Abstract
Objective
To examine the relation of phobic anxiety to late-life cognitive trajectory.
Design
Prospective cohort.
Setting
Nurses’ Health Study – U.S. registered nurses.
Participants
16,351 women among whom phobic anxiety symptoms were assessed in 1988 (mean age=63 years).
Measurements
Beginning a decade after phobic anxiety ascertainment (mean age=74 years), three assessments of general cognition, word and paragraph immediate and delayed recall, category fluency, and attention/working memory were administered over an average of 4.4 years; global cognitive and verbal memory composite scores were generated from the component tests. General linear models of response profiles were used to evaluate relations of phobic anxiety to initial cognitive performance and subsequent change.
Results
Higher phobic anxiety was associated with poorer initial performance: e.g., comparing women with the highest anxiety to those with no/minimal symptoms, the multivariate-adjusted mean difference (95% confidence interval) in scores was −0.10 (−0.13,−0.06) standard units for the global score summarizing all tests, and −0.08 (−0.11,−0.04) standard units for verbal memory (summarizing 4 word- and paragraph-recall tasks). Mean differences between extreme categories of phobic anxiety were equal to those for participants aged 1.5–2 years apart: i.e., cognitively equivalent to being about two years older. There were no relations of phobic anxiety to subsequent cognitive change.
Conclusions
Higher mid-life phobic anxiety was related to worse later-life overall cognition and verbal memory. Yet, profiles of poorer cognition with higher anxiety remained parallel over time, suggesting phobic anxiety may impose impact on cognition earlier in life, rather than ongoing impact in later-life.
INTRODUCTION
Anxiety may be related to poorer late-life cognition. However, results from the few large-sample studies relating anxiety, distress-proneness or neuroticism to late-life cognition have been mixed: some reports indicated significant associations with cognitive performance(1–4) and subsequent decline(3, 5), at least one report found no impact of neuroticism on late-life cognitive performance(6), and some reported no impact on subsequent change(2, 6). Moreover, few large-scale investigations have addressed relations of anxiety, using specific scales of anxiety symptoms, to late-life cognition(7, 8); there has been only one longitudinal study(8).
The hypothesis of an association between phobic anxiety and poor late-life cognition has particular biologic plausibility. Phobic anxiety has been associated with higher levels of inflammatory mediators implicated in development of cognitive impairment(9–11); it has also been related to incidence of major vascular disease(12) – itself linked to late-life cognitive dysfunction(13). Furthermore, a methodologic advantage of addressing phobic anxiety is that phobic symptoms tend to have earlier onset and persistent course(14); thus, there are fewer concerns of potential reverse causation than may occur when addressing associations between other mental health exposures (e.g., depression) and cognition. Therefore, in this prospective study of over 16,000 participants we examined relations of mid-life phobic anxiety to performance on serial assessments of late-life cognition that began 10 years later.
METHODS
Study sample
The Nurses’ Health Study (NHS) included 121,700 U.S., female registered nurses, aged 30 to 55 years at the study’s inception in 1976. Since then, participants have completed biennial mailed questionnaires updating information on lifestyle factors and health outcomes; total follow-up >90%. On the 1988 follow-up questionnaire, participants were asked to complete the phobic anxiety scale of the Crown-Crisp Index (CCI)(15). Subsequently, between 1995 and 2001, NHS participants aged 70 years and over, and free of diagnosed stroke, were invited to participate in a telephone-based study of cognitive function, and 19,415 (93% of those eligible) completed initial cognitive assessments. Two additional waves of assessments were performed an average of 2 years apart; participation remained near 90%. The sample for analysis consisted of 16,351 women who had information on the CCI (i.e., at least 6 of the 8 items) and were NHS cognitive study participants. The NHS, the NHS cognitive study and the current study were all approved by the Institutional Review Board of Brigham and Women’s Hospital, Boston, MA.
Assessment of phobic anxiety: the Crown-Crisp index
The CCI measures common symptoms of phobic anxiety; it has been validated in psychiatric outpatient settings and found to discriminate patients with general anxiety and phobias from healthy comparison participants, as well as from those with other conditions (i.e., obsessive-compulsive or depressive)(15, 16). The index features 8 self-rated questions on phobias and desire for avoidance; scores range from 0 to 16 (higher scores indicate higher anxiety). For those with missing data on 1 or 2 items, the total score was divided by the fraction of questions answered and rounded to the nearest whole number(12, 17). In keeping with previous work(17), we categorized CCI scores into 5 groups as follows: 0 or 1 (reference group); 2; 3; 4 or 5; and 6 or above (highest phobic anxiety group). Of note, the CCI is reliable in this sample. The Cronbach coefficient alpha for phobic scores in 1988 was 0.61 – comparable to the coefficient of 0.69 originally reported by Crown and Crisp among diagnosed patients and comparison participants(15); item-total correlations were also in the desirable range(18): 0.25–0.43. Furthermore, identical phobic items were featured in the 2004 NHS questionnaire; the correlation between 1988 and 2004 scores in our sample was high (r=0.60; p<0.0001) – an indication that scores reliably represent long-term phobic anxiety levels.
Assessment of cognitive function
Participants were administered: the Telephone Interview for Cognitive Status (TICS)(19), a test of general cognition similar to the Mini-Mental State Examination (MMSE)(20) and for which scores<31 points are consistent with cognitive impairment(19); immediate and delayed recall trials of the East Boston Memory Test paragraph(21); a category fluency test (naming as many different animals as possible during 60 seconds), capturing language and executive function (abstract conceptualization and use of grouping strategies)(22); a delayed recall trial of the TICS 10-word list; and a digit span backwards task (repeating backward an increasingly long string of digits), evaluating attention and working memory. As described in detail elsewhere(23), testing consisted of the TICS as the first wave of the cognitive study began in 1995; starting in 1997, the additional tests (EBMT, fluency, delayed 10-word recall, and digit span) were added.
Primary outcomes were general cognition and verbal memory. To address general cognition, we considered the TICS, as well as a global score calculated by averaging the z-scores of all six individual tests. To evaluate verbal memory, we combined the results of the immediate and delayed recall trials of the EBMT and the TICS 10-word list, by averaging z-scores of those four tests. Such composite scores are regularly used in cognition research because they integrate information from a variety of sources and provide a more stable representation of cognition than a single test(24, 25). Global and verbal memory scores were only calculated where participants had completed all component tests.
Reliability and validity of telephone-based cognitive assessments
To evaluate instrument test-retest reliability, we administered the TICS twice to a sample of women at an interval of one month; the Pearson r=0.70 (p<0.001). Examining inter-rater reliability, we found intraclass correlations >0.95 on each test.
In a validity study, 61 similarly well-educated, high-functioning women who had completed extensive in-person neuropsychological testing were administered our telephone-based interview; overall performance on telephone-based tests was correlated with overall performance on in-person tests (r=0.81). Furthermore, among 88 older health professionals, cognitive impairment as determined by our telephone method was associated with clinically-diagnosed dementia three years later: poor performance on the TICS and in verbal memory was associated with significant 8- and 12-fold increased risks, respectively, of dementia – demonstrating clinical validity(25). Finally, additional evidence of validity is the consistent usefulness of this assessment in identifying meaningful relations of major health and genetic factors to late-life cognition in this cohort(23, 26).
Statistical analysis
To evaluate the relation of mid-life phobic anxiety to trajectories of late-life cognition we examined mean performance at each assessment using repeated measures analysis of means (which permits examination of each time point, taking into account the correlations between assessments). We treated scores as continuous outcomes, and modeled the effect of phobic anxiety on change over time using a time-by-phobic anxiety level interaction term. Because the paths of mean scores over time were non-linear, we used the method of response profiles, modeling time nominally rather than linearly(27, 28). This approach imposes minimal structure on outcome trends and permits valid estimation of effects in non-linear data; another advantage of this general linear model formulation is that it can handle settings in which data for some participants are missing (as in the present analysis), thus allowing for analyses to be based on all available data(27). All models were by maximum likelihood, incorporating the longitudinal correlation within participants, using unstructured covariance structures; for statistical testing, we used Wald tests(27). All statistical analyses were conducted in SAS version 9.2 (SAS Institute, Cary, NC, USA), and all models were fitted using PROC MIXED.
In basic models we include time, age, education (associate’s, bachelor’s, or master’s/doctoral degree) and phobic anxiety level, as well as interaction terms for each of these variables with time. Multivariate-adjusted models added the following potential confounders, along with terms for the interaction of each covariate with time: body mass index (BMI) (normal [BMI<25 kg/m2] or overweight/obese [BMI≥25 kg/m2]), cigarette smoking (current/past/never), postmenopausal hormone use (current/past/never), history of hypertension (yes/no), history of elevated cholesterol (yes/no), history of heart disease (myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention; yes/no), history of diabetes (yes/no), alcohol use (0, 0.1–14.9, or 15+ grams/day), and physical activity (metabolic equivalents/week). All covariates were determined as of the 1988 questionnaire (when phobic anxiety was measured), except age, which was determined as of initial cognitive assessment. Covariate information was obtained from the questionnaires; validation studies of these self-reports demonstrate high accuracy(29).
We conducted a key secondary analysis additionally accounting for depression status: depression is an important factor to examine due to the known high degree of comorbidity of anxiety with depression, including among older women(30). Although it is difficult to know whether depression was coincident or subsequent to anxiety in our participants, mediation is likely the key pattern to address, given the relation between anxiety and development of depression; thus, we focused on depression as of initial cognitive assessment. To classify depression status, we considered scores on the Mental Health Index-5 (MHI-5) from the Medical Outcomes Short Form-36 (SF-36), as well as self-reports of regular antidepressant use and physician-diagnosed clinical depression. The MHI-5 (scaled from 0–100; higher scores indicate better mental health), was on questionnaires in 1992, 1996 and 2000; the MHI-5 has high reliability, and a cutoff of 52/53 has high sensitivity and specificity for severe depressive symptoms and high validity for major depression against a diagnostic gold standard(31, 32). Participants began reporting antidepressant use in 1996; in 2000, the Nurses were first asked whether they ever had a lifetime physician diagnosis of depression (1996 or before, 1997–1998, 1999, on or after 2000). Information on antidepressants and physician-diagnosed depression was updated biennially thereafter. Thus, we defined depression status (yes/no) as having one of: MHI-5≤52, regular antidepressant use, or physician-diagnosed depression.
We also conducted a separate analysis in which all potential confounders, including depression, were updated as of cognitive assessment. Thus, we addressed the extent to which relations of mid-life phobic anxiety to late-life cognition may be due to influences of phobic anxiety on subsequent status of covariates.
Finally, given the possibility that anxiety may have differential impact on executive function and attention/working memory – e.g., through preemptory utilization by anxious thoughts of the processing and information storage resources fundamental to performance in these domains(2), as predicted by Eysenck(33) – we separately examined the category fluency and digit span backward tasks. For category fluency, we used the method of response profiles, as described for the primary outcomes. However, because digit span scores over time showed a clearly linear pattern of decline, we constructed random-effects models(28) treating time as a continuous variable (in years). These models incorporated fixed effects for time, all covariates from the primary analyses, interaction terms of time by each covariate, and two person-specific random effects: baseline level (random intercept) and rate of change (random slope)(28); the effect estimate was the annualized rate of cognitive change associated with phobic anxiety level.
RESULTS
Table 1 shows characteristics of the study sample in 1988, across phobic anxiety levels. Women in the highest phobic category were generally less healthy than those in the lowest phobic category: e.g., higher phobic anxiety was associated with higher BMI, higher prevalence of severe depressive symptoms, hypertension, heart disease, diabetes, elevated cholesterol and current smoking, and lower physical activity. Another notable difference was the pattern of lower educational attainment with higher phobic anxiety: only half as many women in the highest phobic category had achieved bachelor’s or above education, compared to the lowest phobic category.
Table 1.
Participant characteristics and unadjusted cognitive test scores by phobic anxiety categories, from lowest to highest (n=16,351)*
| CHARACTERISTICS AT MID-LIFE | Crown-Crisp Index Phobic Anxiety Scores | ||||
|---|---|---|---|---|---|
| 0 or 1 | 2 | 3 | 4 or 5 | ≥6 | |
| Number of participants | 5,068 | 2,916 | 2,519 | 3,454 | 2,394 |
| Median Crown-Crisp phobic index | 1.0 | 2.0 | 3.0 | 4.0 | 7.0 |
| Mean age (years) | 63.0 | 63.0 | 63.0 | 63.0 | 63.0 |
| Bachelor’s level education (%) | 19.0 | 18.0 | 15.5 | 14.3 | 11.3 |
| Master’s or doctoral level education (%) | 8.0 | 7.4 | 5.3 | 5.1 | 3.2 |
| Mental health Index-5 (MHI-5) ≤52 (%) | 2.6 | 3.3 | 4.1 | 7.3 | 12.6 |
| Hypertension (%) | 33.1 | 34.8 | 37.1 | 39.9 | 46.2 |
| Diabetes (%) | 4.1 | 4.9 | 4.6 | 4.8 | 6.2 |
| Heart disease (%) | 2.2 | 2.3 | 2.5 | 2.3 | 3.1 |
| Elevated cholesterol (%) | 30.4 | 33.1 | 32.3 | 34.9 | 37.9 |
| Current smoking (%) | 13.9 | 14.7 | 14.1 | 15.2 | 15.9 |
| Current postmenopausal hormone use (%) | 27.5 | 26.4 | 27.1 | 24.4 | 24.4 |
| Mean body mass index (kg/m2) | 25.3 | 25.4 | 25.6 | 25.8 | 26.2 |
| Mean alcohol intake (g/day) | 5.2 | 5.2 | 5.1 | 5.4 | 4.7 |
| Mean physical activity (METS/week) | 17.9 | 16.6 | 16.5 | 15.7 | 13.8 |
| COGNITIVE TEST SCORES† | |||||
| TICS (points), range: 0–41 | 33.9 (2.8) | 33.9 (2.7) | 33.8 (2.6) | 33.6 (2.8) | 33.5 (2.7) |
| EBMT, immediate recall (story items), range: 0–12 | 9.5 (1.7) | 9.4 (1.7) | 9.4 (1.7) | 9.4 (1.8) | 9.3 (1.8) |
| EBMT, delayed recall (story items), range: 0–12 | 9.0 (2.1) | 9.0 (1.9) | 9.0 (2.0) | 9.0 (2.1) | 8.9 (2.0) |
| Word list, immediate recall (words), range: 0–10 | 4.7 (1.7) | 4.7 (1.7) | 4.6 (1.7) | 4.6 (1.7) | 4.5 (1.7) |
| Word list, delayed recall (words), range: 0–10 | 2.4 (2.1) | 2.4 (2.0) | 2.3 (2.0) | 2.3 (1.9) | 2.2 (1.9) |
| Category fluency (words) | 17.3 (4.7) | 17.2 (4.8) | 16.9 (4.6) | 16.8 (4.6) | 16.4 (4.6) |
| Digit span backward (correct lines), range: 0–12 | 6.8 (2.4) | 6.8 (2.4) | 6.8 (2.5) | 6.7 (2.4) | 6.5 (2.5) |
| Global score (standard units) | 0.04 (0.61) | 0.02 (0.60) | −0.01 (0.60) | −0.03 (0.61) | −0.09 (0.61) |
| Verbal memory (standard units) | 0.02 (0.70) | 0.01 (0.68) | −0.02 (0.69) | −0.02 (0.70) | −0.08 (0.68) |
Characteristics at mid-life appear as of the 1988 questionnaire cycle, with the exception of alcohol use (1990) and MHI-5 score (1992).
Cognitive test scores are provided as means (standard deviations). TICS = Telephone Interview of Cognitive Status; global score combines results of TICS, category fluency, digit span backwards, immediate and delayed recall trials of East Boston Memory Test (EBMT), and delayed recall trial of the TICS 10-word list; verbal memory score combines results of the immediate and delayed recall trials of EBMT and the TICS 10-word list.
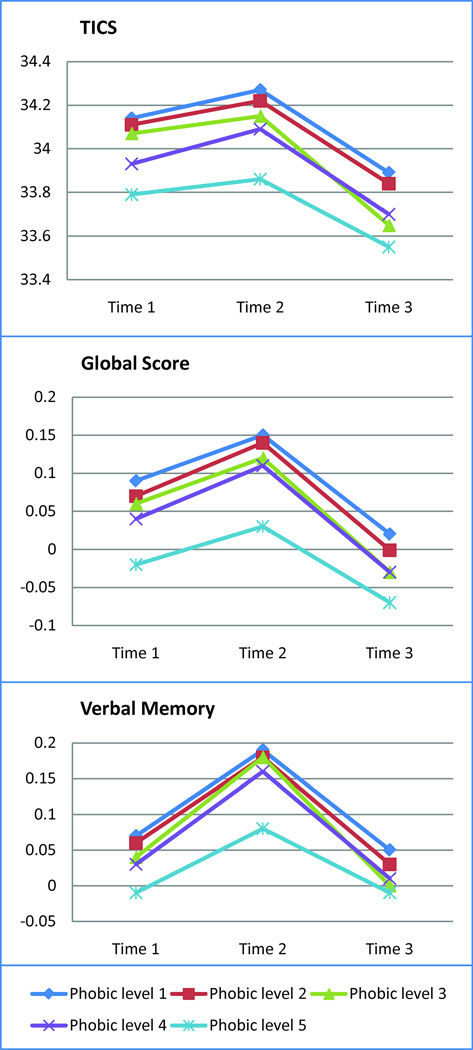
Results from the age- and education-adjusted models are presented in Figure 1, which provides the adjusted mean scores over time on primary outcomes in each phobic anxiety group. At initial assessment, higher phobic anxiety levels were associated with lower cognitive scores. For example, women with the highest phobic anxiety level scored a mean −0.35 (95% confidence interval [CI]: −0.48,−0.22) points lower on the TICS than those with the lowest anxiety, with a statistically significant p-for-linear trend across phobic categories: p<0.0001; Wald F(1, 16346)=37.75 (data not shown in Figure). Findings for the TICS were mildly attenuated but remained statistically significant after multivariate-adjustment (Table 2). Similar age- and education-adjusted relations of higher phobic anxiety to worse initial performance were observed for the global score (p-for-linear trend<0.0001; Wald F(1, 16002)=51.45) and verbal memory (p-for-linear trend<0.0001; Wald F(1, 16005)=27.10); estimates were similar after multivariate-adjustment (data not shown in Figure). To help interpret effect estimates, we compared the association of phobic anxiety with cognition to the association of age with cognition in this cohort: the mean differences in cognitive performance for extreme categories of phobic anxiety were equivalent to those observed for women aged 1.5–2 years apart – i.e., these differences were cognitively comparable to being about 2 years older.
Figure 1. Age- and education-adjusted mean cognitive scores over time, by mid-life levels of phobic anxiety*.
* TICS=Telephone Interview for Cognitive Status, in points; global score is in standard units; verbal memory is in standard units. Lowest phobic anxiety level=1; highest phobic anxiety level=5.
Table 2.
Multivariate-adjusted mean differences in cognitive scores at each follow-up, across mid-life levels of phobic anxiety *
| COGNITIVE TEST |
Crown-Crisp Index Phobic Anxiety Scores | F; p-value† | |||||
|---|---|---|---|---|---|---|---|
| 0 or 1 | 2 | 3 | 4 or 5 | ≥6 | |||
| TICS‡ | 0.81; 0.59 | ||||||
| TIME | 1 (n=16351) | 0.0 | −0.02 (−0.15, 0.10) | −0.06 (−0.18, 0.07) | −0.19 (−0.30, −0.07) | −0.29 (−0.43, −0.16) | |
| 2 (n=14586) | 0.0 | −0.03 (−0.17, 0.11) | −0.10 (−0.25, 0.04) | −0.15 (−0.28, −0.02) | −0.34 (−0.49, −0.19) | ||
| 3 (n=12595) | 0.0 | −0.03 (−0.19, 0.13) | −0.21 (−0.38, −0.04) | −0.15 (−0.30, 0.01) | −0.26 (−0.44, −0.08) | ||
| GLOBAL‡ | 1.27; 0.26 | ||||||
| TIME | 1 (n=14096) | 0.0 | −0.02 (−0.04, 0.01) | −0.03 (−0.06, 0.00) | −0.04 (−0.07, −0.02) | −0.10 (−0.13, −0.06) | |
| 2 (n=13958) | 0.0 | −0.00 (−0.04, 0.03) | −0.02 (−0.05, 0.01) | −0.03 (−0.06, −0.00) | −0.10 (−0.14, −0.07) | ||
| 3 (n=12566) | 0.0 | −0.02 (−0.06, 0.02) | −0.05 (−0.09, −0.01) | −0.04 (−0.08, −0.01) | −0.08 (−0.11, −0.04) | ||
| VERBAL‡ | 1.74; 0.08 | ||||||
| TIME | 1 (n=14111) | 0.0 | −0.01 (−0.04, 0.02) | −0.03 (−0.07, 0.00) | −0.03 (−0.07, −0.00) | −0.08 (−0.11, −0.04) | |
| 2 (n=13961) | 0.0 | −0.01 (−0.04, 0.03) | −0.01 (−0.05, 0.03) | −0.03 (−0.06, 0.01) | −0.10 (−0.14, −0.06) | ||
| 3 (n=12595) | 0.0 | −0.01 (−0.05, 0.03) | −0.04 (−0.08, 0.01) | −0.03 (−0.07, 0.01) | −0.04 (−0.09, 0.00) | ||
Adjusted for age at initial cognitive interview; education level; cigarette smoking, postmenopausal hormone use, hypertension, diabetes, elevated cholesterol, heart disease, body mass index, alcohol intake and physical activity level at mid-life. Mean interval between first and third wave of cognitive tests = 4.4 years.
All p-values are from the Wald test (numerator df=8) of interaction between phobic anxiety categories and time; the denominator degrees of freedom were 16331 for the TICS, 15984 for global score, and 15986 for verbal memory.
TICS = Telephone Interview of Cognitive Status; global score combines results of TICS, category fluency, digit span backwards, immediate and delayed recall trials of East Boston Memory Test (EBMT), and delayed recall trial of the TICS 10-word list; verbal memory score combines results of the immediate and delayed recall trials of EBMT and the TICS 10-word list.
With regard to cognitive change, the profiles of mean performance across phobic anxiety categories remained parallel over time, for all outcomes (Figure 1). Although at every assessment we observed poorer cognitive performance with higher phobic anxiety, all tests for interactions of phobic anxiety with time were statistically non-significant (Table 2).
In a key secondary analysis, we adjusted for depression status as of cognitive testing. Although estimates were slightly further attenuated, the results were not materially changed: e.g., multivariate-adjusted mean differences at initial assessment indicated worse performance on all outcomes for those with the highest vs. lowest phobic anxiety (Table 3).
Table 3.
Multivariate- and depression-adjusted mean differences in cognitive scores at each follow-up, across mid-life levels of phobic anxiety *
| COGNITIVE TEST |
Crown-Crisp Index Phobic Anxiety Scores | F; p-value† | |||||
|---|---|---|---|---|---|---|---|
| 0 or 1 | 2 | 3 | 4 or 5 | ≥6 | |||
| TICS‡ | 0.97; 0.46 | ||||||
| TIME | 1 (n=16351) | 0.0 | −0.01 (−0.13, 0.11) | −0.05 (−0.17, 0.08) | −0.17 (−0.29, −0.05) | −0.25 (−0.39, −0.12) | |
| 2 (n=14586) | 0.0 | −0.02 (−0.16, 0.11) | −0.10 (−0.24, 0.05) | −0.14 (−0.27, −0.01) | −0.31 (−0.47, −0.16) | ||
| 3 (n=12595) | 0.0 | −0.01 (−0.17, 0.14) | −0.19 (−0.36, −0.02) | −0.12 (−0.27, 0.03) | −0.19 (−0.37, −0.02) | ||
| GLOBAL‡ | 1.45; 0.17 | ||||||
| TIME | 1 (n=14096) | 0.0 | −0.01 (−0.04, 0.02) | −0.02 (−0.05, 0.01) | −0.04 (−0.07, −0.01) | −0.09 (−0.12, −0.05) | |
| 2 (n=13958) | 0.0 | −0.00 (−0.03, 0.03) | −0.02 (−0.05, 0.02) | −0.03 (−0.06, 0.00) | −0.09 (−0.13, −0.06) | ||
| 3 (n=12566) | 0.0 | −0.01 (−0.05, 0.02) | −0.04 (−0.08, −0.01) | −0.03 (−0.07, 0.00) | −0.06 (−0.10, −0.02) | ||
| VERBAL‡ | 1.89; 0.06 | ||||||
| TIME | 1 (n=14111) | 0.0 | −0.01 (−0.04, 0.03) | −0.03 (−0.06, 0.01) | −0.03 (−0.06, 0.00) | −0.06 (−0.10, −0.03) | |
| 2 (n=13961) | 0.0 | −0.00 (−0.04, 0.03) | −0.01 (−0.04, 0.03) | −0.02 (−0.05, 0.01) | −0.09 (−0.13, −0.05) | ||
| 3 (n=12595) | 0.0 | −0.01 (−0.05, 0.03) | −0.03 (−0.08, 0.01) | −0.02 (−0.06, 0.02) | −0.03 (−0.07, 0.02) | ||
Adjusted for age at initial cognitive interview; education level; current (vs. never/past) cigarette smoking, current (vs. never/past) postmenopausal hormone use, hypertension, diabetes, elevated cholesterol, heart disease, body mass index, alcohol intake and physical activity level at mid-life; and depression status as of initial cognitive interview. Mean interval between first and third wave of cognitive tests = 4.4 years.
All p-values are from the Wald test (numerator df=8) of interaction between phobic anxiety categories and time; the denominator degrees of freedom were 16330 for the TICS, 15986 for global score, and 15988 for verbal memory.
TICS = Telephone Interview of Cognitive Status; global score combines results of TICS, category fluency, digit span backwards, immediate and delayed recall trials of East Boston Memory Test (EBMT), and delayed recall trial of the TICS 10-word list; verbal memory score combines results of the immediate and delayed recall trials of EBMT and the TICS 10-word list.
Findings again remained similar after adjusting for all covariates, including depression, where the status of each covariate was updated as of initial cognitive testing. However, the relation of the highest vs. lowest anxiety group on cognition was further, albeit mildly, attenuated – suggesting that some of the impact of phobic anxiety on late-life cognition could have been mediated through these factors: e.g., the mean differences in global score comparing highest vs. lowest phobic levels were −0.08 (95% CI: −0.11,−0.05) units at time 1, −0.09 (95% CI: −0.13,−0.06) units at time 2, and −0.05 (95% CI: −0.09,−0.02) units at time 3 (data not shown).
Finally, multivariate-adjusted models analyzing secondary outcomes of category fluency and digit span backwards yielded findings comparable to those from primary analyses: higher phobic anxiety was associated with statistically significantly worse initial performance; mean differences comparing extreme categories of phobic anxiety were cognitively equivalent to being 3.5–4 years older. However, there was no evidence of non-parallel profiles over time for category fluency, or of differences in rates of decline in working memory (data not shown).
DISCUSSION
In this study of 16,351 community-dwelling older women, we observed modest but statistically significant associations between higher mid-life levels of phobic anxiety, measured by a validated symptom scale, and worse later-life performance in overall cognitive function and in verbal memory. Specifically, being in the highest phobic anxiety category was cognitively equivalent to almost two years of aging. Associations remained statistically significant after adjustment for depression and numerous potential confounders, including after updating the status of confounders as of cognitive testing. Secondarily, higher phobic anxiety was associated with worse performance on executive and attention tasks. Patterns of worse performance among those with the highest vs. lowest phobic anxiety were evident at each assessment; however, there was no indication that paths of cognitive change over time were non-parallel across levels of phobic anxiety – suggesting that phobic anxiety may impair cognition earlier in life but may not exert ongoing impact during aging. Finally, although we observed a striking inverse association between phobic anxiety and educational attainment, results for the association between phobic anxiety and cognition remained after education adjustment – indicating that associations were not driven primarily by lower educational attainment among highly phobic women. Of note, although few studies have examined this issue, the relation of phobic anxiety to education in our cohort is highly consistent with the recent finding from a large-scale, multi-national study(34) of a statistically significant 40% higher relative risk of non-initiation or non-completion of tertiary education among young persons (in high-income nations such as the U.S.) who have anxiety disorders – specifically, panic disorder and agoraphobia.
Potential mechanisms through which phobic anxiety may influence late-life cognition include effects of inflammation(9, 10) and activation of the hypothalamic-pituitary-adrenal (HPA) axis/hypercortisolism(35, 36). Specifically, it has been hypothesized that prolonged stress in anxiety disorders might lead to chronically elevated levels of pro-inflammatory cytokines and stress hormones and, consequently, to injury or atrophy of key brain regions, such as the hippocampus, involved in memory and cognition. However, most studies in humans that have addressed this concept directly have focused on post-traumatic stress disorder and did not focus on older participants; furthermore, directionality of the stress-hippocampal volume association remains unclear(37). Finally, another intriguing potential mechanism involves shared genetic vulnerability. For example, the Val158Met polymorphism (Val/Val) in the catechol-O-methyltransferase gene that has been associated with late-life cognitive impairment(38) was also associated with phobic anxiety in Nurses’ Health Study participants(17). However, there are no data in the present analysis to determine whether phobic anxiety-cognition associations in this sample were explained by COMT polymorphism or other genetic or non-genetic factors.
Findings in our study of a statistically significant association between higher phobic anxiety and worse cross-sectional working memory are consistent with those of Wetherell et al.(2), who examined the related construct of neuroticism and late-life cognition (n=704); also consistent with our study, there were no associations between neuroticism and subsequent cognitive decline(2). Wilson et al.(3) found associations between higher neuroticism and worse performance on all domains tested, including verbal memory, verbal fluency and working memory, among approximately 800 elders (mean baseline age=75.2 years); there was a statistically significant relation of neuroticism to 5-year decline on verbal memory, but not on any other cognitive outcomes. In another study by Wilson and colleagues(4), of over 4,000 community-dwelling elders, distress-proneness was associated with 5-year decline on a global cognitive score (p=0.059). In more recent work(5), this group found statistically significant relations of the anxiety facet on a neuroticism scale to decline in global cognition, processing speed and working memory over an average of 3.4 years among 785 persons (mean=80.7 years); the neuroticism-anxiety facet was not related to decline in episodic or semantic memory in those subjects. Lastly, among 2,615 participants of the Longitudinal Aging Study Amsterdam (LASA) cohort (mean age=70.2 years), Bierman and colleagues(7) identified cross-sectional associations (p=0.03) of anxiety symptoms, using a validated anxiety symptom scale, with verbal memory (on the Auditory Verbal Learning Test [AVLT]). Intriguingly, the association with AVLT was curvilinear: mildly anxious persons performed better than those with no or severe anxiety – a pattern similar to that first proposed by Yerkes and Dodson(39). This inverse-U-shaped relation has not been observed by us or other groups. However, it is notable that the AVLT involves 5 successive learning trials – unlike the immediate and delayed word and paragraph recall trials utilized in this study and other studies(3, 4); thus, Bierman and co-workers speculated that the AVLT may be more sensitive to mental effort than other tests(7), such that persons with slightly increased arousal due to mild anxiety may perform optimally. Results from a follow-up study(8) of 9-year change in these same LASA participants were similar to ours: anxiety was not related to subsequent decline on any cognitive measure.
Overall, there has been a scarcity of data on associations of anxiety – in particular, phobias, panic or generalized anxiety – with cognition, even when including research focused on younger adults(40); there is still less evidence from larger-scale studies (e.g., n>500) among older people. Considering the current report in the context of the available literature, anxiety appears related to worse initial performance in multiple cognitive domains at late-life, but evidence for relations to subsequent decline has been either lacking or inconsistent across cognitive domains.
Strengths of the current study include: use of a validated anxiety symptom scale; a large, well-characterized sample; prospective design with almost 5 years of repeated cognitive assessments; and adjustment for numerous major potential confounders, including a depression status variable incorporating mood scores and clinical indicators (i.e., physician-diagnosed depression, antidepressant use). Also, this was the first report to examine anxiety symptoms as of mid-life with regard to late-life cognition. Finally, reverse causation problems, which commonly arise in research involving psychological predictors, were likely minimized by the design of the study. Phobic anxiety is relatively unique as a predictor because of its young onset and persistence through life; overall median age-of-onset for phobic disorders is <12 years(14). Thus, mid-life phobic anxiety levels in our participants likely reflected symptoms that had been present for decades, reducing concerns of possible reverse causation (i.e., cognitive impairment led to increased anxiety).
Potential limitations of this study should also be considered. First, we did not have information on cognitive function prior to late-life; thus, we cannot comment on when relative deficits in cognition potentially attributable to phobic anxiety may first have emerged. Second, generalizability is a potential concern: although there is no apparent reason to believe that phobic anxiety might act differently in other demographic groups, further research is necessary to address potential differences in associations of anxiety with late-life cognition among men and ethnic minorities. Finally, although we were able to adjust for numerous potential confounders, residual confounding by unmeasured factors is possible. However, the relative homogeneity of our cohort reduces potential influences of many unmeasured confounders, such as those related to health knowledge or access to care.
In conclusion, higher mid-life phobic anxiety was related to worse later-life cognition in this study of over 16,000 women; these associations were modest (cognitively equivalent to 1.5–2 years of age) but statistically significant. Yet, profiles of poorer cognitive performance with higher phobic anxiety remained parallel over time. Thus, while anxiety may be related to worse initial cognition, we found no evidence that it is associated with continued decline throughout later-life. Our results suggest that identifying phobic anxiety in younger persons may be important in terms of targeting a high-risk group for later cognitive impairment. Furthermore, because anxiety disorders, such as phobia and panic, have known treatments, future research might address directly whether prompt identification and treatment of phobic anxiety can mitigate potential detrimental impacts on cognition.
ACKNOWLEDGEMENTS
The project was supported by grants CA87969 and AG015424 from the National Institutes of Health. Dr. Okereke is supported by Career Development Award K08-AG029813 and received a grant from the Harvard Medical School Eleanor and Miles Shore Scholars Fund. The funding sources were not involved in the data collection, data analysis, manuscript writing or publication. We are indebted to the participants in the Nurses’ Health Study for their continuing outstanding support, and our many colleagues working in the Study for their valuable help.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous Presentation: Portions of this work were presented on November 20, 2011 as a paper presentation during a symposium at the Gerontological Society of America annual meeting.
Disclosures: No Disclosures to Report.
Author Contributions:
Study concept and design: Okereke, Grodstein.
Analysis and interpretation of data: Okereke, Grodstein.
Drafting of the manuscript: Okereke, Grodstein.
Critical revision of the manuscript for important intellectual content: Okereke, Grodstein.
Statistical analysis: Okereke.
Obtaining funding and Study supervision: Okereke, Grodstein.
Final approval: Okereke, Grodstein.
Disclosures and Conflicts of Interest: The authors have no conflicts of interest to declare.
Data Access and Responsibility: The authors (Drs. Okereke and Grodstein) had full access to the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Jorm AF, Mackinnon A, Christensen H, Henderson S, Scott R, Korten A. Cognitive functioning and neuroticism in an elderly community sample. Pers Individ Diff. 1993;15:721–723. [Google Scholar]
- 2.Wetherell JL, Reynolds CA, Gatz M, Pedersen NL. Anxiety, cognitive performance, and cognitive decline in normal aging. J Gerontol B Psychol Sci Soc Sci. 2002;57(3):P246–P255. doi: 10.1093/geronb/57.3.p246. [DOI] [PubMed] [Google Scholar]
- 3.Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer's disease. Neurology. 2003;61(11):1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- 4.Wilson RS, Bennett DA, Mendes de Leon CF, Bienias JL, Morris MC, Evans DA. Distress proneness and cognitive decline in a population of older persons. Psychoneuroendocrinology. 2005;30(1):11–17. doi: 10.1016/j.psyneuen.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RS, Begeny CT, Boyle PA, Schneider JA, Bennett DA. Vulnerability to stress, anxiety, and development of dementia in old age. Am J Geriatr Psychiatry. 2011;19(4):327–334. doi: 10.1097/JGP.0b013e31820119da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jelicic M, Bosma H, Ponds RW, Van Boxtel MP, Houx PJ, Jolles J. Neuroticism does not affect cognitive functioning in later life. Exp Aging Res. 2003;29(1):73–78. doi: 10.1080/03610730303704. [DOI] [PubMed] [Google Scholar]
- 7.Bierman EJ, Comijs HC, Jonker C, Beekman AT. Effects of anxiety versus depression on cognition in later life. Am J Geriatr Psychiatry. 2005;13(8):686–693. doi: 10.1176/appi.ajgp.13.8.686. [DOI] [PubMed] [Google Scholar]
- 8.Bierman EJ, Comijs HC, Rijmen F, Jonker C, Beekman AT. Anxiety symptoms and cognitive performance in later life: results from the longitudinal aging study Amsterdam. Aging Ment Health. 2008;12(4):517–523. doi: 10.1080/13607860802224276. [DOI] [PubMed] [Google Scholar]
- 9.Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol. 2004;61(5):668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, Harris T. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 11.Brennan AM, Fargnoli JL, Williams CJ, Li T, Willett W, Kawachi I, Qi L, Hu FB, Mantzoros CS. Phobic anxiety is associated with higher serum concentrations of adipokines and cytokines in women with diabetes. Diabetes Care. 2009;32(5):926–931. doi: 10.2337/dc08-1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albert CM, Chae CU, Rexrode KM, Manson JE, Kawachi I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;111(4):480–487. doi: 10.1161/01.CIR.0000153813.64165.5D. [DOI] [PubMed] [Google Scholar]
- 13.Román GC, Sachdev P, Royall DR, Bullock RA, Orgogozo JM, López-Pousa S, Arizaga R, Wallin A. Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci. 2004;226:81–87. doi: 10.1016/j.jns.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 15.Crown S, Crisp AH. A short clinical diagnostic self-rating scale for psychoneurotic patients. The Middlesex Hospital Questionnaire (M.H.Q.) Br J Psychiatry. 1966;112:917–923. doi: 10.1192/bjp.112.490.917. [DOI] [PubMed] [Google Scholar]
- 16.Burgess PM, Mazzocco L, Campbell IM. Discriminant validity of the Crown-Crisp experimental index. Br J Psychiatry. 1987;60:61–69. [PubMed] [Google Scholar]
- 17.McGrath M, Kawachi I, Ascherio A, Colditz GA, Hunter DJ, De Vivo I. Association between catechol-O-methyltransferase and phobic anxiety. Am J Psychiatry. 2004;161:1703–1705. doi: 10.1176/appi.ajp.161.9.1703. [DOI] [PubMed] [Google Scholar]
- 18.Streiner EL, Norma GR. Selecting the Items. Oxford: Oxford University Press; 1989. Health Measurement Scales: A Practical Guide to their development and Use; pp. 39–52. [Google Scholar]
- 19.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsych, Neuropsychol, Behav Neurol. 1988;1(2):111–117. [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's Disease. Intern J Neuroscience. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- 22.Royall DR, Lauterbach EC, Cummings JL, Reeve A, Rummans TA, Kaufer DI, LaFrance WC, Jr, Coffey CE. Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2002;14:377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- 23.Stampfer MJ, Kang JH, Chen J, Cherry R, Grodstein F. Effects of moderate alcohol consumption on cognitive function in women. N Engl J Med. 2005;352(3):245–253. doi: 10.1056/NEJMoa041152. [DOI] [PubMed] [Google Scholar]
- 24.Wilson RS, Schneider JA, Barnes LL, Beckett LA, Aggarwal NT, Cochran EJ, Berry-Kravis E, Bach J, Fox JH, Evans DA, Bennett DA. The apolipoprotein E epsilon 4 allele and decline in different cognitive systems during a 6-year period. Arch Neurol. 2002;59:1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- 25.Kang JH, Cook N, Manson J, Buring JE, Grodstein F. A randomized trial of vitamin E supplementation and cognitive function in women. Arch Intern Med. 2006;166:2462–2468. doi: 10.1001/archinte.166.22.2462. [DOI] [PubMed] [Google Scholar]
- 26.Kang JH, Logroscino G, De Vivo I, Hunter D, Grodstein F. Apolipoprotein E, cardiovascular disease and cognitive function in aging women. Neurobiol Aging. 2005;26(4):475–484. doi: 10.1016/j.neurobiolaging.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Fitzmaurice GM, Laird NM, Ware JH. Modelling the mean: analyzing response profiles. In: Fitzmaurice GM, Laird NM, Ware JH, editors. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons; 2004. pp. 103–139. [Google Scholar]
- 28.Laird NM, Ware JE. Random-effects models for longitudinal data. Biometrics. 1982;38:968–974. [PubMed] [Google Scholar]
- 29.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner B, Hennekens CH, Speizer FE. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 30.Byers AL, Yaffe K, Covinsky KE, Friedman MB, Bruce ML. High occurrence of mood and anxiety disorders among older adults: The National Comorbidity Survey Replication. Arch Gen Psychiatry. 2010;67(5):489–496. doi: 10.1001/archgenpsychiatry.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berwick DM, Murphy JM, Goldman PA, Ware JE, Jr, Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Med Care. 1991;29(2):169–176. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki S, Fukuhara S, Green J. Usefulness of five-item and three-item Mental Health Inventories to screen for depressive symptoms in the general population of Japan. Health Qual Life Outcomes. 2005;3:48. doi: 10.1186/1477-7525-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eysenck MW, Calvo MG. Anxiety and performance: the processing-efficiency theory. Cognition and Emotion. 1992;6:409–434. [Google Scholar]
- 34.Lee S, Tsang A, Breslau J, Aguilar-Gaxiola S, Angermeyer M, Borges G, Bromet E, Bruffaerts R, de Girolamo G, Fayyad J, Gureje O, Haro JM, Kawakami N, Levinson D, Oakley Browne MA, Ormel J, Posada-Villa J, Williams DR, Kessler RC. Mental disorders and termination of education in high-income and low- and middle-income countries: epidemiological study. Br J Psychiatry. 2009;194(5):411–417. doi: 10.1192/bjp.bp.108.054841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aguilera G. HPA axis responsiveness to stress: Implications for healthy aging. Exp Gerontol. 2011;46(2–3):90–95. doi: 10.1016/j.exger.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee BK, Glass TA, McAtee MJ, Wand GS, Bandeen-Roche K, Bolla KI, Schwartz BS. Associations of salivary cortisol with cognitive function in the Baltimore memory study. Arch Gen Psychiatry. 2007;64(7):810–818. doi: 10.1001/archpsyc.64.7.810. [DOI] [PubMed] [Google Scholar]
- 37.Sapolsky RM. Chickens, eggs and hippocampal atrophy. Nat Neurosci. 2002;5(11):1111–1113. doi: 10.1038/nn1102-1111. [DOI] [PubMed] [Google Scholar]
- 38.Starr JM, Fox H, Harris SE, Deary IJ, Whalley LJ. COMT genotype and cognitive ability: a longitudinal aging study. Neurosci Lett. 2007;421:57–61. doi: 10.1016/j.neulet.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 39.Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurol Psychol. 1908;18:459–482. [Google Scholar]
- 40.Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lönnqvist J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106:1–27. doi: 10.1016/j.jad.2007.06.006. [DOI] [PubMed] [Google Scholar]