Abstract
Piper longum L. (Piperaceae) commonly known as “long pepper” is a well known medicinal plant in ayurveda. Different parts of this plant, such as root, seed, fruit, whole plant etc. are used traditionally in various ailments. Here we have investigated the antidermatophytic activity of sequentially extracted petroleum ether, chloroform, methanol and water extracts from P. longum leaf against Trichophytonmentagrophytes, T. rubrum, T. tonsurans, Microsporum fulvum and M. gypseum. Better activity of chloroform and methanol extracts was observed. The chloroform extract was selected for further study and the MIC value was recorded as 5.0 mg ml−1 against the test organisms. In the chloroform extract, tannins and phenolic compounds were detected. Further activity-guided fractionation of chloroform extract by silica gel column chromatography yielded nine major fractions. Among these, fraction-1, 4, 5 and 7 showed higher antidermatophytic activity. Fraction-4 on further purification by repeated column chromatography yielded a potential antidermatophytic fraction showing MIC value of 0.625 mg ml−1 against T. mentagrophytes and T. rubrum as determined by broth microdilution method. The major compounds were identified as 1,2-benzenedicarboxylic acid, bis(2-ethylhexyl) ester (C24H38O4] (41.45 %), 2,2-dimethoxybutane (C6H14O2] (13.6 %) and β-myrcene (C10H16) (6.75 %) based on GC–MS data.
Keywords: Piper longum, Activity-guided fractionation, Antidermatophytic bioactive molecules
Prevalence of dermatophyte infections has been reported from various parts of the world [1, 2]. In Northeast India, dermatophytoses predominate among various the skin diseases [3–5]. Since the majority of the antifungal drugs used in treatment of such infections have several drawbacks, research on novel and effective antifungals from alternative sources is urgently needed. The beneficial role of herbs in therapeutic treatment is an important breakthrough in the history of mankind. Traditional folk medicines are the best examples for phytoremedy of fungal infections [6–8]. It is therefore pertinent to test plants used in traditional medicines for exploring the scientific rationale and to search for a potent source for antifungal drug. The northeastern part of India including Eastern Himalaya is very rich in medicinal plants and one of the two centres of species diversity of Indian Piper (Piperaceae) [9, 10]. Forty species of Piper were reported from this region. Piper species are of great interest owing to their biological properties and a number of isolated bioactive molecules [11–13]. The local people use 10 species of Piper as insecticidal, larvicidal, fungicidal, carminative, antidote etc. [9]. Piper longum L., a wildly abundant species in this region is a highly valuable drug and is essential ingredient in many ayurvedic formulations. Medicinal uses of P. longum essential oils, roots, fruits and whole plants were reported earlier [14]. In spite of its numerous medicinal properties there is no report on P. longum leaves related to antidermatophytic activity. The methanol extract from P. longum (leaf) showed significant antidermatophytic property. Hence the present study was undertaken to isolate and characterize the active antidermatophytic components present in P. longum leaves through bioassay-guided fractionation.
Materials and Methods
Dermatophyte Culture
The cultures of dermatophyte species namely Trichophyton mentagrophytes (MTCC 8476), T. rubrum (MTCC 8477), T. tonsurans (MTCC 8475), Microsporum fulvum (MTCC 8478) and M. gypseum (MTCC 8469) were maintained on sabouraud dextrose agar (SDA, Himedia) and sabouraud dextrose broth (SDB, Himedia).
Plant Material
Fresh leaves of P. longum were collected from the foothills of the Himalayan range in Assam-Arunachal Pradesh border. The plant was identified by Dr. P.R. Gajurel, North Eastern Research Institute of Science and Technology, Itanagar, Arunachal Pradesh, India and further authenticated at Botanical Survey of India, Kolkata, India. The voucher specimen was preserved at DRL, Tezpur, India.
Extraction
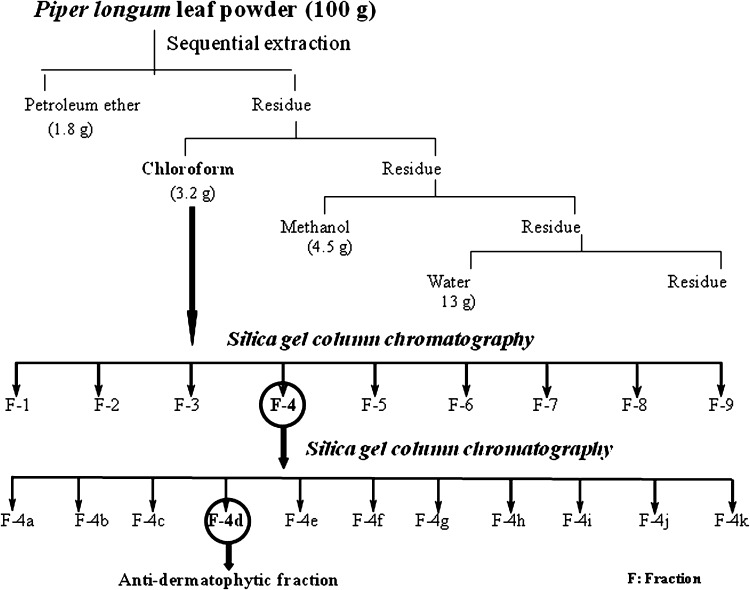
Shade dried powdered leaves (100 g) were extracted sequentially with petroleum ether, chloroform, methanol and water (Fig. 1) [15]. The extracts were filtered using Whatman filter paper (No-1). Solvents were evaporated under reduced pressure at 40 °C using rotary evaporator, lyophilized and kept in glass vials at −20 °C till further use.
Fig. 1.
Schematic representation of extraction and isolation of antidermatophytic constituents
Fractionation of Chloroform Extract
Preactivated silica gel (230–400 mesh, 125 g) was loaded as slurry prepared in petroleum ether into a glass column (400 × 40 mm). The chloroform extract (1.25 g) was dissolved in little amount of chloroform and adsorbed on preactivated silica gel (12.5 g] and loaded into the column and successively eluted stepwise by gradient of petroleum ether, ethyl acetate and methanol (40 %) (10 % increase/step) and 45 fractions were collected. Fractions with similar TLC profile (Silica gel F254 aluminium backed sheet, Merck) were pooled into nine major fractions (F-1–F-9) and evaluated against T. mentagrophytes by agar well diffusion method.
Purification of Fraction
The active fraction (F-4), among the nine fractions was further purified using silica gel column chromatography (silica gel: 230–400 mesh, fraction: 33 g, column size: 150 × 20 mm) using petroleum ether, dichloromethane, ethyl acetate, methanol with stepwise gradient and 72 sub fractions were obtained. The fractions were pooled into 11 major sub fractions (F-4a–F-4k) on the basis of their TLC profiles. The active fraction (F-4d) was analysed by GC–MS for identification of active compounds. The steps involved in isolation of antidermatophytic components are shown in Fig. 1.
In Vitro Evaluation for Antidermatophytic Activity
Test solutions of extracts/fractions were prepared in dimethyl sulphoxide (DMSO, w/v), filter sterilized (Millipore filter, 0.22 μm) and tested for antidermatophytic activity by agar well diffusion [16] and agar dilution [17] methods. In agar well diffusion method, SDA plate (80 mm dia.) was swabbed with 150 μl of the inoculum (2.5 × 104 c.f.u. ml−1). A well of 8 mm diameter was made in each plate, loaded with 150 μl of the test sample (5 mg ml−1). The plates were incubated at 28 ± 2 °C for 15–20 days. The activity was determined by measuring the diameter of the zone of inhibition. In agar dilution method test extract was incorporated in sterilized molten SDA medium at the concentration of 5, 2, 1, 0.5 mg ml−1) and inoculated with the dermatophytes. The plates were incubated at 28 ± 2 °C for 15–20 days and observed the mycelial growth. The percentage of mycelial inhibition (I %) was calculated as I % = [(dc − dt)/dc] × 100 (dc = Colony diameter in control, dt = Colony diameter in treatment) [17]. The lowest concentration of the extract showing no visible mycelial growth was considered as MIC.
The MIC of the isolated fraction obtained from second step column chromatography was determined by broth microdilution assay with some modifications [18]. Twofold serial dilutions (0.0039–5 mg ml−1) of the fraction were prepared in DMSO in 96-well microtiter plate with RPMI 1640 (Rosewell Park Memorial Institute, Himedia). Inoculum concentration of approximately 2.5 × 104 c.f.u. ml−1 was adjusted in each well. The plates were incubated at 28 ± 2 °C for 15–20 days. DMSO was used as negative control while clotrimazole and griseofulvin were used as positive control. Each experiment was performed in triplicate and repeated twice. The MIC was interpreted as the lowest concentration of the test samples showing no visible growth.
Determination of Longevity of the Chloroform Extract
The longevity of the chloroform extract was observed by storing it at 4 °C and room temperature (25–34 °C) for 180 days and evaluating the activity of the extract (5 mg ml−1) against T. mentagrophytes by determining the radial growth at 1 month interval.
Phytochemical Analysis
Chloroform extract of P. longum leaves was analyzed for the presence of alkaloids, phenolic compounds, tannins and saponins. Test solution of 50 mg/ml was prepared in acetone. For alkaloids, 1 ml test solution was treated with a few drops of Dragendorfs reagent and Mayer’s reagent separately [19]. For the presence of phenolic compounds, 1 ml of the test solution was treated with 1 % ethanolic ferric chloride [20]. The test for tannins was carried out with 0.1 % FeCl3 [21]. Test solution (1 ml) was mixed with 5 ml water and shaken and observed for stable froth, further mixed with olive oil (3 drops) and shaken to observe formation of emulsion [19].
Gas Chromatography Mass Spectroscopy (GC–MS) Analysis of the Isolated Fraction
The GC–MS analysis was performed in EI mode on a GCMS, Perkin Elmer, Turbomass gold, GC-Autosample xL (Perkin Elmer International, Boesch, Huenenberg, Switzerland) system with fused capillary column Elite-1, dimethylpolysiloxane, 30 m × 0.25 mm × 0.25 μm directly coupled to mass detector. The mass spectrometer was operated at 70 eV. Injection conditions were as follows: Column temperature 40–250 °C at a rate of 4 °C/1 min; carrier gas was He: 1 ml/min; sample injection volume 1 μl. The bioactive constituents were identified based on the comparison of mass spectra with those of data available in the National Institute of Standards and Technology libraries (NIST/EPA/NIH mass spectral library).
Results and Discussion
The extractive value of each solvent extract from P. longum (leaf) was recorded (Fig. 1). It seems that the polarity of the solvent played an important role in extracting the plant components as well as their activity. In preliminary agar dilution assay, the chloroform and methanol extracts were found to be more active than its petroleum ether and water extracts. The differential activity might be due to either difference in plant constituents in each extract or tolerance of the pathogens to different plant constituents. Similar observations were reported earlier [15–17]. The MIC of the chloroform extracts was recorded as 5.0 mg ml−1 for all the test dermatophytes, however this extract showed some activity at 0.5 mg ml−1 causing 38.4, 37.3 and 44.7 % inhibition of T. mentagrophytes, T. rubrum and T. tonsurans respectively. In case of M. fulvum and M. gypseum the inhibition was 38.3 %.
Chloroform extract retained its activity up to 150 and 90 days respectively at 4 °C and room temperature (25–34 °C). The extract was soluble in coconut oil, DMSO, methanol, ethyl acetate and hexane, while insoluble in water. These findings would be useful in various biological experiments. Phytochemical analysis of the chloroform extracts of P. longum leaf revealed the presence of tannins and phenolic compounds and absence of alkaloids and saponins.
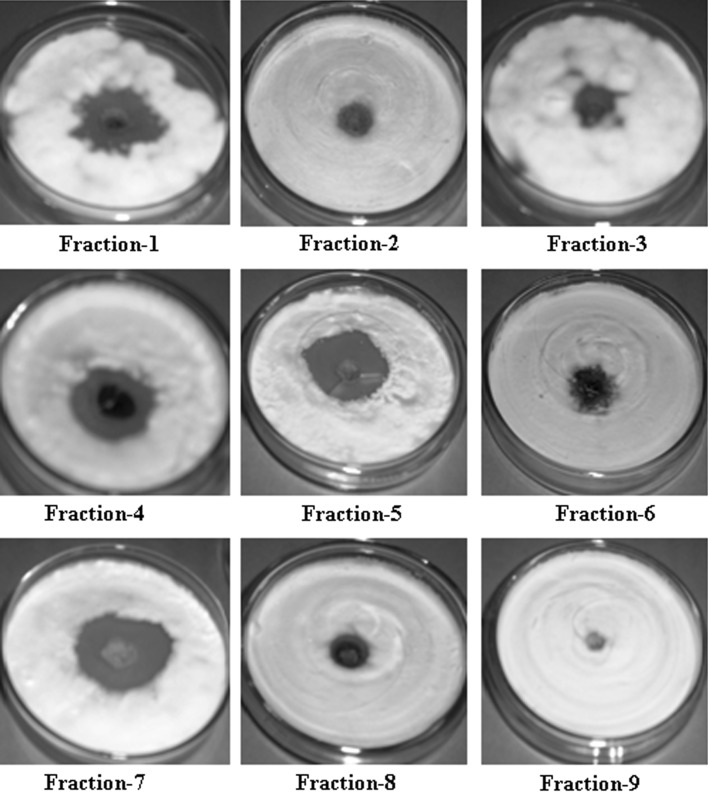
Fractionation leading to isolation of active compounds should result higher activity than the original extract. This approach forms the basis of discovery of many important natural bioactive compounds [22]. Since plant materials are highly complex profiles of phytochemicals, isocratic separation cannot achieve satisfactory separation, therefore multiple mobile phases in increasing polarity were used to separate target components from the chloroform extract. Among the fractions, separated from the chloroform extract, fraction 1, 4, 5 and 7 were found to be highly promising (inhibition zone: 25–32 mm at 5 mg ml−1) against T. mentagrophytes (Table 1; Fig. 2).
Table 1.
Zone of inhibition caused by column fractions separated from chloroform extract of P. longum leaf
| T. mentagrophytes | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Combined column fraction (Conc: 5 mg ml−1) | F-1 (1) | F-2 (2–7) | F-3 (8–12) | F-4 (13) | F-5 (14) | F-6 (15–16) | F-7 (17–32) | F-8 (33–41) | F-9 (42–45) |
| Zone of inhibition (mm) | 25 | 11 | 11 | 27 | 28 | 10 | 32 | 11 | 0 |
F Combined column fraction
Fig. 2.
Zone of inhibition caused by column fractions (5 mg ml−1) separated from P. longum leaf against T. mentagrophytes
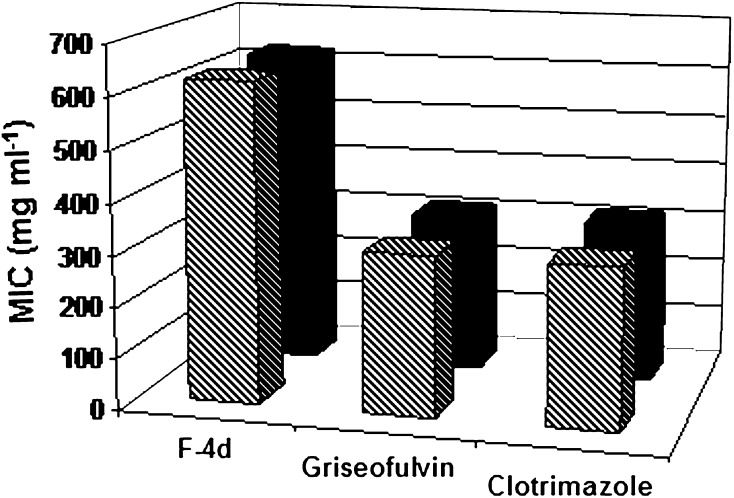
Fraction-4 obtained from first step column chromatography on further fractionation resulted into successful isolation of antidermatophytic components. Among 11 major fractions, the forth fraction (F-4d) eluted with 70 % petroleum ether in dichloromethane was obtained as a deep orange coloured material (3 mg) and found to be potentially active (MIC value: 0.625 mg ml−1) against T. mentagrophytes and T. rubrum. Griseofulvin and clotrimazole showed MIC value of 0.313 mg ml−1 against the two dermatophytes tested (Fig. 3). The better antidermatophytic profile as observed in purified fractions might be due to the presence of many undesirable compounds in the crude extract that probably got removed during fractionation.
Fig. 3.
MIC of the isolated fraction (F-4d] and standard antifungal
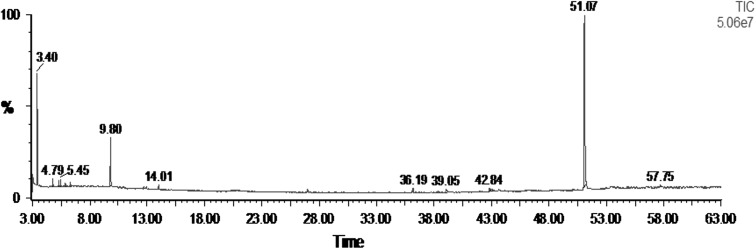
The sub fraction-4 was soluble in hexane, dichloromethane and partially soluble in methanol and water. Analysis of GC–MS spectrum of the fraction (F-4d) indicated the presence of three major compounds such as 2-benzenedicarboxylic acid, bis (2-ethylhexyl) ester, 2,2-dimethoxybutane and β-myrcene apart from ten other compounds in traces (Table 2; Fig. 4). It is difficult to attribute the activity of a complex mixture to a particular component. Nevertheless, it is reasonable to speculate that the activity of sub fraction-4(d) might be due to major compounds, since in most cases, the inhibitory activity of extracts or fractions has been attributed to the most dominant components [23]. In our study we observed the antidermatophytic effect of these compounds in combined form. However, purification of the major compounds is being carried out to ascertain the activity of the compounds in pure form. The major compound, 1,2-benzenedicarboxylic acid, bis(2-ethylhexyl) ester was previously isolated from rice (Var-Taichung Native-1) [24]. The presence of β-myrcene was also recorded in the essential oil from Artemisia herba and lemon grass [25, 26] and leaves and flower of Bidens pilosa [27]. A number of bioactive compounds including fungicidal compound, pipernonaline have been isolated from P. longum [11, 13, 28, 29]. However, most of the compounds were isolated from the fruits and other parts of P. longum. There are no reports on isolation of antidermatophytic compound from P. longum leaves. It may be presumed that phytochemicals identified in P. longum in the present study may play major roles in the antidermatophytic activities.
Table 2.
Compounds identified in fraction (F-4d] of P. longum leaf extract by GC-MS analysis
| S. no. | RT | Mass | Compound name | % Area |
|---|---|---|---|---|
| 1 | 3.40 | 118 | 2,2-Dimethoxybutane (C6H14O2] | 13.4 |
| 2 | 4.79 | 244 | 2-Hydroxy myristic acid (C14H28O3] | 1.08 |
| 3 | 5.28 | 161 | 4-Nitro-3-oxo-butyric acid methyl ester (C5H7NO5] | 0.87 |
| 4 | 5.45 | 159 | 2-Hydroxy-N,2,3,3-tetramethyl butanamide (C7H16NO2] | 0.95 |
| 5 | 6.34 | 161 | 2-Methyl-N-phenyl-2-propenamide (C10H11NO] | 0.55 |
| 6 | 9.80 | 136 | β-Myrcene (C10H16] | 6.75 |
| 7 | 14.01 | 268 | 6-Methyl octadecane (C19H40] | 0.58 |
| 8 | 36.19 | 283 | N-methyl-1- octadecanamine (C19H41N] | 0.66 |
| 9 | 39.05 | 232 | Piperazine adipate (C10H20N2O4] | 1.07 |
| 10 | 42.84 | 154 | 2-Nonynoic acid (C9H14O2] | 1 |
| 11 | 43.02 | 184 | Dodecanal (CH3(CH2]10CHO] | 0.89 |
| 12 | 51.07 | 390 | 1,2-Benzenedicarboxylic acid, bis(2-ethylhexyl] ester (C24H38O4] | 41.45 |
| 13 | 57.75 | 207 | 2-Amino-4-hydroxypteridine-6-carboxylic acid (C7H5N5O3] | 0.89 |
RT retention time
Fig. 4.
GC–MS spectrum of fraction (F-4d] of P. longum leaf extract
Conclusion
The potent antidermatophytic fraction consists of three major compounds, might be responsible for the activity. Hence, further investigation on these compounds for in vivo efficacy and safety to determine the viable prospects of the compounds as antidermatophytic agents is highlighted. Further studies on isolation of the compounds from the active fractions reported here in under progress.
Acknowledgments
The authors convey their thanks to Sri Sivaswamy CIFS, Central Food Technology and Research Institute, Mysore for his help in GC–MS analysis of sample.
References
- 1.Prasad PVS, Priya K, Kaviarasan PK, et al. A study of chronic dermatophyte infection in a rural hospital. Indian J Dermatol Venereol Leprol. 2005;71:129–130. doi: 10.4103/0378-6323.14003. [DOI] [PubMed] [Google Scholar]
- 2.Kannan P, Janaki C, Selvi GS. Prevalence of dermatophytes and other fungal agents isolated from clinical samples. Indian J Med Microbiol. 2006;24:212–215. [PubMed] [Google Scholar]
- 3.Jaiswal AK. Ecologic perspective of dermatologic problems in North Eastern India. Indian J Dermatol Venereol Leprol. 2002;68:206–207. [PubMed] [Google Scholar]
- 4.Devi T, Zamzachin G. Pattern of skin diseases in Imphal. Indian J Dermatol. 2006;51:149–150. doi: 10.4103/0019-5154.26943. [DOI] [Google Scholar]
- 5.Sharma S, Borthakur AK. A clinico epidemiological study of dermatophytoses in Northeast India. Indian J Venereol Dermatol Leprol. 2007;73:427–428. doi: 10.4103/0378-6323.37068. [DOI] [PubMed] [Google Scholar]
- 6.Seneviratne CJ, Wong RWK, Samaranayake LP. Potent anti-microbial activity of traditional Chinese medicine herbs against Candida species. Mycoses. 2007;51:30–34. doi: 10.1111/j.1439-0507.2007.01431.x. [DOI] [PubMed] [Google Scholar]
- 7.Li A, Zhu Y, He X, Tian X, Xu L, Ni W, et al. Evaluation of antimicrobial activity of certain Chinese plants used in folkloric medicine. World J Microbiol Biotechnol. 2008;24:569–572. doi: 10.1007/s11274-007-9494-4. [DOI] [Google Scholar]
- 8.Webster D, Taschereau P, Belland RJ, et al. Antifungal activity of medicinal plant extracts: preliminary screening studies. J Ethnopharmacol. 2008;115:140–146. doi: 10.1016/j.jep.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Rahiman BA, Nair MK (1994) The genus Piper Linn. in Karnataka. Indian J Bombay Nat Hist Soc 91:66
- 10.Gajurel PR, Rethy P, Singh B, Kumar Y (2001) Importance of systemic investigation for proper utilization and conservation of Piper species of north-east India. Proceeding NEI National Seminar, pp 305–313
- 11.Reddy PS, Jamil K, Madhusudhan P, Anjani G, Das B. Antibacterial activity of isolates from Piper longum and Taxus baccata. Pharm Biol. 2001;39:236–238. doi: 10.1076/phbi.39.3.236.5926. [DOI] [Google Scholar]
- 12.Yang YC, Lee SG, Lee HK, et al. A Piperidine amide extracted from Piper longum L. fruit shows activity against Aedes aegypti mosquito larvae. J Agric Food Chem. 2002;50:3765–3767. doi: 10.1021/jf011708f. [DOI] [PubMed] [Google Scholar]
- 13.Prasad AK, Kumar V, Arya P, et al. Investigations toward new lead compounds from medicinally important plants. Pure Appl Chem. 2005;77:25–40. doi: 10.1351/pac200577010025. [DOI] [Google Scholar]
- 14.Zaveri M, Khandhar A, Patel S, Patel A. Chemistry and pharmacology of Piper longum L. Int J Pharm Sci Rev Res. 2010;5:67–76. [Google Scholar]
- 15.Das J, Lahan JP, Srivastava RB. Solanummelongena: a potential source of antifungal agent. Indian J Microbiol. 2010;5(Suppl 1):62–69. doi: 10.1007/s12088-010-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaushik P, Goyal P. In vitro evaluation of Datura innoxia (thorne apple) for potential antibacterial activity. Indian J Microbiol. 2008;48:353–357. doi: 10.1007/s12088-008-0020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali-Shtayeh MS, Ghdeib SIA. Antifungal activity of plant extracts against dermatophytes. Mycoses. 1999;42:665–672. doi: 10.1046/j.1439-0507.1999.00499.x. [DOI] [PubMed] [Google Scholar]
- 18.Hammer KA, Carson CF, Riley TV. In vitro activity of Melaleuca alternifolia (tea tree) oil against dermatophytes and other filamentous fungi. J Antimicrob Chemother. 2002;50:195–199. doi: 10.1093/jac/dkf112. [DOI] [PubMed] [Google Scholar]
- 19.Falodun A, Ali S, Quadir IM, Choudhary IMI. Phytochemical and biological investigation of chloroform and ethylacetate fractions of Euphorbia heterophylla leaf (Euphorbiaceae) J Med Plant Res. 2008;2:365–369. [Google Scholar]
- 20.Harborne JB. Phytochemical methods: a guide to modern techniques of plant analysis. 3. London: Chapman & Hall; 1998. [Google Scholar]
- 21.Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4:685–688. [Google Scholar]
- 22.McRae J, Yang Q, Crawford R, Palombo E. Review of the methods used for isolating pharmaceutical lead compounds from traditional medicinal plants. Environmentalist. 2007;27:165–174. doi: 10.1007/s10669-007-9024-9. [DOI] [Google Scholar]
- 23.Farag RS, Daw ZY, Abo-raya SH. Influence of some spice essential oils on Aspergillus parasiticus growth and production of aflatoxins in a synthetic medium. J Food Sci. 1989;54:74–76. doi: 10.1111/j.1365-2621.1989.tb08571.x. [DOI] [Google Scholar]
- 24.Rimando AM, Olofsdotter M, Dayan FE, Duke SO. Searching for rice allelochemicals: an example of bioassay-guided isolation. Agron J. 2001;93:16–20. doi: 10.2134/agronj2001.93116x. [DOI] [Google Scholar]
- 25.Silva VA, Freitas JC, Mattos AP, et al. Neurobehavioral study of the effect of beta-myrcene on rodents. Braz J Med Biol Res. 1991;24:827–831. [PubMed] [Google Scholar]
- 26.Nezhadali A, Akbarpour M, Shirvan BZ. Chemical composition of the essential oil from the aerial parts of Artemisiaherba. E-J Chem. 2008;5:557–561. doi: 10.1155/2008/730453. [DOI] [Google Scholar]
- 27.Deba F, Xuan TD, Yasuda M, Tawata S. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control. 2008;19:346–352. doi: 10.1016/j.foodcont.2007.04.011. [DOI] [Google Scholar]
- 28.Parmar VS, Jain SC, Gupta S, et al. Polyphenols and alkaloids from Piper species. Phytochemistry. 1998;49:1069–1078. doi: 10.1016/S0031-9422(98)00208-8. [DOI] [Google Scholar]
- 29.Lee SE, Park BS, Kim MK, et al. Fungicidal activity of pipernonaline, a piperidine alkaloid derived from long pepper, Piper longum L., against phytopathogenic fungi. Crop Prot. 2001;20:523–528. doi: 10.1016/S0261-2194(00)00172-1. [DOI] [Google Scholar]