Abstract
The wound infection is one of the frequent complications in patients undergoing surgical operations. Staphylococcus aureus is the most common cause of surgical wounds. Artemisia absinthium has been shown to bear strong antimicrobial activity, especially against Gram-positive pathogens. This study was designed to investigate the antimicrobial effects of A. absinthium against surgical wounds infected by S. aureus in a rat model. Twenty male Sprague–Dawley rats were divided randomly into two equal groups of treated and control rats. A circular incision was created on the dorsal inter-scapular region of each rat. After skin wounding, rats were inoculated locally with 1 × 104 CFU of S. aureus at sites of skin wounds. The extract was applied topically twice a day throughout the experiment. Animals of the control group were left untreated. Results have revealed that topical application of A. absinthium extract on the infected wound sites produced significant antibacterial activity against S. aureus.
Keywords: Artemisia absinthium, Surgical wounds, Staphylococcus aureus
Introduction
The infection of wounds is one of the frequent complications in patients undergoing surgeries. Infections of organs, tissues or cavities, exposed by surgeons during invasive procedures, cause significant postoperative morbidity and mortality and prolong hospital stay [1]. Although the total elimination of wound infections seems impossible, minimizing the infection rate results significant benefits such as increased patient comfort and decreased medical costs [2]. Gram-positive bacteria are the predominant organisms on the skin and the Staphylococcus aureus is the most common cause of surgical wound and nosocomial infections [3]. It forms a part of the normal flora and can be isolated from the noses of up to 60 % of the healthy individuals. It is readily transmitted from person to person, onto the hands and clothes of healthcare staff, onto objects and into the air [4].
In recent decades, antimicrobial herbal products have been included in special interests of the researchers because of a rapid increase in antibiotic resistance in microorganisms [5]. Many members of the Genus Artemisia are important medicinal plants. Previously, the antibacterial effects of the Artemisia species have been reported [6–8]. Artemisia absinthium, a species of wormwood, grows in temperate regions of Eurasia and Northern Africa. Extracts of the plant have been shown to exhibit strong antimicrobial activity, especially against Gram-positive pathogenic bacteria [9]. This paper presents the first detailed investigation of in vivo antibacterial properties of A. absinthium against surgical wounds infected by S. aureus.
Materials and Methods
Animal Model
Twenty male Sprague–Dawley rats weighing 243 ± 17.1 g were housed in the Faculty of Veterinary Medicine, Islamic Azad University, Garmsar Branch. Before the experiment, the rats were left for seven days at room conditions for acclimatization. They were supplied with standard pellet diet with tap water ad libitum throughout the experiment. All animals received sufficient care according to “Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health.
Organisms and Preparation of Inoculum
In this study, S. aureus (ATCC 29213) was used as a standard strain for inoculation and determination of antibacterial activity of A. absinthium. The 1 × 104 inoculum was used as in previous studies by Stratford et al. [10] and Barker et al. [11] in animal wound infection models.
Minimum Inhibitory Concentration (MIC)
To assess the MIC of the extract using a broth macrodilution assay, a 30,000 ppm stock solution of A. absinthium extract was made in phosphate buffer saline (PBS). The MIC was assessed by the preparation of two-fold dilutions (up to 1875 ppm) of the extract in nutrient broth, and the adjusted inoculum of S. aureus (1 × 104 CFU/ml) was subsequently added to each tube. The tube contents were mixed and incubated at 35 °C for 18–20 h and then the turbidity of the tubes was checked by unaided eyes. The last dilution at which the growth of the organism was inhibited was reported as MIC of the extract [12].
Circular Excision Wound Model
For the evaluation of the antibacterial activity, a circular excision wound model was used. The rats were anaesthetized intraperitoneally with a combination of 10 % ketamine hydrochloride (50 mg/kg) and 2 % xylazine hydrochloride (5 mg/kg) and then the animals’ back hair were shaved. The sites of surgery were sterilized by povidone iodine followed by 70 % ethanol solution. A nearly 15-mm circular incision was made on the dorsal inter-scapular region of each animal. The skin carefully dissected out and the wounds were left open. After skin wounding, rats were inoculated locally with 1 × 104 CFU of S. aureus at sites of skin wounds (Fig. 1). The extract was applied topically twice a day throughout the experiment. Animals of the control group were not treated with any materials.
Fig. 1.
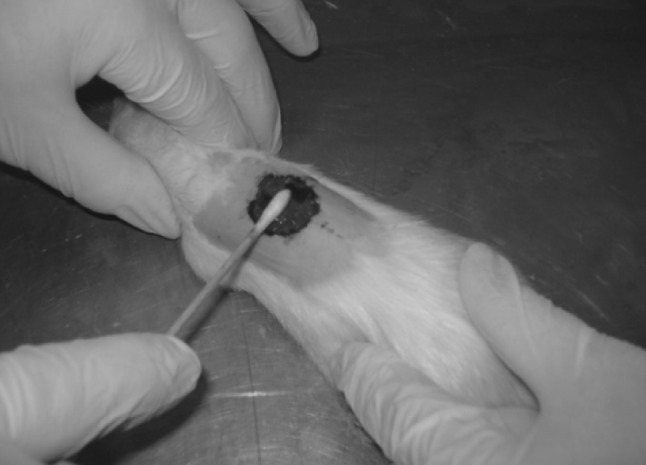
A circular incision was made on the dorsal inter-scapular region of each animal. Rats were inoculated locally with S. aureus at sites of skin wounds
Tissue Preparation and Culture
Preparation of tissues and antimicrobial screening was carried out as described by Stratford et al. [10]. Tissue specimens were weighed and homogenized in broth. The mixture of broth and tissue was subsequently used for two 1:10 serial dilutions in Mueller–Hinton broth and transferred to sterile test tubes at 4 °C. The samples (each 100 μl), including serial dilutions, were then transferred to tryptone soy agar plates and incubated at 35 °C for 24 h. Colony counts were considered accurate for numbers from 30 to 300 and were compared with the serial dilutions and duplicate plates for reproducibility. Colony counts were then translated to colony forming units per gram using the following formula.
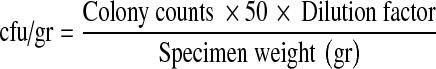 |
Statistical Analysis
Statistical analysis was performed using independent t test and analysis of variance. Data considered significant when P ≤ 0.05.
Results and Discussion
The mean ± SD of the bacterial count in the two groups are shown in Table 1. Following infection with S. aureus ATCC 29213 (8 days post infection), the bacterial number in untreated control animals was 7 × 106 ± 6 CFU/wound. These values were significantly different from those obtained from animals treated with extract of A. absinthium (3 × 105 ± 1 CFU/wound; P ≤ 0.05). Since past decades, the prevalence of surgical wound infections is high. S. aureus has been frequently mentioned as the most important bacterial species in the primary etiology of surgical wound infections. The results of the present study have revealed that topical application of A. absinthium extract at the infected wound site produced significant antibacterial activity against S. aureus. The antimicrobial activity of this extract may be due to the major components or synergy between major and minor compounds. Nezhadali and Parsa [13] have analyzed compounds of A. absinthium from Iran and identified 72 components, representing more than 97 % of the volatiles. The major constituents were camphor (14.83 %), p-cymene (10.35 %) and caryophyllene (6.92 %). These compounds have been previously reported to exhibit antibacterial against S. aureus. Many researchers have reported that camphor, as the major constituent of A. absinthium, has a strong antibacterial activity against Gram positive bacteria [14–16]. Karuppusamy et al. [17] and Mishra et al. [18] studied the antibacterial activity of some essential oils on Gram-positive bacteria and showed that the p-cymene (an essential oil) has significant antibacterial activity against S. aureus. Furthermore, various in vitro studies have suggested antibacterial activity of caryophyllene [19, 20]. Pinene-type monoterpenes (α-pinene and β-pinene), as minor components of this plant, are also well-known chemicals having antimicrobial potentials. Enantiomers of α-pinene and β-pinene have a strong antibacterial activity [21–24].
Table 1.
The mean ± SD of the bacterial count in the two groups
| Group | Experiment | Control |
|---|---|---|
| Bacterial counta | 3 × 105 ± 1 | 7 × 106 ± 6 |
aCFU/wound
These compounds (camphor, p-cymene, caryophyllene, α-pinene and β-pinene) are classified as monoterpene hydrocarbons. The site of action of terpenoids is at the cell membrane [25, 26]. They were found to affect structural and functional properties of artificial membranes. These compounds were shown to permeabilize the membranes making them swell and to increase membrane fluidity. These inhibited respiratory enzymes which lead to a partial dissipation of the pH gradient and electrical potential due to the increased permeability to H+ ions [27, 28]. The effect of terpenoids on microbial oxygen uptake and oxidative phosphorylation has also been studied. Most of terpenoids tested were found to inhibit both processes [29, 30].
Based on the literature review, it can be concluded that the hydroalcoholic extract of Iranian A. absinthium possesses antibacterial activity. In the current study, the reported antimicrobial activity of A. absinthium can be attributed to the presence of the major (camphor, p-cymene, caryophyllene) or minor (α-pinene, β-pinene) components of the plant or synergy between these compounds.
Acknowledgments
The authors are grateful to Dr. A. Koochakzadeh, Mr K. Khosravi, Mr M. Yazdani and A. Keshmiri for excellent technica assistance.
References
- 1.Paocharoen V, Mingmalairak C, Apisarnthanarak A. Comparison of surgical wound infection after preoperative skin preparation with 4 % chlorhexidine and povidone iodine: a prospective randomized trial. J Med Assoc Thail. 2009;92:898–902. [PubMed] [Google Scholar]
- 2.Nandi PL, Soundara Rajan S, Mak KC, Chan SC, So YP. Surgical wound infection. Hong Kong Med J. 1999;5:82–86. [PubMed] [Google Scholar]
- 3.Onche I, Adedeji O. Microbiology of post-operative wound infection in implant surgery. Niger J Surg Res. 2004;6:37–40. [Google Scholar]
- 4.Naik G, Deshpande S. A study on surgical site infections caused by Staphylococcus aureus with a special search for methicillin-resistant isolates. J Clin Diagn Res. 2011;5:502–508. [Google Scholar]
- 5.Essawi T, Srour M. Screening of some Palestinian medicinal plants for antibacterial activity. J Ethnopharmacol. 2000;70:343–349. doi: 10.1016/S0378-8741(99)00187-7. [DOI] [PubMed] [Google Scholar]
- 6.Guangrong H, Jiaxin J, Dehui D. Antioxidative and antibacterial activity of the methanol extract of Artemisia anomala S. Moore. Afr J Biotechnol. 2008;7:1335–1338. [Google Scholar]
- 7.Hedi M, Hajlaoui H, Akrout A, Najjaa H, Neffati M. Antimicrobial and antioxidant activities of Artemisia herba-alba essential oil cultivated in Tunisian arid zone. C R Chim. 2010;13:380–386. doi: 10.1016/j.crci.2009.09.008. [DOI] [Google Scholar]
- 8.Karabegovic I, Nikolova M, Velickovic D, Stojicevic S, Veljkovic V, Lazic M. Comparison of antioxidant and antimicrobial activities of methanolic extracts of the Artemisia sp. recovered by different extraction techniques. Chin J Chem Eng. 2011;19:504–519. doi: 10.1016/S1004-9541(11)60013-X. [DOI] [Google Scholar]
- 9.Fiamegos YC, Kastritis PL, Exarchou V, Han H, Bonvin AM, Vervoort J, Lewis K, Hamblin MR, Tegos GP. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against Gram-positive pathogenic bacteria. PLoS One. 2011;6:1–12. doi: 10.1371/journal.pone.0018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stratford AF, Zoutman DE, Davidson JSD. Effect of lidocaine and epinephrine on Staphylococcus aureus in a guinea pig model of surgical wound infection. Plast Reconstr Surg. 2002;110:1275–1279. doi: 10.1097/00006534-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Barker W, Rodeheaver GT, Edgerton MT, Edlich RF. Damage to tissue defenses by a topical anesthetic agent. Ann Emerg Med. 1982;11:307–310. doi: 10.1016/S0196-0644(82)80129-7. [DOI] [PubMed] [Google Scholar]
- 12.Coyle MB. Manual of antimicrobial susceptibility testing. Washington, DC: American Society for Microbiology; 2005. pp. 53–62. [Google Scholar]
- 13.Nezhadali A, Parsa M. Study of the volatile compounds in Artemisia absinthium from Iran using HS/SPME/GC/MS. Adv Appl Sci Res. 2010;1:174–179. [Google Scholar]
- 14.Jeong-Dan C. Chemical composition and antibacterial activity against oral bacteria by the essential oil of Artemisia iwayomogi. J Bacteriol Virol. 2007;37:129–136. doi: 10.4167/jbv.2007.37.3.129. [DOI] [Google Scholar]
- 15.Moghtader M, Afzali D. Study of the antibacterial properties of the essential oil of Rosemary. Am Eurasian J Agric Environ Sci. 2009;5:393–397. [Google Scholar]
- 16.Izadi Z, Esna-Ashari M, Piri K, Davoodi P. Chemical composition and antimicrobial activity of feverfew (Tanacetum parthenium) essential oil. Int J Agric Biol. 2010;12:759–763. [Google Scholar]
- 17.Karuppusamy S, Muthuraja G, Rajasekaran KM. Chemical composition and antimicrobial activity of essential oil from fruits of Vanasushava pedata (Apiaceae) Adv Biol Res. 2009;3:196–200. [Google Scholar]
- 18.Mishra D, Joshi S, Bisht G, Pilkhwal S. Chemical composition and antimicrobial activity of Solidago Canadensis Linn. Root essential oil. J B Clin Pharm. 2010;1:187–190. [PMC free article] [PubMed] [Google Scholar]
- 19.Essien E, Aboaba S, Ogunwande I. Constituents and antimicrobial properties of the leaf essential oil of Gossypium barbadense (Linn.) J Med Plants Res. 2011;5:702–705. [Google Scholar]
- 20.Hammami I, Triki MA, Rebai A. Chemical compositions, antibacterial and antioxidant activities of essential oil and various extracts of Geranium sanguineum L. flowers. Arch Appl Sci Res. 2011;3:135–144. [Google Scholar]
- 21.Magiatis P, Melliou E, Skaltsounis A, Chinou I, Mitaku S. Chemical composition and antimicrobial activity of the essential oils of Pistacia lentiscus var. chia. Plant Med. 1999;65:749–752. doi: 10.1055/s-2006-960856. [DOI] [PubMed] [Google Scholar]
- 22.Dorman H, Deans S. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 23.Filipowicz N, Kaminski M, Kurlenda J, Asztemborska M. Antibacterial and antifungal activity of juniper berry oil and its selected components. Phytother Res. 2003;17:227–231. doi: 10.1002/ptr.1110. [DOI] [PubMed] [Google Scholar]
- 24.Leite AM, Lima EO, Souza EL, Diniz MF, Trajano VN, Medeiros IA. Inhibitory effect of β-pinene, α-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Rev Bras Cienc Farm. 2007;43:121–126. doi: 10.1590/S1516-93322007000100015. [DOI] [Google Scholar]
- 25.Helander I, Alakomi H, Latva-kala K, Mattila-sandholm T, Pol I, Smid E, Gorris L, Wright A. Characterization of the action of selected essential oil components on Gram-negative bacteria. J Agric Food Chem. 1998;46:3590–3595. doi: 10.1021/jf980154m. [DOI] [Google Scholar]
- 26.Sikkema J, Debont J, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sikkema J, Debont J, Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem. 1994;269:8022–8028. [PubMed] [Google Scholar]
- 28.Sikkema J, Poolman B, Konings W, Debont J. Effects of the membrane action of tetralin on the functional and structural properties of artificial and bacterial membranes. J Bacteriol. 1992;174:2986–2992. doi: 10.1128/jb.174.9.2986-2992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weis N, Weigand H, Knobloch K. On the enfluence of terpene and phenylpropane derivatives on bacterial respiration and oxidative phosphorylation. Biol Chem. 1985;366:866. [Google Scholar]
- 30.Knobloch K, Weis N, Weigand H. Metabolism of antimicrobial activity of essential oils. Plant Med. 1986;52:556. doi: 10.1055/s-2007-969370. [DOI] [PubMed] [Google Scholar]


