Abstract
Mycobacterium leprae (M. leprae) lives and replicates within macrophages in a foamy, lipid-laden phagosome. The lipids provide essential nutrition for the mycobacteria, and M. leprae infection modulates expression of important host proteins related to lipid metabolism. Thus, M. leprae infection increases the expression of adipophilin/adipose differentiation-related protein (ADRP) and decreases hormone-sensitive lipase (HSL), facilitating the accumulation and maintenance of lipid-rich environments suitable for the intracellular survival of M. leprae. HSL levels are not detectable in skin smear specimens taken from leprosy patients, but re-appear shortly after multidrug therapy (MDT). This study examined the effect of MDT components on host lipid metabolism in vitro, and the outcome of rifampicin, dapsone and clofazimine treatment on ADRP and HSL expression in THP-1 cells. Clofazimine attenuated the mRNA and protein levels of ADRP in M. leprae-infected cells, while those of HSL were increased. Rifampicin and dapsone did not show any significant effects on ADRP and HSL expression levels. A transient increase of interferon (IFN)-β and IFN-γ mRNA was also observed in cells infected with M. leprae and treated with clofazimine. Lipid droplets accumulated by M. leprae-infection were significantly decreased 48 h after clofazimine treatment. Such effects were not evident in cells without M. leprae infection. In clinical samples, ADRP expression was decreased and HSL expression was increased after treatment. These results suggest that clofazimine modulates lipid metabolism in M. leprae-infected macrophages by modulating the expression of ADRP and HSL. It also induces IFN production in M. leprae-infected cells. The resultant decrease in lipid accumulation, increase in lipolysis, and activation of innate immunity may be some of the key actions of clofazimine.
Author Summary
Leprosy, caused by Mycobacterium leprae (M. leprae), is an ancient infectious disease that remains the leading infectious cause of disability. After infection, M. leprae lives inside host macrophages that contain a large amount of lipids, which is thought to be an essential microenvironment for M. leprae to survive in host cells. M. leprae infection increases lipid accumulation in macrophages and decreases the metabolic breakdown of lipids (catabolism). In addition, the treatment of leprosy with multidrug therapy (MDT) reverses the effect of infection on the modulation of lipid metabolism. We therefore aimed to use cultured human macrophage cells to determine which of the three MDT drugs (clofazimine, dapsone, or rifampicin) is responsible for this effect. We found that only clofazimine affects lipid accumulation and catabolism in M. leprae-infected cells in vitro. The amounts of lipids accumulated in the cells decreased when clofazimine was added to the cell culture medium. Clofazimine also activated immune responses in M. leprae-infected cells. These results suggest that the effectiveness of clofazimine against leprosy is due to the modulation of lipid metabolism and activation of immune reactions in M. leprae-infected host cells.
Introduction
Leprosy is a chronic infectious disease caused by Mycobacterium leprae (M. leprae), which is a typical intracellular pathogen that parasitizes tissue macrophages (histiocytes) and Schwann cells of the peripheral nerves of the dermis. Although its prevalence has declined over the last several decades due to the introduction of multi-drug therapy (MDT) by the World Health Organization (WHO), leprosy remains a major public health problem in many developing countries: In 2010, 228,474 new cases were registered worldwide [1]. Based on their clinical, histological and immunological manifestations, leprosy patients are classified into five groups that comprise one continuous spectrum: Tuberculoid (TT), Borderline Tuberculoid (BT), Borderline (BB), Borderline Lepromatous (BL) and Lepromatous (LL) [2]. LL is characterized by widespread skin lesions containing numerous bacilli that live in the foamy or enlarged lipid-filled phagosome within macrophages. Schwann cells in LL nerves also have the foamy, lipid-laden appearance that favors mycobacterial survival and persistence. In Schwann cells, M. leprae infection-induced biogenesis of lipid droplets correlates with increased prostaglandin E2 (PGE2) and interleukin-10 (IL-10) secretion, which is essential for leprosy pathogenesis [3], [4]. Although lipid-laden macrophages are also observed in other mycobacterial infections, including tuberculosis [5], [6], the amount of lipid and the number of infected macrophages are most prominent in cases of LL [7], [8].
The PAT protein family is named after three of its members: perilipin, adipophilin/adipose differentiation-related protein (ADRP), and tail-interacting protein of 47 kDa (TIP47). PAT family members are responsible for the transportation of lipids and the formation of lipid droplets in a variety of tissues and cultured cell lines, including adipocytes [9]–[12]. ADRP selectively increases the uptake of long chain fatty acids and has an essential role in fatty acid transport [13], [14]. Hormone-sensitive lipase (HSL), as the first enzyme identified in the induction of lipo-catabolic action initiated by hormones, is the predominant lipase effector of catecholamine-stimulated lipolysis in adipocytes [15]. Therefore, ADRP and HSL have opposing functions, i.e., lipid accumulation vs. its degradation. ADRP and HSL also play important roles in lipid accumulation in M. leprae-infected macrophages [8], [16]. M. leprae infection increased the expression of ADRP mRNA and protein, facilitating the accumulation and maintenance of a lipid-rich environment suitable for intracellular survival [8]. Conversely, HSL expression was suppressed in macrophages infected with M. leprae [16]. These results suggest that both ADRP and HSL influence the lipid-rich environment that favors M. leprae parasitization and survival in infected host cells. In our previous study, HSL expression was not detectable in slit-skin smear specimens from non-treated LL and BL patients, but it re-appeared shortly after MDT treatment [16]. However, how treatment modulates HSL expression is not clear. In the present study, we determine the effect of MDT components on host lipid metabolism by investigating the influence of rifampicin, dapsone and clofazimine on the expression of ADRP and HSL in THP-1 cells.
Materials and Methods
Ethic statement
Human specimens were used according to the guidelines approved by the Ethical Committee of the National Institute of Infectious Diseases (Tokyo, Japan). All samples were anonymized before use.
Drugs
Clofazimine (Sigma-Aldrich Co., St. Louis, MO), rifampicin (Wako Pure Chemical Industries Ltd., Osaka, Japan) and dapsone (Wako Pure Chemical Industries Ltd.) were dissolved in dimethyl sulfoxide (DMSO) and stored at 4°C. The final concentration used in the culture medium was 8.0 µg/ml rifampicin, 5.0 µg/ml dapsone or 2.0 µg/ml clofazimine.
M. leprae isolation and cell culture
Hypertensive nude rats (SHR/NCrj-rnu), infected with the Thai53 strain of M. leprae [17], [18] were kindly provided by Dr. Y. Yogi of the Leprosy Research Center, National Institute of Infectious Diseases. Japan. The protocol was approved by the Experimental Animal Committee, of the National Institute of Infectious Diseases, Tokyo, Japan (Permit Number: 206055). Animal studies were carried out in strict accordance with the recommendations from Japan's Animal Protection Law. M. leprae was isolated as previously described [19], [20]. The human premonocytic cell line THP-1 was obtained from the American Type Culture Collection (ATCC; Manassas, VA). The cells were cultured in six-well plates in RPMI medium supplemented with 10% charcoal-treated fetal bovine serum (FBS), 2% non-essential amino acids, 100 IU/ml penicillin and 100 µg/ml streptomycin at 37°C in 5% CO2 [7], [8]. Typically, 3×107 bacilli were added to 3×106 THP-1 cells (multiplicity of infection: MOI = 10).
RNA preparation and reverse transcription polymerase chain reaction (RT-PCR)
Total RNA from cultured cells was prepared using RNeasy Mini Kits (Qiagen Inc., Valencia, CA) as described previously [7], [8]. Total RNA preparation from slit-skin smear samples was performed as described [8], [16]. Briefly, stainless steel blades (Feather Safety Razor Co., Osaka, Japan) used to obtain slit-skin smear specimens were rinsed in 1 ml of sterile 70% ethanol. The tube was then centrifuged at 20,000×g for 1 min at 4°C. After removing the supernatant, RNA was purified with the same protocol that was used for cultured cells. The RNA was eluted in 20 µl of elution buffer and treated with 0.1 U/µl DNase I (TaKaRa Bio, Kyoto, Japan) at 37°C for 60 min to degrade any contaminating genomic DNA. All RNA samples had an OD260/280 of 1.8–2.0 and an OD260/230 >1.8. RNA sample quality was also confirmed using denaturing agarose gel electrophoresis and the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) (Fig. S1). Total RNA from each sample was reverse-transcribed to cDNA using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) with random primers [8], [16]. The following primers were used to amplify specific cDNAs: ADRP: 5′-TGTGGAGAAGACCAAGTCTGTG-3′ (forward) and 5′-GCTTCTGAACCAGATCAAATCC-3′ (reverse); HSL: 5′-CTCCTCATGGCTCAACTCCTTCC-3′ (forward) and 5′-AGGGGTTCTTGACTATGGGTG-3′ (reverse); interferon (IFN)-β: 5′-TGCTCTCCTGTTGTGCTTCTCCAC-3′ (forward) and 5′-CAATAGTCTCATTCCAGCCAGTGC-3′ (reverse); IFN-γ: 5′-GCAGAGCCAAATTGTCTCCTTTTAC-3′ (forward) and 5′-ATGCTCTTCGACCTCGAAACAGC-3′ (reverse) and actin: 5′-AGCCATGTACGTAGCCATCC-3′ (forward) and 5′-TGTGGTGGTGAAGCTGTAGC-3′ (reverse). Touchdown PCR was performed using a PCR Thermal Cycler DICE (TaKaRa Bio, Tokyo, Japan) [7], [8]. Briefly, the PCR mixture was first denatured for 5 min at 94°C, followed by 20 cycles of three-temperature PCR consisting of a 30-sec denaturation at 94°C, a 30-sec annealing that started at 65°C and decreased 0.5°C every cycle to 55°C, and a 45-sec extension at 72°C. An additional 10 cycles were performed for ADRP and β-actin, and 14 cycles for HSL with a fixed annealing temperature of 55°C. The products were analyzed by 2% agarose gel electrophoresis.
Protein preparation and Western blot analysis
Cellular protein was extracted and analyzed as previously described [16], [21]. Briefly, cells were lysed in a lysis buffer containing 50 mM HEPES, 150 mM NaCl, 5 mM EDTA, 0.1% NP40, 20% glycerol, and protease inhibitor cocktail (Complete Mini, Roche, Indianapolis, IN) for 1 h. After centrifugation, the supernatant was transferred and 10 µg of protein was used for analysis. Cellular proteins were mixed with 4× LDS sample buffer and 10× reducing agent (Invitrogen, Life Technologies, Carlsbad, CA) and incubated for 10 min at 70°C prior to electrophoresis. Proteins were separated on NuPage 4–12% Bis Tris Gels and transferred using an iBlot Gel Transfer Device (Invitrogen). The membrane was washed with PBST (phosphate buffered saline (PBS) with 0.1% Tween 20), blocked in blocking buffer (PBST containing 5% skim milk) overnight, and then incubated with either rabbit anti-ADRP antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA; 1∶2,000 dilution), rabbit anti-HSL antibody (Cell Signaling Technology, Danvers, MA; 1∶1,000 dilution) or goat anti-β-actin antibody (Santa Cruz; dilution 1∶2,000). After washing with PBST, the membrane was incubated for 1 h with biotinylated donkey anti-rabbit antibody for ADRP and HSL (GE Healthcare, Fairfield, CT; 1∶2,000 dilution) or biotinylated donkey anti-goat antibody for β-actin (Millipore, Billerica, MA; dilution 1∶10,000) followed by streptavidin-HRP (GE Healthcare; 1∶10,000 dilution) for 1 h. The signal was developed using ECL Plus Reagent (GE Healthcare).
Lipid staining
THP-1 cells were grown on glass coverslips in 24-well plates for 24 h, before the culture medium was exchanged with RPMI 1640 containing M. leprae and clofazimine. Control and drug-treated THP-1 cells were fixed in 10% formalin for 10 min. They were then washed with Dulbecco's PBS (DPBS) and balanced with 60% isopropanol for 1 min before staining with oil-red-O (Muto Pure Chemicals, Tokyo, Japan) for 10 min. The cells were counterstained with hematoxylin for 5 min followed by ethanol dehydration and coverslip sealing.
Immunohistochemistry
Archived formalin-fixed, paraffin-embedded tissue sections were subjected to immunohistochemical staining as described [7]. Briefly, deparaffinized sections were heated in 1 mM NaOH at 120°C for 5 min for antigen retrieval. They were then washed with PBST and blocked in blocking buffer (DAKO, Carpinteria, CA) for 10 min, and then incubated with either anti-ADRP antibody (Santa Cruz Biotechnology Inc.; 1∶200 dilution) or anti-HSL antibody (Cell Signaling Technology; 1∶100 dilution), for 1 h at room temperature. After washing the slides with PBST, peroxidase-labeled streptavidin-biotin method was employed using the LSAB2 kit (DAKO) and 3,3-diaminobenzidine tetrahydrochloride (DAB) for the staining of ADRP. Tyramide signal amplification (TSA)-HRP method was utilized to amplify HSL staining signals using the TSA Biotin System (PerkinElmer, Inc., Waltham, MA) according to the manufacturer's protocol. Sections were then stained using carbol fuchsin to visualize acid-fast mycobacteria and counterstained with hematoxylin.
Others
All experiments were repeated at least three times. Since the replicates produced essentially the same outcomes, representative results from these independent experiments are shown in the figures.
Results
Clofazimine decreases ADRP and increases HSL mRNA levels in macrophages infected with M. leprae
The effect of MDT drugs on lipid metabolism in M. leprae-infected macrophages was examined by infecting human premonocytic THP-1 cells with M. leprae (MOI = 10) in the presence of 8.0 µg/ml rifampicin, 5.0 µg/ml dapsone or 2.0 µg/ml clofazimine for 24 h. Total RNA was isolated and RT-PCR analysis was performed to evaluate possible changes in ADRP and HSL mRNA levels. In our previous studies, M. leprae infection has been shown to increase ADRP and decrease HSL expression, which will in turn increase the lipid accumulation that is thought to contribute to maintaining a phagosome environment which permits M. leprae to parasitize tissue macrophages [8], [16]. However, when M. leprae-infected THP-1 cells were treated with clofazimine, ADRP expression levels decreased and HSL expression increased (Fig. 1). Rifampicin and dapsone did not show significant effects on the mRNA expression of ADRP, while they decreased HSL expression by augmenting the effect of M. leprae infection.
Figure 1. Expression of ADRP and HSL is modulated by clofazimine in THP-1 cells infected with M. leprae.
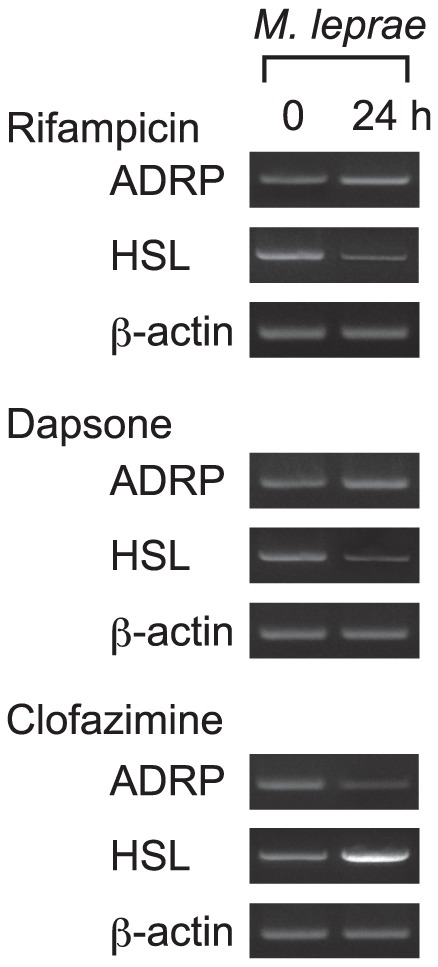
THP-1 cells were cultured in six-well plates with culture medium containing either 8.0 µg/ml rifampicin, 5.0 µg/ml dapsone or 2.0 µg/ml clofazimine with M. leprae infection (MOI = 10). After incubating for 24 h, total RNA was purified and RT-PCR analysis of ADRP, HSL and β-actin was performed. Representative results from three independent experiments are shown.
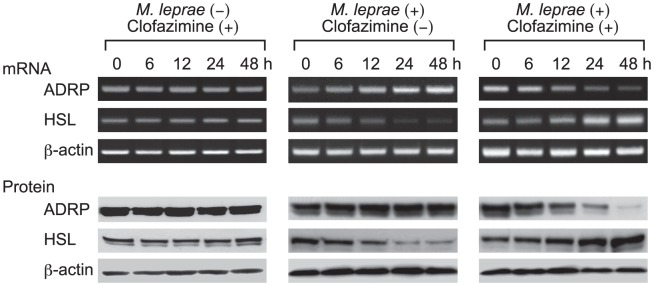
The effect of clofazimine is specific only for M. leprae-infected cells
To further evaluate the effect of clofazimine on ADRP and HSL expression, THP-1 cells were treated with clofazimine in the presence or absence of M. leprae infection for 6, 12, 24 and 48 h. Total RNA and cellular protein were extracted and used for RT-PCR analysis and Western blot analysis, respectively. Linearity of the RT-PCR amplifications of ADRP, HSL and β-actin was confirmed by serial dilution of RNA samples and densitometric analysis of the bands (Fig. S2). RT-PCR showed that clofazimine alone had no effect on ADRP and HSL mRNA levels in control THP-1 cells (Fig. 2, left panel). Consistent with previous reports, ADRP mRNA expression was increased and HSL mRNA expression was decreased when THP-1 cells were infected with M. leprae (Fig. 2, middle panel) [8], [16]. However, simultaneous clofazimine treatment and M. leprae infection of THP-1 cells led to decreased ADRP and increased HSL mRNA levels (Fig. 2, right panel). The decrease of ADRP and increase of HSL mRNA expression were further confirmed by quantitative real-time PCR (Fig. S3), which also supports the linearity of our RT-PCR data. Thus, it was shown that clofazimine modulated expression of ADRP and HSL only in M. leprae-infected cells. Similar results were observed for ADRP and HSL protein expression levels in each experiment.
Figure 2. Only M. leprae-infected THP-1 cells are susceptible to clofazimine.
THP-1 cells were cultured in six-well plates with or without 2.0 µg/ml clofazimine in the presence or absence of M. leprae infection (MOI = 10). After incubating for the indicated period of time, total RNA and total cellular protein were purified and RT-PCR and Western blot analyses of ADRP, HSL and β-actin were performed. Representative results from three independent experiments are shown.
Clofazimine antagonizes the effects of M. leprae to modulate ADRP and HSL expression
In the above studies, THP-1 cells were simultaneously treated with clofazimine and infected with M. leprae. Therefore, there was a possibility that clofazimine might have modulated the cellular environments of THP-1 cells before engulfing M. leprae. To eliminate this possibility and to imitate clinical situations, THP-1 cells were first infected with M. leprae for 24 h, to allow cells to engulf enough bacilli, before they were treated with clofazimine. M. leprae infection enhanced ADRP expression and suppressed HSL expression for up to 72 h (Fig. 3, left panel), which is consistent with the results shown in Fig. 2, middle panel. However, adding clofazimine 24 h after M. leprae infection produced lower levels of ADRP expression, but increased HSL expression (Fig. 3, right panel). Interestingly, ADRP expression fell even lower than the original level, and HSL rose higher than original levels, following clofazimine treatment. These results suggest that the lipid catabolic activity once suppressed by M. leprae infection was reactivated by clofazimine treatment, which in turn would promote lipolysis in infected macrophages and decrease cellular lipids. Also, these results are consistent with clinical situations in which HSL mRNA levels were recovered following successful treatment with MDT in LL and BL patients [16].
Figure 3. Clofazimine counteracts M. leprae to modulate ADRP and HSL expression levels.
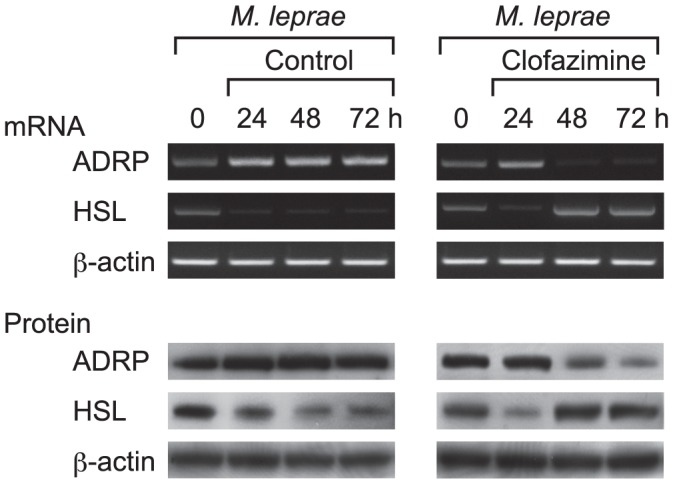
THP-1 cells were cultured in six-well plates and infected with M. leprae (MOI = 10) for 24 h. Clofazimine (2.0 µg/ml) was added and incubation continued another 24 and 48 h (48 and 72 h from M. leprae infection). Total RNA and total cellular protein were purified and RT-PCR and Western blot analyses of ADRP, HSL and β-actin were performed. Representative results from three independent experiments are shown.
Clofazimine increases expression of IFN-β and IFN-γ mRNA in M. leprae-infected THP-1 cells
The decrease in ADRP expression and increase in HSL expression produced by clofazimine treatment were also observed when M. leprae-infected cells were further treated with peptidoglycan (PGN), a ligand for Toll-like receptor (TLR)-2, to activate innate immunity [8], [16]. We therefore hypothesized that clofazimine treatment might activate the innate immune response of THP-1 cells, which also confers bactericidal activities. To assess activation of innate immunity, production of interferon IFN-β and IFN-γ mRNA was evaluated in control and M. leprae-infected THP-1 cells treated with clofazimine. A transient increase of IFN-β and induction of IFN-γ were observed only in THP-1 cells infected with M. leprae and treated with clofazimine (Figs. 4A and 4B). Transient induction of IFNs as a result of macrophage activation is consistent with previous reports [22]–[24]. Innate immune activation of infected cells will further contribute to the elimination of intracellular bacilli, which is also consistent with the observation that the active form of vitamin D suppresses CORO1A expression in THP-1 cells [21].
Figure 4. Clofazimine increases mRNA expression of IFN-β and IFN-γ in M. leprae-infected THP-1 cells.
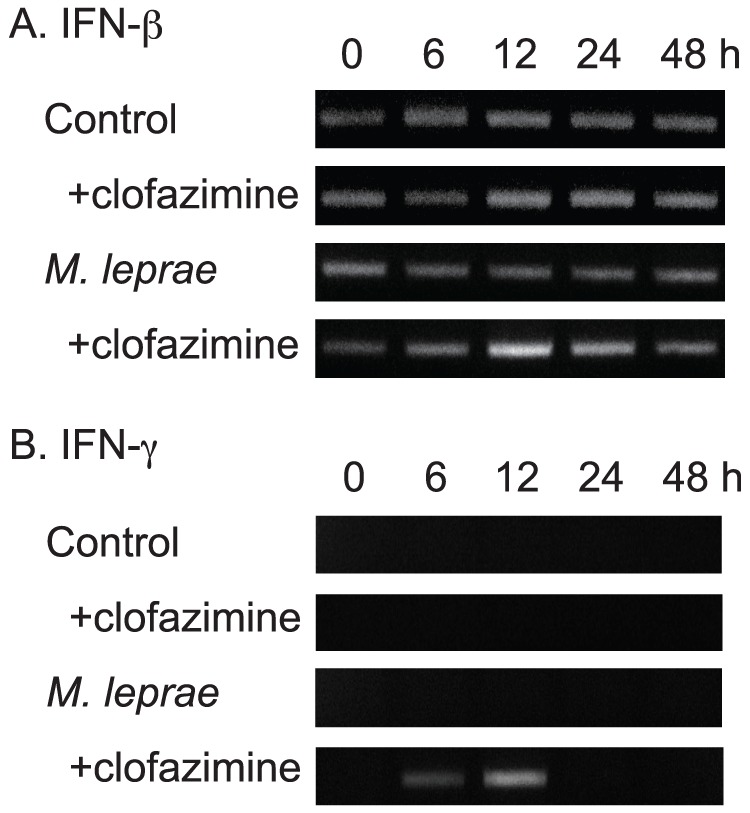
THP-1 cells were cultured in six-well plates with or without 2.0 µg/ml clofazimine in the presence or absence of M. leprae infection (MOI = 10). After incubating for the indicated period of time, total RNA was purified and RT-PCR analysis of IFN-β (A) and IFN-γ (B) was performed. Representative results from three independent experiments are shown.
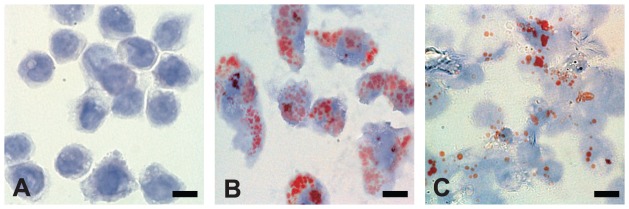
Clofazimine treatment decreases the cellular lipid droplets in M. leprae-infected THP-1 cells
To test whether the decrease in ADRP expression and increase in HSL expression after clofazimine treatment would result in less accumulation of cellular lipids after M. leprae infection, THP-1 cells were infected with M. leprae (MOI = 10) in the presence or absence of 2.0 µg/ml clofazimine for 48 h. Oil-red-O staining clearly demonstrated the accumulation of cellular lipid droplets following M. leprae infection (Fig. 5B vs. Fig. 5A). In M. leprae-infected cells treated with clofazimine, the amount of lipid droplets in the cell had significantly decreased by 48 h (Fig. 5C vs. 5B). The decrease in cellular lipid droplets is in agreement with the results shown in this study in which clofazimine decreased ADRP and increased HSL expression in M. leprae-infected cells.
Figure 5. Clofazimine decreases cellular lipid accumulation in M. leprae-infected THP-1 cells.
THP-1 cells were grown on glass coverslips in 24-well plates. Cells with no treatment (A), infected with M. leprae (MOI = 10) (B), and infected with M. leprae (MOI = 10) and treated with clofazimine (2.0 µg/ml) (C) were cultured for 48 h. Oil-red-O staining followed by brief hematoxylin counter staining was performed and observed under a microscope. Representative results from three independent experiments are shown. Bars = 10 µm.
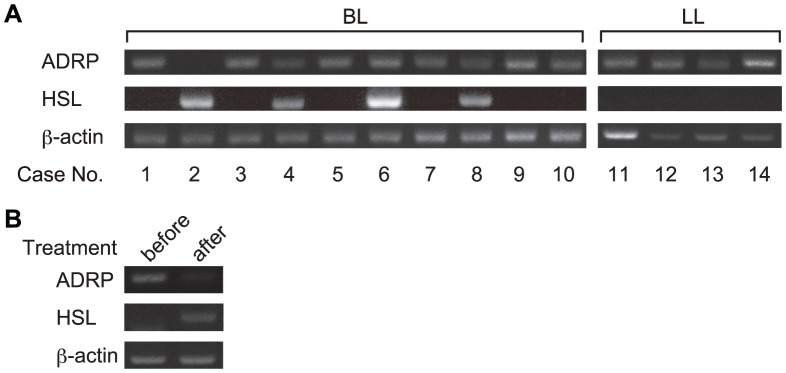
ADRP and HSL expression levels in skin lesions correlate with the clinical course of leprosy before and after treatment of leprosy patients
To confirm the expression pattern of ADRP and HSL in clinical courses of leprosy, ADRP and HSL mRNA levels were evaluated in slit-skin smear specimens by RT-PCR analysis. ADRP mRNA was detected in all LL and most BL cases tested (Fig. 6A, right panel). HSL mRNA was detected in four BL cases; however, ADRP mRNA expression in these cases was absent or weaker than in other BL samples (Fig. 6A, cases 2, 4, 6 and 8). In one case, from which serial samples were obtained, the expression of ADRP mRNA decreased and HSL mRNA levels increased after treatment (Fig. 6B).
Figure 6. Detection of ADRP and HSL mRNA in slit-skin smear samples from leprosy patients.
Total RNA was isolated from slit-skin smear specimens taken from ten BL and four LL patients (A) or from one patient before and after treatment (B). Total RNA was purified and RT-PCR analysis of ADRP, HSL and β-actin was performed. Representative results from three independent experiments are shown.
To further confirm changes in ADRP and HSL expression following treatment, immunohistochemical and acid-fast staining were performed using formalin-fixed paraffin-embedded skin tissue sections. Consistent with a previous report, ADRP localized to phagosome membranes that contains solid-shaped M. leprae (Fig. 7A) [8]. HSL staining was not evident before treatment (Fig. 7C). Three months after treatment, staining of the bacilli showed a dotted pattern with no solid-staining, indicating degeneration of M. leprae (Fig. 7 B and 7D). At this point, ADRP staining was faint (Fig. 7B), but strong HSL staining was observed along the phagosomal membrane (Fig. 7D). These staining patterns correlate with changes in mRNA levels of ADRP and HSL in the skin smears (Fig. 6B).
Figure 7. Immunostaining of ADRP and HSL proteins in skin biopsy specimens before and after treatment.
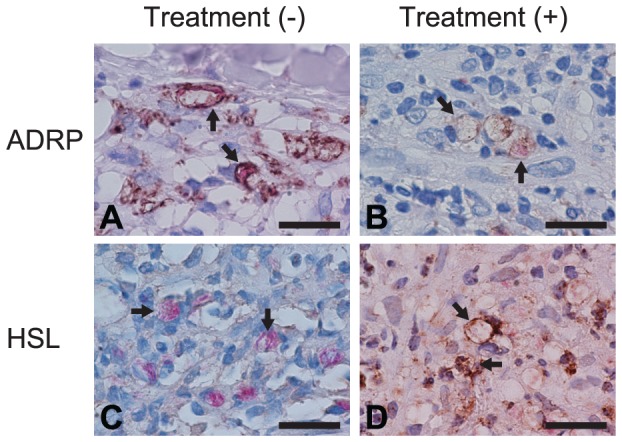
Sections of skin biopsy specimens taken from one patient before (A and C) and after (B and D) treatment were subjected to immunostaining of ADRP (A and B) and HSL (C and D), followed by acid-fast staining for M. leprae and hematoxylin counterstaining. Arrows indicate phagosome membrane that contains M. leprae. Representative results from three independent experiments are shown. Bars = 20 µm.
Discussion
In previous studies, we showed that M. leprae infection increases ADRP expression and decreases HSL expression in host macrophages [8], [16]. The results of the present study demonstrate that clofazimine, one of the three major drugs used to treat leprosy, counteracts the effect of M. leprae to reduce ADRP and increase HSL expression of both mRNA and protein levels. These results are consistent with our observations in clinical samples obtained from leprosy patients, in which HSL levels were not detectable in skin smear specimens before treatment, but re-appeared shortly after MDT [8], [16]. The other two MDT drugs, dapsone and rifampicin, revealed no effects on the expression of either ADRP or HSL.
Mycobacteria survive by evading the host immune system and accessing host metabolic pathways to obtain nutrients for growth. M. leprae has undergone reductive evolution and pseudogenes now occupy half of its genome [25]–[27], thus M. leprae is thought to be the mycobacterium most dependent on host metabolic pathways, including host-derived lipids. As we previously reported, PGN can activate TLR2 to increase the expression of HSL [16] and suppress ADRP and perilipin expression [7], [8], [21]. These effects mediated by the TLR-initiated signaling pathway will induce lipid degradation, which makes it difficult for M. leprae to survive within host cells. M. leprae infection not only suppresses HSL expression, but also invalidates all effects of PGN on ADRP and perilipin, thus ensuring a phagosome environment that is favorable for mycobacterial survival [16]. In the present study clofazimine increased HSL expression and decreased ADRP expression only in M. leprae-infected cells. The amounts of lipids accumulated in the cells decreased when clofazimine was added to the cell culture medium. The decrease of the lipid-rich environment against the survival of M. leprae may be one of the key actions of clofazimine.
Clofazimine was the first clinically developed riminophenazine for the treatment of tuberculosis [28]. Its use has been extended to many Gram-positive bacterial infections as well as mycobacterial diseases [28]–[30]. The drug is now widely used for the treatment of leprosy, but its mechanism remains unclear [31]–[33]. The drug is extremely lipophilic and is also active in membrane destabilization and possible promotion of antigen processing. Stimulated phospholipase A2 activity and subsequent accumulation of arachidonic acid and lysophospholipids were confirmed in clofazimine-induced membrane destabilization [29], [34]. Increased major histocompatibility complex (MHC) class II expression in peripheral blood monocytes [35], up-regulated lysosomal enzyme activity of cultured macrophages [36] and decreased suppressor T-cell activity in mycobacteria-infected mice [37] reveal the potential role of clofazimine in facilitating immune recognition.
Although the underlying molecular mechanisms are not clear, clofazimine suppressed ADRP and induced HSL, IFN-β and IFN-γ expression only in cells infected with M. leprae, the same effects products by PGN [8], [16], [21]. Therefore, it is possible that clofazimine revives at least some of the activities of PGN, which is normally shielded by redundant mycolic acid at the M. leprae cell wall. Given the extreme lipophilicity of clofazimine and its activity against many Gram-positive bacteria, clofazimine may interact with the mycolic acid in the M. leprae cell wall that facilitates the exposure of PGN, which in turn activates TLR2-mediated signaling cascades, subsequently decreasing ADRP and increasing HSL [8], [16], [21]. Furthermore, since most lepra reactions, a cell-mediated, delayed-type hypersensitivity immune response, occur during or after MDT [38], [39], the prospect that clofazimine rescues shielded PGN activities, promoting lysosomal fusion and antigen processing, would be a plausible explanation for the trigger of lepra reactions.
The results from present and previous studies may explain the underlying mechanisms, at least in part, of successful parasitization of M. leprae and the effects of MDT treatment observed in patients. In conclusion, we have shown that clofazimine devastates the lipid-rich environment in M. leprae-infected host macrophages by modulating the expression of ADRP and HSL and activates the innate immune response of infected cells, both of which would be important in fighting mycobacterial infection.
Supporting Information
Quality of RNA samples purified from THP-1 cells infected with M. leprae . RNA samples were purified from THP-1 cells infected with M. leprae as described in the Materials and Methods. Ten samples were analyzed using a 1% denatured agarose gel (A) and four were analyzed with theAgilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) (B).
(EPS)
Linearity of RT-PCR analysis. RNA samples were serially diluted and RT-PCR analysis of ADRP, HSL and β-actin was performed. Specific bands on the agarose gel were quantified using ImageJ64 software.
(EPS)
The effect of simultaneous clofazimine treatment and M. leprae infection on mRNA levels in THP-1 cells. THP-1 cells were cultured in six-well plates with or without 2.0 µg/ml clofazimine in the presence of M. leprae infection (MOI = 10). After incubating for the indicated period of time, total RNA was purified and real-time PCR analyses of ADRP (A), HSL (B) and β-actin were performed as previously described (reference 8). The same primers that were used for RT-PCR analysis were utilized with SYBER Green PCR Master Mix (Applied Biosytems). All samples were amplified in triplicate from the same RNA preparation. Each result is expressed as the mean ± SE. The Student's t-test was used for statistical analysis. One asterisk indicates a value of P<0.05; two asterisks indicate a value of P<0.01; and three asterisks indicate a value of P<0.001.
(EPS)
Funding Statement
This work was supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Research Promotion of Science (#23803000; http://www.jsps.go.jp/j-grantsinaid/index.html). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. World Health Organization (2011) Leprosy update, 2011. Wkly Epidemiol Rec 86: 389–399. [PubMed] [Google Scholar]
- 2. Ridley DS, Jopling WH (1966) Classification of leprosy according to immunity: A five-group system. Int J Lepr Other Mycobact Dis 34: 255–273. [PubMed] [Google Scholar]
- 3. Mattos KA, Lara FA, Oliveira VG, Rodrigues LS, D'Avila H, et al. (2011) Modulation of lipid droplets by Mycobacterium leprae in Schwann cells: a putative mechanism for host lipid acquisition and bacterial survival in phagosomes. Cell Microbiol 13: 259–273. [DOI] [PubMed] [Google Scholar]
- 4. Mattos KA, Oliveira VG, D'Avila H, Rodrigues LS, Pinheiro RO, et al. (2011) TLR6-driven lipid droplets in Mycobacterium leprae-infected Schwann cells: immunoinflammatory platforms associated with bacterial persistence. J Immunol 187: 2548–2558. [DOI] [PubMed] [Google Scholar]
- 5. Cardona PJ, Llatjos R, Gordillo S, Diaz J, Ojanguren I, et al. (2000) Evolution of granulomas in lungs of mice infected aerogenically with Mycobacterium tuberculosis . Scand J Immunol 52: 156–163. [DOI] [PubMed] [Google Scholar]
- 6. Kondo E, Kanai K (1976) Accumulation of cholesterol esters in macrophages incubated with mycobacteria in vitro. Jpn J Med Sci Biol 29: 123–137. [DOI] [PubMed] [Google Scholar]
- 7. Suzuki K, Takeshita F, Nakata N, Ishii N, Makino M (2006) Localization of CORO1A in the macrophages containing Mycobacterium leprae . Acta Histochem Cytochem 39: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanigawa K, Suzuki K, Nakamura K, Akama T, Kawashima A, et al. (2008) Expression of adipose differentiation-related protein (ADRP) and perilipin in macrophages infected with Mycobacterium leprae . FEMS Microbiol Lett 289: 72–79. [DOI] [PubMed] [Google Scholar]
- 9. Blanchette-Mackie EJ, Dwyer NK, Barber T, Coxey RA, Takeda T, et al. (1995) Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res 36: 1211–1226. [PubMed] [Google Scholar]
- 10. Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, et al. (1997) Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res 38: 2249–2263. [PubMed] [Google Scholar]
- 11. Miura S, Gan JW, Brzostowski J, Parisi MJ, Schultz CJ, et al. (2002) Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J Biol Chem 277: 32253–32257. [DOI] [PubMed] [Google Scholar]
- 12. Wolins NE, Rubin B, Brasaemle DL (2001) TIP47 associates with lipid droplets. J Biol Chem 276: 5101–5108. [DOI] [PubMed] [Google Scholar]
- 13. Gao J, Ye H, Serrero G (2000) Stimulation of adipose differentiation related protein (ADRP) expression in adipocyte precursors by long-chain fatty acids. J Cell Physiol 182: 297–302. [DOI] [PubMed] [Google Scholar]
- 14. Serrero G, Frolov A, Schroeder F, Tanaka K, Gelhaar L (2000) Adipose differentiation related protein: expression, purification of recombinant protein in Escherichia coli and characterization of its fatty acid binding properties. Biochim Biophys Acta 1488: 245–254. [DOI] [PubMed] [Google Scholar]
- 15. Zimmermann R, Lass A, Haemmerle G, Zechner R (2009) Fate of fat: the role of adipose triglyceride lipase in lipolysis. Biochim Biophys Acta 1791: 494–500. [DOI] [PubMed] [Google Scholar]
- 16. Tanigawa K, Degang Y, Kawashima A, Akama T, Yoshihara A, et al. (2012) Essential role of hormone-sensitive lipase (HSL) in the maintenance of lipid storage in Mycobacterium leprae-infected macrophages. Microb Pathog 52: 285–291. [DOI] [PubMed] [Google Scholar]
- 17. Yogi Y, Banba T, Kobayashi M, Katoh H, Jahan N, et al. (1999) Leprosy in hypertensive nude rats (SHR/NCrj-rnu). Int J Lepr Other Mycobact Dis 67: 435–445. [PubMed] [Google Scholar]
- 18. Yogi Y, Endoh M, Banba T, Kobayashi M, Katoh H, et al. (2002) Susceptibility to Mycobacterium leprae of congenic hypertensive nude rat (SHR/NCrj-rnu) and production of cytokine from the resident peritoneal macrophages. Jpn J Lepr 71: 39–45. [DOI] [PubMed] [Google Scholar]
- 19. Akama T, Suzuki K, Tanigawa K, Kawashima A, Wu H, et al. (2009) Whole-genome tiling array analysis of Mycobacterium leprae RNA reveals high expression of pseudogenes and noncoding regions. J Bacteriol 191: 3321–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akama T, Tanigawa K, Kawashima A, Wu H, Ishii N, et al. (2010) Analysis of Mycobacterium leprae gene expression using DNA microarray. Microb Pathog 49: 181–185. [DOI] [PubMed] [Google Scholar]
- 21. Tanigawa K, Suzuki K, Kimura H, Takeshita F, Wu H, et al. (2009) Tryptophan aspartate-containing coat protein (CORO1A) suppresses Toll-like receptor signalling in Mycobacterium leprae infection. Clin Exp Immunol 156: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akhtar LN, Qin H, Muldowney MT, Yanagisawa LL, Kutsch O, et al. (2010) Suppressor of cytokine signaling 3 inhibits antiviral IFN-beta signaling to enhance HIV-1 replication in macrophages. J Immunol 185: 2393–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, et al. (2011) NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappa B signaling pathways. Immunity 34: 854–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun Q, Sun L, Liu HH, Chen X, Seth RB, et al. (2006) The specific and essential role of MAVS in antiviral innate immune responses. Immunity 24: 633–642. [DOI] [PubMed] [Google Scholar]
- 25. Singh P, Cole ST (2011) Mycobacterium leprae: genes, pseudogenes and genetic diversity. Future Microbiol 6: 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akama T, Suzuki K, Tanigawa K, Nakamura K, Kawashima A, et al. (2010) Whole-genome expression analysis of Mycobacterium leprae and its clinical application. Jpn J Infect Dis 63: 387–392. [PubMed] [Google Scholar]
- 27. Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, et al. (2001) Massive gene decay in the leprosy bacillus. Nature 409: 1007–1011. [DOI] [PubMed] [Google Scholar]
- 28. Barry VC, Belton JG, Conalty ML, Denneny JM, Edward DW, et al. (1957) A new series of phenazines (rimino-compounds) with high antituberculosis activity. Nature 179: 1013–1015. [DOI] [PubMed] [Google Scholar]
- 29. Cholo MC, Steel HC, Fourie PB, Germishuizen WA, Anderson R (2012) Clofazimine: current status and future prospects. J Antimicrob Chemother 67: 290–298. [DOI] [PubMed] [Google Scholar]
- 30. Reddy VM, O'Sullivan JF, Gangadharam PR (1999) Antimycobacterial activities of riminophenazines. J Antimicrob Chemother 43: 615–623. [DOI] [PubMed] [Google Scholar]
- 31. Rodrigues LC, Lockwood D (2011) Leprosy now: epidemiology, progress, challenges, and research gaps. Lancet Infect Dis 11: 464–470. [DOI] [PubMed] [Google Scholar]
- 32. Britton WJ, Lockwood DN (2004) Leprosy. Lancet 363: 1209–1219. [DOI] [PubMed] [Google Scholar]
- 33. Shepard CC, Chang YT (1964) Activity of Antituberculosis Drugs against Mycobacterium Leprae . Int J Lepr 32: 260–271. [PubMed] [Google Scholar]
- 34. Krajewska MM, Anderson R (1993) An in vitro comparison of the effects of the prooxidative riminophenazines clofazimine and B669 on neutrophil phospholipase A2 activity and superoxide generation. J Infect Dis 167: 899–904. [DOI] [PubMed] [Google Scholar]
- 35. Wadee AA, Kuschke RH, Dooms TG (1995) The inhibitory effects of Mycobacterium tuberculosis on MHC class II expression by monocytes activated with riminophenazines and phagocyte stimulants. Clin Exp Immunol 100: 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sarracent J, Finlay CM (1982) The action of Clofazimine on the level of lysosomal enzymes of cultured macrophages. Clin Exp Immunol 48: 261–267. [PMC free article] [PubMed] [Google Scholar]
- 37. Watson SR, Auclair LK, Collins FM (1981) The effect of combined chemotherapy on suppressor T-cell activity in Mycobacterium simiae-infected mice. Immunology 43: 459–465. [PMC free article] [PubMed] [Google Scholar]
- 38. Shen J, Liu M, Zhou M, Wengzhong L (2009) Occurrence and management of leprosy reaction in China in 2005. Lepr Rev 80: 164–169. [PubMed] [Google Scholar]
- 39. Walker SL, Nicholls PG, Dhakal S, Hawksworth RA, Macdonald M, et al. (2011) A phase two randomised controlled double blind trial of high dose intravenous methylprednisolone and oral prednisolone versus intravenous normal saline and oral prednisolone in individuals with leprosy type 1 reactions and/or nerve function impairment. PLoS Negl Trop Dis 5: e1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quality of RNA samples purified from THP-1 cells infected with M. leprae . RNA samples were purified from THP-1 cells infected with M. leprae as described in the Materials and Methods. Ten samples were analyzed using a 1% denatured agarose gel (A) and four were analyzed with theAgilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) (B).
(EPS)
Linearity of RT-PCR analysis. RNA samples were serially diluted and RT-PCR analysis of ADRP, HSL and β-actin was performed. Specific bands on the agarose gel were quantified using ImageJ64 software.
(EPS)
The effect of simultaneous clofazimine treatment and M. leprae infection on mRNA levels in THP-1 cells. THP-1 cells were cultured in six-well plates with or without 2.0 µg/ml clofazimine in the presence of M. leprae infection (MOI = 10). After incubating for the indicated period of time, total RNA was purified and real-time PCR analyses of ADRP (A), HSL (B) and β-actin were performed as previously described (reference 8). The same primers that were used for RT-PCR analysis were utilized with SYBER Green PCR Master Mix (Applied Biosytems). All samples were amplified in triplicate from the same RNA preparation. Each result is expressed as the mean ± SE. The Student's t-test was used for statistical analysis. One asterisk indicates a value of P<0.05; two asterisks indicate a value of P<0.01; and three asterisks indicate a value of P<0.001.
(EPS)