Abstract
Background
Insomnia is associated with increased risk of coronary heart disease (CHD), but the underlying mechanisms are not understood. To our knowledge, no previous studies have examined insomnia in relation to endothelial function, an indicator of preclinical atherosclerosis. Our aim was to assess the association of insomnia with endothelial function in a large population based study of healthy individuals.
Methods
A total of 4 739 participants free from known cardiovascular or pulmonary diseases, cancer, and sarcoidosis, and who were not using antihypertensive medication were included in the study. They reported how often they had experienced difficulties falling asleep at night, repeated awakenings during the night, early awakenings without being able to go back to sleep, and daytime sleepiness. Endothelial function was measured by flow mediated dilation (FMD) derived from the brachial artery.
Results
We found no consistent association between the insomnia symptoms and endothelial function in multiadjusted models, but individual insomnia symptoms may be related to endothelial function. Among women who reported early awakenings, endothelial function may be lower than in women without this symptom (p = 0.03).
Conclusions
This study provided no evidence that endothelial function, an early indicator of atherosclerosis, is an important linking factor between insomnia and CHD. Further studies are needed to explore the complex interrelation between sleep and cardiovascular pathology.
Introduction
Insomnia is a subjective feeling of having difficulties initiating or maintaining sleep or having a feeling of non-restorative sleep [1]. Increasing evidence suggests that insomnia is associated with subsequent coronary heart disease (CHD) in populations initially free of heart disease [2]–[4]. Previously, we found a dose-dependent association between number of insomnia symptoms and the risk of acute myocardial infarction which was stronger in women than in men [3]. However, the nature of this association is not yet clear. Atherosclerosis, the underlying pathophysiological mechanism of CHD, is known to develop decades before the first clinical symptoms. It is possible that both insomnia and subsequent CHD are associated with subclinical manifestations of atherosclerosis. Since insomnia is a common and potentially treatable condition, it is important to understand if insomnia is a causal factor or only a correlate of CHD.
The endothelium is a thin layer of cells that forms the inner surface of the blood vessel, forming an interface between circulating blood and the rest of the vessel wall. Healthy endothelial cells control the passage of materials and white blood cells in and out of the blood stream, prevent blood clotting and control blood pressure by releasing nitric oxide (NO) promoting vasodilation [5]. Impaired endothelial function is an indicator of preclinical atherosclerosis [6]–[8] and is characterized by decreased activity of endothelium-derived NO, causing vasoconstriction, platelet aggregation, and thrombus formation, all of which are important factors in the development of atherosclerosis [9]. Endothelial function has also consistently been shown to predict CHD [10]–[14].
To our knowledge, no previous studies have examined insomnia in relation to endothelial function. If an association was found, it would provide a plausible pathway linking insomnia to CHD. Accordingly, our aim was to assess the association of insomnia with endothelial function when controlled for potentially confounding factors, including age, marital status, education, sleep-disordered breathing, and snoring, established cardiovascular risk factors such as smoking, alcohol consumption, physical activity and BMI, and measures of psychological distress like depression or anxiety.
Materials and Methods
Ethics Statement
All participants gave written informed consent. This research was approved by the Norwegian research ethics committee.
Data Collection
Between October 2006 and June 2008, the entire adult population of Nord-Trøndelag County in Norway was invited to participate in the third wave of the Nord-Trøndelag Health Study (HUNT-3). Approximately 54% of those who were invited, attended the study (n = 51 000). Information was collected by self-administered questionnaires, a clinical examination and blood samples. Self-reported health status, use of tobacco and alcohol, dietary items, use of medication, and information on sleep, physical activity, and education were included in the questionnaire. Anthropometry, including measurements of height, weight, and waist and hip circumference, was recorded, and blood pressure and serum lipids were measured.
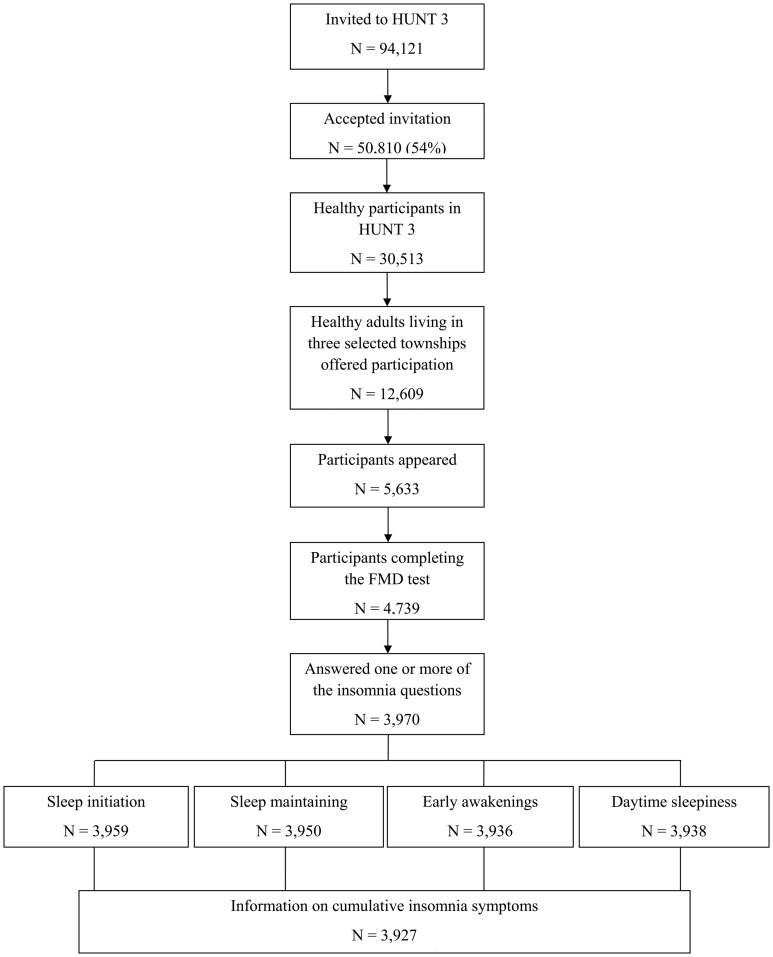
After excluding 20 533 participants (39%) known to have cardiovascular or pulmonary diseases, cancer, and sarcoidosis, and who were using antihypertensive medication, 30 513 participants were eligible for a separate study called the Fitness study [15], [16]. Among them, 12 609 participants from three selected towns were invited, and a total of 5 633 participants (44.6%) appeared at the test site. After further exclusion of 97 participants (1.7%) with cardiovascular disease or hypertension and 797 participants for other reasons (e.g. pain, illness or reluctance to carry out the test), 4 739 participants completed the endothelial function test. The participants also responded to a physical activity questionnaire. The selection process is illustrated in Figure 1.
Figure 1. The selection process.
Insomnia
The participants answered how often during the last three months they had experienced difficulties falling asleep at night, repeated awakenings during the night, and early awakenings without being able to go back to sleep, and daytime sleepiness. The response categories were “never/almost never”, “sometimes” or “several times a week”. These questions differed slightly from the insomnia questions asked in HUNT-2 (1996–1997) which our previous study [3] was based upon. The questionnaire in HUNT-2 included three items related to insomnia and the participants answered how often the last month they had difficulties falling asleep at night, early awakenings without being able to go back to sleep (with the response options never/occasionally, often/almost every night), in addition to how often they suffered from poor sleep, with the response options “never or a few times a year/1–2 times per month/about once a week/more than once a week”.
In total, 82.9% of the participants in the fitness study (n = 3 927) answered all the insomnia questions. The separate response rates for the questions about difficulties falling asleep at night, repeated awakenings during the night, early awakenings without being able to go back to sleep, and daytime sleepiness, were 83.5% (n = 3 959), 83.4% (n = 3 950), 83.1% (n = 3 936), and 83.1% (n = 3 938), respectively. These response rates largely reflect the overall response rate of the HUNT-3 questionnaire that included the insomnia questions.
Information was also collected on other aspects of sleep: loud snoring, sleep-disordered breathing, sweating while asleep, waking up with a headache and having an uncomfortable feeling in the legs.
Flow Mediated Dilation (FMD)
All the participants were asked to refrain from food and coffee, smoking and dipping tobacco during the last four hours before testing. The test was performed with the participant lying down, in a dark room with neutral temperature and minimal noise. Measurements were performed by ultrasonography (Vivid-ι, GE healthcare, USA) with 3 point ECG. A 12 Mhz linear array transducer visualized the left brachial artery in the longitudinal plane above the antecubital fossa. Baseline diameter was measured after 10 minutes of supine rest. Arterial occlusion was created by a cuff placed at the forearm [17], [18], inflated at 250 mmHg for 5 minutes, before abruptly released. Blood flow was estimated by pulsed Doppler velocity and recorded ten seconds after cuff deflation. Post diameter was measured 60 sec after cuff deflation. All arterial diameters were recorded at the peak of the R-wave in ECG, to avoid confounding of cyclic changes in the arterial dimension. The mean of three measurements (intima to intima) was recorded using optical callipers with 0.1-mm resolution. Shear rate was calculated as blood velocity (cm/s) divided by vessel diameter (cm). The difference in baseline diameter and post diameter was used as maximal dilation of the artery; yielding FMD expressed as per cent change.
Physical Activity
The participants answered a physical activity questionnaire that included questions about frequency, intensity and duration. The frequency question was stated as “How often do you exercise?” with the response options “Never”, “Less than once a week”, “Once a week”, “Two to three times a week” or “Almost every day”. The question related to intensity was stated as “How hard do you exercise?” with the response options “No sweat or heavy breathing”, “Sweat and heavy breathing”, or “Push myself to exhaustion”. Duration of the exercise was stated as “How long does each session last?” with the response options “Less than 15 minutes”, “Between 15 and 29 minutes”, “Between 30 and 60 minutes”, or “More than 60 minutes”. Frequency, intensity and duration were combined to form a physical activity index. We recoded the frequency scale to approximate number of times per week (i.e. “0”, “0.5”, “1”, “2.5”, or “5”), the intensity scale to “1”, “2”, or “3”, and the duration scale to the approximate number of hours per session (i.e. “0.10”, “0.38”, “0.75”, and “1.00”). The physical activity index was the product of the recoded frequency, intensity and duration scales. This method of calculating physical activity level has been reported and validated previously [19].
Clinical Information
Clinical information on weight, height, and blood pressure was collected by trained nurses. Systolic and diastolic blood pressure was measured three times (Dinamap 845XT Criticon) and the average of the second and third measurement was used in the analysis. Height was measured to the nearest 1 cm and weight to the nearest 0.5 kg. The participants wore light clothes and no shoes during these measurements. Body mass index (BMI) was calculated as weight (in kilograms) divided by height squared (in meters).
Socio-demographic (i.e. sex, age, and marital status) and lifestyle factors (i.e. smoking and alcohol intake) were collected by questionnaires. Marital status was categorised into never married, married, or separated/divorced/widowed, and education was categorised into whether they had completed primary and lower secondary school, upper secondary school, or university. The participants were defined as either never smokers, previous smokers or current smokers. In relation to alcohol consumption, the participants reported how many glasses of beer, wine and spirits they usually consumed over a two-week period. From this information, we categorised the participants into abstainers, light drinkers (0–1 drinks per day), moderate drinkers (above 1 but below 2 drinks per day), or heavy drinkers (2 or more drinks per day).
Anxiety and Depression
To assess symptoms of anxiety and depression, the Hospital Anxiety and Depression Scale (HADS) was used. The questionnaire consists of 14 questions (7 for anxiety and 7 for depression) with a four point scale ranging from 0 (not at all) to 3 (very often). Responses are summed to provide separate scores for symptoms of anxiety and depression with possible scores ranging from 0 to 21 for each scale. Higher scores indicate greater likelihood of depression or anxiety [20]. The Hospital Anxiety and Depression Scale is a useful tool in the assessment of symptom severity both in hospital settings and in primary health care [21]. The psychometric properties of the scale have been validated previously in the HUNT study [22].
Participant Characteristics
Characteristics of the participants according to cumulative number of insomnia symptoms are presented in Table 1 and Table 2. For both women and men, participants with insomnia symptoms tended to be older and heavier than participants without insomnia symptoms. They also had lower physical activity level and were less educated. In both sexes, depression and anxiety scores increased with increasing number of insomnia symptoms. Compared to women without insomnia symptoms, women with insomnia symptoms were more likely to be heavy drinkers and separated, divorced or widowed. For men, but not in women, the prevalence of insomnia symptoms increased with increasing severity of sleep-disordered breathing and snoring.
Table 1. Characteristics according to cumulative number of insomnia for women.
| Cumulative insomnia symptoms | ||||
| Variable | n | 0 | 1–2 | 3–4 |
| N = 2,144 | N = 1,468 | N = 566 | N = 110 | |
| Age (years) | 2,144 | 49.1 (13.4) | 49.5 (13.6) | 51.6 (13.1) |
| BMI (kg/m2) | 2,142 | 25.5 (3.7) | 25.5 (4.1) | 26.0 (4.4) |
| HADS anxiety score | 2,139 | 3.5 (2.7) | 5.4 (3.4) | 6.7 (3.9) |
| HADS depression score | 2,141 | 2.2 (2.2) | 3.2 (2.8) | 4.5 (3.3) |
| Physical activity index | 1.986 | 3.7 (2.5) | 3.6 (2.5) | 3.3 (3.0) |
| FMD (%) | 2,144 | 5.3 (4.5) | 5.3 (4.4) | 5.1 (4.5) |
| Smoking (%) | ||||
| Never | 1,052 | 53.1 | 43.0 | 48.8 |
| Previous | 581 | 26.3 | 31.5 | 27.4 |
| Current | 483 | 20.6 | 25.6 | 23.8 |
| Marital status (%) | ||||
| Never married | 460 | 21.0 | 23.3 | 18.2 |
| Married | 1,316 | 62.7 | 59.5 | 54.6 |
| Separated/divorced/widowed | 365 | 16.3 | 17.1 | 27.3 |
| Sleep-disordered breathing (%) | ||||
| Never/almost never | 2,042 | 97.5 | 94.5 | 96.6 |
| Sometimes | 63 | 2.3 | 4.6 | 3.0 |
| Several times a week | 8 | 0.1 | 0.9 | 0.4 |
| Snoring (%) | ||||
| Never/almost never | 1,416 | 69.6 | 62.9 | 61.8 |
| Sometimes | 537 | 25.0 | 25.9 | 31.8 |
| Several times a week | 147 | 5.5 | 11.2 | 6.7 |
| Alcohol intake (%) | ||||
| Abstainers | 865 | 50.7 | 52.5 | 48.9 |
| Light drinkers | 652 | 39.7 | 35.3 | 38.6 |
| Moderate drinkers | 139 | 7.8 | 9.5 | 6.8 |
| Heavy drinkers | 37 | 1.7 | 2.7 | 5.7 |
| Education (%) | ||||
| Primary | 277 | 11.7 | 14.0 | 24.8 |
| Secondary | 987 | 45.3 | 47.8 | 49.5 |
| Tertiary | 873 | 43.0 | 38.2 | 25.7 |
Table 2. Characteristics according to cumulative number of insomnia for men.
| Cumulative insomnia symptoms | ||||
| Variable | n | 0 | 1–2 | 3–4 |
| N = 1,783 | n = 1,345 | n = 380 | n = 58 | |
| Age (years) | 1,783 | 50.0 (13.2) | 51.4 (13.5) | 51.6 (11.3) |
| BMI (kg/m2) | 1,782 | 26.6 (3.0) | 26.8 (3.4) | 27.1 (3.1) |
| HADS anxiety score | 1,769 | 2.9 (2.5) | 4.8 (3.4) | 6.5 (4.1) |
| HADS depression score | 1,769 | 2.6 (2.5) | 4.1 (3.0) | 5.7 (3.6) |
| Physical activity index | 1,557 | 3.7 (2.8) | 3.4 (2.6) | 3.4 (3.1) |
| FMD (%) | 1,783 | 4.3 (3.8) | 4.0 (3.9) | 5.0 (4.1) |
| Smoking (%) | ||||
| Never | 901 | 53.0 | 45.7 | 49.1 |
| Previous | 488 | 26.6 | 32.1 | 29.1 |
| Current | 366 | 20.4 | 22.2 | 21.8 |
| Marital status (%) | ||||
| Never married | 444 | 25.2 | 23.3 | 31.0 |
| Married | 1,127 | 63.8 | 63.1 | 55.2 |
| Separated/divorced/widowed | 208 | 11.1 | 13.5 | 13.8 |
| Sleep-disordered breathing (%) | ||||
| Never/almost never | 1,543 | 88.9 | 83.2 | 80.7 |
| Sometimes | 164 | 8.8 | 11.0 | 10.5 |
| Several times a week | 58 | 2.3 | 5.9 | 8.8 |
| Snoring (%) | ||||
| Never/almost never | 735 | 42.1 | 38.8 | 44.8 |
| Sometimes | 700 | 40.9 | 36.7 | 25.9 |
| Several times a week | 337 | 17.0 | 24.5 | 29.3 |
| Alcohol intake (%) | ||||
| Abstainers | 285 | 16.1 | 18.8 | 25.5 |
| Light drinkers | 710 | 42.7 | 41.6 | 36.4 |
| Moderate drinkers | 430 | 25.7 | 24.5 | 30.9 |
| Heavy drinkers | 255 | 15.5 | 15.1 | 7.3 |
| Education (%) | ||||
| Primary | 191 | 10.2 | 12.5 | 12.1 |
| Secondary | 989 | 53.8 | 50.8 | 63.8 |
| Tertiary | 595 | 33.0 | 36.7 | 24.1 |
Statistical Analysis
The data were analysed using general linear models. We assessed each insomnia symptom using the original response categories. First, we adjusted for both age and square of age (Model 1). In the next model, we further adjusted for marital status, education, smoking, alcohol consumption, BMI, physical activity index, sleep-disordered breathing, and snoring (Model 2). Lastly, we included depression score, and anxiety score in addition to factors included in Model 2 (Model 3). We calculated least square means of FMD with corresponding 95% confidence intervals for each category of insomnia symptoms. Participants with no insomnia complaints were the reference group. In analyses of trend, we assigned a value from 1–3 to the insomnia variables representing “never/almost never”, “sometimes”, and “several times a week”, respectively. We treated these variables as continuous variables to test for linear trend. In separate analyses, we included a quadratic term to assess non-linear trends.
The insomnia symptoms were also dichotomized so we could assess the association of cumulative number of symptoms with FMD. Participants with the most frequent symptoms (i.e. those experiencing the symptom several times a week) were defined as having the respective symptom. To test the cumulative association of insomnia symptoms, we calculated the least square mean associated with increasing number of dichotomized insomnia symptoms (i.e. 0, 1–2, and 3–4). In this analysis, we excluded participants with missing information on one or more of the insomnia variables.
As a sensitivity analysis we repeated the original analysis after excluding participants who reported sleep-disordered breathing.
To assess possible effect modification we conducted stratified analyses as well as performed formal tests of interactions with sex, age (i.e. below and above 50 years), and BMI (i.e. below and above 30 kg/m2).
In these analyses, we found indication for a possible sex difference in the association between some of the insomnia symptoms and FMD. The p-values for an interaction by gender in relation to sleep initiation, frequent awakenings, early awakenings and daytime sleepiness were 0.565, 0.172, 0.002 and 0.036, respectively. Because of these findings, and due to the well-known sex differences in endothelial function and in the prevalence of insomnia [16], [23], [24] all models were run separately for women and men.
All statistical analyses were conducted using STATA 12 for Windows (Stata corp., College Station Texas).
Results
We found no evidence for an association of having difficulties initiating sleep or maintaining sleep with FMD in either women (Table 3) or men (Table 4).
Table 3. Least square means and 95% confidence intervals for the insomnia symptoms and flow mediated dilation (%) in women.
| Model 1 | Model 2 | Model 3 | ||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||
| Sleep initiation | ||||||||
| Never/almost never | 5.42 | 5.13–5.70 | 5.43 | 5.10–5.76 | 5.40 | 5.07–5.74 | ||
| Occasionally | 5.18 | 4.91–5.47 | 5.30 | 4.96–5.63 | 5.33 | 4.99–5.66 | ||
| Several times/week | 5.25 | 4.69–5.81 | 5.50 | 4.81–6.20 | 5.57 | 4.86–6.28 | ||
| P- value for linear trend | 0.35 | 0.89 | 0.88 | |||||
| P- value for quadratic trend | 0.49 | 0.51 | 0.54 | |||||
| Repeated awakenings | ||||||||
| Never/almost never | 5.40 | 5.07–5.73 | 5.54 | 5.16–5.92 | 5.52 | 5.13–5.91 | ||
| Occasionally | 5.29 | 5.03–5.56 | 5.27 | 4.95–5.59 | 5.29 | 4.97–5.61 | ||
| Several times/week | 5.19 | 4.76–5.61 | 5.35 | 4.83–5.87 | 5.38 | 4.86–5.91 | ||
| P- value for linear trend | 0.44 | 0.45 | 0.59 | |||||
| P- value for quadratic trend | 0.99 | 0.45 | 0.49 | |||||
| Early awakenings | ||||||||
| Never/almost never | 5.47 | 5.20–5.73 | 5.58 | 5.27–5.89 | 5.57 | 5.26–5.90 | ||
| Occasionally | 5.17 | 4.88–5.46 | 5.28 | 4.93–5.64 | 5.30 | 4.94–5.66 | ||
| Several times/week | 5.12 | 4.50–5.75 | 4.56 | 3.78–5.34 | 4.57 | 3.78–5.36 | ||
| P- value for linear trend | 0.15 | 0.02 | 0.03 | |||||
| P- value for quadratic trend | 0.59 | 0.44 | 0.42 | |||||
| Daytime sleepiness | ||||||||
| Never/almost never | 5.27 | 4.91–5.62 | 5.26 | 4.83–5.69 | 5.19 | 4.76–5.63 | ||
| Occasionally | 5.26 | 5.02–5.50 | 5.28 | 5.00–5.57 | 5.29 | 5.01–5.58 | ||
| Several times/week | 5.68 | 5.15–6.22 | 6.04 | 5.43–6.65 | 6.17 | 5.54–6.80 | ||
| P- value for linear trend | 0.33 | 0.10 | 0.04 | |||||
| P- value for quadratic trend | 0.28 | 0.12 | 0.10 | |||||
Model 1: Adjusted for age and age squared.
Model 2: Adjusted for age, age squared, marital status, education, smoking, alcohol consumption, physical activity index, BMI, sleep-disordered breathing, snoring.
Model 3: Adjusted for the same variables as in model 2, and depression score and anxiety score.
Table 4. Least square means and 95% confidence intervals for the insomnia symptoms and flow mediated dilation (%) in men.
| Model 1 | Model 2 | Model 3 | ||||||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |||||||
| Sleep initiation | ||||||||||||
| Never/almost never | 4.29 | 4.10–4.52 | 4.35 | 4.10–4.61 | 4.34 | 4.09–4.60 | ||||||
| Occasionally | 4.27 | 3.97–4.57 | 4.33 | 3.99–4.66 | 4.33 | 3.99–4.67 | ||||||
| Several times/week | 3.65 | 2.88–4.41 | 3.35 | 2.46–4.25 | 3.40 | 2.48–4.31 | ||||||
| P- value for linear trend | 0.28 | 0.17 | 0.24 | |||||||||
| P- value for quadratic trend | 0.33 | 0.11 | 0.12 | |||||||||
| Repeated awakenings | ||||||||||||
| Never/almost never | 4.15 | 3.87–4.42 | 4.18 | 3.88–4.48 | 4.18 | 3.88–4.48 | ||||||
| Occasionally | 4.33 | 4.10–4.60 | 4.39 | 4.08–4.69 | 4.39 | 4.08–4.69 | ||||||
| Several times/week | 4.34 | 3.86–4.81 | 4.38 | 3.85–4.91 | 4.38 | 3.85–4.91 | ||||||
| P- value for linear trend | 0.38 | 0.40 | 0.29 | |||||||||
| P- value for quadratic trend | 0.66 | 0.63 | 0.65 | |||||||||
| Early awakenings | ||||||||||||
| Never/almost never | 4.20 | 3.96–4.45 | 4.21 | 3.94–4.47 | 4.16 | 3.89–4.43 | ||||||
| Occasionally | 4.19 | 3.90–4.49 | 4.26 | 3.93–4.59 | 4.29 | 3.96–4.63 | ||||||
| Several times/week | 4.88 | 4.02–5.50 | 4.99 | 4.31–5.67 | 5.13 | 4.43–5.82 | ||||||
| P- value for linear trend | 0.18 | 0.10 | 0.04 | |||||||||
| P- value for quadratic trend | 0.12 | 0.18 | 0.16 | |||||||||
| Daytime sleepiness | ||||||||||||
| Never/almost never | 4.45 | 4.13–4.77 | 4.48 | 4.13–4.83 | 4.45 | 4.09–4.80 | ||||||
| Occasionally | 4.16 | 3.93–4.39 | 4.17 | 3.91–4.43 | 4.17 | 3.91–4.43 | ||||||
| Several times/week | 4.14 | 3.58–4.70 | 4.40 | 3.75–5.06 | 4.48 | 3.80–5.16 | ||||||
| P- value for linear trend | 0.18 | 0.41 | 0.58 | |||||||||
| P- value for quadratic trend | 0.50 | 0.24 | 0.21 | |||||||||
Model 1: Adjusted for age and age squared.
Model 2: Adjusted for age, age squared, marital status, education, smoking, alcohol consumption, physical activity index, BMI, sleep-disordered breathing, snoring.
Model 3: Adjusted for the same variables as in model 2, and depression score and anxiety score.
Women experiencing early awakenings several times/week had an estimated FMD of 4.56% (95% CI 3.78–5.34) compared to 5.58% (95% CI 5.27–5.89) in women without the symptom after adjusting for age, marital status, education, smoking, alcohol consumption, self-reported physical activity, body mass index (BMI), sleep-disordered breathing, and snoring (p for trend = 0.02). Further adjustment for anxiety and depression did not change the estimates.
Among men, there was no apparent association between daytime sleepiness and estimated FMD in any of the models. For women however, we found that those who reported a feeling of daytime sleepiness several times/week had an estimated FMD of 6.17% (95% CI 5.54–6.80) compared to 5.19% (95% CI 4.76–5.63) in other women (fully adjusted model, p for trend = 0.04).
For both women and men, we found no consistent associations between the cumulative number of insomnia symptoms and FMD in any of the models (Table 5). We obtained similar results after excluding participants who reported sleep-disordered breathing sometimes and several times/week (n = 293, results not shown).
Table 5. Least square means and 95% confidence intervals for the cumulative insomnia symptoms and flow mediated dilation (%).
| Model 1 | Model 2 | Model 3 | |||||
| Insomnia symptoms | n | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI |
| Women | |||||||
| 0 | 1,468 | 5.31 | 5.08–5.53 | 5.33 | 5.06–5.59 | 5.32 | 5.05–5.59 |
| 1–2 | 566 | 5.29 | 4.93–5.65 | 5.57 | 5.12–6.01 | 5.61 | 5.15–6.06 |
| 3–4 | 110 | 5.28 | 4.46–6.11 | 5.16 | 4.17–6.14 | 5.26 | 4.26–6.26 |
| P- value for linear trend | 0.92 | 0.72 | 0.55 | ||||
| P- value for quadratic trend | 0.99 | 0.35 | 0.35 | ||||
| Men | |||||||
| 0 | 1,345 | 4.28 | 4.08–4.49 | 4.30 | 4.07–4.52 | 4.27 | 4.04–4.50 |
| 1–2 | 380 | 4.04 | 3.65–4.42 | 4.14 | 3.69–4.58 | 4.20 | 3.75–6.66 |
| 3–4 | 58 | 5.01 | 4.03–5.99 | 5.15 | 4.04–6.26 | 5.32 | 4.18–6.46 |
| P- value for linear trend | 0.96 | 0.65 | 0.39 | ||||
| P- value for quadratic trend | 0.06 | 0.11 | 0.11 | ||||
Model 1: Adjusted for age and age squared.
Model 2: Adjusted for age, age squared, marital status, education, smoking, alcohol consumption, physical activity index, BMI, sleep-disordered breathing, snoring.
Model 3: Adjusted for the same variables as in model 2, and depression score and anxiety score.
We found no evidence of effect modification of age or BMI for any of the symptoms in any of the models (results not shown).
Discussion
To the best of our knowledge, this is the first study of insomnia and endothelial function. Overall, we found no consistent associations between insomnia and endothelial function. Thus, we could not provide clear evidence for the early indicator of atherosclerosis acting as a link between insomnia and CHD.
A few small-scale experimental studies have examined the possible vascular effects of acute sleep deprivation [25], [26]. In those studies, the investigators observed a decrease in vascular reactivity after short term sleep deprivation.
Some previous studies have examined the association of more chronic sleep deprivation with endothelial function [27], [28]. One study [28] reported that mean endothelial function had decreased from 7.4% to 3.7% before and after a 4-week period with chronic sleep deprivation. Another study [27] found an inverse relation between mean sleep duration and forearm blood flow responses to infusion of an endothelin receptor antagonist, indicating that habitual short sleep duration is associated with impaired endothelial function. However, both studies were small and did not provide sex-specific results. Also, there was no available information on anxiety and depression, two factors that are potentially important confounders in relation to insomnia and cardiovascular diseases, since anxiety and depression are both related to insomnia [29], [30] and to the risk of cardiac events [31].
In our previous analyses of the HUNT-2 study, we found that experiencing insomnia symptoms increased the risk of incident AMI [3]. Recently, endothelial function was collected in HUNT-3, and we were able to explore whether endothelial dysfunction could be a mechanism behind the previously observed association between insomnia and AMI. However, the HUNT-3 study does not have an adequate follow time yet to investigate insomnia and AMI risk in this population.
Our analyses of individual insomnia symptoms suggest that some of the symptoms might be related to endothelial function, and that this relation might differ by sex. Among women we observed an inverse association of early awakenings with endothelial function, but there was an opposite trend among men. Also, women that reported daytime sleepiness had a higher FMD than other women. Of note, a large study reported a reduction in all-cause mortality associated with increasing frequency of insomnia [32]. Therefore, some aspects of insomnia might actually have a protective effect in certain subgroups. Nevertheless, the positive association between daytime sleepiness and endothelial function that we found among women was unexpected and warrants further evaluation in future studies.
The nature of the association of insomnia and CHD is unclear, and it is unknown whether insomnia is a cause or only a marker of increased risk. Endothelial dysfunction, an indicator of atherosclerosis in its earlier, preclinical stage, had no overall consistent association with insomnia in our study. However, insomnia might have an impact on CHD through other pathways, including influence on plaque stability via increasing the level of pro-inflammatory cytokines or on thrombosis via increasing the level of pro-coagulatory factors [33]–[38]. The latter two mechanisms, in contrast to endothelial dysfunction refer to a late stage of atherosclerosis. The increased CHD risk apparent in persons suffering from insomnia [2]–[4], [39], regardless of its exact nature, suggests that insomniacs and their doctors should be aware of increased cardiovascular risk and should be able to recognize symptoms and early manifestations of CHD in an effort to forestall its serious consequences.
Limitations
Despite of its clear strengths that include the large sample size, the population based design and the use of directly measured FMD, our study also has some important limitations.
It was based on self-reported measures of sleep, and objective measures (i.e. polysomnography) were not available. Insomnia is defined as a subjective feeling of having difficulties falling asleep, remaining asleep or receiving restorative sleep [1], [24] and thus, such problems are not routinely evaluated by polysomnography [40]. However, our evaluation largely reflects the DSM-IV criteria of insomnia [1].
We did not evaluate chronic insomnia symptoms. If an association with endothelial function only result from prolonged exposure to insomnia symptoms, not to only recent exposure, and subjects in our study belonged mainly to the latter group, the association between insomnia and vascular dysfunction might be diluted and difficult to detect.
Information about sleep duration was not available in our study. However, insomnia is a different condition than short sleep duration [32] and people with insomnia symptoms could have normal or long duration of sleep [41]. Also, some people with short sleep duration may not have any insomnia symptoms because there is a large variation between individuals in the duration of sleep that is needed for physiological and psychological restoration [42], [43]. It is also important to recognize that insomnia provides additional information on the quality of sleep. From one study it was reported that the increased risk of CHD associated with short duration of sleep was greatest among those with a sleep disturbance [44]. Thus, only accounting for sleep duration will miss information about subjective quality of sleep that may be important in the assessment of its harmful effects.
In our study, we restricted to individuals free of cardiovascular diseases (CVD) and hypertension. This restriction, as a conditioning on common consequences of cardiovascular risk factors, might introduce a collider stratification bias, which is a frequent problem in similar cross sectional studies [45], [46].
This study may also be subject to self-selection bias. Only 54% of the eligible population responded to the HUNT-3 study and only 44.6% of those invited to the Fitness study chose to participate in the FMD testing. Thus, it might be suspected that only the healthiest accepted the invitation. However, by comparing the participants in the fitness study with a healthy sample of the total HUNT population (i.e. free from cardiovascular or pulmonary diseases, cancer, or sarcoidosis), it was confirmed that the fitness participants were not considerably different from other healthy participants in the HUNT Study with regard to self-reported and measured health variables [15].
We cannot exclude the possibility that the reported insomnia symptoms are caused by underlying pathology and that the insomnia symptoms are secondary to that condition. Because participants in the HUNT-3 study with known cardiovascular or pulmonary diseases, cancer, sarcoidosis, and users of antihypertensive medication were ineligible for our study however, this possibility has been minimised.
It is possible that some of the covariates included in models 2 and 3 may act as mediators in the association of insomnia with endothelial function. For example alcohol consumption near bed time may be used as sleep aid. However, adjustment for these factors had very limited impact on the regression coefficients.
Our study was performed on an apparently healthy, socio-economically homogenous population and the results cannot be directly generalized to less healthy populations or to countries on different latitudes, different socioeconomic status or with different sleeping habits.
Conclusions
In this large population based study of apparently healthy individuals, we found no consistent association of insomnia with FMD. Thus, endothelial function did not provide a clear link between insomnia and subsequent CHD. As it is an early indicator of preclinical atherosclerosis and a significant determinant of cardiovascular diseases, our findings may be important in the complex interrelation between sleep and the cardiovascular system. The sex-specific differences related to some of the insomnia symptoms warrant further investigation.
Acknowledgments
The Nord-Trøndelag Health Study (The HUNT Study) is a collaboration between HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology NTNU), Nord-Trøndelag County Council, Central Norway Health Authority, and the Norwegian Institute of Public Health.
Funding Statement
This work was supported by the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology (LBS, IJ, LEL), the Swedish Research Council and by the Swedish Council of Working Life and Social Research (IJ). FMD-data was collected in the Fitness – study and this work was supported by grants from the Norwegian Council on Cardiovascular Disease, the Foundation for Cardiovascular Research at St. Olav’s Hospital; the Norwegian State Railways; and Roche Norway Incorporated, and a Norwegian Research Council Grant for Outstanding Young Investigators (UW) and the K.G. Jebsen Foundation, Norwegian Council of Cardiovascular Disease. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.American Psychiatric Association (2004) Diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV). Arlington, United States.
- 2. Elwood P, Hack M, Pickering J, Hughes J, Gallacher J (2006) Sleep disturbance, stroke, and heart disease events: evidence from the Caerphilly cohort. J Epidemiol Commun H 60: 69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laugsand LE, Vatten LJ, Platou C, Janszky I (2011) Insomnia and the risk of acute myocardial infarction. Circulation 124: 2073–2081. [DOI] [PubMed] [Google Scholar]
- 4. Spiegelhalder K, Scholtes C, Riemann D (2010) The association between insomnia and cardiovascular disease. Nat Sci Sleep 2: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Behrendt D, Ganz P (2002) Endothelial function: From vascular biology to clinical applications. Am J Cardiol 90: L40–L48. [DOI] [PubMed] [Google Scholar]
- 6. Drexler H (1997) Endothelial dysfunction: clinical implications. Prog Cardiovasc Dis 39: 287–324. [DOI] [PubMed] [Google Scholar]
- 7. Davignon J, Ganz P (2004) Role of Endothelial Dysfunction in Atherosclerosis. Circulation 109: III–27–III-32. [DOI] [PubMed] [Google Scholar]
- 8. Zeiher AM, Drexler H, Wollschlager H, Just H (1991) Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation 83: 391–401. [DOI] [PubMed] [Google Scholar]
- 9. Egashira K (2002) Clinical importance of endothelial function in arteriosclerosis and ischemic heart disease. Circ J 66: 529–533. [DOI] [PubMed] [Google Scholar]
- 10. Widlansky ME, Gokce N, Keaney JF Jr, Vita JA (2003) The clinical implications of endothelial dysfunction. J Am Coll Cardiol 42: 1149–1160. [DOI] [PubMed] [Google Scholar]
- 11. Halcox JPJ, Schenke WH, Zalos G, Mincemoyer R, Prasad A, et al. (2002) Prognostic Value of Coronary Vascular Endothelial Dysfunction. Circulation 106: 653–658. [DOI] [PubMed] [Google Scholar]
- 12. Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, et al. (2000) Long-Term Follow-Up of Patients With Mild Coronary Artery Disease and Endothelial Dysfunction. Circulation 101: 948–954. [DOI] [PubMed] [Google Scholar]
- 13. Kuvin JT, Patel AR, Sliney KA, Pandian NG, Rand WM, et al. (2001) Peripheral vascular endothelial function testing as a noninvasive indicator of coronary artery disease. J Am Coll Cardiol 38: 1843–1849. [DOI] [PubMed] [Google Scholar]
- 14. Yeboah J, Crouse JR, Hsu F-C, Burke GL, Herrington DM (2007) Brachial Flow-Mediated Dilation Predicts Incident Cardiovascular Events in Older Adults. Circulation 115: 2390–2397. [DOI] [PubMed] [Google Scholar]
- 15. Aspenes ST, Nilsen TIL, Skaug E, Bertheussen GF, Ellingsen Ø, et al. (2011) Peak oxygen uptake and cardiovascular risk factors in 4631 healthy women and men. Med Sci Sports Exerc 43: 1465–1473. [DOI] [PubMed] [Google Scholar]
- 16.Skaug E-A, Aspenes ST, Oldervoll L, Mørkedal B, Vatten L, et al.. (2012) Age and gender differences of endothelial function in 4739 healthy adults- The HUNT 3 Fitness study. European Journal of Preventive Cardiology Published online. [DOI] [PubMed]
- 17. Peretz A, Leotta DF, Sullivan JH, Trenga CA, Sands FN, et al. (2007) Flow mediated dilation of the brachial artery: an investigation of methods requiring further standardization. BMC cardiovascular disorders 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, et al. (2002) Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39: 257–265. [DOI] [PubMed] [Google Scholar]
- 19. Kurtze N, Rangul V, Hustvedt B-E, Flanders WD (2008) Reliability and validity of self-reported physical activity in the Nord-Trøndelag Health Study – HUNT 1. Scand J Public Health 36: 52–61. [DOI] [PubMed] [Google Scholar]
- 20.Zigmond A, Snaith R (1994) The HADS: Hospital Anxiety and Depression Scale. Windsor: NFER Nelson.
- 21. Bjelland I, Dahl AA, Haug TT, Neckelmann D (2002) The validity of the Hospital Anxiety and Depression Scale: An updated literature review. J Psychosom Res 52: 69–77. [DOI] [PubMed] [Google Scholar]
- 22. Mykletun A, Stordal E, Dahl AA (2001) Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry 179: 540. [DOI] [PubMed] [Google Scholar]
- 23. Schillaci G, Marchesi S, Siepi D, Lupattelli G, Vaudo G, et al. (2001) Gender differences in postprandial endothelial function. Am J Cardiol 87: 1323–1325. [DOI] [PubMed] [Google Scholar]
- 24. Ohayon MM (2002) Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev 6: 97–111. [DOI] [PubMed] [Google Scholar]
- 25. Sauvet F, Leftheriotis G, Gomez-Merino D, Langrume C, Drogou C, et al. (2010) Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol 108: 68–75. [DOI] [PubMed] [Google Scholar]
- 26. Amir O, Alroy S, Schliamser JE, Asmir I, Shiran A, et al. (2004) Brachial artery endothelial function in residents and fellows working night shifts. Cardiol 93: 947–949. [DOI] [PubMed] [Google Scholar]
- 27. Weil BR, Mestek ML, Westby CM, Van Guilder GP, Greiner JJ, et al. (2010) Short sleep duration is associated with enhanced endothelin-1 vasoconstrictor tone. Can J Physiol Pharmacol 88: 777–781. [DOI] [PubMed] [Google Scholar]
- 28. Takase B, Akima T, Uehata A, Ohsuzu F, Kurita A (2004) Effect of chronic stress and sleep deprivation on both flow-mediated dilation in the brachial artery and the intracellular magnesium level in humans. Clin Cardiol 27: 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor DJ (2008) Insomnia and depression. Sleep 31: 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carney CE, Edinger JD (2010) Insomnia and Anxiety. New York: Springer.
- 31. Janszky I, Ahnve S, Lundberg I, Hemmingsson T (2010) Early-Onset Depression, Anxiety, and Risk of Subsequent Coronary Heart Disease: 37-Year Follow-Up of 49,321 Young Swedish Men. J Am Coll Cardiol 56: 31–37. [DOI] [PubMed] [Google Scholar]
- 32. Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR (2002) Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry 59: 131. [DOI] [PubMed] [Google Scholar]
- 33. Hattori M, Azami Y (2001) Searching for preventive measures of cardiovascular events in aged Japanese taxi drivers–the daily rhythm of cardiovascular risk factors during a night duty day. J Hum Ergol (Tokyo) 30: 321. [PubMed] [Google Scholar]
- 34. Miller MA, Kandala N-B, Kumari M, Marmot MG, Cappuccio FP (2010) Relationships Between Sleep Duration and von Willebrand Factor, Factor VII, and Fibrinogen. Arterioscler Thromb Vasc Biol 30: 2032–2038. [DOI] [PubMed] [Google Scholar]
- 35. Tofler GH, Brezinski D, Schafer AI, Czeisler CA, Rutherford JD, et al. (1987) Concurrent Morning Increase in Platelet Aggregability and the Risk of Myocardial Infarction and Sudden Cardiac Death. N Engl J Med 316: 1514–1518. [DOI] [PubMed] [Google Scholar]
- 36. Meier-Ewert HK, Ridker PM, Mullington JM, Dinges DF, Price NJ, et al. (2004) Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 43: 678–683. [DOI] [PubMed] [Google Scholar]
- 37. Vgontzas A, Zoumakis E, Bixler E, Lin HM, Follett H, et al. (2004) Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 89: 2119–2126. [DOI] [PubMed] [Google Scholar]
- 38. Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, et al. (2008) Sleep loss activates cellular inflammatory signaling. Biol Psychiatry 64: 538–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Y, Tanaka H (2002) Overtime work, insufficient sleep, and risk of non-fatal acute myocardial infarction in Japanese men. Occup Environ Med 59: 447–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Littner M, Hirshkowitz M, Kramer M, Kapen S, Anderson WMD, et al. (2003) Practice parameters for using polysomnography to evaluate insomnia: an update. Sleep 26: 754–760. [DOI] [PubMed] [Google Scholar]
- 41. Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A (2009) Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep 32: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Dongen H, Vitellaro KM, Dinges DF (2005) Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep 28: 479–496. [DOI] [PubMed] [Google Scholar]
- 43. Benoit O, Foret J, Merle B, Reinberg A (1981) Circadian rhythms (temperature, heart rate, vigilance, mood) of short and long sleepers: Effects of sleep deprivation. Chronobiologia 8: 341–350. [PubMed] [Google Scholar]
- 44. Chandola T, Ferrie JE, Perski A, Akbaraly T, Marmot MG (2010) The effect of short sleep duration on coronary heart disease risk is greatest among those with sleep disturbance: a prospective study from the Whitehall II cohort. Sleep 33: 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Janszky I, Ahlbom A, Svensson AC (2010) The Janus face of statistical adjustment: confounders versus colliders. Eur J Epidemiol 25: 361–363. [DOI] [PubMed] [Google Scholar]
- 46.Rothman KJ, Greenland S, Lash TL (2008) Modern Epidemiology. Philadelphia, USA: Lippincott, Williams & Wilkins.