Abstract
Background
The purpose of this study was to determine the efficacy of besifloxacin ophthalmic suspension 0.6% when used in the treatment of bacterial conjunctivitis infections due to Pseudomonas aeruginosa.
Methods
We undertook a post hoc analysis of clinical outcomes in patients with bacterial conjunctivitis due to P. aeruginosa across four prospective, multicenter, double-masked, randomized, controlled, clinical studies of besifloxacin ophthalmic suspension 0.6%. Efficacy outcomes included bacterial eradication and clinical resolution of the baseline infection at follow-up visits. Bacterial eradication was defined as the absence of ocular bacterial species present at or above threshold at baseline, while clinical resolution was defined as grade 0 ocular discharge and bulbar conjunctival injection. Safety outcomes included the incidence of adverse events, changes in visual acuity, and biomicroscopy and ophthalmoscopy findings. Patient outcomes were summarized and bacterial eradication and clinical resolution rates integrated.
Results
Of 1317 patients with culture-confirmed bacterial conjunctivitis across four clinical studies, nine (0.7%) were infected with P. aeruginosa at baseline, and of these, five were randomized to treatment with besifloxacin ophthalmic suspension 0.6%. Bacterial eradication of the baseline infection was observed at both follow-up visits in all five patients. Clinical resolution was achieved in two of five patients by the first follow-up visit and four of five patients by the second follow-up visit. There were no adverse events reported in these patients. There were no clinically meaningful biomicroscopy findings or changes in ophthalmoscopy or visual acuity.
Conclusion
The incidence of bacterial conjunctivitis due to P. aeruginosa was low. Treatment of patients with P. aeruginosa infections with besifloxacin ophthalmic suspension 0.6% led to bacterial eradication of P. aeruginosa by the first follow-up visit and high rates of clinical resolution.
Keywords: bacterial conjunctivitis, besifloxacin ophthalmic suspension, besifloxacin, conjunctivitis, Pseudomonas aeruginosa
Introduction
Bacterial conjunctivitis is a common eye infection characterized by marked hyperemia and mild-to-moderate purulent conjunctival discharge.1–4 Symptoms often include tearing, ocular irritation (foreign-body sensation), and matting of the eyelids, particularly in the morning.1,3,4 Bacterial conjunctivitis is usually self-limited and can resolve spontaneously without specific treatment in immune-competent individuals.5 The use of topical antibacterial therapy hastens clinical and microbiological remission6 which can translate into less work and school time losses, lower risk of disease transmission, and a minimized potential for sight-threatening complications. The choice of antibiotic is usually made empirically.
Bacterial conjunctivitis is most commonly caused by Haemophilus influenzae, Streptococcus pneumoniae, and Staphylococcus aureus. However, Staphylococcus epidermidis, Enterococcus spp, Moraxella spp, Streptococci viridans groups, Escherichia coli, Serratia marcescens, Proteus mirabilis, and Pseudomonas aeruginosa have also been identified as causative, albeit less frequently.1,7–9 Of infections caused by the less prevalent pathogens, bacterial conjunctivitis due to P. aeruginosa can be particularly concerning to eye care practitioners because these may be severe and because P. aeruginosa is a risk factor for keratitis, in particular for patients who wear contact lenses.10–14 Both Cavuoto et al and Adebayo et al reported P. aeruginosa as the etiological agent in approximately 5% of bacterial conjunctivitis cases in their respective studies.8,9 Among bacterial keratitis infections, up to one third of cases have been attributed to P. aeruginosa.15
The pathogenicity of P. aeruginosa is attributed to its large genome, which is capable of producing numerous toxins and proteases that help it initiate and maintain ocular surface infection, including exoenzymes S and U, elastase, alkaline protease, and protease IV.12,14,16 In addition to biofilm formation and quorum sensing, certain strains of P. aeruginosa, known as invasive strains, secrete exoenzyme S, enabling them to invade epithelial cells and replicate within them, while others strains, known as cytotoxic strains, secrete exoenzyme U, directly killing epithelial cells without first invading them.12,14,17 In keratitis, the net result of these virulence factors is that P. aeruginosa is able to penetrate the epithelial layer, cross the basal lamina, and gain access to the corneal stroma where it produces further damage and a severe inflammatory response. Indeed, it appears to be the host inflammatory response to the pathogen, both innate and adaptive, which results in damage to the cornea that leads to loss of visual acuity in keratitis.12,14,18
Besifloxacin ophthalmic suspension 0.6% (Besivance®, Bausch and Lomb Inc) is a topical chlorofluoroquinolone formulated with DuraSite (InSite Vision, Alameda, CA), a mucoadhesive polycarbophil polymer designed to prolong a drug’s residence time on the ocular surface and improve bioavailability.19–22 It is currently approved for the treatment of bacterial conjunctivitis in the US, Canada, and some Latin American and Asian countries.23,24 Besifloxacin ophthalmic suspension was found to be safe and effective in the treatment of bacterial conjunctivitis in multicenter, randomized, double-masked, controlled clinical studies whether administered three times daily for 5 days25–27 or twice daily for 3 days.28,29 Besifloxacin has potent broad spectrum in vitro activity, including activity against P. aeruginosa. Haas et al reported an MIC90 (minimum inhibitory concentration required to inhibit 90% of isolates) for besifloxacin of 4 μg/mL against ciprofloxacin-susceptible P. aeruginosa isolates collected in the 2009 ARMOR (Antibiotic Resistance Monitoring in Ocular micRorganisms) surveillance study (n = 89)30 and those obtained from a repository of isolates of ocular and respiratory origin (n = 105).31 This MIC90 value is lower than besifloxacin tear concentrations measured 12 hours after a single topical dose,32 suggesting that besifloxacin ophthalmic suspension could be clinically effective against this pathogen.
The aim of the current analysis was to determine the incidence of bacterial conjunctivitis caused by P. aeruginosa in clinical studies of besifloxacin ophthalmic suspension 0.6% to date, and to assess both bacterial eradication and clinical resolution rates in patients with P. aeruginosa infections treated with besifloxacin ophthalmic suspension 0.6%. Safety outcomes in these patients were also reviewed.
Materials and methods
Study design
This post hoc study analyzed cases of bacterial conjunctivitis caused by P. aeruginosa across four prospective, randomized, multicenter, double-masked clinical studies of besifloxacin ophthalmic suspension: two vehicle-controlled studies employing three times daily treatment for 5 days (Clinical- Trials.gov identifiers NCT00622908 and NCT00347932), one active-comparator three times daily study with 5 days of treatment (NCT00348348), and one vehicle-controlled study using twice daily treatment for 3 days (NCT00972777). The active-comparator study utilized moxifloxacin ophthalmic solution 0.5% as the comparator. All trial protocols were conducted in accordance with Good Clinical Practices, the International Conference on Harmonization guidelines, the Declaration of Helsinki, and the Health Insurance Portability and Accountability guidelines. Individual study results have been published previously.25–27,29 The two vehicle-controlled studies of three times daily treatment25,26 were conducted at 35 and 58 US sites and included 269 and 957 patients, respectively. The active comparator study was conducted at 73 US sites and 11 Asian sites and included 1161 patients,27 while the vehicle-controlled study with twice daily treatment was conducted at 32 US sites and included 474 patients.29
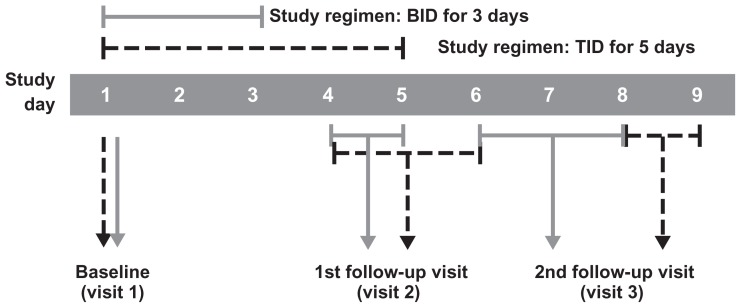
Patient inclusion and exclusion criteria and detailed study procedures have been described previously.25–29 In all four studies, patients aged one year and older were eligible for participation if they had a clinical diagnosis of bacterial conjunctivitis as evidenced by grade 1 or greater severity of both purulent ocular discharge and bulbar conjunctival injection (each on a four-point scale) in at least one eye and had pinhole visual acuity of 20/200 or better in both eyes using age-appropriate testing. Ocular discharge severity was graded as 0 = absent, 1 = mild, 2 = moderate, and 3 = severe. Grading of bulbar conjunctival injection severity was also done on a 0–3 scale and utilized validated photographic standards (Ophthalmic Research Associates, North Andover, MA). Patients who met eligibility criteria completed three study visits. At the first visit (day 1 or baseline), patients underwent an eye examination that included pinhole visual acuity, biomicroscopy, and ophthalmoscopy in both eyes. Samples for microbial cultures were taken from the conjunctival cul de sac of the affected eye(s), avoiding contact with eyelids, and patients were randomized to study treatment. Patients were instructed to administer one drop of study medication in the affected eye(s) three times daily at approximately 6-hour intervals for 5 days25–27 or twice daily at approximately 8-hour intervals during waking hours for a total of 3 days.28,29 Patients returned to the study site at or near the end of treatment (visit 2) and ≥48 hours after treatment ended (visit 3) for clinical assessment of ocular signs and symptoms, visual acuity testing, biomicroscopy, ophthalmoscopy (visit 3 only), and culture of infected eye(s). Ocular and nonocular adverse events were recorded at each visit. Figure 1 shows the timing of study visits in the various trials.
Figure 1.
Timing of study visits in bacterial conjunctivitis trials that included patients with Pseudomonas aeruginosa infections at baseline.
Note: Visit 2 occurred on day 5 ± 126,27 or day 4 or 5,29 while visit 3 occurred on day 8 or 926,27 or day 7 ± 1.29
Abbreviations: BID, twice daily; TID, three times daily.
At each visit, cultures were taken from the conjunctival cul de sac of the affected eye(s) prior to instillation of any medication using a saline moistened sterile swab, and samples were analyzed by a central laboratory to enumerate and identify bacterial pathogens (Covance Central Laboratory Services, Indianapolis, IN). Briefly, serial dilutions of test samples were plated onto bacteriological media, and the resulting colony-forming units (cfu) were enumerated and speciated by standard biochemical and/or molecular identification methods. Patients were considered culture positive if the count for a particular species (in cfu/mL) equaled or exceeded threshold values on the Cagle list, as modified by Leibowitz.33,34 For infections attributed to P. aeruginosa, the threshold criterion was 1 cfu/mL. For all species that met the bacterial threshold, antibacterial susceptibility testing was performed for besifloxacin and comparator agents following the recommended procedures of the Clinical and Laboratory Standards Institute.35–39
Outcomes
In all four studies, the primary efficacy endpoints included bacterial eradication of the baseline bacterial infection and clinical resolution of the signs of conjunctivitis in patients with culture-confirmed bacterial conjunctivitis at visit 226,27,29 or visit 3.25 Bacterial eradication was defined as the absence of ocular bacterial species that were present at or above the Cagle threshold at baseline or visit 1. Clinical resolution was defined as the absence (grade 0) of both ocular discharge and bulbar conjunctival injection.
For each randomized patient, a single eye was represented in the analysis of efficacy endpoints. When the conjunctivitis was bilateral, the study eye was the treated eye that had at least one bacterial species at or above threshold and a minimum severity of grade 1 for both conjunctival discharge and bulbar conjunctival injection at baseline. If both eyes had at least one bacterial species at or above threshold, the study eye was the one with the highest combined severity of conjunctival discharge and bulbar conjunctival injection at baseline; if severity was the same in each eye, then the right eye was considered the study eye.
Safety endpoints included rates of ocular and nonocular adverse events, changes in visual acuity and biomicroscopy findings at each follow-up visit, and changes in ophthalmoscopy findings at visit 3. All adverse events were recorded, whether observed by the investigator or reported by the patient, assessed for their relationship to the study drug, and rated as mild, moderate, or severe. Biomicroscopy was used to evaluate the lids (hyperemia and swelling), conjunctiva (chemosis), cornea (staining/erosion, edema, infiltrate), anterior chamber (cells and flare), lens, and vitreous ( pathology). Direct, undilated ophthalmoscopy was used to evaluate fundus pathology. Biomicroscopy and ophthalmoscopy findings were assessed on a four-point scale (0 = none, 1 = mild, 2 = moderate, and 3 = severe).
For the current analysis, patients with P. aeruginosa infections were identified across the four clinical studies, and, of these, cases of patients presenting with P. aeruginosa infections at baseline and treated with besifloxacin were reviewed. Narratives summarizing patient baseline characteristics, clinical and microbiological outcomes for each case, and safety findings were generated based on information contained in case report forms and microbiological results. Bacterial eradication rates and clinical resolution rates were further pooled for these patients by combining individual study data from each follow-up visit.
Results
A total of 2859 patients across the four studies were randomized and treated, and 1317 patients were culture-confirmed with a bacterial pathogen(s) above the threshold criteria. Among patients with culture-confirmed bacterial conjunctivitis, there were nine patients (0.7%) who were infected with P. aeruginosa at baseline, while an additional two patients (0.2%) presented with P. aeruginosa at follow-up visits. One study25 did not have any patients infected with P. aeruginosa at any study visit. Table 1 presents patient characteristics and baseline pathogens for all 11 patients. Patients ranged in age from 2 to 86 years, and all but two patients were female. None of the 11 patients were coinfected with virus, but most (n = 7) were infected with additional bacterial pathogens. The MIC90 for besifloxacin against the 11 P. aeruginosa isolates from these patients was 2 (range 1–4) μg/mL.
Table 1.
Characteristics and baseline pathogens of bacterial conjunctivitis patients infected with P. aeruginosa across four clinical studies
| Patient age/gender | Baseline pathogen(s) at or above threshold criteria | Besifloxacin MICa (μg/mL) | Treatment allocation | Treatment regimen/study | |
|---|---|---|---|---|---|
|
| |||||
| OD | OS | ||||
| 72-year-old female |
P. aeruginosa (150 cfu/mL) S. aureus (750 cfu/mL) |
NS | 2 | Vehicle | TID 5 days Tepedino et al26 |
| 50-year-old female |
P. aeruginosa (5 cfu/mL) S. marcescens (35 cfu/mL) |
No growth | 4 | BES | TID 5 days Tepedino et al26 |
| 40-year-old female | NS |
P. aeruginosa (250 cfu/mL) S. marcescens (125 cfu/mL) |
2 | BES | TID 5 days Tepedino et al26 |
| 62-year-old female | No growth | P. aeruginosa (1750 cfu/mL) | 2 | BES | TID 5 days McDonald et al27 |
| 86-year-old female | NS |
E. faecalis (450 cfu/mL) P. aeruginosa (30 cfu/mL) S. aureus (1000 cfu/mL) S. epidermidis (700 cfu/mL) |
2 | MOX | TID 5 days McDonald et al27 |
| 80-year-old female* | S. aureus (2125 cfu/mL) | NS | 1 | MOX | TID 5 days McDonald et al27 |
| 63-year-old female |
P. aeruginosa (30 cfu/mL) S. epidermidis (575 cfu/mL) S. pneumoniae (140 cfu/mL) |
S. epidermidis (240 cfu/mL) S. pneumoniae (2875 cfu/mL) |
2 | MOX | TID 5 days McDonald et al27 |
| 3-year-old female† |
S. pneumoniae (100 cfu/mL) C. pseudodiphtheriticum (925 cfu/mL) |
S. pneumoniae (5675 cfu/mL) S. epidermidis (65 cfu/mL) |
2 | BES | TID 5 days McDonald et al27 |
| 64-year-old female | NS | P. aeruginosa (6000 cfu/mL) | 2 | MOX | TID 5 days McDonald et al27 |
| 81-year-old male | No growth | P. aeruginosa (1350 cfu/mL) | 1 | BES | TID 5 days McDonald et al27 |
| 2-year-old male | P. aeruginosa (50 cfu/mL) | No growth | 2 | BES | BID 3 days DeLeon et al29 |
Notes:
P. aeruginosa cultured at visit 3 (140,000 cfu/mL, OD);
P. aeruginosa cultured at visit 2 (100 cfu/mL, OS) and at visit 3 (15 cfu/mL, OD);
minimum inhibitory concentration (MIC) of besifloxacin needed to inhibit the growth of the P. aeruginosa isolate from that patient.
Abbreviations: BID, twice daily; TID, three times daily; NS, no conjunctival sample collected; BES, besifloxacin ophthalmic suspension 0.6%; MIC, minimum inhibitory concentration; MOX, moxifloxacin ophthalmic solution 0.5%; P. aeruginosa, Pseudomonas aeruginosa; C. pseudodiphtheriticum, Corynebacterium pseudodiphtheriticum; S. epidermidis, Staphylococcus epidermidis; S. pneumoniae, Streptococcus pneumoniae; S. marcescens, Serratia marcescens; E. faecalis, Enterococcus faecalis; S.aureus, Staphylococcus aureus.
Case summaries
Five of the nine patients with baseline P. aeruginosa infections were treated with besifloxacin ophthalmic suspension 0.6%. All five patients presented with bilateral conjunctivitis at baseline. Baseline MICs of besifloxacin against the P. aeruginosa isolates from these patients were in the range of 1–4 μg/mL. Case summaries for these patients follow.
Case 1 was a 50-year-old white, non-Hispanic female with a history of obesity requiring gastric bypass surgery and abdominoplasty, but with no relevant ocular medical history. At baseline she presented with mild ocular discharge and mild bulbar conjunctival injection OD and moderate ocular discharge and moderate bulbar conjunctival injection OS. Additional biomicroscopy findings included mild swelling of the lids and chemosis of the conjunctiva OU. There was no fundus pathology and visual acuity was 20/40 in both eyes. Conjunctival samples from the right eye yielded P. aeruginosa at 5 cfu/mL and S. marcescens at 35 cfu/mL, while no bacterial species were recovered from the left eye. The patient was randomized to bilateral treatment with besifloxacin ophthalmic suspension 0.6% three times daily for 5 days. By visit 2, ocular discharge was absent in both eyes. Bulbar conjunctival injection was rated as mild in both eyes by visit 2 and absent in the study eye (OD) but remaining mild in the fellow eye at visit 3. There were no additional biomicroscopy findings at either follow-up visit for either eye. Conjunctival samples taken at visits 2 and 3 failed to recover any P. aeruginosa or S. marcescens from the study eye (OD) although Achromobacter xylosoxidans (30 cfu/mL) was recovered at visit 3. No bacteria were recovered from the fellow eye at either follow-up visit. No adverse events were reported at any study visit, and visual acuity did not change from baseline at any follow-up visit. There were no findings of fundus pathology at visit 3. Although A. xylosoxidans was present in the study eye at visit 3, the patient exited the study with no follow-up needed.
Case 2 was a 40-year-old white, non-Hispanic female with a history of hypertension, managed through treatment with spironolactone, and a history of allergy to erythromycin. Additional concomitant medication included ethinyl estradiol and levonorgestrel. She did not have any relevant ocular medical history. Ocular discharge at baseline was moderate in both eyes, while bulbar conjunctival injection was mild OD and moderate OS. Additional biomicroscopy findings at baseline included lid hyperemia (mild OD, moderate OS), hyperemia of the limbus (mild OD, moderate OS), and mild limbal and conjunctival chemosis OU. Baseline ophthalmoscopy was unremarkable; visual acuity was 20/30 OD and 20/40 OS. No baseline conjunctival sample was collected from the right eye, while baseline conjunctival samples from the left eye yielded P. aeruginosa (250 cfu/mL) and S. marcescens (125 cfu/mL). The patient was randomized to bilateral treatment with besifloxacin ophthalmic suspension 0.6% three times daily for 5 days. By visit 2, ocular discharge was absent OD and mild in the study eye (OS), while bulbar conjunctival injection was mild OD and absent in the study eye. Additional biomicroscopy findings at visit 2 included limbal hyperemia and chemosis, all mild in severity in the nonstudy eye. No bacteria were recovered from either eye at visit 2. Ocular discharge and bulbar conjunctival injection were absent in both eyes at visit 3 with no further biomicroscopy findings. Conjunctival samples taken at visit 3 recovered S. marcescens (590 cfu/mL) from the nonstudy eye (OD) but failed to recover any bacteria from the study eye. No adverse events were reported at any study visit. There was no change from baseline in the nonstudy eye, while visual acuity improved to 20/30 at visit 2 and 20/25 at visit 3 in the study eye. There were no ophthalmoscopy findings at visit 3. Although S. marcescens was present in the nonstudy eye at visit 3, the patient exited the study with no further follow-up needed.
Case 3 was a 62-year-old white, non-Hispanic female with a history of hypertension, arthritis, headaches, irritable bowel syndrome, overactive bladder, uterine fibroid tumors, and fibromyalgia. The patient was allergic to sulfa, penicillin, and tetracycline antibiotics. She was pseudophakic in both eyes with a history of Yag capsulotomy (OD). Concomitant medications included naproxen, dicyclomine, tolterodine, and cyclobenzaprine. At baseline, she presented with mild ocular discharge and bulbar conjunctival injection OD, and moderate ocular discharge and bulbar conjunctival injection OS. Additional baseline observations included lid hyperemia and lid swelling (mild OD, moderate OS), and mild limbal hyperemia (OS). No ophthalmoscopy findings were reported at baseline. Visual acuity was 20/20 in both eyes. Baseline conjunctival samples from the left eye yielded P. aeruginosa (1750 cfu/mL), while no bacteria were recovered from the right eye. The patient was randomized to bilateral treatment with besifloxacin ophthalmic suspension 0.6% three times daily for 5 days. By visit 2, ocular discharge and bulbar conjunctival injection were absent in both eyes, remaining absent through visit 3. Mild conjunctival chemosis was reported for the study eye (OS) at visit 2. No bacteria were recovered from either eye at visit 2. Conjunctival samples taken at visit 3 failed to recover bacteria in the nonstudy eye (OD), but recovered S. epidermidis (25 cfu/mL) from the study eye (OS). No adverse events were reported at any study visit and visual acuity remained 20/20 for both eyes at each follow-up visit. No ophthalmoscopy findings were reported at visit 3. The patient exited the study with no follow-up needed.
Case 4 was an 81-year-old white, non-Hispanic male with a history of cataract extraction in both eyes, macular degeneration in both eyes, and branch retinal vein occlusion OD. Concomitant medications included multivitamins and tramadol for pain. Ocular discharge and bulbar conjunctival injection were mild OD and severe OS. Mild lid hyperemia and mild corneal guttata were observed in both eyes at baseline. Visual acuity was 20/50 OD and 20/40 OS. No bacteria were recovered from the right eye, while P. aeruginosa (1350 cfu/mL) was recovered from the left eye. The patient was randomized to bilateral treatment with besifloxacin ophthalmic suspension 0.6% three times daily for 5 days. By visit 2, both ocular discharge and bulbar conjunctival injection had improved to mild in the study eye (OS), and remained mild in the nonstudy eye. Limbal hyperemia remained mild at visit 2 in both eyes. No bacteria were recovered from either eye at visit 2 or visit 3. At visit 3, bulbar conjunctival injection was absent in both eyes, although ocular discharge remained mild in both eyes. No adverse events were reported at any study visit. Visual acuity worsened to 20/60 OD and 20/50 OS at visit 2 improving to 20/50 OD and 20/30 OS at visit 3. There were no changes in fundus pathology findings at visit 3. The patient exited the study with no follow-up needed.
Case 5 was a 2-year-old white, non-Hispanic male with a history of amoxicillin allergy. At baseline, he presented with ocular discharge characterized as severe in both eyes and bulbar conjunctival injection of moderate severity in both eyes. In addition, moderate lid hyperemia and mild lid swelling was observed in both eyes. Baseline ophthalmoscopy did not reveal any fundus pathology. The young age of the patient prevented measurement of visual acuity, but the child could fix and follow in both eyes. Baseline conjunctival specimens yielded P. aeruginosa (50 cfu/mL) from the right eye; no bacteria were recovered from the left eye. The patient was randomized to bilateral treatment with besifloxacin ophthalmic suspension 0.6% twice daily for 3 days. By visit 2, both ocular discharge and bulbar conjunctival injection was absent OU with no further biomicroscopy findings. Conjunctival samples taken at visit 2 and visit 3 failed to recover any bacterial pathogens in either eye. No adverse events were reported at any study visit, visual acuity did not change from baseline at any follow-up visit, and visit 3 ophthalmoscopy findings remained unremarkable.
Integrated clinical outcomes
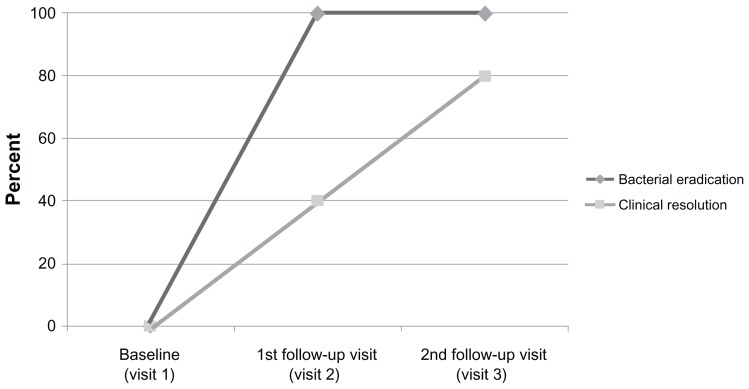
Clinical outcome data for the five patients with P. aeruginosa infections at baseline randomized to treatment with besifloxacin ophthalmic suspension were integrated. Bacterial eradication of the baseline infection with P. aeruginosa was achieved by the first follow-up visit in all five patients; clinical resolution was achieved in two of five (40%) patients by the first follow-up visit and four of five (80%) patients by the second follow-up visit (Figure 2). The only patient without clinical resolution at the second follow-up visit was the 81-year-old male with persistent mild ocular discharge.
Figure 2.
Bacterial eradication and clinical resolution at each follow-up visit for patients with Pseudomonas aeruginosa infections at baseline treated with besifloxacin ophthalmic suspension 0.6%.
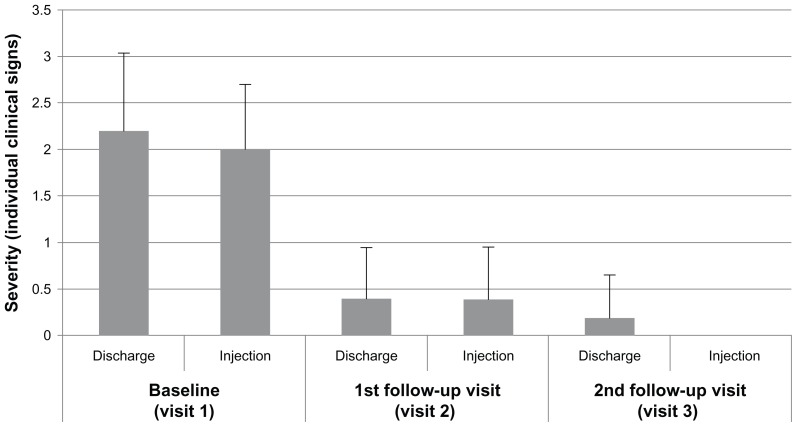
Figure 3 summarizes individual clinical signs (ocular discharge and bulbar conjunctival injection) for the study eyes. Mean (SD) severity of ocular discharge decreased from 2.2 ± 0.84 to 0.4 ± 0.55 by visit 2 and 0.2 ± 0.45 by visit 3. Mean severity of bulbar conjunctival injection decreased from 2.0 ± 0.71 to 0.4 ± 0.55 by visit 2 and 0.0 ± 0.0 by visit 3. There were no adverse events reported in these patients, and there were no clinically meaningful biomicroscopy findings or changes in ophthalmoscopy or visual acuity.
Figure 3.
Severity of clinical signs at each visit for patients with Pseudomonas aeruginosa infections at baseline treated with besifloxacin ophthalmic suspension, 0.6%.
Note: Data are the mean (+ standard deviation) severity score for ocular discharge and bulbar conjunctival injection in the study eye rated on a scale of 0–3.
Discussion
P. aeruginosa is an opportunistic Gram-negative rod commonly associated with burn, wound, and systemic infections. 40 Usually found in soil, water, plants, and animals, P. aeruginosa rarely causes disease in healthy persons, but infects those who are sick or have weakened immune systems, such as hospitalized patients. Indeed, recent estimates indicate that 10% of nosocomial infections are caused by P. aeruginosa. In the eye, P. aeruginosa has been identified as the most frequent etiologic agent in bacterial keratitis associated with contact lens wear.10–14 The American Academy of Ophthalmology estimates that 3%–33% of bacterial keratitis cases in the US are due to infection with P. aeruginosa, while approximately one-third of bacterial keratitis cases associated with contact lens wear are due to P. aeruginosa.15P. aeruginosa is considered an uncommon etiologic agent in bacterial conjunctivitis, but is always of concern to eye care practitioners due to its association with keratitis.12–14
The objective of this post hoc analysis of data from four clinical studies was to assess the clinical efficacy of besifloxacin ophthalmic suspension 0.6% when used in the treatment of patients with bacterial conjunctivitis caused by P. aeruginosa. As expected, few patients (n = 9) had infections due to P. aeruginosa at baseline, resulting in an incidence rate of 0.7% across four clinical studies in bacterial conjunctivitis, lower than rates reported by Cavuoto et al (4.5%)8 and Adebayo et al (4.8%).9 These latter studies were retrospective analyses using banked isolates collected during routine medical practice and likely limited to infections of relatively greater clinical severity, with resulting smaller isolate “denominators” for frequency calculations. In contrast, the current analysis was based on prospective, multicenter studies in which all conjunctivitis infections were cultured.25–29 Few patients with P. aeruginosa infections at baseline were infected with P. aeruginosa only. Concurrent bacterial pathogens at baseline included S. aureus, S. marcescens, S. epidermidis, Enterococcus faecalis, and S. pneumoniae (Table 1). Two additional patients developed infection with P. aeruginosa at follow-up visits, one with unilateral conjunctivitis treated with moxifloxacin ophthalmic solution 0.5% for a S. aureus infection and one with bilateral conjunctivitis treated with besifloxacin ophthalmic solution 0.6% for an S. pneumoniae infection. These findings suggest that P. aeruginosa may have been opportunistic in these individuals. The MIC90 for besifloxacin against all 11 isolates was 2 μg/mL, well below reported mean besifloxacin concentrations in human tears following a single topical ocular administration (610 μg/g 10 minutes following administration and remaining over 10 μg/g at 12 hours following administration).32
Five patients with P. aeruginosa infections at baseline were randomized to treatment with besifloxacin ophthalmic suspension 0.6%. Patient ages ranged from 2 years to 81 years, three were female and two were male, and the severity of conjunctivitis was moderate on average. Eradication of the P. aeruginosa cultured at baseline was achieved by the first follow-up visit in all five patients treated with besifloxacin. The first follow-up visit occurred on day 5 ± 1 in the studies with 5-day three times daily treatment regimens and on day 4 or 5 in the study with a 3-day twice-daily treatment regimen (Figure 1). Besifloxacin has consistently shown rapid bactericidal activity in vitro,30,31 so it is likely that bacterial eradication occurred prior to these visits. Clinical resolution, which has been reported to be delayed compared with bacterial eradication,25,26,28,29,41–44 was achieved in two of five (40%) patients by the first follow-up visit and four of five (80%) patients by the second follow-up visit. Treatment with besifloxacin ophthalmic suspension 0.6% did not present any safety concerns in these five patients. There were no adverse events reported in any of these patients. No patient had an increase in severity of any biomicroscopy findings. There were no incident fundus pathologies in any of the patients, and visual acuity was the same or improved relative to baseline. Although not the focus of this study, comparative outcomes among non-besifloxacin-treated patients with baseline P. aeruginosa infection are worthy of mention, of which there were four such cases. Three patients randomized to treatment with moxifloxacin ophthalmic solution 0.5% all achieved bacterial eradication by the first follow-up visit, while the one patient randomized to treatment with vehicle (formulation without active) failed to achieve bacterial eradication. Clinical resolution was observed in one moxifloxacin-treated patient at the first follow-up visit and in two of three patients at the final follow-up visit.
There are few reports in the literature on the treatment of P. aeruginosa ocular infections with besifloxacin ophthalmic suspension 0.6%. Michaud reported successful use of besifloxacin ophthalmic suspension in the treatment of a corneal ulcer due to presumed Pseudomonas infection in a 33-year-old North African soft contact lens wearer.45 Aggressive treatment with besifloxacin ophthalmic suspension (hourly for the first 2 days during waking hours, every 3 hours overnight along with ciprofloxacin ointment, tapered thereafter) led to a full recovery by the patient. Turaka et al described the successful use of besifloxacin ophthalmic suspension in the treatment of a 75-year-old woman with giant fornix syndrome and a soft contact lens lodged in the upper fornix.46 She had dry eye symptoms and a history of chronic conjunctivitis in the right eye. Blepharitis, diffuse mucoid discharge, conjunctival injection, and follicular and papillary reaction were observed OD and a conjunctival culture grew P. aeruginosa. Upper eyelid eversion revealed a soft contact lens lodged in the upper fornix, a thick pseudomembrane, and a deep superior conjunctival fornix. She had diffuse superficial punctate keratitis in the right eye. Following removal of the contact lens and debris, she was treated with topical besifloxacin ophthalmic suspension (dose regimen not stated). One month later, her visual acuity had improved to normal and she was free of infection. Finally, Sanders et al reported a reduction in P. aeruginosa cfus in rabbit models of keratitis induced by quinolone-susceptible or quinolone-resistant P. aeruginosa.47 In these models, keratitis was induced by intrastromal injection with 103 cfu/eye quinolone-susceptible or quinolone-resistant P. aeruginosa into rabbit corneas, and the rabbits were treated 16 hours later with besifloxacin ophthalmic suspension 0.6% or a comparator antimicrobial every 15 minutes for 5 doses and then every 30 minutes for 14 doses. Control animals were treated with phosphate-buffered saline. None of the active treatments produced a significant reduction in clinical severity of infection. However, treatment with besifloxacin ophthalmic suspension resulted in a 72% reduction of Pseudomonas cfus in the cornea in the quinolone-susceptible P. aeruginosa model and a 61% reduction in Pseudomonas cfus in the cornea in the quinolone-resistant P. aeruginosa model compared with treatment with phosphate-buffered saline (P < 0.01 for both).
In conclusion, the results of this post hoc analysis show that while the incidence of bacterial conjunctivitis due to P. aeruginosa was low across four bacterial conjunctivitis studies, treatment of patients with P. aeruginosa infections using besifloxacin ophthalmic suspension 0.6% led to bacterial eradication of P. aeruginosa by the first follow-up visit and high rates of clinical resolution by the second follow-up visit. These results are consistent with previous reports on the use of besifloxacin ophthalmic suspension for the treatment of ocular infections due to P. aeruginosa, and suggest that besifloxacin may be an appropriate choice for ocular surface infections with this pathogen, although large, prospective studies are needed.
Footnotes
Disclosure
This post hoc analysis was sponsored by Bausch and Lomb Inc, which designed and conducted the original studies. Bausch and Lomb sponsored publication of this article. BES is a speaker for Bausch and Lomb. TWM, LSG, HHD, and TLC are employees of Bausch and Lomb. The authors indicated that they have no other conflicts of interest with regard to the content of this article. The authors thank Churchill Communications, Maplewood, NJ, for editorial assistance.
References
- 1.Høvding G. Acute bacterial conjunctivitis. Acta Ophthalmol. 2008;86:5–17. doi: 10.1111/j.1600-0420.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 2.Tarabishy AB, Jeng BH. Bacterial conjunctivitis: a review for internists. Cleve Clin J Med. 2008;75:507–512. doi: 10.3949/ccjm.75.7.507. [DOI] [PubMed] [Google Scholar]
- 3.Pichichero MP. Bacterial conjunctivitis in children: antibacterial treatment options in an era of increasing drug resistance. Clin Pediatr (Phila) 2011;50:7–13. doi: 10.1177/0009922810379045. [DOI] [PubMed] [Google Scholar]
- 4.Golde KT, Gardiner MF. Bacterial conjunctivitis in children: a current review of pathogens and treatment. Int Ophthalmol Clin. 2011;51:85–92. doi: 10.1097/IIO.0b013e31822d66a1. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Ophthalmology Cornea/External Disease Panel. Conjunctivitis – Limited Revision. San Francisco, CA: American Academy of Ophthalmology; 2011. [Accessed June 6, 2012]. Preferred Practice Pattern© Guidelines. Available from: http://one.aao.org/CE/PracticeGuidelines/PPP.aspx?sid=9955f101-a94b-4f8f-a3c9-15d014f613b9. [Google Scholar]
- 6.Sheikh A, Hurwitz B. Antibiotics versus placebo for acute bacterial conjunctivitis. Cochrane Database Syst Rev. 2006;2:CD001211. doi: 10.1002/14651858.CD001211.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Weiss A. Acute conjunctivitis in childhood. Curr Probl Pediatr. 1994;24:4–11. doi: 10.1016/0045-9380(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 8.Cavuoto K, Zutshi D, Karp CL, Miller D, Feuer W. Update on bacterial conjunctivis in South Florida. Ophthalmology. 2008;115:51–56. doi: 10.1016/j.ophtha.2007.03.076. [DOI] [PubMed] [Google Scholar]
- 9.Adebayo A, Parikh JG, McCormick SA, et al. Shifting trends in in vitro antibiotic susceptibilities for common bacterial conjunctival isolates in the last decade at the New York Eye and Ear Infirmary. Graefes Arch Clin Exp Ophthalmol. 2011;249:111–119. doi: 10.1007/s00417-010-1426-6. [DOI] [PubMed] [Google Scholar]
- 10.Dutta D, Cole N, Willcox M. Factors influencing bacterial adhesion to contact lenses. Mol Vis. 2012;18:14–21. [PMC free article] [PubMed] [Google Scholar]
- 11.Fleiszig SM, Evans DJ. The pathogenesis of bacterial keratitis: studies with Pseudomonas aeruginosa. Clin Exp Optom. 2002;85:271–278. doi: 10.1111/j.1444-0938.2002.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 12.Fleiszig SM. The Glenn A. Fry award lecture 2005. The pathogenesis of contact lens-related keratitis. Optom Vis Sci. 2006;83:866–873. doi: 10.1097/01.opx.0000250045.85499.55. [DOI] [PubMed] [Google Scholar]
- 13.Green M, Apel A, Stapelton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27:22–27. doi: 10.1097/ICO.0b013e318156caf2. [DOI] [PubMed] [Google Scholar]
- 14.Willcox MDP. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci. 2007;84:273–278. doi: 10.1097/OPX.0b013e3180439c3e. [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Ophthalmology Cornea/External Disease Panel. Bacterial keratitis – Limited Revision. San Francisco, CA: American Academy of Ophthalmology; 2011. [Accessed June 6, 2012]. Preferred Practice Pattern© Guidelines. Available from: http://one.aao.org/CE/PracticeGuidelines/PPP.aspx?sid=9955f101-a94b-4f8f-a3c9-15d014f613b9. [Google Scholar]
- 16.Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 17.Choy MH, Stapelton F, Wilcox MD, Zhu H. Comparison of virulence factors in Pseudomonas aeruginosa strains isolated from contact lens and non-contact lens-related keratitis. J Med Microbiol. 2008;57(Pt 12):1539–1546. doi: 10.1099/jmm.0.2008/003723-0. [DOI] [PubMed] [Google Scholar]
- 18.Hazlett LD. Role of innate and adaptive immunity in the pathogenesis of keratitis. Ocul Immunol Inflamm. 2005;13:133–138. doi: 10.1080/09273940490912362. [DOI] [PubMed] [Google Scholar]
- 19.Friedlaender MH, Protzko E. Clinical development of 1% azithromycin in DuraSite, a topical azalide anti-infective for ocular surface therapy. Clin Ophthalmol. 2007;1:3–10. [PMC free article] [PubMed] [Google Scholar]
- 20.Bowman LM, Si E, Pang J, Archibald R, Friedlander M. Development of a topical polymeric mucoadhesive ocular delivery system for azithromycin. J Ocul Pharmacol Ther. 2009;25:133–139. doi: 10.1089/jop.2008.0066. [DOI] [PubMed] [Google Scholar]
- 21.Akpek EK, Vittitow J, Verhoeven RS, et al. Ocular distribution and pharmacokinetics of a novel ophthalmic 1% azithromycin formulation. J Ocul Pharmacol Ther. 2009;25:433–439. doi: 10.1089/jop.2009.0026. [DOI] [PubMed] [Google Scholar]
- 22.Si EC, Bowman LM, Hosseini K. Pharmacokinetic comparisons of bromfenac in DuraSite and Xibrom. J Ocular Pharmacol Ther. 2011;27:61–66. doi: 10.1089/jop.2010.0103. [DOI] [PubMed] [Google Scholar]
- 23.Bausch and Lomb Incorporated. Besivance™ (besifloxacin ophthalmic suspension) 0.6%: US prescribing information [online] [Accessed June 14, 2012]. Available from: http://www.besivance.com/site/cms/includes/assets/documents/Besivance-Full-Prescribing-Info.pdf.
- 24.Bausch and Lomb Incorporated. Besifloxacin ophthalmic suspension 0.6% (Besivance): Canadian product monograph. Vaughan, ON: Bausch & Lomb Incorporated; 2009. [Google Scholar]
- 25.Karpecki P, Depaolis M, Hunter JA, et al. Besifloxacin ophthalmic suspension 0.6% in patients with bacterial conjunctivitis: a multicenter, prospective, randomized, double-masked, vehicle-controlled, 5-day efficacy and safety study. Clin Ther. 2009;31:514–526. doi: 10.1016/j.clinthera.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Tepedino ME, Heller WH, Usner DW, et al. Phase III efficacy and safety study of besifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis. Curr Med Res Opin. 2009;25:1159–1169. doi: 10.1185/03007990902837919. [DOI] [PubMed] [Google Scholar]
- 27.McDonald MB, Protzko EE, Brunner LS, et al. Efficacy and safety of besifloxacin ophthalmic suspension 0.6% compared with moxifloxacin ophthalmic solution 0.5% for treating bacterial conjunctivitis. Ophthalmology. 2009;116:1615–1623. doi: 10.1016/j.ophtha.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Silverstein BE, Allaire C, Bateman KM, Gearinger LS, Morris TW, Comstock TL. Efficacy and tolerability of besifloxacin 0.6% ophthalmic suspension administered twice daily for 3 days in the treatment of bacterial conjunctivitis: a multicenter, double-masked, vehicle controlled, parallel group study in adults and children. Clin Ther. 2011;33:13–26. doi: 10.1016/j.clinthera.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 29.DeLeon J, Silverstein BE, Allaire C, et al. Besifloxacin ophthalmic suspension 0.6% administered twice daily for 3 days in the treatment of bacterial conjunctivitis in adults and children. Clin Drug Investig. 2012;32:303–317. doi: 10.2165/11632470-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Haas W, Pillar CM, Torres M, Morris TM, Sahm DF. Monitoring antibiotic resistance in ocular microorganisms: results from the Antibiotic Resistance Monitoring in Ocular MicRorganisms (ARMOR) 2009 surveillance study. Am J Ophthalmol. 2011;152:567–574.e3. doi: 10.1016/j.ajo.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Haas W, Pillar CM, Zurenko GE, Lee JC, Brunner LS, Morris TW. Besifloxacin, a novel fluoroquinolone, has broad-spectrum in vitro activity against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 2009;53:3552–3560. doi: 10.1128/AAC.00418-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Proksch JW, Granvil CP, Siou-Mermet R, Comstock TL, Paterno MR, Ward KW. Ocular pharmacokinetics of besifloxacin following topical administration to rabbits, monkeys, and humans. J Ocul Pharm Ther. 2009;25:335–344. doi: 10.1089/jop.2008.0116. [DOI] [PubMed] [Google Scholar]
- 33.Cagle G, Davis S, Rosenthal A, Smith J. Topical tobramycin and gentamicin sulfate in the treatment of ocular infections: multicenter study. Curr Eye Res. 1981;1:523–534. doi: 10.3109/02713688109069178. [DOI] [PubMed] [Google Scholar]
- 34.Leibowitz HM. Antibacterial effectiveness of ciprofloxacin 0.3% ophthalmic solution in the treatment of bacterial conjunctivitis. Am J Ophthalmol. 1991;112(Suppl 4):29S–33S. [PubMed] [Google Scholar]
- 35.Clinical and Laboratory Standards Institute. CLSI document M07-A6. Wayne, PA: CLSI; 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-sixth edition. [Google Scholar]
- 36.Clinical and Laboratory Standards Institute. CLSI document M07-A8. Wayne, PA: CLSI; 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-eighth edition. [Google Scholar]
- 37.Clinical and Laboratory Standards Institute. CLSI document M100-S14. Wayne, PA: CLSI; 2004. Performance standards for antimicrobial susceptibility testing: Fourteenth Informational Supplement. [Google Scholar]
- 38.Clinical and Laboratory Standards Institute. CLSI document M100-S16. Wayne, PA: CLSI; 2006. Performance standards for antimicrobial susceptibility testing: Sixteenth Informational Supplement. [Google Scholar]
- 39.Clinical and Laboratory Standards Institute. CLSI document M100-S19. Wayne, PA: CLSI; 2009. Performance standards for antimicrobial susceptibility testing: Nineteenth Informational Supplement. [Google Scholar]
- 40.Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA. Manual of Clinical Microbiology. Washington DC: ASM Press; 2007. [Google Scholar]
- 41.Abelson MB, Heller W, Shapiro AM, et al. for the AzaSite Clinical Study Group. Clinical cure of bacterial conjunctivitis with azithromycin 1%: vehicle-controlled, double-masked clinical trial. Am J Ophthalmol. 2008;145:959–965. doi: 10.1016/j.ajo.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 42.Gigliotti F, Hendley JO, Morgan J, Michaels R, Dickens M, Lohr J. Efficacy of topical antibiotic therapy in acute conjunctivitis in chidren. J Pediatr. 1984;104:623–626. doi: 10.1016/s0022-3476(84)80566-1. [DOI] [PubMed] [Google Scholar]
- 43.Rose PW, Harnden A, Brueggemann AB, et al. Chloramphenicol treatment for acute infective conjunctivitis in children in primary care: a randomized double-blind placebo-controlled trial. Lancet. 2005;366:37–43. doi: 10.1016/S0140-6736(05)66709-8. [DOI] [PubMed] [Google Scholar]
- 44.Hwang DJ, Schanzlin DJ, Rotberg MH, et al. A Phase III, placebo controlled clinical trial of 0.5% levofloxacin ophthalmic solution for the treatment of bacterial conjunctivitis. Br J Ophthalmol. 2003;87:1004–1009. doi: 10.1136/bjo.87.8.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michaud L. Efficacy of besifloxacin in the treatment of corneal ulcer. Clinical and refractive optometry. 2011;22:90–93. [Google Scholar]
- 46.Turaka K, Penne RB, Rapuano CJ, et al. Giant fornix syndrome: a case series. Ophthal Plast Reconstr Surg. 2012;28:4–6. doi: 10.1097/IOP.0b013e3182264440. [DOI] [PubMed] [Google Scholar]
- 47.Sanders ME, Moore QC, III, Norcross EW, et al. Comparison of besifloxacin, gatifloxacin, and moxifloxacin against strains of pseudomonas aeruginosa with different quinolone susceptibility patterns in a rabbit model of keratitis. Cornea. 2011;30:83–90. doi: 10.1097/ICO.0b013e3181e2f0f3. [DOI] [PubMed] [Google Scholar]