Abstract
Objective
This was a first pilot study evaluating the acute phase (8-week) efficacy of the antidepressant medication mirtazapine for the treatment of depressive symptoms and drinking of subjects with comorbid major depressive disorder and alcohol dependence (MDD/AD). We hypothesized that mirtazapine would demonstrate within-group efficacy for the treatment of both depressive symptoms and drinking in these subjects.
Methods
We conducted a first open label study of the second generation antidepressant mirtazapine in 12 adult outpatient subjects with comorbid major depressive disorder/alcohol dependence. The pharmacological profile of that medication is unique among antidepressants, unrelated to tricyclics or selective serotonin reuptake inhibitors.
Results
Mirtazapine was well tolerated in this treatment population. Self-reported depressive symptoms decreased from 31.8 to 8.3 on the Beck Depression Inventory, a 74.0% decrease (p<0.001), and drinking decreased from 33.9 to 13.3 drinks per week, a 60.8% decrease (p<0.05). None of the subjects were employed full-time at baseline, but 9 of the 12 (75%) were employed full-time at end-of-study.
Conclusions
These preliminary findings suggest efficacy for mirtazapine for treating both the depressive symptoms and excessive alcohol use of comorbid major depressive disorder and alcohol dependence. Double-blind studies are warranted to further clarify the efficacy of mirtazapine in this population.
Keywords: mirtazapine, comorbid, major depression, alcohol dependence
To date, most studies of antidepressant medications among persons with comorbid major depressive disorder in combination with alcohol dependence have focused on selective serotonin reuptake inhibitors or tricyclic medications, and the results of those trials have been disappointing (Salloum et al., 2008; Cornelius et al., 2009). Nunes and Levin (2004) conducted a meta-analysis of 14 clinical trials of persons with comorbid major depressive disorder and a substance use disorder, 8 of which involved alcohol dependence. Those 14 clinical trials included 5 studies with tricyclic antidepressants, 7 studies of selective serotonin reuptake inhibitors, and 2 from other classes (vioxazine, nefazodone) with a total of 848 subjects. None of those studies involved the antidepressant medication mirtazapine. Few of those studies demonstrated efficacy for treating depression, and even fewer demonstrated efficacy for treating alcohol or other substance use. Similarly, a meta-analysis by Lovieno et al. (2011) concluded that the selective serotonin reuptake inhibitors have not been shown to demonstrate efficacy in comorbid populations. Those same authors (Lovieno et al., 2011) also noted the complete lack of study data on a number of newer antidepressants for treating comorbid populations. Thus, to date, no medications have consistently demonstrated efficacy for treating the large population of persons with comorbid major depressive disorder and alcohol dependence. However, findings from studies by Altintoprak et al. (2008) and by Yoon et al. (2006), elaborated below, suggested that mirtazapine treatment resulted in a decrease in craving for alcohol, which raised the possibility that mirtazapine might be shown to decrease alcohol consumption in future studies involving comorbid populations, though neither of those studies assessed level of alcohol consumption. Thus, comorbid major depression and alcohol dependence currently represent a considerable unmet treatment need.
Mirtazapine is classified as a second generation antidepressant medication with a tetracyclic structure. It is unique in its pharmacological profile among the currently available antidepressants, unrelated to tricyclic antidepressants or selective serotonin reuptake inhibitors. Mirtazapine is approved by the U.S. Food and Drug Administration (FDA) for the treatment of major depressive disorder. In a recent review (Watanabe et al., 2008), mirtazapine demonstrated a faster onset of therapeutic action compared to other antidepressant medications. Cipriani et al. (2009) conducted a meta-analysis involving 12 new-generation antidepressants, which demonstrated that mirtazaine was more commonly found to be among the most efficacious medications than the other antidepressant medications.
Mirtazapine’s potential benefit for treating the dual disorders of alcohol dependence and depression has recently been explored by two investigators (Altintoprak et al, 2008; Yoon et al., 2006), though neither of those two studies assessed level of drinking. Yoon and colleagues (2006) completed an 8 week open-label, naturalistic multicenter study using mirtazapine in a trial involving 184 subjects with comorbid alcohol dependence and depressive disorder. Subjects were prescribed a flexible dose schedule of 15-45 mg/day of mirtazapine based on the clinician’s judgment. No subjects reported serious adverse events; all adverse events related to mirtazapine were reported to be minimal to moderate. No subjects in the study dropped out due to side effects of mirtazapine. The study showed a statistically significant reduction of depressive and anxiety-related symptoms and craving, as shown on the scores on the Obsessive Compulsive Drinking Scale, the Visual Analog Scale for Craving, Hamilton Depression Rating Scale, and the Hamilton Anxiety Rating Scale. Altintoprak and colleagues (2008) conducted a study comparing the effectiveness and tolerability of two serotonergic/noradrenergic antidepressant drugs, mirtazapine and amitriptyline, for the treatment of subjects with comorbid alcohol dependence and major depressive disorder in a randomized, double-blind study. Mirtazapine was better tolerated than amitriptyline. The findings of that study indicated that both medications resulted in a reduction of depression and alcohol craving. Mirtazapine demonstrated a large effect size for treating depression and a moderate effect size for treating alcohol craving. Efficacy for decreasing level of alcohol use could not be assessed, because neither of those two studies assessed level of drinking.
In the current report, we present the findings from a first open-label study to assess the efficacy of mirtazapine for treating the depressive symptoms and the excessive alcohol consumption of adults with comorbid major depressive disorder and alcohol dependence. We hypothesized that mirtazapine would demonstrate within-group efficacy for decreasing both the depressive symptoms and level of alcohol use of our subjects.
METHODS
Subjects
Before entry into this treatment protocol, the study was explained, and written informed consent was obtained from all subjects after all procedures had been fully explained. The study was approved by the University of Pittsburgh Institutional Review Board. This study was conducted at the Western Psychiatric Institute and Clinic of the University of Pittsburgh Medical Center. Subjects were recruited for participation in the treatment study through posters and by responding to newspaper or radio advertisements.
Twelve participants were recruited for this pilot study. Participants were required to be outpatients between 18 and 55 years of age at baseline to be included in the study. At the baseline assessment, participants were evaluated for the DSM-IV diagnoses of alcohol dependence and major depressive disorder, using an instrument called the MINI International Neuropsychiatric Interview (Sheehan et al., 1997). The MINI has demonstrated good reliability, validity, and clinical utility (Sheehan et al., 2008). The comorbid presence of both current alcohol dependence and current major depressive disorder was required for inclusion in the treatment study. The MINI provides guidelines for identifying substance-induced mood disorders. Persons with substance-induced mood disorders were excluded from participation in the current study. Other exclusion criteria included a DSM-IV diagnosis of bipolar disorder, schizoaffective disorder, or schizophrenia. Persons with any substance abuse or dependence other than nicotine dependence or cannabis abuse or dependence were excluded from the study. Persons with hyper- or hypo-thyroidism, significant cardiac, neurological, or renal impairment, and significant liver disease (SGOT, SGPT, or gamma-GTP greater than 3 times normal levels) were also excluded from the study. Persons who had received antipsychotic or antidepressant medication in the month prior to baseline assessment were excluded. Persons with any history of intravenous drug use were excluded from the study. Persons who exhibited any evidence of withdrawal or need for detoxification were excluded from the study. Persons who complained of suicidal thoughts in the previous year or who had made a suicide attempt at any point during their lifetime were excluded from participation in the study. Other exclusion criteria were pregnancy, inability or unwillingness to use contraceptive methods, and an inability to read or understand study forms. Potential subjects were recruited into the study regardless of race, ethnicity, or gender.
Treatment and assessment
Following completion of the baseline assessment, participants were treated using an open-label study design. The study medication was taken once per day at bedtime. Subjects were given 15 mg of mirtazapine for the first two weeks of the trial and 30 mg for the last six weeks of the medication trial. Protocol assessments were conducted weekly in the first month and biweekly in the second month. Brief (about 10 to 15 minutes) Motivation Enhancement Therapy was also provided at each assessment, which focused on medication compliance and compliance with study procedures (Miller, Zweben, DiClemente, & Rychtarik, 1992). Pill counts were also used to ensure compliance with protocol medication. To ensure a high level of participation for these evaluations, a $20.00 payment was made to patients completing each assessment (Festinger, Marlowe, Dugosh, Croft & Arabia, 2008). Participant-rated depressive symptoms were assessed with the Beck Depression Inventory (BDI) (Beck, Ward, Mendelson, Mock & Erbaugh, 1961). The Beck Depression Inventory has demonstrated good reliability and validity (Beck et al., 2008). Drinking behavior was evaluated using the timeline follow-back method (TLFB) (Sobell LC, Sobell MB, Leo, & Cancilla, 1988). All of these assessment instruments were completed at every study visit.
Statistical Analysis
Descriptive statistics were calculated for all variables. Continuous baseline measures were compared by paired, 2-tailed t tests for continuous variables. Categorical baseline measures were compared by chi-square analysis, corrected for continuity. Statistical analyses were completed on an intent-to-treat study group. All tests of significance were 2-tailed. An alpha level of less than or equal to 0.05 was used in the study to indicate statistical significance. All analyses were conducted using the Statistical Package for the Social Sciences, version 15.0 (Norusis, 1992).
RESULTS
A total of 12 subjects entered the study. All subjects participated in protocol ratings and provided data at all data collection times throughout the study, and none dropped out of the study. Subjects were 5 women and 7 men, who included 9 Caucasians, 1 African American, 1 Native American, and 1 Asian American. The mean age of study subjects was 36.1 years (SD=13.4). At baseline, subjects demonstrated prominent depressive symptoms and drinking behavior, with a mean BDI of 31.8 (SD=8.3), and TLFB of 33.9 (SD=14.9) drinks per week. Two of the twelve subjects (17%) demonstrated a current cannabis use disorder, though in both of those cases the alcohol use disorder was deemed to be the primary substance use disorder.
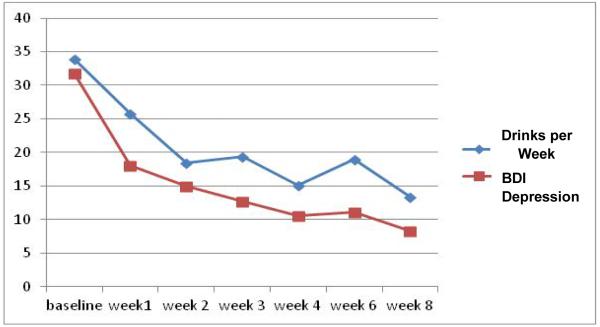
During the 8-week course of the study, statistically significant improvements (decreases) were noted for both the depressive symptoms and the level of alcohol use of the study population. Self-reported depressive symptoms, as measured on the BDI, decreased from a mean of 31.8 (SD=8.2) at baseline to an end-of-study level of 8.3 (SD=7.0) (t=6.7, df=11, p<0.001), which was an average of a 74.0% decrease in depressive symptoms. Alcohol consumption, as measured on the TLFB, decreased from a mean of 33.9 (SD=??)drinks per week at baseline to an end-of-study level of 13.3 (SD=11.5) drinks per week (t=3.86, df=11, p=0.003), which was an average of a 60.8% decrease in level of drinking. Figure 1 shows a graphic representation of the changes in depressive symptoms and in drinking during the course of the study.
Fig. 1.
Level of drinking (number of drinks/week on the Timeline Follow-Back) and self-reported level of depressive symptoms (Beck Depression Inventory, BDI).
Mirtazapine was well tolerated in the study. There were no serious adverse events during the study. None of the subjects underwent any alcohol treatment or other treatment outside the study. Three persons complained of mild sedation when they first started the medication, but the sedation resolved over a few days. At baseline, 4 subjects were unemployed, 7 had part-time jobs (less than 40 hours of work per week), and none had a full-time job. However, at the end of the medication trial, all 12 were employed, - 3 held part-time jobs, and 9 held full time jobs. In other words, at the beginning of the medication trial, none of the 12 subjects were employed full time, but at the end of the medication trial 9 of the 12 subjects (75%) were employed full time. The two subjects with a cannabis use disorder in addition to their alcohol use disorder fared no better or worse than those who demonstrated only an alcohol use disorder.
DISCUSSION
This report provides data from what we believe is the first open-label study evaluating the efficacy of the second generation antidepressant medication mirtazapine for the treatment of both the depressive symptoms and the level of alcohol use of persons with comorbid major depressive disorder and alcohol dependence. The two previous studies involving mirtazapine in this comorbid population did not evaluate the level of drinking of subjects in their studies (Yoon et al., 2006; Altintoprack et al., 2008). Mirtazapine was well tolerated our subjects with comorbid major depressive disorder and alcohol dependence. During the course of the medication trial, study participants demonstrated significant within-group improvement in both depressive symptoms and in level of alcohol consumption. The magnitude of those clinical improvements was large, especially for the clinical improvement in depressive symptoms. These preliminary findings suggest efficacy for mirtazapine for treating both the depressive symptoms and the alcohol use of comorbid major depressive disorder and alcohol dependence. The increase in number of employed subjects noted during this treatment trial suggests an increase in level of functioning associated with the decreases in depressive symptoms and level of drinking. These clinical improvements occurred relatively quickly after starting mirtazapine, which is consistent with the rapid onset of response noted by previous authors (Watanbe et al., 2008).
The significant decrease in depressive symptoms during the course of the current treatment trial is consistent with the finding of Yoon and colleagues (2006) and of Altintoprak and colleagues (2008), both of whom reported significant decreases in depressive symptoms in their studies of comorbid subjects involving mirtazpine. However, those two previous studies did not evaluate the level of alcohol use during the course of their studies, though they both reported a significant decrease in level of alcohol craving during the course of their studies. Consequently, we cannot compare the results of the current study to the results from those other two studies on the important outcome variable of level of alcohol use. Therefore, we believe that our current study is the first study to report a significant decrease in level of drinking in a comorbid major depressive disorder/alcohol dependence population treated with mirtazapine. The reason for the effect of mirtazapine on alcohol consumption in the current trial is not clear, though mirtazapine is known to affect both the serotonergic system and the noradrenergic system, as opposed to selective serotonin reuptake inhibitors which affect only the serotonergic system. The norepinepherine system may be involved in the modulation of brain reward circuit, which can be related to level of drinking.
The results of this study should be interpreted in light of some limitations. First, the sample size in this pilot study was limited, as was the number of assessment instruments. Also, no placebo control group was used, so we cannot rule out the possibility that some (or all) of the therapeutic effect that was noted in this clinical trial may have resulted from the brief motivation enhancement therapy used in study, or from the extra attention and monitoring afforded by the study. In addition, it is unclear to what extent the results of this study generalize to inpatient populations or populations using cocaine, opiates, etc., in addition to their depression and their alcohol use disorder. Therefore, the exclusion criteria used in the current study may have contributed to a selection bias, and thus may limit the generalizability of the findings to other populations. Double-blind placebo-controlled trials of mirtazapine appear to be warranted in to clarify the role of mirtazapine vs. therapy in the treatment of persons with comorbid disorders.
ACKNOWLEDGMENTS
This research was supported in part by grants from the National Institute on Alcohol Abuse and Alcoholism (R01 AA013370, R01 AA015173, K24 AA15320, K02 AA018195, and K02 AA00291); and from the National Institute on Drug Abuse (R01 DA019142, P50 DA05605, K02 DA017822, and the NIDA Clinical Trials Network (CTN)). The authors thank Jeanine Hayes and Maribeth Wesesky for their assistance in data collection and in the preparation of this paper. The authors also thank Levent Kirisci, Ph.D. for his consultation on data analyses.
Footnotes
DISCLOSURES Drs. Cornelius, Douaihy, Clark, Chung, Wood, and Daley report no financial relationships with commercial interests.
Presented in part at the 22nd Annual Meeting of the American Academy of Addiction Psychiatry (AAAP), Scottsdale, Arizona, December 10, 2011; at the 165th Annual Meeting of the American Psychiatric Association (APA), Philadelphia, Pennsylvania, May 5-9, 2012; and at the 35th Annual Scientific Meeting of the Research Society on Alcoholism (RSA), San Francisco, California, June 23-27, 2012.
Contributor Information
Jack R. Cornelius, Department of Psychiatry University of Pittsburgh School of Medicine Pittsburgh, PA corneliusjr@upmc.edu.
Antoine B. Douaihy, Department of Psychiatry University of Pittsburgh School of Medicine Pittsburgh, PA douaihya@upmc.edu
Duncan B. Clark, Department of Psychiatry University of Pittsburgh School of Medicine Pittsburgh, PA clarkdb@upmc.edu
Tammy Chung, Department of Psychiatry University of Pittsburgh School of Medicine Pittsburgh, PA chungta@upmc.edu
D. Scott Wood, University of Pittsburgh Medical Center Pittsburgh, PA woodds@upmc.edu
Dennis Daley, Department of Psychiatry University of Pittsburgh School of Medicine Pittsburgh, PA daleydc@upmc.edu
REFERENCES
- Altintoprak AE, Zorlu N, Coskunol H, Akdeniz F, Kitapcioglu G. Effectiveness and tolerability of mirtazapine and amitriptyline in alcoholic patients with co-morbid depressive disorder: A randomized, double-blind study. Human Psychopharmacology. 2008;23(4):313–319. doi: 10.1002/hup.935. doi:10.1002/hup.935. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. doi:10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. Mood disorders measures. In: Rush A. John, Jr., First Michael B., Blacker Deborah., editors. Handbook of Psychiatric Measures. Second Edition. American Psychiatric Publishing, Inc.; Washington, D.C.: 2008. pp. 504–506. M.D. M.D. M.D., Sc.D. [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Barbui C. Comparative efficacy and acceptability of 12 new-generation antidepressants: A multiple-treatments meta-analysis. Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. doi:10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Bukstein OG, Wood DS, Kirisci L, Douaihy AB, Clark DB. Double-blind placebo-controlled trial of fluoxetine in adolescents with comorbid major depression and an alcohol use disorder. Addictive Behaviors. 2009;34:905–909. doi: 10.1016/j.addbeh.2009.03.008. doi:10.1016/j.addbeh.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovieno N, Tedeschini E, Bentley KH, Evins AE, Papakostas GI. Antidepressants for major depressive disorder and dysthymic disorder in patients with comorbid alcohol use disorders: A meta-analysis of placebo-controlled randomized trials. Journal of Clinical Psychiatry. 2011;72:1144–1151. doi: 10.4088/JCP.10m06217. doi:10.4088/JCP.10m06217. [DOI] [PubMed] [Google Scholar]
- Miller WR, Zweben A, DiClemente CC, Rychtarik RG. Motivational Enhancement Therapy Manual. Volume 2. National Institute on Alcohol Abuse and Alcoholism; Washington, DC: 1992. (Project MATCH Monograph Series). [Google Scholar]
- Norusis MJ. Norusis Statistical Package for the Social Sciences. McGraw-Hill; New York, NY: 1992. [Google Scholar]
- Nunes EV, Levin FB. Treatment of depression in patients with alcohol or other drug dependence. A meta-analysis. JAMA: Journal of the American Medical Association. 2004;291:1887–1896. doi: 10.1001/jama.291.15.1887. doi:10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- Salloum IM, Jones YO. Efficacy of pharmacotherapy for comorbid major depression and substance use disorders: A review. Current Psychiatry Reviews. 2008;4(1):14–27. doi:10.2174/157340008783743785. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan H, Amorim P, Janars J, Weiller E, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(s20):22–33. [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan H, Amorim P, Janars J, Weiller E, Hergueta MS, Baker R, Dunbar GC. Diagnostic measures for adults. In: Rush A. John, Jr., First Michael B., Blacker Deborah., editors. Handbook of Psychiatric Measures, Second Edition. American Psychiatric Publishing, Inc.; Washington, D.C.: 2008. pp. 48–51. M.D. M.D. M.D., Sc.D. [Google Scholar]
- Sobell LD, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: a Assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. British Journal of Addiction. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. dx.doi.org/10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Omori IM, Nakagawa A, Cipriani A, Barbui C, McGuire H, Furukawa TA. Mirtazapine versus other antidepressants in the acute-phase treatment of adults with major depression: Systematic review and meta-analysis. Journal of Clinical Psychiatry. 2008;69(9):1404–1415. doi: 10.4088/jcp.v69n0908. doi:10.4088/JCP.v69n0908. [DOI] [PubMed] [Google Scholar]
- Yoon SJ, Pae CU, Kim DJ, Namkoong K, Lee E, Oh DY, Lee CT. Mirtazapine for patients with alcohol dependence and comorbid depressive disorders: A multicentre, open label study. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2006;30(7):1196–1201. doi: 10.1016/j.pnpbp.2006.02.018. doi:10.1016/j.pnpbp.2006.02.018. [DOI] [PubMed] [Google Scholar]