Abstract
Sweet taste receptors may enhance glucose absorption.
AIM
To explore the cell biology of sweet taste receptors on glucose uptake.
HYPOTHESIS
Artificial sweeteners increase glucose uptake via activating sweet taste receptors in the enterocyte to translocate GLUT2 to the apical membrane through the PLC βII pathway.
METHODS
Caco-2, RIE-1, and IEC-6 cells, starved from glucose for 1 h were pre-incubated with 10 mM acesulfame potassium (AceK). Glucose uptake was measured by incubating cells for 1 to 10 min with 0.5–50 mM glucose with or without U-73122, chelerythrine, and cytochalasin B.
RESULTS
In Caco-2 and RIE-1 cells, 10 mM AceK increased glucose uptake by 20~30% at glucose ≥ 25 mM, but not in lesser glucose concentrations (≤10 mM), nor at 1 min or 10 min incubations. U-73122 inhibited uptake at glucose ≥ 25 mM and for 5 min incubation; chelerythrine and cytochalasin B had similar effects. No effect occurred in IEC-6 cells.
SUMMARY
Activation of sweet taste receptors had no effect on glucose uptake in low (<25 mM) glucose concentrations but increased uptake at greater concentrations (≥ 25 mM).
CONCLUSIONS
Role of artificial sweeteners on glucose uptake appears to act in part by effects on the enterocyte itself.
Keywords: Sweet taste receptor, acesulfame potassium, GLUT2, PLC βII
INTRODUCTION
Two major pathways mediate glucose absorption in small intestine. At low concentrations, the predominant classic pathway is an active absorption mediated by the Na+-glucose cotransporter SGLT1 [1–4]. When the glucose level is > 30 mM in the lumen after a meal, however, SGLT1 is fully saturated and absorption of glucose is augmented markedly by a second mechanism mediated by the facilitative glucose transporter GLUT2 (glucose transporter 2) [5, 6]. Of note, SGLT1 itself is an important and required mediator of GLUT2 uptake of glucose by providing the induction signal to generate additional transport capacity for glucose absorption through rapid translocation of GLUT2 from preformed, cytoplasmic vesicles into the apical membrane. This translocation of GLUT2 increases markedly the capacity of glucose uptake by the enterocyte [7–18].
Recent studies have suggested that translocation of GLUT2 into the apical membrane is also dependent on a second signaling mechanism provided by the activation of sweet taste receptors in the enterocyte as well as in the enteroendocrine cells of the gut [19–21]. The activation of sweet taste receptors in the apical membrane of the enterocyte by glucose is a heterologous expression system that appears to play a role in GLUT2 translocation to apical brush border membrane in the intestine at luminal concentrations of glucose of > 30 mM. At these greater concentrations, apical GLUT2 a low affinity, high capacity transporter then provides the major and dominant pathway of absorption. Alternatively, one hypothesis is that apical GLUT2 translocation at low luminal concentrations of glucose (<20 mM) may be also induced rapidly by artificial sweeteners [20].
The T1R taste receptor family comprises three, G-protein coupled receptors (GPCRs) that act through α-gustducin and /or transducin to active a PLC βII-dependent pathway [19,22,23]. The T1R2+T1R3 heterodimer senses sweet taste (glucose, fructose, and sucrose) at concentrations in the 100 mM range and the artificial sweeteners (acesulfame potassium, sucralose, and saccharin) at much lesser concentrations (1–10 mM). The T1R1+T1R3 heterodimer senses the “bitter” amino acids, notably L-aspartate and L-glutamate. Mace et al. found that simple sugars and artificial sweeteners act synergistically through a T1R2+T1R3- α -gusducin-PLC βII pathway to stimulate activation of PKC BII, which appears to be essential for apical translocation of GLUT2 [20].
In our previous studies, we established in vitro cell culture models in three different intestinal cell lines to study the cell biology of glucose transporter function in attempt to complement our studies of glucose uptake in in vivo animal models [24, 25]. Our previous studies used Caco-2 (from humans) and RIE-1 (from rats) cells that express active GLUT2 that is recruited to the apical membrane when exposed to high concentrations of glucose (≥30 mM) and also IEC-6 cells (also from rats) that do not express active GLUT2 and do not increase carrier-mediated glucose uptake at greater concentrations of glucose. The aim of the current study was to explore the mechanisms of sweet taste receptor activation on glucose uptake in our established, in vitro, cell culture models. We chose to study acesulfame potassium (AceK) rather than sucralose or saccharin, because AceK is more potent than the other two artificial sweeteners [20]. We hypothesized that the artificial sweetener AceK increases glucose uptake via activating sweet taste receptors on the enterocyte to translocate GLUT2 to the apical membrane through a PLC βII-dependent pathway, even when the glucose concentration was <25 mM.
MATERIALS AND METHODS
Chemicals and Supplies
Twenty-four-well, cell culture plates were purchased from Corning Life Sciences (Lowell, MA). Acesulfame K, cytochalasin B, chelerythrine, U-73122 (a PLC βII inhibitor), and insulin were purchased from Sigma (St. Louis, MO). Dulbecco’s modified Eagle medium (DMEM), non-essential amino acids, sodium pyruvate (100 mM), and streptomycin/penicillin solution from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was obtained from PAA Laboratories (Dartmouth, MA). Solvable™ and Opti-Fluor were purchased from Perkin-Elmer (Waltham, MA). 14C-d-glucose and 3H-l-glucose was obtained from Moravek Biochemicals (Brea, CA), while D-glucose and the BCA Protein Assay Kit (#23225) were purchased from Thermo Fisher Scientific Inc. (Rockford, IL).
Cell Cultures
Caco-2 and IEC-6 cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, Virginia). RIE-1 cells were a gift from Dr. Laurence Egan. Caco-2, RIE-1, and IEC-6 cell lines were used between passages 20 to 40, 5 to 40, and 5 to 40, respectively and were grown at 37°C in a 95% O2 and 5% CO2 atmosphere with 90% humidity in 35 × 10-mm Petri dishes containing DMEM with penicillin (10,000 U/ml) and streptomycin (10,000 µg/ml). Caco-2 cells were grown in 25 mM glucose supplemented with 20% FBS, 1% nonessential amino acids, and 1% sodium pyruvate. RIE-1 cells were grown in 5 mM glucose supplemented with 5% FBS. IEC-6 cells were grown in 25 mM glucose supplemented with 10% FBS and 10 µg/ml insulin. Stock cells were subcultured once a week at a 1:10 ratio; media for all cells was changed two to three times weekly as needed [24].
Glucose Uptake Assay
Glucose uptake was evaluated as described in our previous publications [24, 25]. In brief, cells were seeded on 24-well plates and left to differentiate/polarize for 10 days (RIE-1 and IEC-6) or 15 days (Caco-2) after reaching confluence. Cells were starved from glucose by incubation with 500 µl of Krebs buffer (30 mM HEPES, 130 mM NaCl, 4 mM KH2PO4, 1 mM MgSO4, 1 mM CaCl2, pH 7.4, 290 mOsm) at 37°C for 1 h and pre-incubated with 10 mM acesulfame K (AceK) for 30 min. Glucose uptake studies were performed by incubating cell monolayers in 200 µl of Krebs buffer with varying concentrations of d-glucose (0.5–50 mM) and 10 mM AceK; NaCl was replaced to maintain isosmolarity among solutions. AceK was chosen rather than sucralose or saccharin, because AceK is a more potent artificial sweetener [20]. Cells were incubated for 1, 5, and 10 min. To allow calculation of carrier-mediated (stereo-specific) and passive (non-stereo-specific) uptake, 0.5–1 µCi/ml of 14C-d-glucose and 3H-l-glucose respectively were added simultaneously to the glucose-containing test solutions. 14C-d-glucose was used to measure total glucose uptake, both stereospecific, carrier-mediated uptake (SGLT1 and GLUT2) and non-stereospecific passive uptake; 3H-l-glucose was used to measure only passive (non-carrier-mediated) uptake (see below). Glucose uptake was stopped by washing twice with ice-cold phosphate buffer solution (PBS). Cells were solubilized with 300 µl of 0.1N NaOH at 37°C for 30 min. Aliquots of 10 µl of the lysate were used for protein measurement, and 200 µl were mixed into 4.5 ml of liquid scintillation cocktail (Opti-Fluor) with 0.5 ml distilled H2O and counted using dual isotope, liquid scintillation techniques on a Beckman LS6000SC (Beckman Coulter, Inc, Brea, CA). Stereo-specific, carrier-mediated glucose uptake was calculated as total uptake (14C-d-glucose) minus passive uptake (3H-l-glucose) and expressed in nmol/mg protein per duration of incubation (see below).
Calculation of glucose uptake
In all studies, values for carrier-mediated uptake were determined by subtracting passive uptake (3H-l-glucose) from total uptake (14C-d-glucose). We used the method of nonlinear regression of carrier-mediated uptake to calculate the Michaelis-Menton affinity constant (Km) and maximal transport rate (Vmax) using Michaelis-Menton kinetics (GraphPad Prism version 4.03). The best fit curve of the Michaelis-Menten equation was determined for carrier-mediated uptake values using the following equation:
where Vo is the initial uptake velocity, Vmax is the maximal uptake velocity at saturating substrate concentrations, S is the substrate concentration, and Km is a constant analogous to the Michaelis-Menten constant. To summarize individual curves of glucose uptake over the range of different concentrations (0.5–50 mM) evaluated under different experimental conditions (with/without inhibitors, starved/non-starved cells, etc.), we measured the total area under the uptake curve. The effects of the various stimulators or inhibitors were evaluated by percent stimulation or inhibition (of area under the curve) over the range of concentrations evaluated. In addition, glucose uptakes at various glucose concentrations were also compared.
Statistical Analysis
Values are presented as mean±standard deviation and were analyzed by paired or unpaired Student’s t-test as appropriate. A p-value of <0.05 was considered significant. All experiments were carried out in triplicate and repeated at least three times on different days in different cell passages.
RESULTS
Enhanced Glucose Uptake by Acesulfame K
In our previous studies, we showed that glucose uptake in Caco-2 and RIE-1 cells was significantly increased by glucose concentrations ≥25 mM and by greater durations of incubations (≥5 min). This increase in glucose uptake was associated with rapid translocation of GLUT2 to the apical membrane. In our current study, we tested if the artificial sweetener AceK could increase carrier-mediated glucose uptake in our cell culture system. When exposed to 10 mM AceK, carrier-mediated glucose uptake in Caco-2 and RIE-1 cells was increased at 25 and 50 mM glucose concentrations at 5-min duration of incubation (Fig 1B and 1E). There was no increase at lesser glucose concentrations (≤10 mM) at any duration of incubation; similarly, no effect of AceK on glucose uptake was seen in the 1-, and 10-min durations of incubations (Fig 1A and C, and 1D and F). In IEC-6 cells, no effect of AceK on glucose uptake was detected at any of the 3 durations of incubations (Fig 2).
Figure 1.
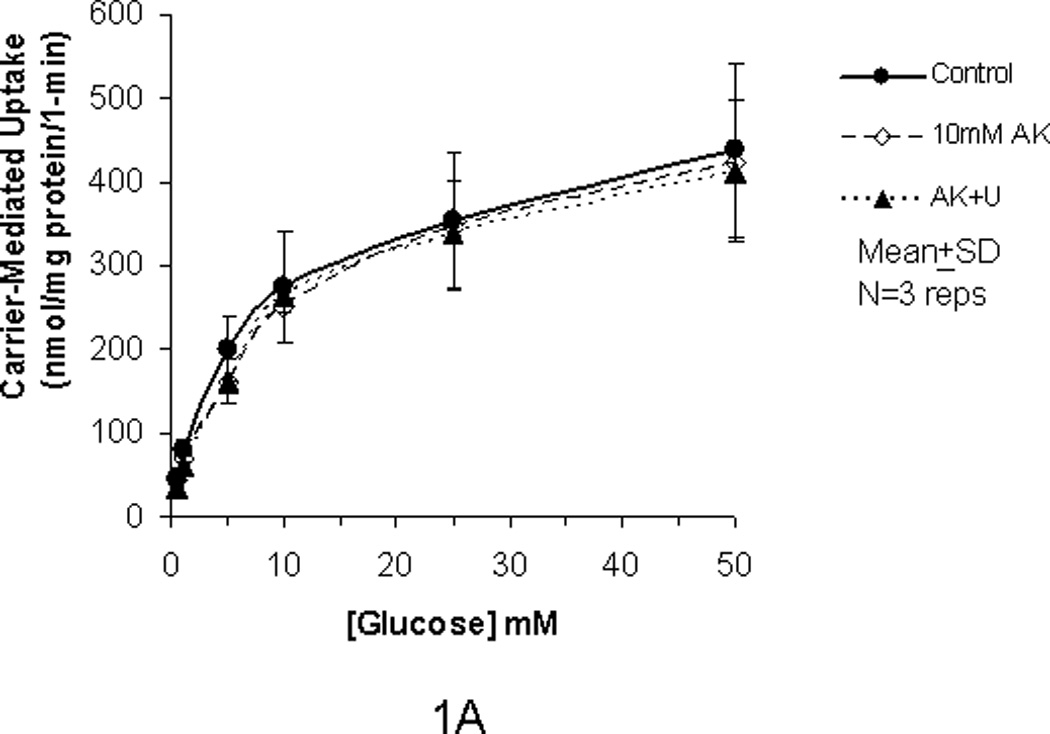
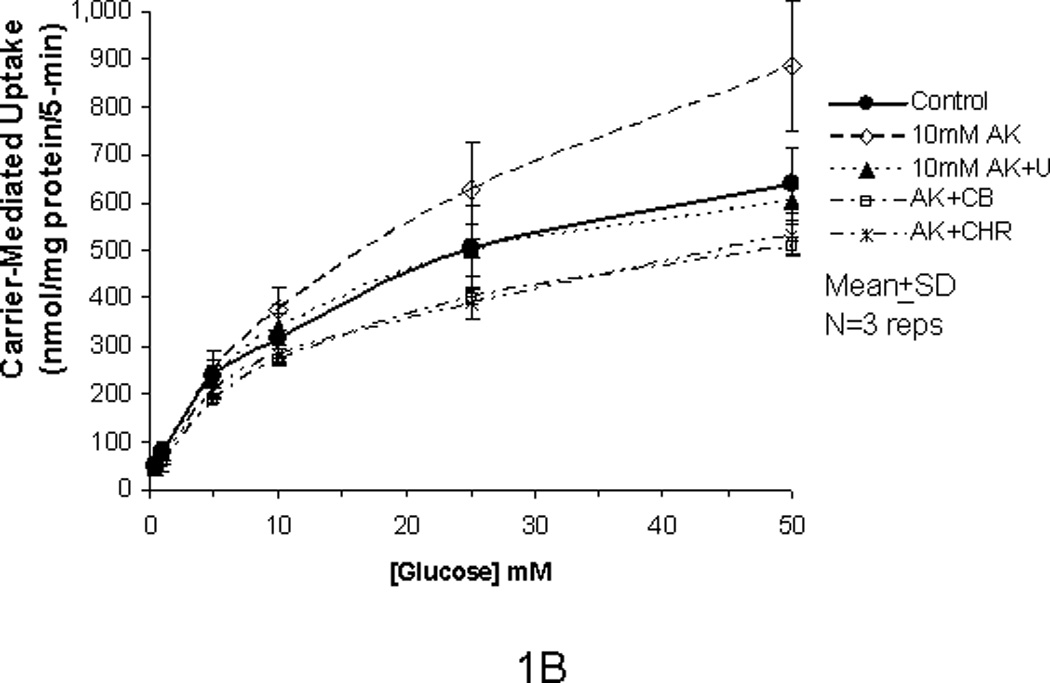
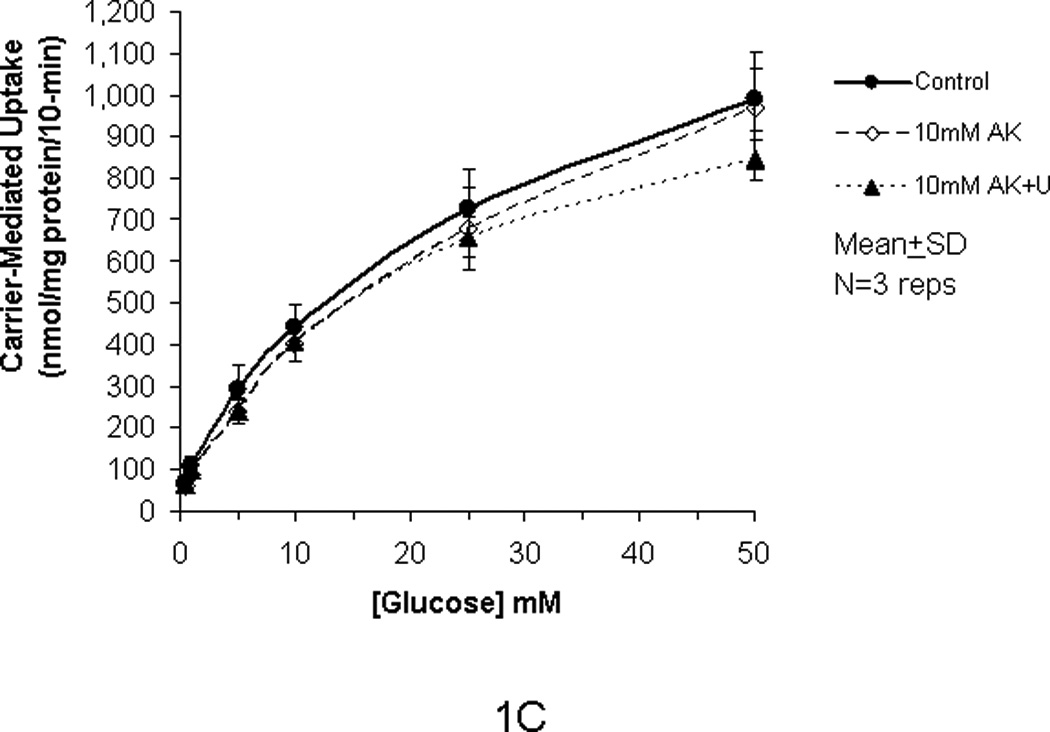
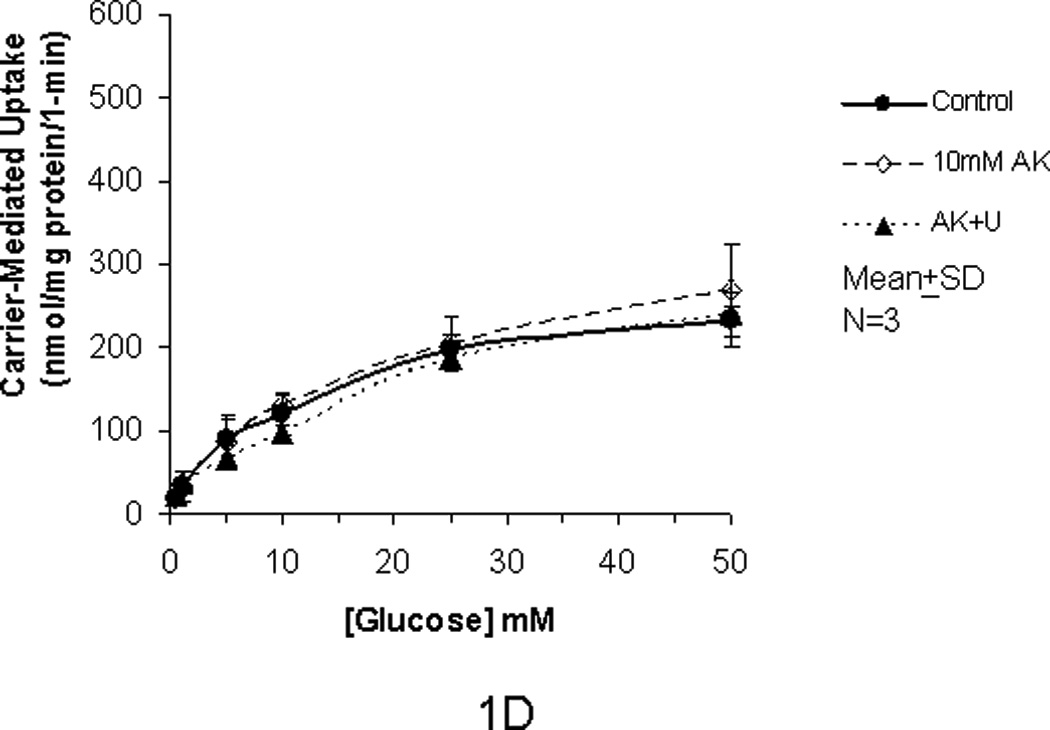
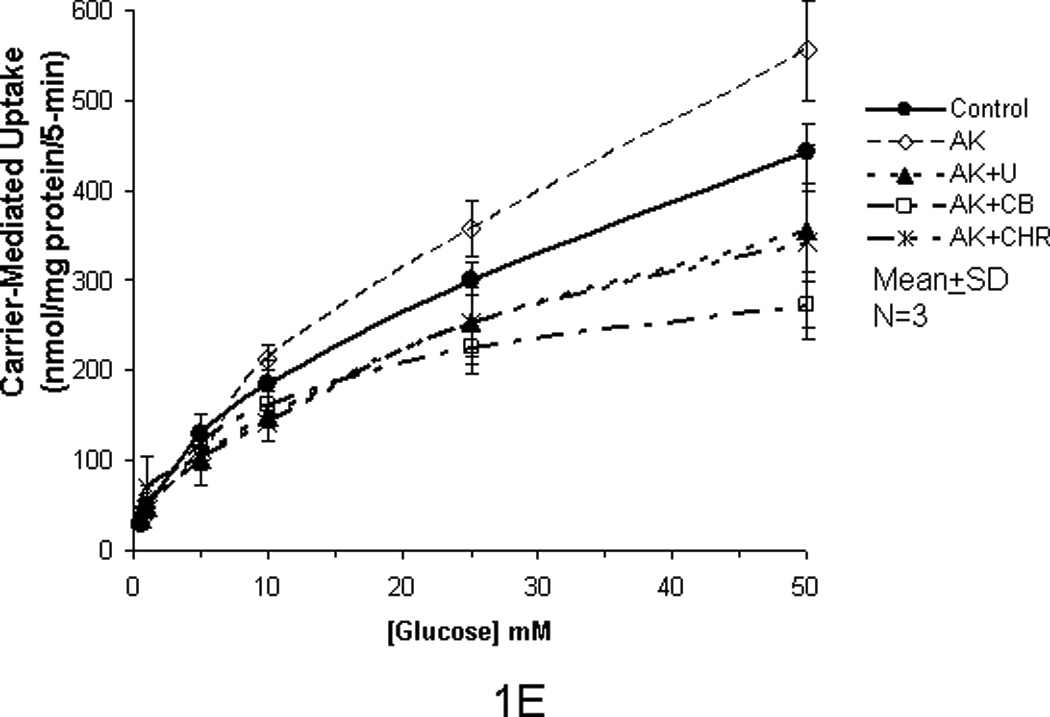
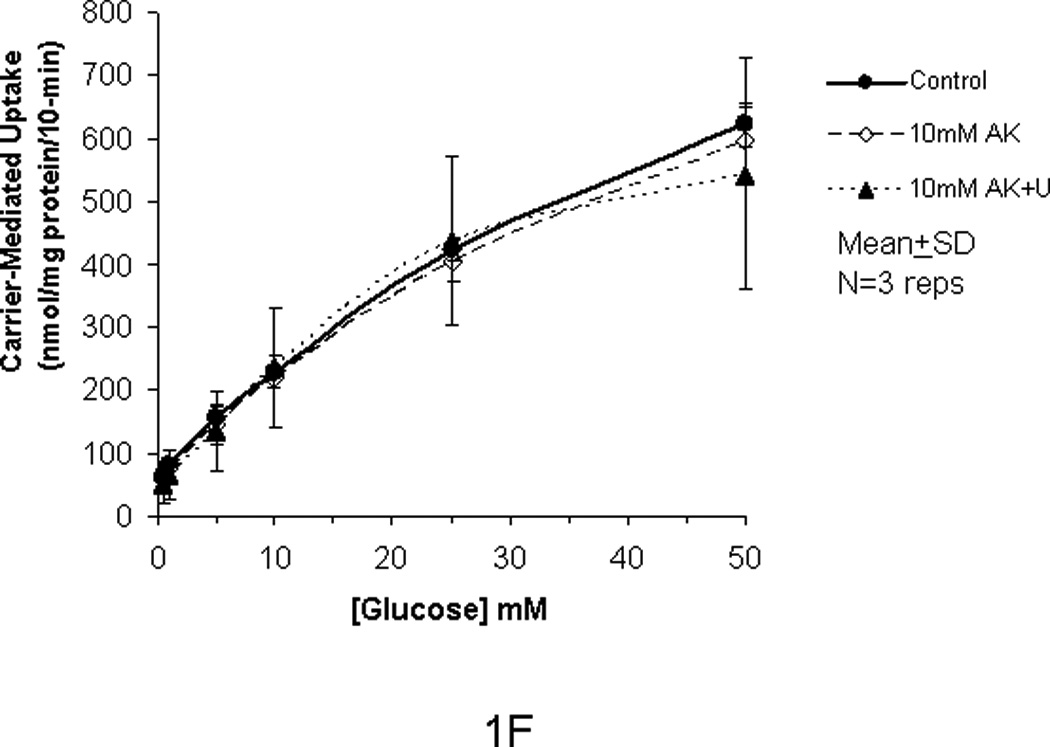
Carrier-mediated glucose uptake in Caco-2 cells (panels A, B, C) and RIE-1 cells (panels D, E, F). Differentiated cells were starved from glucose for 1 h and pre-incubated with 10 mM AceK for 30 min. Glucose uptakes in Caco-2 cells were measured at 1- (A), 5- (B) and 10-min (C) durations of exposure and in RIE-1 cells at 1- (D), 5- (E), and 10-min (F) durations of exposure with various concentrations of glucose in the media with or without AceK or inhibitors of PKC βII and PLC βII. Values are presented as mean±SD of triplicates repeated three times.
Figure 2.
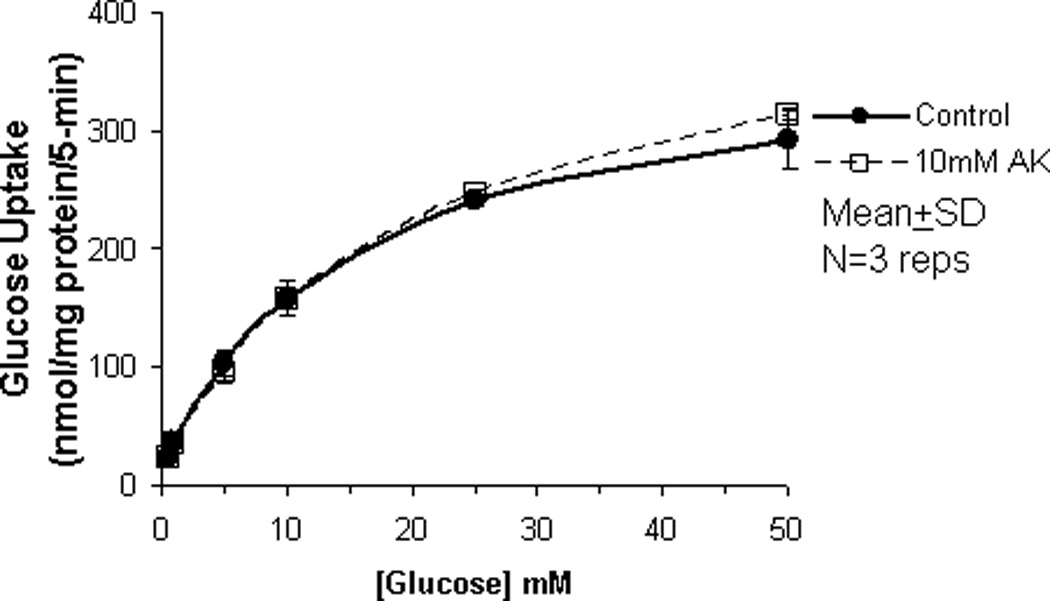
Carrier-mediated glucose uptake in IEC-6 cells. IEC-6 cells were chosen specifically because this cell line lacks functional GLUT2. IEC-6 cells were differentiated for 10 days, after which the cells were starved from glucose for 1 h, and pre-incubated with 10 mM AceK for 30 min. Glucose uptake in IEC-6 were measured at 5-min duration of exposure with various concentrations of glucose with or without AceK. Values are presented as mean±SD of triplicates repeated three times.
Effect of PLC βII and PKC Inhibition, and Disruption of the Intracellular Cytoskeleton
In order to confirm that the enhanced glucose uptake was related to the AceK, we used U-73122, a PLC βII antagonist, to inhibit both the glucose uptake at lesser concentrations of glucose (<25 mM) as well as any enhanced glucose uptake by AceK noted at the greater concentrations of glucose (≥25 mM) during the incubation times of 1-, 5- and 10-min. U-73122 inhibited the enhanced glucose uptake back to the control level in Caco-2 and RIE-1 cells at glucose concentrations ≥25 mM during the 5-min duration of incubation (Fig 1B and 1E) but had no effects at glucose concentrations ≤10 mM at the 5-min incubation, nor at 1- and 10-min durations of incubations (Fig 1A and C and 1D and F). Similarly, 0.5 µM cytochalasin B (a disruptor of intracellular cytoskeleton) and 10 µM chelerythrine (an inhibitor of PKC) showed similar inhibitory effects (Fig 1B and 1E). We did not study the effects of cytochalasin B or chelerythrine at the 1 and 10 minute incubations because AceK had no effect at these incubations and both inhibit downstream mechanisms in the sweet taste receptor signaling pathway.
DISCUSSION
The concept of sweet taste receptors that regulate glucose uptake has received considerable attention. Recently, Mace et al. [19] reported that in the rat, sucralose acted synergistically with glucose to activate the T1R2+T1R3 heterodimer receptors to increase the rate of glucose absorption by translocation of GLUT2 into the apical membrane of the enterocyte through a PLC βII-dependent pathway [20], AceK acted similarly but was much more potent. In contrast, several other studies have failed to show a synergistic interaction of intraluminal sucralose with glucose in relation to small intestinal glucose absorption and subsequent concentrations of blood glucose concentrations in healthy human beings and in patients with diabetes [26–28].
The goal of our study was to determine if the artificial sweetener AceK could increase glucose uptake in three models of intestinal cell lines (Caco-2: human colonic cell line derived from colon cancer; RIE-1: rat intestinal cell line; and IEC-6: neonatal rat intestinal cell line) when the apical glucose concentrations were < 25 mM and whether the increases in glucose uptake were related to GLUT2 via activation of PLC βII. In our previous studies, we have showed that GLUT2 translocation and GLUT2-mediated glucose uptake occurred when glucose concentrations were ≥ 25 mM and the incubation times were ≥ 5 min [24, 25]. Using the same in vitro cell culture system, we showed that AceK increased glucose uptake by 30% in Caco-2 and RIE-1 cells when glucose concentrations were ≥ 25mM for a 5-min incubation. This enhanced glucose uptake was inhibited by the PLC βII inhibitor U-73122, suggesting that PLC βII plays an important role during this activation of increased glucose uptake via sweet taste receptor signaling. We were unable to show any increased glucose uptake in these two cell lines when exposed to glucose concentrations of < 25 mM, suggesting that AceK was not sufficient to induce increased glucose uptake by GLUT2 translocation when glucose concentrations were low; the lack of effect may have been related to the much greater affinity of SGLT1 at these lesser concentrations of glucose in the media. At greater glucose concentration when SGLT1 transporter would be saturated, however, the sweet taste receptor agonist AceK would have the ability to enhance the glucose uptake if GLUT2 was translocated apically, because SGLT1 would be saturated. We also tested whether pre-incubation of AceK would cause apical translocation of GLUT2 to apical membrane even without the presence of glucose in the medium. Therefore, we incubated these cell lines with glucose for 1 min after pre-incubation of the cells with AceK, but with no glucose in the medium, thereby starving the cells; no increase in glucose uptake occurred, suggesting that AceK alone in the absence of luminal glucose could not augment glucose uptake via a GLUT2-mediated mechanism, presumably by translocation of GLUT2. Interestingly, in the 10-min incubations, AceK did not increase glucose uptake further, suggesting that during the 10-min incubation, cells had maximized the process of GLUT2 translocation, and AceK had no further synergistic effect on translocating more GLUT2 transporters to the apical membrane. As would be expected, in the IEC-6 cells that do not express functional GLUT2, AceK did not increase the glucose uptake in the 5-min incubation.
Our study offers several potentially important observations. First, these cell lines may offer a model to more fully study the cell biology of the regulation of GLUT2 translocation to the apical membrane of the enterocyte (signaling, translocation, docking, membrane insertion, etc.). Second, the enterocyte itself appears to respond directly to artificial sweeteners to augment carrier-mediated uptake of glucose independent of sweet taste receptors on the tongue or elsewhere and independent of enteroendocrine cells that also express sweet taste receptors. Third, further augmentation of glucose absorption at higher concentrations of glucose delivery may not be possible by adding artificial sweeteners.
In summary, activation of sweet taste receptors by AceK in cell culture models of the enterocyte (Caco-2 and RIE-1 cells) augmented glucose uptake at 5-min incubations via a GLUT2-dependent mechanism that was mediated by a PLC βII pathway. In contrast, when incubated for 10 min, there was no added effect of AceK. This observation suggests that sweet taste receptors on the apical surface of the enterocyte “sense” luminal glucose concentrations and at least initially, augment glucose absorption via a GLUT2-dependent mechanism mediated through PLC βII.
ACKNOWLEDGEMENTS
The authors wish to thank Deborah Frank for her expertise in the preparation of the manuscript.
This work was supported in part by NIH grant R01 DK039337 (MGS).
Footnotes
This work will be presented in part at the SSAT in Digestive Disease Week, on May 22, 2012.
REFERENCES
- 1.Hediger MA, Coady MJ, Ikeda TS, Wright EM. Expression cloning and cDNA sequencing of the Na+/glucose cotransporter. Nature. 1987;330:379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- 2.Tavakkolizadeh A, Berger UV, Shen KR, Levitsky LL, Zinner MJ, Hediger MA, Ashley SW, Whang EE, Rhoads DB. Diurnal rhythmicity in intestinal SGLT-1 function, V(max), and mRNA expression topography. Am J Physiol Gastrointest Liver Physiol. 2001;280:G209–G215. doi: 10.1152/ajpgi.2001.280.2.G209. [DOI] [PubMed] [Google Scholar]
- 3.Maenz DD, Cheeseman CI. The Na+-independent D-glucose transporter in the enterocyte basolateral membrane: orientation and cytochalasin B binding characteristics. J. Membr Biol. 1987;97:259–266. doi: 10.1007/BF01869228. [DOI] [PubMed] [Google Scholar]
- 4.Turner JR, Lencers WI, Carlson S, Madara JL. Carboxyl-terminal vesicular stomatitis virus G protein-tagged intestinal Na+-dependent glucose cotransporter (SGLT1) J Biological Chemistry. 1996;271:7738–7744. doi: 10.1074/jbc.271.13.7738. [DOI] [PubMed] [Google Scholar]
- 5.Cheeseman CI. GLUT2 is the transporter for fructose across the rat intestinal basolateral membrane. Gastroenterology. 1993;105:1050–1056. doi: 10.1016/0016-5085(93)90948-c. [DOI] [PubMed] [Google Scholar]
- 6.Thorens B, Sarkar HK, Kaback HR, Lodish HF. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988;330:281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- 7.Au A, Gupta A, Schembri P, Cheeseman CI. Rapid insertion of GLUT2 into the rat jejunal brush-border membrane promoted by glucagon-like peptide 2. Biochem J. 2002;367:247–254. doi: 10.1042/BJ20020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellett GL. The facilitated component of intestinal glucose absorption. J Physiol. 2001;531:585–595. doi: 10.1111/j.1469-7793.2001.0585h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boudry G, Cheeseman CI, Perdue MH. Psychological stress impairs Na+-dependent glucose absorption and increases GLUT2 expression in the rat jejunal brush-border membrane. Am J Physiol Regul Integr Comp Physiol. 2007;292:R862–R867. doi: 10.1152/ajpregu.00655.2006. [DOI] [PubMed] [Google Scholar]
- 10.Mace OJ, Morgan EL, Affleck JA, Lister N, Kellett GL. Calcium absorption by Cav1.3 induces terminal web myosin II phosphorylation and apical GLUT2 insertion in rat intestine. J Physiol. 2007;580:605–616. doi: 10.1113/jphysiol.2006.124784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Affleck JA, Helliwell PA, Kellett GL. Immunocytochemical detection of GLUT2 at the rat intestinal brush-border membrane. J Histochem Cytochem. 2003;51:1567–1574. doi: 10.1177/002215540305101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helliwell PA, Richardson M, Affleck J, Kellett GL. Stimulation of fructose transport across the intestinal brush-border membrane by PMA is mediated by GLUT2 and dynamically regulated by protein kinase C. Biochem J. 2000;350(Pt 1):149–154. [PMC free article] [PubMed] [Google Scholar]
- 13.Helliwell PA, Richardson M, Affleck J, Kellett GL. Regulation of GLUT5, GLUT2 and intestinal brush-border fructose absorption by the extracellular signal-regulated kinase, p38 mitogen-activated kinase and phosphatidylinositol 3-kinase intracellular signalling pathways: implications for adaptation to diabetes. Biochem J. 2000;350(Pt 1):163–169. [PMC free article] [PubMed] [Google Scholar]
- 14.Helliwell PA, Rumsby MG, Kellett GL. Intestinal sugar absorption is regulated by phosphorylation and turnover of protein kinase C betaII mediated by phosphatidylinositol 3- kinase- and mammalian target of rapamycin-dependent pathways. J Biol Chem. 2003;278:28644–28650. doi: 10.1074/jbc.M301479200. [DOI] [PubMed] [Google Scholar]
- 15.Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-board membrane. Biochem J. 2000;350:155–162. [PMC free article] [PubMed] [Google Scholar]
- 16.Turner JR, Lencers WI, Carlson S, Madara JL. Carboxyl-terminal vesicular stomatitis virus G protein-tagged intestinal Na+-dependent glucose cotransporter (SGLT1) J Biological Chemistry. 1996;271:7738–7744. doi: 10.1074/jbc.271.13.7738. [DOI] [PubMed] [Google Scholar]
- 17.Shepherd EJ, Helliwell PA, Mace OJ, Morgan EL, Patel N, Kellett GL. Stress and glucocorticoid inhibit apical GLUT2-trafficking and intestinal glucose absorption in rat small intestine. J Physiol. 2004;560:281–290. doi: 10.1113/jphysiol.2004.072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellett GL, Helliwell PA. The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush boards. Biochem J. 2000;350:155–162. [PMC free article] [PubMed] [Google Scholar]
- 19.Mace OJ, Lister N, Morgan E, Shepherd E, Affleck S, Helliwell P, Bronk JR, Kellet GL, Mereditu D, Boyd R, Piere M, Bailen PD, Perrerew R, Foley D. An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestinal. J Physiol. 2009;587(Part 1):195–210. doi: 10.1113/jphysiol.2008.159616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mace OJ, Affleck S, Patel N, Kellet GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582(Part 1):379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gall ML, Tobin V, Stolarczyk E, Dalet V, Leturque A, Brot-Laroche E. Sugar sensing by enterocytes combines polarity, membrane bound detectors and sugar metabolis. J. Cell. Physiol. 2007;213:834–843. doi: 10.1002/jcp.21245. [DOI] [PubMed] [Google Scholar]
- 22.Nelson G, Chandrashekar J, Hoon MS, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y, Scow JS, Duenes JA, Sarr MG. Mechanisms of glucose uptake in intestinal cell lines: role of GLUT2. Surgery. 2012;151(1):13–25. doi: 10.1016/j.surg.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y, Sarr MG. Translocation of transfected GLUT2 to the apical membrane in rat intestinal IEC-6 cells. Digestive Disease & Science. 2012 doi: 10.1007/s10620-011-1984-4. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grotz VL, Henry RR, McGill JB, Prince MJ, Ahamoon H, Trout JR, Pi-Sunyer FX. Lack of effect of sucrose on glucose homeostasis in subjects with type 2 diabetes. J Am Diet Asoc. 2003;103:1607–1612. doi: 10.1016/j.jada.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Mezitis NH, Maggio CA, Koch P, Quddoos A, Allison DB, Pi-Sunyer FX. Glycemic effect of a single high oral dose of the novel sweetener sucralose in patients with diabetes. Diabetes Care. 1996;19:1004–1005. doi: 10.2337/diacare.19.9.1004. [DOI] [PubMed] [Google Scholar]
- 28.Ma J, Chand J, Checklin HL, Young RL, Jones KL, Horowitz M, Rayner CK. Effect of artificial sweetener, sucralose, on small intestinal glucose absorption in healthy human subjects. British J Nutrition. 2010;104:803–806. doi: 10.1017/S0007114510001327. [DOI] [PubMed] [Google Scholar]


