Abstract
Edwardsiella ictaluri is a Gram-negative pathogenic bacterium in the family Enterobacteriaceae that causes enteric septicemia of catfish, which has become a significant problem in the aquaculture of striped catfish (Pangasianodon hypophthalmus) in Vietnam. In this study, a bacterium designated as Ei-151 was isolated from diseased striped catfish and proved to be virulent. Based on 16S rDNA sequencing and phenotypic tests, the pathogenic bacterium was identified as Edw. ictaluri. The presence of quorum sensing signal molecules in Edw. ictaluri Ei-151 was detected with different biosensor strains. The results showed that Ei-151 produced at least three kinds of acylated homoserine lactone (AHL) signal molecules as detected with the biosensor Agrobacterium tumefaciens KYC55, and the AHLs fingerprint was similar to that of Edw. tarda. During its entire growth, the levels of AHLs and autoinducer-2 produced by Ei-151 peaked at the stationary phase (OD600 1.8), which suggested that both of them may function at the stationary phase. No Cholerae autoinducer-1-like activity (including Edw. ictaluri LMG7860T) was detected.
Electronic supplementary material
The online version of this article (doi:10.1007/s12088-012-0312-9) contains supplementary material, which is available to authorized users.
Keywords: Edwardsiella ictaluri, Quorum sensing, N-acylhomoserine lactones (AHLs), Autoinducer-2 (AI-2), Cholerae autoinducer-1 (CAI-1)
Introduction
Edwardsiella ictaluri, the causative agent of enteric septicemia of catfish (ESC), typically causes ESC in channel catfish (Ictalurus punctatus Rafinesque) and infrequently in other species thriving in freshwater habitats such as green knife fish (Eigenmannia virens), tadpole madtom (Noturus gyrinus) and rainbow trout (Oncorhynchus mykiss) [1]. Edw. ictaluri is responsible for big economic losses to the catfish industry across the world. It is estimated that ESC costs about $19 million every year in direct fish losses in the USA, where channel catfish production accounts for more than half of its total aquaculture products [2].
Quorum sensing (QS) is a cell-to-cell signaling mechanism that refers to the ability of bacteria for responding to extracellular chemical signal molecules called autoinducers, whose external concentration increases according to the increasing cell density [3]. Bacterial processes such as biofilm formation, virulence factor secretion, bioluminescence, antibiotic production and sporulation, which are all shown to be regulated by QS [4–8]. N-acylhomoserine lactones (AHLs) are the most intensively investigated family of QS signal molecules used by Gram-negative bacteria, and they are composed of homoserine lactone (HSL) rings with acyl chains of C4 to C18 in length. Two proteins belonging to the LuxI/LuxR protein families are used as synthase and transcriptional regulator, respectively [3]. This kind of system is used predominantly for intraspecies communication as to a large extent specificity exists between the LuxR proteins and their cognate AHL signals [9]. Another type of QS system which is found in a wide range of both Gram-negative and Gram-positive bacteria termed as the LuxS or autoinducer-2 (AI-2) system, which is for interspecies communication, and the structure of AI-2 was reported to be a furanosyl-borate diester [10]. A third type of QS system is based on CAI-1, which was recently purified from Vibrio cholerae and identified as (S)-3-hydroxytridecan-4-one. Its synthesis depends on CqsA, an enzyme similar to aminotransferases, and the CqsA/CqsS system is considered to be used for inter-Vibrio communication [5].
Previous study demonstrated that Edw. tarda, which has a close genetic relationship with Edw. ictaluri, produced AHLs and AI-2 signal molecules [11]. Some virulence-associated elements such as the type III secretion system (TTSS) and biofilm formation of Edw. tarda were found to be regulated by a LuxS system [12]. However, the information about the QS system in Edw. ictaluri remained undocumented. In this study, an Edw. ictaluri Ei-151 was obtained from diseased striped catfish cultured in Vietnam, and investigated for the production of different QS signal molecules during the entire growth cycle with different biosensor strains.
Materials and Methods
Bacterial Strains, Media and Cultivation Conditions
Bacterial strains used in this study were listed in Supplementary material Table S1. Edw. tarda LTB-4, Chromobacterium violaceum CV026 and Escherichiacoli were grown in Luria–Bertani (LB) medium. Edw. ictaluri strains and Vibrio strains were grown in BHI (Brain Heart Infusion Broth) medium and LBN medium (LB medium with 2 % NaCl), respectively. Agrobacterium tumefaciens KYC55 was grown in AT minimal broth with appropriate antibiotics according to Tempe et al. [13], while V. harveyi mutant strains were grown in Autoinducer bioassay (AB) medium prepared according to Greenberg et al. [14]. All these strains were incubated at 28 °C with exception of E. coli which was grown at 37 °C. Spectrophotometry (WFJ2100, Shanghai) was used to measure the bacterial growth (OD600). Bioluminescence was measured with a BPCL-4 Ultra-Weak Luminescence Analyzer (China) at emission of 474 nm.
Isolation of Bacterial Pathogen
During June of 2006, an epizootic white spot disease occurred among cultured striped catfish in Chau Thanh District, Dong Thap Province, Vietnam. Hemorrhaging at the base of fins, sluggish behavior, and abundant white spots on kidney, liver and spleen were the main macroscopic lesions observed in these diseased stripes catfish with 22–24 cm body length (Fig. 1). Three to five diseased fish from each farm were taken for microbiological examination immediately. Samples taken aseptically from kidney, liver and spleen of the diseased fish were put separately into sterile Eppendorf tubes, kept at 4 °C and transported to the laboratory. Within 24 h of collection, the samples were directly spread on Blood Agar (BA) plates and incubated for 24–48 h at 30 °C. The dominant isolates were purified by streaking and restreaking on to fresh media, with storage at −80 °C in BHI supplemented with 15 % (v/v) glycerol.
Fig. 1.
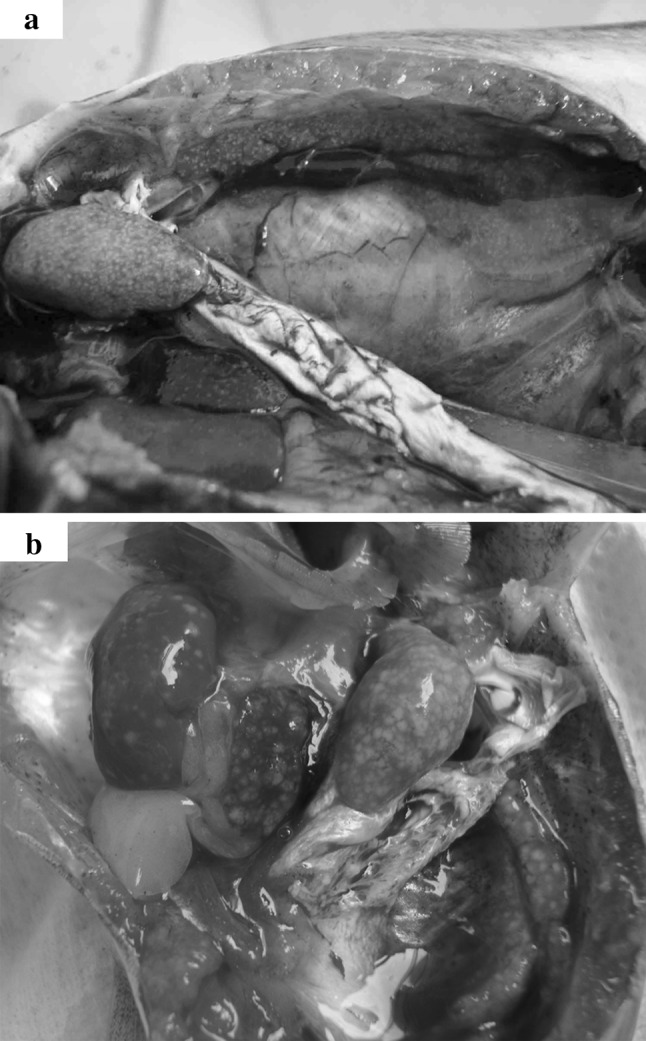
Typically clinical signs of white spot disease, which were swollen and pale internal organs (kidney, liver and spleen) contain various necrotic white spots. a Experimentally infected catfish by Edw. ictaluri Ei-151 in Nov 2009. b Naturally infected catfish by Edw. ictaluri in May 2010
Experimental Infection
Overnight cultures on BHI broth were used to prepare suspensions in 0.9 % (w/v) saline. The viable cell concentration was established by preparing serial tenfold dilutions of the bacterial suspension in saline and spreading 0.1 ml volumes onto duplicate plates of BHI with incubation at 30 °C for 48 h. After sedation (0.2 ppt ethylene glycol monophenyl ether for 3–5 min), groups of 20 catfish (average weight = 30 g) from quarantined stocks recognized as disease-free were infected by intraperitoneal (ip) injection with 200 μl volumes of the bacterial suspensions containing 8 × 103 to 8 × 106 cells ml−1, in triplicate. The fish were maintained in tanks containing freshwater which was continuously filtered through spongy at 28–30 °C. Controls were injected ip with 200 μl volumes of saline. The infected fish were maintained for two weeks, and mortalities were recorded. The lethal doses at 50 % were calculated using method of Reed and Muench [15]. Bacterial re-isolation was made on catfish following the methods described above.
16S rDNA PCR, Sequencing and Phylogenic Analysis
The 16S rDNA of bacterial isolates from diseased fish was amplified by PCR with bacterial universal primers P11: 5′-AGAGTTTGATCCTGGCTCAG-3′ and P12: 5′-GGTTACCTTGTTACGACTT-3′) [16]. After purification, the 16S rDNA fragment (1,503 bp) was cloned into pUC vector and sequenced (Sangon, Shanghai). The 16S rDNA sequence of Ei-151 (GenBank accession number JF274254) was aligned and compared with available sequences in species of family Enterobacteriaceae in the NCBI GenBank with BLAST.
Morphological, Physiological and Biochemical Tests
The morphology and motility of the bacterial pathogen was determined microscopically by Gram stain. Physiological and biochemical results were obtained by inoculating the bacteria into API 20E test strips (BioMrieux Inc., France) following the method described in the product manual. The bacterial isolate was identified based on the taxonomic criteria in the Bergey’s Manual of Systematic Bacteriology [17], and the 16S rDNA sequence and phylogenetic analysis.
Preparation of Cell-Free Culture Fluids of the Strains
Two millilitres overnight culture of Ei-151 was inoculated into 400 ml fresh BHI medium, and cultured under constant agitation for 48 h. Ten ml aliquots were taken every 3 h and centrifuged at 1,000×g for 10 min to remove cells from the growth media. After filtration with 0.22 μm filter, the obtained cell-free fluids were stored at −20 °C for further examination. Bacterial growth was determined by turbidity measurements (OD600). The cell-free culture fluids of Edw. ictaluri LMG7860T, Edw. tarda LTB-4 and Vibrio strains were prepared as Ei-151.
Detection of AHL Molecules in Edw. ictaluri Ei-151
Screening for AHLs in Agar System
Double-layer plate method with KYC55 and cross-streaking method with CV026 were used to detect AHL molecules produced by Ei-151[11]. Edw. tarda LTB-4 was used as the positive control, while BHI plate without any supplements was used as the negative control.
Growth Phase-Dependent Analysis of AHLs Production in Liquid System
The level of AHLs in cell-free culture fluids of Ei-151 at various time points was quantified by examining the ability of samples to activate TraR in KYC55, as described by Zhu et al. [18]. Fifty microliters of oxo-C8-HSL (OOHL) (50 μg ml−1) was used as a positive control, while the fresh BHI media was the negative control.
Thin Layer Chromatography (TLC)
The presence of AHLs in the supernatants of Edw. ictaluri strains was tested by C18 reversed-phase TLC (aluminum sheets 20 × 20 cm2, Merck) with KYC55 [11]. AHLs standards (3-oxo-C6-HSL, C4-HSL and C6-HSL) with various retention factors (Rf) were used as references.
Detection of AI-2 Activity in Edw. ictaluri Ei-151
The presence of AI-2 activity in the supernatants of Edw. ictaluri Ei-151 at various time points was assessed with V. harveyi reporter strain BB170 [19]. Sterile culture supernatants from V. harveyi BB152 (at OD600 around 2.0) and E. coli DH5α served as positive and negative controls, respectively. Statistical analysis was performed using the SPSS software, ver. 17.0.
Detection of CAI-1 Activity in Edw. ictaluri Ei-151
The presence of CAI-1 activity in the supernatants of Ei-151 (at 12, 15, 21 and 24 h), Edw. ictaluri LMG7860T, Edw. tarda LTB-4, V. campbellii VIB285 and V. parahaemolyticus VIB304 was assessed with V. harveyi report strain JAF375 [11]. Overnight-culture supernatant of V. harveyi BB120, Listonella anguillarum VIB72 and E. coli DH5α served as two positive controls and a negative control respectively. Statistical analysis was performed using the SPSS software, ver. 17.0.
Results and Discussion
White spot disease has recurrently occurred each production cycle of striped catfish and caused severe damages for farmers each year in Vietnam. Colonies of Edw. ictaluri Ei-151 was smooth circular (1–2 mm in diameter) and non-pigmented on BHI plates. It was motile at 28 °C but not at 37 °C, no growth was observed at 40 °C. Biochemically, Ei-151 was identical to known Edw. ictaluri strains. Compared with Edw. tarda, Ei-151 was negative for ornithine decarboxylase, indole and H2S production (see Supplementary material Table S2), which was in accordance with previous report [20]. The 16S rDNA sequence of Ei-151 was most similar (99 %) to those members of Edw. ictaluri. In combination with its temperature limitation and the different biochemical characteristics from Edw. tarda, Ei-151 was identified as Edw. ictaluri.
Pathogenicity
Ei-151 was virulent to striped catfish with massive mortalities occurring between 3 and 8 days after ip injection. Most infected catfish revealed numerous white spots on swollen kidney, spleen and liver (Fig. 1). All fish were killed when 200 μl volumes of Ei-151 containing 8 × 106 CFU per fish were administered. In comparison, the 101-, 102- and 103- fold-diluted suspension of Ei-151 killed 93, 73, and 32 % of the striped catfish, respectively. Thus, the LD50 was established as 8.3 × 102 CFU g−1 of fish (2.5 × 104 CFU per fish). In addition, Ei-151 was re-isolated as pure cultures from internal organs of the infected fish.
Detection of AHL Activity in Edw. ictaluri Ei-151
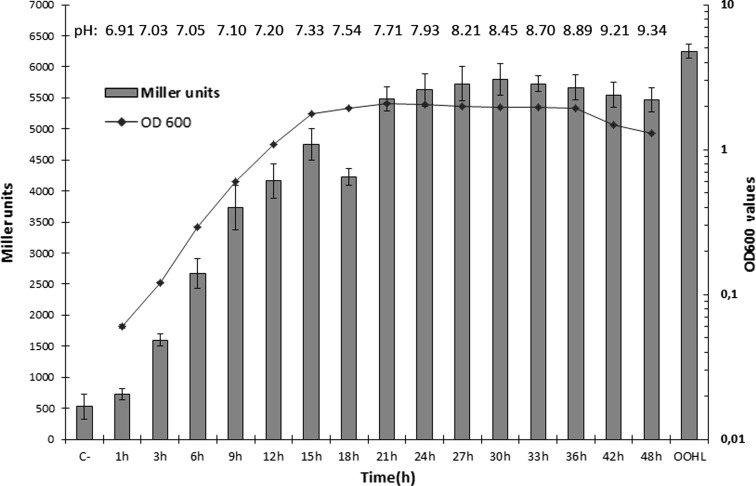
Both the blue colouration by KYC55 and the purple pigment formation by CV026 proved that Ei-151 produced AHL molecules. Ei-151 produced AHL molecules during its whole growth phase (Fig. 2). The level of AHLs recognized by KYC55 gradually increased until the stationary phase (at 15 h, OD600 1.8), then decreased sharply when it reached up to the maximal cell density (OD600 2.1) and subsequently increased to maintain a higher level. The same phenomenon has been observed in Edw. tarda LTB-4 [11] and warrants further investigation. It might be attributed to the fact that QS systems in some bacteria is likely to involve in the transition into stationary phase, which is directed by key transcription factors, the activity or production of which is stimulated by starvation [8]. Low level AHL activity may prolong the transition into stationary phase, which might be one reason for the significant decrease of AHLs level at 18 h. In a number of bacteria species, QS and starvation-sensing pathways have been found to co-regulate each other in regulation of the transition into stationary phase [21]. The key transcription factor σS of Pseudomonas aeruginosa is positively regulated by an AHL [22]. Therefore, the high AHLs level of Ei-151 in stationary phase is probably associated with its transition and resistance to adverse environment in stationary phase. After 42 h, EI-151 entered into the decline phase, the level of AHLs did not show a significant fall, though the pH values of bacterial cultures increased to 9.34.
Fig. 2.
N-acylhomoserine lactones production by Edw. ictaluri Ei-151 during the entire growth cycle. C− negative control, OOHL positive control, Bars = SD
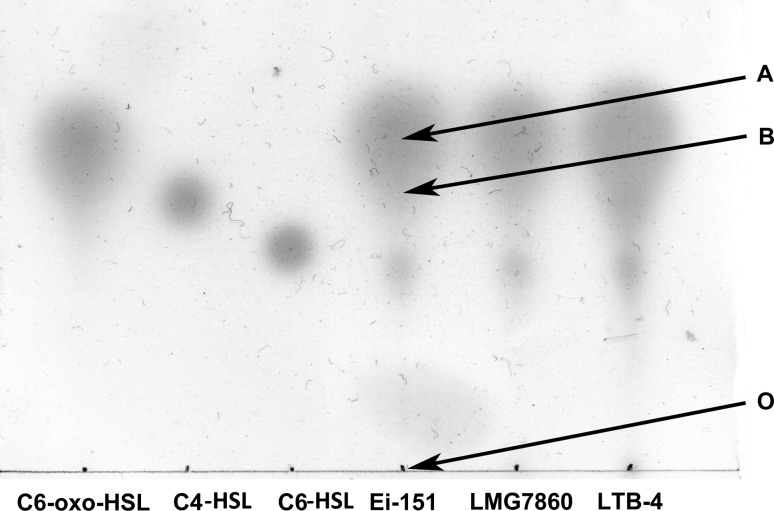
Edw. ictaluri Ei-151 and its type strain LMG7860T were found to have a same AHLs profile, which corresponded with the profile of Edw. tarda, and produced three kinds of AHL molecules as detected with KYC55. The first spot with a tail (Rf value 0.696) migrated as fast as 3-oxo-C6-HSL (Rf value 0.702), the second spot (Rf value 0.578) could be C4-HSL (Rf value 0.585) and the third spot was uncharacterized (Fig. 3). The sequences of putative AHL synthase [Genbank: YP_002934276] and AHL-dependent transcriptional regulator [Genbank: YP_002934275] of Edw. ictaluri obtained from Genbank database showed 93 % and 96 % similarity with those of EdwI [Genbank: YP_003296640] and EdwR [Genbank: YP_003296639] from Edw. tarda, respectively. Whether Edw. ictaluri Ei-151 produces the same AHLs as Edw. tarda will be interesting to further analyse by HPLC–mass spectrometry.
Fig. 3.
The AHL fingerprints of Edw. ictaluri strains were detected by thin-layer chromatography (TLC) with Ag. tumefaciens KYC55. The origin (O) of TLC was marked. Synthetic standards: Lane 1 oxo-C6-HSL (0.0001 μg), lane 2 C4-HSL (0.5 μg), lane 3 C6-HSL (0.08 μg). Lane 4–5 acidified ethyl acetate extracts of Edw. ictaluri Ei-151, Edw. ictaluri LMG7860T and Edw. tarda LTB-4, respectively. Position A had the same retention factor as oxo-C6-HSL. Position B had the same retention factor as C4-HSL
AHL-based QS in some pathogenic species are well studied and have been shown to relate to their virulence [6]. It has been reported that some prokaryotes and a few eukaryotic organisms, especially Bacillus spp., can produce AHL-degrading enzymes such as AHL-lactonases and AHL-acylases to degrade these signals, which is called quorum quenching (QQ) [23, 24]. QQ can be exploited as potentially novel antibiotic-free therapeutics to protect from bacterial infections [25].
Detection of AI-2 Activity in the Edw. ictaluri Ei-151
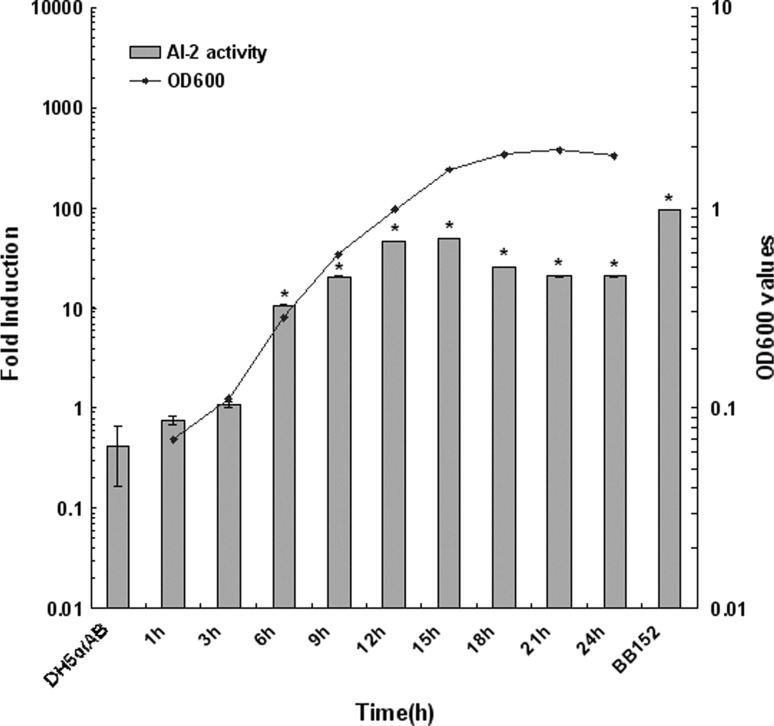
The production of AI-2 signal molecules by Ei-151 was growth phase-dependent. AI-2 activity began to rapidly increase at 6 h (OD600 0.5), and reached the maximum level at 15 h (OD600 1.8), then maintained a 2.4-fold lower level when compared with the stationary phase (OD600 1.8) (Fig. 4). E. coli and Sal. typhimurium have both been reported to produce AI-2 during early exponential growth, but later apparently degrade the autoinducer in the absence of glucose, however, in the presence of glucose, AI-2 activity remains in the culture supernatant. It suggested that the degradation might be subject to catabolism repression [26]. Various biological functions have been reported to be regulated by AI-2 either directly or indirectly, such as expression of virulence factors, biofilm formation, swarming motility and toxin production [27]. Further investigation is necessary to find out whether AI-2 in Edw. ictaluri regulated its virulence.
Fig. 4.
Induction of bioluminescence in V. harveyi BB170 by the cell-free fluids of. Edw. ictaluri Ei-151 throughout growth. Results presented as fold induction were obtained by dividing the luminescence of the samples by the luminescence of the sterile AB medium. DH5α negative control, BB152 positive control, Bars = SD. Asterisk significantly different from bioluminescence of V. harveyi BB170 induced by cell-free culture fluids of E. coli DH5α (p < 0.05, Tukey’s test)
Interestingly, the levels of AHLs and AI-2 produced by Ei-151 all increased rapidly at OD600 around 0.5 and peaked in the stationary phase (OD600 1.8), which suggested that both of them may function in the stationary phase.
Detection of CAI-1-like Activity in the Edw. ictaluri Ei-151
Data analysed statistically showed that neither Edw. ictaluri Ei-151 in lag, log and stationary phase nor its type strain LMG7860T was shown to possess any CAI-1 activities, or the molecules they produced were not recognized by V. harveyi JAF375, as well as the Edw. tarda LTB-4 (see Supplementary material Table S3). Vibrio campbellii VIB285 and V. parahaemolyticus VIB304 could significantly induce bioluminescence in V. harveyi JAF375 (p < 0.05; Table S3), which is in accordance with previous reports [6, 28].
In conclusion, Edw. ictaluri Ei-151 isolated from kidney of diseased striped catfish was identified as Edw. ictaluri based on the 16S rDNA sequencing and phenotypic tests. The LD50 was established as 8.3 × 102 CFU g−1 of fish (2.5 × 104 CFU per fish). It is the first report that Edw. ictaluri Ei-151 produces AHLs and AI-2 QS signals, which is similar to Edw. tarda. Moreover, the two kinds of QS signals of Edw. ictaluri may play an equal role in regulation. No CAI-1 activity is detected in Edw. ictaluri Ei-151.
Electronic supplementary material
Acknowledgments
The work was supported by grants from the National Natural Science Foundation of China (No. 31072241).
References
- 1.Panangala VS, Shoemaker CA, Klesius PH, Mitra A, Russo R. Cross-protection elicited in channel catfish (Ictalurus punctatus Rafinesque) immunized with a low dose of virulent Edwardsiella ictaluri strains. Aquac Res. 2009;40:915–926. doi: 10.1111/j.1365-2109.2009.02185.x. [DOI] [Google Scholar]
- 2.Part II: reference of foodsize catfish health and production practices in the United States, USDA. Fort Collins: N.A.H.M.S; 2003. [Google Scholar]
- 3.Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR–LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176(2):269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irie Y, Parsek MR. Quorum sensing and microbial biofilms. Curr Top Microbiol Immunol. 2008;322:67–84. doi: 10.1007/978-3-540-75418-3_4. [DOI] [PubMed] [Google Scholar]
- 5.Higgins DA, Pomianek ME, Kraml CM, Taylor RK, Semmelhack MF, Bassler BL. The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature. 2007;450:883–886. doi: 10.1038/nature06284. [DOI] [PubMed] [Google Scholar]
- 6.Defoirdt T, Verstraete W, Bossier P. Luminescence, virulence and quorum sensing signal production by pathogenic Vibrio campbellii and Vibrio harveyi isolates. J Appl Microbiol. 2008;104:1480–1487. doi: 10.1111/j.1365-2672.2007.03672.x. [DOI] [PubMed] [Google Scholar]
- 7.Kleerebezem M. Quorum sensing control of lantibiotic production; nisin and subtilin autoregulate their own biosynthesis. Peptides. 2004;25:1405–1414. doi: 10.1016/j.peptides.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Lazazzera BA. Quorum sensing and starvation: signals for entry into stationary phase. Curr Opin Microbiol. 2000;3:177–182. doi: 10.1016/S1369-5274(00)00072-2. [DOI] [PubMed] [Google Scholar]
- 9.Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Schauder S, Potier N, Dorssealaer A, Pelczer I, Bassler BL, Hughson FM. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 11.Han Y, Li X, Qi ZZ, Zhang XH, Bossier P. Detection of different quorum-sensing signal molecules in a virulent Edwardsiella tarda strain LTB-4. J Appl Microbiol. 2010;108(1):139–147. doi: 10.1111/j.1365-2672.2009.04405.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Sun K, Sun L. Regulation of autoinducer 2 production and luxS expression in a pathogenic Edwardsiella tarda strain. Microbiology. 2008;154:2060–2069. doi: 10.1099/mic.0.2008/017343-0. [DOI] [PubMed] [Google Scholar]
- 13.Tempe J, Petit A, Holsters M, Mon Atagu MV, Schell J. Thermosensitive step associated with transfer of the Ti Plasmid during conjugation: possible relation to transformation in crown gall. Proc Natl Acad Sci USA. 1977;74:2848–2849. doi: 10.1073/pnas.74.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenberg EP, Hastings JW, Ulitzur S. Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch Microbiol. 1979;120:87–91. doi: 10.1007/BF00409093. [DOI] [Google Scholar]
- 15.Reed LJ, Muench S. A simple method of estimating fifty percent and point. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 16.Lan J, Zhang XH, Wang Y, Chen J, Han Y. Isolation of an unusual strain of Edwardsiella tarda from turbot and establish a PCR detection technique with the gyrB gene. J Appl Microbiol. 2008;105:644–651. doi: 10.1111/j.1365-2672.2008.03779.x. [DOI] [PubMed] [Google Scholar]
- 17.Garrity GM. Bergey’s manual of systematic bacteriology. 2. New York: Springer; 2005. [Google Scholar]
- 18.Zhu J, Chai Y, Zhong Z, Li S, Winans SC. Agrobacterium bioassay strain for ultrasensitive detection of N-acylhomoserine lactone-type quorum-sensing molecules: detection of autoinducers in Mesorhizobium huakuii. Appl Environ Microbiol. 2003;69:6949–6953. doi: 10.1128/AEM.69.11.6949-6953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vilchez R, Lemme A, Thiel V, Schulz S, Sztajer H, Wagner DI. Analysing traces of autoinducer-2 requires standardization of the Vibrio harveyi bioassay. Anal Bioanal Chem. 2007;387:489–496. doi: 10.1007/s00216-006-0824-4. [DOI] [PubMed] [Google Scholar]
- 20.Panangala VS, Shoemaker CA, McNulty ST, Arias CR, Klesius PH. Intra- and interspecific phenotypic characteristics of fish-pathogenic Edwardsiella ictaluri and E. tarda. Aquac Res. 2006;37:49–60. doi: 10.1111/j.1365-2109.2005.01394.x. [DOI] [Google Scholar]
- 21.Pesci EC, Iglewski BH. The chain of command in Pseudomonas quorum sensing. Trends Microbiol. 1997;5:132–135. doi: 10.1016/S0966-842X(97)01008-1. [DOI] [PubMed] [Google Scholar]
- 22.You Z, Fukushima J, Tanaka K, Kawamoto S, Okuda K. Induction of entry into the stationary growth phase in Pseudomonas by N-acylhomoserine lactone. FEMS Microbiol Lett. 1998;164:99–106. doi: 10.1111/j.1574-6968.1998.tb13073.x. [DOI] [PubMed] [Google Scholar]
- 23.Kalia VC, Raju SC, Purohit HJ. Genomic analysis reveals versatile organisms for quorum quenching enzymes: acyl-homoserine lactone- acylase and -lactonase. Open Microbiol J. 2011;5:1–13. doi: 10.2174/1874285801105010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huma N, Shankar P, Kushwah J, Bhushan A, Joshi J, Mukherjee T, Raju SC, Purohit HJ, Kalia VC. Diversity and polymorphism in AHL-lactonase gene (aiiA) of Bacillus. J Microbiol Biotechnol. 2011;21:1001–1011. doi: 10.4014/jmb.1105.05056. [DOI] [PubMed] [Google Scholar]
- 25.Kalia VC, Purohit HJ. Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol. 2011;37:121–140. doi: 10.3109/1040841X.2010.532479. [DOI] [PubMed] [Google Scholar]
- 26.Vendeville A, Winzer K, Heurlier K, Tang CM, Hardie KR. Making ‘sense’ of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat Rev Microbiol. 2005;3:383–396. doi: 10.1038/nrmicro1146. [DOI] [PubMed] [Google Scholar]
- 27.Lombardía E, Rovetto AJ, Arabolaza AL, Grau RR. A LuxS-dependent cell-to-cell language regulates social behavior and development in Bacillus subtilis. J Bacteriol. 2006;188:4442–4452. doi: 10.1128/JB.00165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henke JM, Bassler BL. Three parallel quorumsensing systems regulate gene expression in Vibrio harveyi. J Bacteriol. 2004;186:6902–6914. doi: 10.1128/JB.186.20.6902-6914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.