences in the response of Sertoli cells from rats of different ages. This conclusion is supported by the fact that Sertoli cells isolated from 20-day-old rats are fully differentiated and functionally similar to those isolated from adult rats (Orth, 1982).
The effects of BPA in vivo to the spermatogenesis and BTB integrity appear to be complicated by other parameters in the animal study as noted above including its bioavailability in adult and immature rats. However, in vitro study was also performed to explore the direct effects of BPA on Sertoli cells. BPA was reported to significantly reduce the steady-state protein levels of occludin, N-cadherin and connexin43 in the SerW3 Sertoli cell line (Fiorini et al., 2004), implicating its disruptive effects on the BTB in vitro. Our findings in vitro using primary cultures of Sertoli cells with the established TJ-permeability barrier support this notion. We speculated that perhaps due to the lower ability of immature rats to metabolize BPA to its non-toxic form, their BTB may be more readily disrupted by this environmental toxicant. These findings illustrate the possibility that through exposing children to BPA, the development of the BTB at puberty may become compromised and their reproductive function may be affected later on in adulthood.
Additionally, we demonstrate that BPA at 200 μM, but not 40 μM, while non-cytotoxic to cells, is capable of inducing reversible disruption of the Sertoli cell TJ-permeability barrier by the redistribution of BTB-associated proteins including occludin, ZO-1, and Cx43 at the Sertoli-Sertoli cell interface. This alteration of the distribution of intergral membrane proteins and their peripheral adaptors is likely due to a significant increase in the turnover from the cell surface. This increase in the protein turnover represents the net result of their internalization, degradation and recycling, which should be vigorously investigated in future studies.
BPA perturbs the Sertoli cell TJ-permeability barrier via its effects on protein endocytosis
The striking observation in this study is the highly reversible effects of BPA on the Sertoli cell TJ-barrier function. Reversibility is preferred due to the need to study the reassembly of disrupted junctions. This thus makes BPA a better choice versus CdCl2 (Chung and Cheng, 2001) to establish an in vitro model to study the BTB dynamics. This was because the damaging effect of CdCl2 on the TJ-barrier was irreversible at 5 μM even though the adverse effect of CdCl2 on the TJ-permeability barrier was more pronounced versus 200 μM of BPA.
More importantly, the disruptive effects of BPA on the Sertoli cell BTB function appears to be mediated via changes in the kinetics of integral junction protein. These include their redistribution at the Sertoli-Sertoli cell interface and an increase in their turnover rate. Indeed, BPA was shown to enhance the turnover of occludin and N-cadherin in Sertoli cells with an established TJ-barrier. Thus, these observations support the notion that the BPA-induced BTB disruption is a suitable model to study the endocytic trafficking in Sertoli cells, mimicking some of the recent findings that Sertoli cells are using this mechanism to regulate BTB dynamics during the seminiferous epithelial cycle (Xia et al., 2009; Yan et al., 2008). This concept is consistent with studies in other epithelia that the clathrin- or caveolin-mediated endocytic pathway, or macropinocytosis, is being used to rapidly regulate the anchoring and/or tight junction proteins at the cell-cell interface and hence the cell adhesion in response to changes in environment and/or external stimuli (Delva and Kowalczyk, 2009; Ivanov et al., 2005; Maxfield and McGraw, 2004).
It was postulated that protein endocytosis coupled with recycling and perhaps transcytosis are being used by the BTB to effectively regulate redistribution of BTB-associated proteins at the site to facilitate the transit of preleptotene spermatocytes at stage VIII-IX of the seminiferous epithelial cycle (Yan et al., 2008). In fact, recent studies using Sertoli cells cultured in vitro with established TJ-permeability barrier have shown that the TGF-β2-, TGF-β3- and TNF-α-induced BTB disruption is mediated via an increase in clathrin-mediated endocytosis of integral membrane protein at the BTB (Xia et al., 2009; Yan et al., 2008). The endocytosed proteins were targeted to the endosome-mediated intracellular degradation (Yan et al., 2008), thereby compromising the BTB. On the other hand, the promoting effects of testosterone on the BTB integrity (Meng et al., 2005; Wang et al., 2006) is mediated by an increase in protein recycling of internalized integral membrane at the BTB site (Yan et al., 2008). In short, at the time of primary preleptotene spermatocytes in transit at the BTB, ‘new’ TJ-fibrils at the basal region of a migrating spermatocyte are formed, perhaps facilitated by androgen-mediated endocytosis, recycling and transcytosis, while ‘old’ TJ-fibrils at the apical region of the spermatocyte are being disrupted as a result of cytokine-mediated endocytosis and endosome-mediated degradation.
However, additional functional studies to support this potentially important concept of BTB regulation to facilitate spermatocytes in transit while maintaining the immunological barrier is difficult to perform without the development of a suitable in vitro model to study these events of protein endocytosis, recycling and transcytosis. In this study, we have unequivocally demonstrated that BPA perturbs the Sertoli cell TJ-permeability barrier reversibly and these effects apparently are mediated via an increase in the protein turnover at the cell surface. Thus these properties make the BPA model a useful one to study the protein trafficking and BTB regulation.
Acknowledgments
We would like to thank Ms. Eleana Sphicas from the Rockefeller University Bio-Imaging Resource Center, for her excellent technical assistance in the electron microscopy.
This work was supported by grants from the National Institutes of Health (NICHD, R01 HD056034, R03 HD051512, and U54 HD029990 Project 5) to CYC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene epxression in rat Leydig cells. Endocrinology. 2004;145:592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- Al-Hiyasat AS, Darmani H. In vivo effects of BISGMA-a component of dental composite-on male mouse reproduction and fertility. J Biomed Mater Res A. 2006;78:66–72. doi: 10.1002/jbm.a.30667. [DOI] [PubMed] [Google Scholar]
- Al-Hiyasat AS, Darmani H, Elbetieha AM. Effects of bisphenol A on adult male mouse fertility. Eur J Oral Sci. 2002;110:163–7. doi: 10.1034/j.1600-0722.2002.11201.x. [DOI] [PubMed] [Google Scholar]
- Ashby J, Tinwell H, Lefevre PA, Joiner R, Haseman J. The effect on sperm production in adult Sprague-Dawley rats exposed by gavage to bisphenol A between postnatal days 91-97. Toxicol Sci. 2003;74:129–38. doi: 10.1093/toxsci/kfg093. [DOI] [PubMed] [Google Scholar]
- Brown WJ, Farquhar MG. Immunoperoxidase methods for the localization of antigens in cultured cells and tissue sections by electron microscopy. Methods Cell Biol. 1989;31:553–69. doi: 10.1016/s0091-679x(08)61626-x. [DOI] [PubMed] [Google Scholar]
- Byers S, Hadley MA, Djakiew D, Dym M. Growth and characterization of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J Androl. 1986;7:59–68. doi: 10.1002/j.1939-4640.1986.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Chapin RE, Adams J, Boekelheide K, Gray LE, Jr, Hayward SW, Lees PSJ, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- Cheng CY, Marther JP, Byer AL, Bardin CW. Identification of hormonally responsive proteins in primary Sertoli cell culture medium by anion-exchange high performance liquid chromatography. Endocrinology. 1986;118:480–8. doi: 10.1210/endo-118-2-480. [DOI] [PubMed] [Google Scholar]
- Chitra KC, Latchoumycandane C, Mathur PP. Induction of oxidative stress by bisphenol A in the epididymal sperm of rats. Toxicology. 2003;185:119–27. doi: 10.1016/s0300-483x(02)00597-8. [DOI] [PubMed] [Google Scholar]
- Chung NPY, Cheng CY. Is cadmium chloride-induced inter-Sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis? Endocrinology. 2001;142:1878–88. doi: 10.1210/endo.142.5.8145. [DOI] [PubMed] [Google Scholar]
- Clermont Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–235. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- Delva E, Kowalczyk AP. Regulation of cadherin trafficking. Traffic. 2009;10:259–67. doi: 10.1111/j.1600-0854.2008.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3:308–26. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- EFSA. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food on a request from the Commission related to 2,2-bis(4-hydroxylphenyl) propane (bisphenol A) The EFSA Journal. 2006:1–75. [Google Scholar]
- Fiorini C, Tilloy-Ellul A, Chevalier S, Charuel C, Pointis G. Sertoli cell junctional proteins as early targets for different classes of reproductive toxicants. Reprod Toxicol. 2004;18:413–21. doi: 10.1016/j.reprotox.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Galdieri M, Ziapro E, Palombi F, Russo M, Stefanini M. Pure Sertoli cell cultures: a new model for the study of somatic-germ cell interactions. J Androl. 1981;2:249–54. [Google Scholar]
- Grima J, Pineau C, Bardin CW, Cheng CY. Rat Sertoli cell clusterin, α2-macroglobulin, and testins: biosynthesis and differential regulation by germ cells. Mol Cell Endocrinol. 1992;89:127–40. doi: 10.1016/0303-7207(92)90219-v. [DOI] [PubMed] [Google Scholar]
- Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin: Landes Bioscience; 2008. pp. 1–15. [DOI] [PubMed] [Google Scholar]
- Hew K, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight-junction associated microfilaments in rat Sertoli cells. Biol Reprod. 1993;49:840–49. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Nusrat A, Parkos CA. Endocytosis of the apical junctional complex: mechanisms and possible roles in regulation of epithelial barriers. BioEssays. 2005;27:356–65. doi: 10.1002/bies.20203. [DOI] [PubMed] [Google Scholar]
- Janecki A, Jakubowiak A, Steinberger A. Effect of cadmium chloride on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment cultures - a new model for toxicological investigations of the “blood-testis” barrier in vitro. Toxicol Appl Pharmacol. 1992;112:51–7. doi: 10.1016/0041-008x(92)90278-z. [DOI] [PubMed] [Google Scholar]
- Kawai K, Nozaki T, Nishikata H, Aou S, Takii M, Kubo C. Aggressive behavior and serum testosterone concentration during the maturation process of male mice: the effects of fetal exposure to bisphenol A. Environ Health Perspect. 2003;111:175–8. doi: 10.1289/ehp.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakind JS, Naiman DQ. Bisphenol A (BPA) daily intakes in the United States: estimates from the 2003-2004 NHANES urinary BPA data. J Expo Sci Environ Epidemiol. 2008;18:608–15. doi: 10.1038/jes.2008.20. [DOI] [PubMed] [Google Scholar]
- LeBlond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann NY Acad Sci. 1952;55:548–73. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- Lee NPY, Mruk DD, Lee WM, Cheng CY. Is the cadherin/catenin complex a functional unit of cell-cell actin based adherens junctions in the rat testis? Biol Reprod. 2003;68:489–508. doi: 10.1095/biolreprod.102.005793. [DOI] [PubMed] [Google Scholar]
- Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0901700106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MWM, Xia W, Mruk DD, Wang CQF, Yan HHN, Siu MKY, et al. Tumor necrosis factor α reversibly disrupts the blood-testis barrier integrity and impairs Sertoli-germ cell adhesion in the seminiferous epithelium of adult rat testes. J Endocrinol. 2006;190:313–29. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- Lui WY, Lee WM, Cheng CY. Transforming growth factor-μ3 perturbs the inter-Sertoli tight junction permeability barrier in vitro possibly mediated via its effects on occludin, zonula occludens-1, and claudin-11. Endocrinology. 2001;142:1865–77. doi: 10.1210/endo.142.5.8116. [DOI] [PubMed] [Google Scholar]
- Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-μ3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003;144:1139–42. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A. 2005;102:16696–70. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program. NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. NTP CERHR MON. 2008;22:i–III1. [PubMed] [Google Scholar]
- Okanlawon A, Dym M. Effect of chloroquine on the formation of tight junctions in cultured immature rat Sertoli cells. J Androl. 1996;17:249–55. [PubMed] [Google Scholar]
- Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: A quantitative autoradiographic study. Anat Rec. 1982;203:485–92. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- Parvinen M. Regulation of the seminiferous epithelium. Endocrine Reviews. 1982;3:404–17. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- Pelletier RM, Byers SW. The blood-testis barrier and Sertoli cell junctions: structural considerations. Microsc Res Tech. 1992;20:3–33. doi: 10.1002/jemt.1070200104. [DOI] [PubMed] [Google Scholar]
- Russell LD. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977;148:313–28. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- Sakaue M, Ohsako S, Ishimura R, Kurosawa S, Kurohmaru M, Hayashi Y, et al. Bisphenol-A affects spermatogenesis in the adult rat even at a low dose. J Occup Health. 2001;43:185–90. [Google Scholar]
- Setchell BP. The functional significance of the blood-testis barrier. J Androl. 1980;1:3–10. [Google Scholar]
- Setchell BP. Blood-testis barrier, junctional and transport proteins and spermatogenesis. In: Cheng CY, editor. Molecular Mechanisms in Spermatogenesis. Austin: Landes Biosciences; 2008. pp. 212–33. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–47. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- Takahashi O, Oishi S. Testicular toxicity of dietary 2,2-bis(4-hydroxyphenly)propane (bisphenol A) in F344 rats. Arch Toxicol. 2001;75:42–51. doi: 10.1007/s002040000204. [DOI] [PubMed] [Google Scholar]
- Toyama Y, Suzuki-Toyota F, Maekawa M, Ito C, Toshimori K. Adverse effects of bisphenol A to spermiogenesis in mice and rats. Arch Histol Cytol. 2004;67:373–81. doi: 10.1679/aohc.67.373. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel M, Frei K, Ehler E, Fontana A, Winterhalter K, Gloor S. Occludin proteolysis and increased permeability in endothelial cells through tyrosine phosphatase inhibition. J Cell Sci. 1999;112:4347–56. doi: 10.1242/jcs.112.23.4347. [DOI] [PubMed] [Google Scholar]
- Wang RS, Yeh S, Chen LM, Lin HY, Zhang C, Ni J, et al. Androgen receptor in Sertoli cell is essential for germ cell nursery and junction complex formation in mouse testes. Endocrinology. 2006;147:5624–33. doi: 10.1210/en.2006-0138. [DOI] [PubMed] [Google Scholar]
- Wiebe JP, Kowalik A, Gallardi RL, Egeler O, Clubb BH. Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in Sertoli cells. J Androl. 2000;21:625–35. [PubMed] [Google Scholar]
- Wong CH, Mruk DD, Lui WY, Cheng CY. Regulation of blood-testis barrier dynamics: an in vivo study. J Cell Sci. 2004;117:783–98. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- Xia W, Mruk DD, Lee WM, Cheng CY. Differential interactions between transforming growth factor-β3/TβR1, TAB1, and CD2AP disrupt blood-testis barrier and Sertoli-germ cell adhesion. J Biol Chem. 2006;281:16799–813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- Xia W, Wong EWP, Mruk DD, Cheng CY. TGF-β3 and TNFα perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–61. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K, Sawaki M, Noda S, Imatanaka N, Takatsuki M. Subacute oral toxicity study of ethynylestradiol and bisphenol A, based on the draft protocol for the “Enhanced OECD Test Guideline no. 407”. Arch Toxicol. 2002;76:65–74. doi: 10.1007/s00204-001-0319-1. [DOI] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008;22:1945–59. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]


